Abstract
We have shown that increased luminal flow induces O2− and nitric oxide (NO) production in thick ascending limbs (TALs). However, the interaction of flow-stimulated NO and O2− in TALs is unclear. We hypothesized that NO inhibits flow-induced O2− production in TALs via cGMP-dependent protein kinase (PKG). We measured flow-stimulated O2− production in rat TALs using dihydroethidium in the absence and presence of l-arginine (0.3 mM), the substrate for NO synthase. The addition of l-arginine reduced flow-induced net O2− production from 68 ± 9 to 17 ± 4 AU/s (P < 0.002). The addition of the NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 5 mM) in the presence of l-arginine stimulated production (l-arginine: 15 ± 4 AU/s vs. l-arginine + l-NAME: 63 ± 7 AU/s; P < 0.002). The guanylate cyclase inhibitor LY-83583 (10 μM) also enhanced flow-induced net O2− production in the presence of l-arginine (l-arginine: 7 ± 4 AU/s vs. l-arginine + LY-83583: 53 ± 7 AU/s; P < 0.01). In the presence of LY-83583, l-arginine only reduced flow-induced net O2− by 36% (LY-83583: 80 ± 7 AU/s vs. LY-83583 + l-arginine: 51 ± 3 AU/s; P < 0.006). The cGMP analog dibutyryl (db)-cGMP reduced flow-induced net O2− from 39 ± 9 to 7 ± 3 AU/s (P < 0.03). The PKG inhibitor KT-5823 (5 μM) partially restored flow-induced net O2− in the presence of l-arginine (l-arginine: 4 ± 4 AU/s vs. l-arginine + KT-5823: 32 ± 9 AU/s; P < 0.03) and db-cGMP (db-cGMP: 9 ± 7 AU/s vs. db-cGMP + KT-5823: 54 ± 5 AU/s; P < 0.01). Phosphodiesterase II inhibition had no effect on arginine-inhibited O2− production. We conclude that 1) NO reduces flow-stimulated O2− production, 2) this occurs primarily via the cGMP/PKG pathway, and 3) O2− scavenging by NO plays a minor role.
Keywords: reactive oxygen species, soluble guanylate cyclase, phosphodiesterase, scavenging
nitric oxide (NO) and superoxide (O2−) regulate kidney function, and consequently blood pressure. NO promotes natriuresis and diuresis, and therefore reductions in blood pressure. O2− promotes salt and water retention, and thus favors increases in blood pressure. Changes in natriuresis and urinary volume caused by these two reactive oxygen species can be due to alterations in renal hemodynamics or ion transport along the nephron. In the thick ascending limb, NO inhibits NaCl reabsorption (27, 30, 33, 34). In contrast, O2− stimulates it (16, 17, 26, 41). Perturbations in production or degradation of NO or O2− can lead to hypertension (1, 4, 36) and/or renal injury (2).
NO is synthesized from l-arginine in the thick ascending limb by NO synthase 3 (NOS3). Thus, in the absence of exogenously added l-arginine, thick ascending limbs do not generate NO (25). Several factors stimulate NO production in this nephron segment, including angiotensin (5, 35), α-2 adrenergic agonists (31, 45), endothelin (13, 32), and luminal flow (28, 29). Once generated, NO activates soluble guanylate cyclase and forms cGMP (21). cGMP, in turn, can activate two signaling cascades which are initiated by distinct enzymes: 1) cGMP-dependent protein kinase (PKG) and 2) cGMP-stimulated phosphodiesterase. Most of the physiological effects of NO are mediated by cGMP (9, 23, 24, 42, 43), but some may be mediated by other processes such as nitrosylation (39).
Under basal conditions, thick ascending limbs generate O2−. Several enzymes may be involved in this process including NADPH oxidase (14, 19, 20, 46). Similar to NO, NADPH oxidase may be activated by luminal flow (11, 14). Luminal flow stimulates O2− production in this nephron segment by causing cellular stretch (11) and increasing NaCl transport (14). NO can reduce O2− levels by a variety of mechanisms. NO has been shown to inhibit NADPH oxidase in human vascular smooth muscle cells (22). It may also scavenge O2− through a chemical reaction forming peroxynitrite (ONOO−) (15). Finally, NO could reduce O2− levels by enhancing degradation, but this has not been described to date. Previously, we showed that O2− reduces NO in the thick ascending limb (25), but whether flow-induced NO reduces flow-stimulated O2− in this segment and the mechanisms involved are unknown. We hypothesized that flow-induced NO diminishes flow-stimulated O2− production via an increase in cGMP and activation of PKG.
MATERIALS AND METHODS
Chemicals and solutions.
Dihydroethidium was purchased from Molecular Probes/Invitrogen (Eugene, OR). LY-83583, a soluble guanylate cyclase inhibitor, and KT-5823, a PKG inhibitor, were obtained from Biomol International (Plymouth Meeting, PA). BAY 60-7550, a phosphodiesterase II inhibitor, was purchased from Axxora (San Diego, CA). l-Arginine, NG-nitro-l-arginine methyl ester (l-NAME), and N2,2′-O-dibutyrylguanosine 3′,5′-cyclic monophosphate [dibutyryl (db)-cGMP] were from Sigma (St. Louis, MO). The composition of physiological saline used to perfuse and bathe thick ascending limbs was (in mM) 130 NaCl, 2.5 NaH2PO4, 4 KCl, 1.2 MgSO4, 6 l-alanine, 1 trisodium citrate, 5.5 glucose, 2 calcium dilactate, and 10 HEPES, pH 7.4 at 37°C. Osmolality was adjusted to 290 ± 3 mosmol/kgH2O. Inhibitors and l-arginine (0.3 mM) were added to the bath.
Isolation and perfusion of rat thick ascending limb.
All protocols involving animals were approved by the Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (Charles River Breeding Laboratories, Wilmington, MA) maintained on a diet containing 0.22% sodium and 1.1% potassium (Purina, Richmond, IN) for at least 5 days and weighing 120–150 g were used. Medullary thick ascending limbs were dissected from the outer medulla of coronal slices of the left kidney at 4–10°C. Tubules were perfused at 20 nl/min as described previously (10).
Measurement of O2− using dihydroethidium.
Thick ascending limbs were loaded by bathing tubules in 10 μM dihydroethidium for 15 min and then washing them in dye-free solution for 20 min. Oxyethidium and dihydroethidium were excited using 490 and 365 nm light, respectively. Emitted fluorescence was measured between 520 and 600 nm (oxyethidium) and 400 and 450 nm (dihydroethidium) from regions of interest (ROI). Fluorescence was recorded using Metafluor version 7 (Universal Imaging, Downingtown, PA). O2− was measured at the beginning of each period once every 5 s for 1 min. Regression analysis was performed and differences in slopes were statistically evaluated. The format for all protocols was that O2− was measured in a tubule in the absence of luminal flow and then again after luminal flow was initiated (control period). Flow was then stopped. In the same tubule, l-arginine and/or an inhibitor was added. After 15 min, O2− measurements were recorded again in the absence and presence of flow (experimental period).
The reaction between dihydroethidium and O2− is driven by O2− concentration and is irreversible. However, O2− concentration is determined by both production and degradation. Consequently, the rate of change of the oxyethidium/dihydroethidium fluorescence ratio is a measure of “net O2− production” (production − degradation), although it is commonly referred to as O2− production (7, 44). Given that net O2− production represents production minus degradation, it may decrease when degradation is enhanced or production is reduced without indicating whether the former, latter, or both has/have occurred. Consequently, O2− measurements in this study will be referred to as net O2− production.
Statistical analysis.
Results are expressed as means ± SE. Data were evaluated with ANOVA for repeated measures with two factors, flow and treatment. The difference in net O2− production for no flow vs. flow during the control period was compared with that during the experimental period, and P < 0.05 was considered significant.
RESULTS
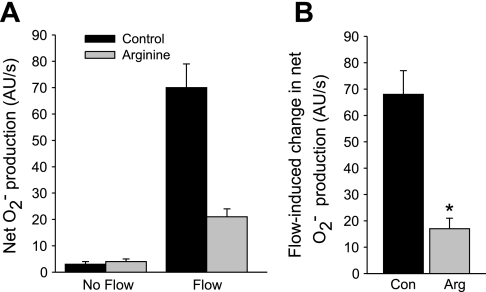
To study whether NO affects the flow-induced increase in net O2− production, we subjected isolated thick ascending limbs to enhanced luminal flow in the absence and presence of the substrate for NOS, l-arginine. Figure 1A shows that in tubules with no luminal flow, net O2− production was 3 ± 1 AU/s. After flow was initiated, it increased to 70 ± 9 AU/s. Thus, flow increased net O2− production by 68 ± 9 AU/s (control). In the presence of l-arginine (0.3 mM), net O2− production was 4 ± 1 AU/s in the absence of flow, and it rose to 21 ± 3 AU/s when flow was increased (P < 0.002; n = 5 vs. control). Thus, arginine decreased flow-induced net O2− production by 75% to 17 ± 4 AU/s (Fig. 1B).
Fig. 1.
A: effect of l-arginine (Arg) on net O2− production by thick ascending limbs in the presence and absence of luminal flow. B: effect of Arg on flow-induced change in net O2− production. *P < 0.002; n = 5 vs. control (Con). Tubules were perfused at 20 nl/min.
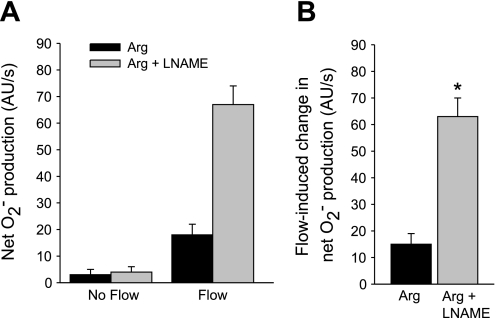
To examine whether the effect of l-arginine on flow-induced net O2− production is due to NO produced by NOS, we used the NOS inhibitor l-NAME. Figure 2 shows that in the presence of l-arginine, flow stimulated net O2− production from 3 ± 2 to 18 ± 4 AU/s, a flow-induced increase of 15 ± 4 AU/s. In the presence of l-arginine plus 5 mM l-NAME, flow enhanced net O2− production from 4 ± 2 to 67 ± 7 AU/s, a flow-induced increase of 63 ± 7 AU/s (P < 0.002; n = 5 vs. l-arginine alone). This value was similar to the effect of flow in the absence of l-arginine. In separate experiments, l-NAME alone had no effect on flow-induced net O2− production vs. nontreated tubules (data not shown). Therefore, the effect of l-arginine on net O2− production is due to endogenously produced NO.
Fig. 2.
A: effect of the nitric oxide (NO) synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME) on Arg-induced inhibition of net O2− production by thick ascending limbs in the absence and presence of luminal flow. B: effect of l-NAME on Arg's effect on flow-stimulated net O2− production. *P < 0.002; n = 5 vs. Arg alone. Tubules were perfused at 20 nl/min.
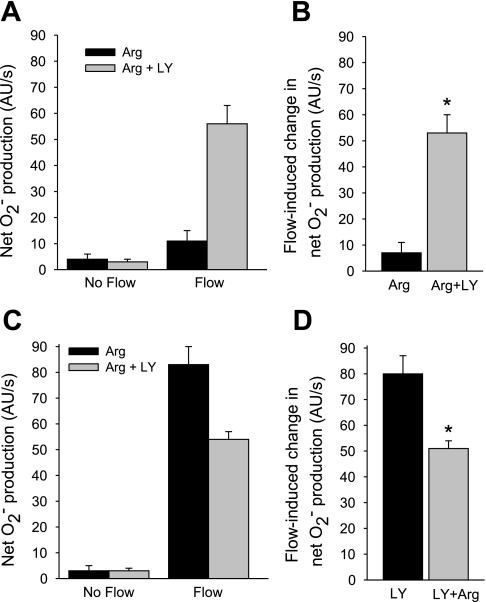
The reduction in O2− caused by NO could be due to either scavenging (15) or inhibition of O2− production by NO via its second messenger cGMP (8, 22). To study which is responsible, we used the soluble guanylate cyclase inhibitor LY-83583. First, we tested the effect of LY-83583 on the change in net O2− production induced by flow in the presence of l-arginine (Fig. 3, A and B). In the presence of l-arginine, flow increased net O2− production from 4 ± 2 to 11 ± 4 AU/s. In the presence of 10 μM LY-83583 plus l-arginine, flow increased net O2− production from 3 ± 1 to 56 ± 7 AU/s, a flow-induced increase of 53 ± 7 AU/s (P < 0.01; n = 5 vs. l-arginine alone).
Fig. 3.
A: effect of the soluble guanylate cyclase inhibitor LY-83583 (LY) on the effect of Arg on net O2− production by thick ascending limbs in the absence and presence of luminal flow. B: effect of LY on Arg's effect on flow-stimulated net O2− production. *P < 0.01; n = 5 vs. Arg alone. C: effect of Arg in the presence of LY on net O2− production by thick ascending limbs in the absence and presence of luminal flow. D: effect of Arg on the flow-induced change in net O2− production in the presence of LY. *P < 0.006; n = 5 vs. LY alone. Tubules were perfused at 20 nl/min.
To rule out the possibility that LY-835823 itself increases O2−, we next tested the effect of l-arginine in the presence of LY-83583 (Fig. 3, C and D). In the presence of LY-83583 (10 μM), flow stimulated net O2− production from 3 ± 2 to 83 ± 7 AU/s, an increase of 80 ± 7 AU/s. When l-arginine was added in the presence of LY-83583, flow stimulated net O2− production from 3 ± 1 to 54 ± 3 AU/s (P < 0.006, n = 5 vs. LY-83583 alone), an increase of 51 ± 3 AU/s. Thus, l-arginine reduced flow-induced net O2− production by 36% in the presence of LY-83583, significantly less than the 75% inhibition caused by l-arginine in the absence of LY-83583 (Fig. 1B).
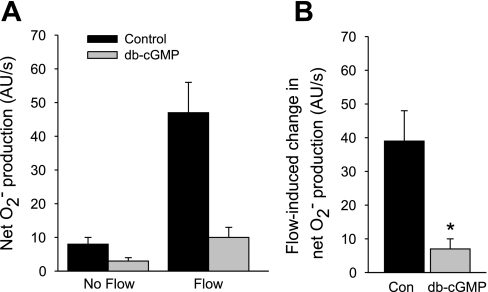
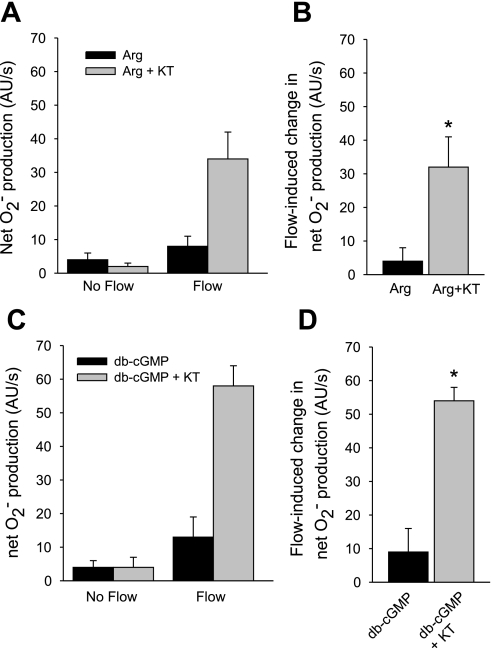
If NO-stimulated cGMP decreases net O2− production, then db-cGMP, a cGMP analog, should mimic this effect. Figure 4 shows that during the control period, flow stimulated net O2− production from 8 ± 2 to 47 ± 9 AU/s, an increase of 39 ± 9 AU/s. In the presence of 0.1 mM db-cGMP, flow stimulated net O2− production from 3 ± 1 to 10 ± 3 AU/s, an increase of only 7 ± 3 AU/s (P < 0.03; n = 5 vs. control).
Fig. 4.
A: effect of dibutyryl (db)-cGMP on net O2− production by thick ascending limbs in the absence and presence of luminal flow. B: effect of db-cGMP on flow-stimulated net O2− production. *P < 0.03; n = 5 vs. Con. Tubules were perfused at 20 nl/min.
Most of the effects of cGMP on thick ascending limb function are mediated by either PKG (23) or phosphodiesterase II (PDE2) (24). Therefore, we investigated which is involved in NO's ability to inhibit flow-induced net O2− production. First, we tested the effect of the PKG inhibitor KT-5823 (Fig. 5, A and B). In the presence of l-arginine, flow stimulated net O2− production from 4 ± 2 to 8 ± 3 AU/s, a flow-induced increase of 4 ± 4 AU/s. After addition of KT-5823 (5 μM), flow stimulated net O2− production from 2 ± 1 to 34 ± 8 AU/s, an increase of 32 ± 9 AU/s (P < 0.03; n = 5 vs. l-arginine alone).
Fig. 5.
A: effect of KT-5823 (KT), a PKG inhibitor, on the effect of Arg on net O2− production by thick ascending limbs in the absence and presence of luminal flow. B: effect of KT on Arg's effect on net O2− production induced by flow. *P < 0.03; n = 5 vs. Arg alone. C: effect of KT, a PKG inhibitor, on the effect of db-cGMP on net O2− production by thick ascending limbs in the absence and presence of luminal flow. D: effect of KT on db-cGMP's effect on net O2− production induced by flow. *P < 0.01; n = 5 vs. db-cGMP alone. Tubules were perfused at 20 nl/min.
Next, we tested whether KT-5823 could also block the effects of db-cGMP (Fig. 5, C and D). In the presence of db-cGMP, flow stimulated net O2− production from 4 ± 2 to 13 ± 6 AU/s, an increase of 9 ± 7 AU/s. In the presence of 5 μM KT-5823, flow stimulated net O2− from 4 ± 3 to 58 ± 6 AU/s, an increase of 54 ± 5 AU/s (P < 0.01; n = 5 vs. db-cGMP alone).
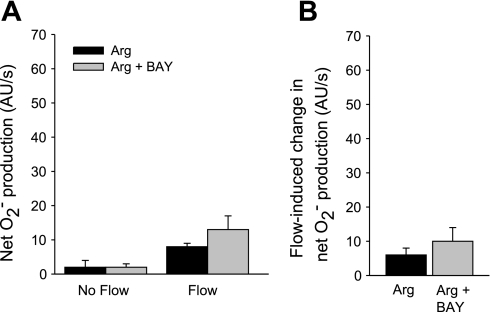
To determine whether PDE2 is involved in the effect of arginine/NO on flow-stimulated net O2− production, we used the PDE2 inhibitor BAY 60–7550 (Fig. 6). BAY 60–7550 (100 nM) did not significantly change arginine's effect on flow-induced net O2− production (l-arginine alone Δ: 6 ± 2 AU/s vs. l-arginine plus BAY 60–7550 Δ: 10 ± 4 AU/s; n = 5).
Fig. 6.
A: effect of Arg in the presence of the phosphodiesterase II inhibitor BAY 60–7550 (BAY) on net O2− production by thick ascending limbs in the absence and presence of luminal flow. B: effect of Arg in the presence of BAY on flow-induced change in net O2− production by thick ascending limbs. P > 0.05; n = 5 vs. Arg alone. Tubules were perfused at 20 nl/min.
DISCUSSION
Our hypothesis was that flow-induced NO diminishes flow-stimulated net O2− production via an increase in cGMP and activation of PKG. To test this hypothesis, we first studied the effect of l-arginine on flow-induced net O2− production. We used l-arginine because we previously showed that addition of arginine to the bath of isolated thick ascending limbs stimulates NO production (25), whereas in its absence thick ascending limbs do not produce NO. We found that l-arginine reduced flow-stimulated net O2− production by ∼70%. However, l-arginine had no effect on O2− in the absence of luminal flow.
To show that this effect was in fact due to NO, we tested whether the competitive inhibitor of NO production, l-NAME, could block the effects of l-arginine. We found that in the presence of l-NAME, l-arginine no longer had any significant effect on net O2− production in the presence or absence of luminal flow. l-NAME alone did not change net O2− production compared with nontreated tubules, suggesting that O2− produced by uncoupled NOS is not involved. Taken together, these data indicate that flow-induced NO diminishes flow-stimulated net O2− production. However, NO does not completely prevent flow from enhancing net O2− production. About 20% of flow-induced O2− appears to be insensitive to the effects of NO. To our knowledge, this is the first study investigating the interaction of NO and O2− in thick ascending limbs.
NO and O2− can react if they are formed simultaneously in the same compartment. This reaction proceeds at a rate of at least 6 × 109 mol·l−1·s−1 (15). This rapid rate is primarily due to the fact that both NO and O2− are free radicals, with an unpaired electron in the outer valence shell. Given that 20% of net O2− is NO insensitive, our data suggest that at least part of flow-induced O2− is generated in a compartment different from flow-stimulated NO.
Most of the effects of NO are mediated by cGMP (9, 23, 24, 42, 43). Thus, NO could reduce flow-induced net O2− production either by scavenging O2− or by affecting degradation or production via cGMP. To investigate which predominates in thick ascending limbs, we first used an inhibitor of soluble guanylate cyclase, LY-83583. When LY-83583 was added to the bath in the presence of l-arginine, it restored the ability of flow to stimulate net O2− production. These data indicate that some of the inhibitory effect of NO on net O2− production is mediated by cGMP. However, this experimental design does not allow one to estimate how much of this effect is mediated by cGMP. To do this, we reversed the experiment and added l-arginine in the presence of LY-83583. Under these conditions, we found that l-arginine only reduced flow-stimulated net O2− production by ∼35%.
Given that inhibition of soluble guanylate cyclase prevented 70% of NO's inhibition, we next tested whether a cGMP analog would mimic the effect of NO. We found that db-cGMP also reduced flow-induced net O2− production. Taken together, these data indicate that cGMP mediates ∼70% of the inhibitory effect of l-arginine on net O2− production. They also indicate that LY-83583 alone does not affect O2− production.
cGMP can activate a number of enzymes, including PKG and PDE2. To test whether either is involved in NO-induced inhibition of net O2− production, we used KT-5823 and BAY-60–7550, inhibitors of PKG and PDE2, respectively. We found that inhibition of PKG with KT-5823 blocked the effects of both arginine and cGMP on net O2− production. In contrast, PDE2 inhibition did not change the effect of arginine on flow-stimulated net O2− production. Taken together, these data indicate that flow-induced net O2− production in the thick ascending limb is reduced by endogenously produced NO and that a cGMP-dependent pathway mediated by PKG, rather than PDE2/cAMP, is involved. To our knowledge, this is the first study to demonstrate that a significant portion of NO's effect on flow-induced net O2− production in the thick ascending limb is due to a cGMP/PKG-mediated process rather than scavenging.
Our data suggest that a majority of the observed decrease in net O2− production caused by NO is due to a PKG-mediated process. However, we did find that a small proportion of the inhibition was not mediated by PKG. The most likely explanation for the non-PKG-mediated inhibition is scavenging of O2− by NO. However, it is also possible that nitrosylation is involved. Nitrosylation could inactivate the source of O2− (39) or a regulatory protein, or activate an enzyme responsible for O2− degradation.
We unexpectedly found that most of the reduction of net O2− production was not due to scavenging. Unfortunately, due to the nature of this study using isolated, perfused tubules, it is technically impossible to measure peroxynitrite production. However, it would be interesting to explore other stimuli of NO and O2− to study the relative contributions of such processes as scavenging, nitrosylation, and cGMP/PKG signaling.
Given that we previously showed that flow-stimulated O2− production is due to activation of NADPH oxidase, our data showing that NO reduces net O2− production are consistent with other studies. Fujii et al. (8) found that arginine inactivated p47phox in the renal cortex of Dahl salt-sensitive rats, which should reduce O2− production by preventing NADPH oxidase assembly. These authors assumed that the effect was due to enhanced NO synthesis, but did not test that hypothesis. Muzaffar et al. (22) showed that NO acutely inhibits O2− production in human vascular smooth muscle cells by blocking translocation of the NADPH oxidase subunit p47phox. They also found that NO's effect on O2− production could be blocked by a PKG inhibitor (22) similar to our results.
The mechanism by which PKG inhibits net O2− production in the thick ascending limb is unknown. Either production, degradation, or both may be affected. To our knowledge, there are currently no known effects of cGMP or PKG on enzymes involved in O2− degradation. Thus, it is likely that PKG-dependent inhibition of net O2− production is due to reduced synthesis. As discussed above, flow-induced O2− is a result of NADPH oxidase activation. Consequently, PKG may reduce net O2− production by blocking p47phox translocation as has been reported in vascular smooth muscle cells (22). Protein kinase C (PKC) may also play a role. PKG phosphorylates (12) and inhibits PKC (18). PKC is required for assembly of p47phox with other subunits to form active NADPH oxidase (3, 40). Finally, PKG may inhibit one or more steps involved with sensing increased luminal flow.
Luminal flow through the thick ascending limb varies over a wide range (6, 37, 38). Production of O2− in response to increased luminal flow is not an on/off process. We previously showed that O2− production is linearly related to flow rate in the physiological range of 0 to 20 nl/min (14). We also observed that NO production is linearly related to flow. Although we did not use different flow rates in this study, we would expect a similar relationship between flow rate and O2− production.
In summary, we found that NO attenuates flow-stimulated O2− production in the thick ascending limb via a cGMP/PKG pathway. The complexity of actions and interactions of these two factors helps regulate important cellular functions in the kidney, such as salt and water reabsorption. In addition to having direct natriuretic effects, NO keeps in check the potential deleterious effects of enhanced O2− induced by increases in flow. O2− stimulates Na absorption in the thick ascending limb. Thus, understanding the processes involved in maintaining NO and O2− balance may give insight into the pathogenesis and treatment of diseases associated with Na retention and oxidative stress.
GRANTS
This work was supported by National Institutes of Health Grants HL-28982 and HL-70985 to J. L. Garvin.
REFERENCES
- 1.Adler S, Huang H. Impaired regulation of renal oxygen consumption in spontaneously hypertensive rats. J Am Soc Nephrol 13: 1788–1794, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Araujo M, Welch WJ. Oxidative stress and nitric oxide in kidney function. Curr Opin Nephrol Hypertens 15: 72–77, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM, Wientjes FB, Cathcart MK. Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol 173: 5730–5738, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Brands MW, Bell TD, Gibson B. Nitric oxide may prevent hypertension early in diabetes by counteracting renal actions of superoxide. Hypertension 43: 57–63, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Dickhout JG, Mori T, Cowley AW Jr. Tubulovascular nitric oxide crosstalk: buffering of angiotensin II-induced medullary vasoconstriction. Circ Res 91: 487–493, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Dwyer TM, Schmidt-Nielsen B. The renal pelvis: machinery that concentrates urine in the papilla. News Physiol Sci 18: 1–6, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol 287: C895–C902, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Fujii S, Zhang L, Igarashi J, Kosaka H. l-Arginine reverses p47phox and gp91phox expression induced by high salt in Dahl rats. Hypertension 42: 1014–1020, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Garcia NH, Stoos BA, Carretero OA, Garvin JL. Mechanism of the nitric oxide-induced blockade of collecting duct water permeability. Hypertension 27: 679–683, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Garvin JL, Burg MB, Knepper MA. Active NH4+ absorption by the thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 255: F57–F65, 1988. [DOI] [PubMed] [Google Scholar]
- 11.Garvin JL, Hong NJ. Cellular stretch increases superoxide production in the thick ascending limb. Hypertension 51: 488–493, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatti A, Wang X, Robinson PJ. Protein kinase C-alpha is multiply phosphorylated in response to phorbol ester stimulation of PC12 cells. Biochim Biophys Acta 1313: 111–118, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Herrera M, Garvin JL. Endothelin stimulates endothelial nitric oxide synthase expression in the thick ascending limb. Am J Physiol Renal Physiol 287: F231–F235, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Hong NJ, Garvin JL. Flow increases superoxide production by NADPH oxidase via activation of Na-K-2Cl cotransport and mechanical stress in thick ascending limbs. Am J Physiol Renal Physiol 292: F993–F998, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun 18: 195–199, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Juncos R, Garvin JL. Superoxide enhances Na-K-2Cl cotransporter activity in the thick ascending limb. Am J Physiol Renal Physiol 288: F982–F987, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Juncos R, Hong NJ, Garvin JL. Differential effects of superoxide on luminal and basolateral Na+/H+ exchange in the thick ascending limb. Am J Physiol Regul Integr Comp Physiol 290: R79–R83, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Kumar R, Cartledge WA, Lincoln TM, Pandey KN. Expression of guanylyl cyclase-A/atrial natriuretic peptide receptor blocks the activation of protein kinase C in vascular smooth muscle cells. Role of cGMP and cGMP-dependent protein kinase. Hypertension 29: 414–421, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP. Production of superoxide through NADH oxidase in thick ascending limb of Henle's loop in rat kidney. Am J Physiol Renal Physiol 282: F1111–F1119, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Li N, Zhang G, Yi FX, Zou AP, Li PL. Activation of NAD(P)H oxidase by outward movements of H+ ions in renal medullary thick ascending limb of Henle. Am J Physiol Renal Physiol 289: F1048–F1056, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Murad F Regulation of cytosolic guanylyl cyclase by nitric oxide: the NO-cyclic GMP signal transduction system. Adv Pharmacol 26: 19–33, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Muzaffar S, Shukla N, Bond M, Sala-Newby G, Angelini GD, Newby AC, Jeremy JY. Acute inhibition of superoxide formation and Rac1 activation by nitric oxide and iloprost in human vascular smooth muscle cells in response to the thromboxane A2 analog, U46619. Prostaglandins Leukot Essent Fatty Acids 78: 247–255, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortiz PA, Garvin JL. Autocrine effects of nitric oxide on HCO3− transport by rat thick ascending limb. Kidney Int 58: 2069–2074, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Ortiz PA, Garvin JL. NO inhibits NaCl absorption by rat thick ascending limb through activation of cGMP-stimulated phosphodiesterase. Hypertension 37: 467–471, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Ortiz PA, Garvin JL. Interaction of O2− and NO in the thick ascending limb. Hypertension 39: 591–596, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol 283: F957–F962, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Ortiz PA, Hong NJ, Garvin JL. Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. Am J Physiol Renal Physiol 287: F274–F280, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz PA, Hong NJ, Garvin JL. Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. II. Role of PI3-kinase and Hsp90. Am J Physiol Renal Physiol 287: F281–F288, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Ortiz PA, Hong NJ, Wang D, Garvin JL. Gene transfer of eNOS to the thick ascending limb of eNOS-KO mice restores the effects of l-arginine on NaCl absorption. Hypertension 42: 674–679, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Plato CF, Garvin JL. Alpha(2)-adrenergic-mediated tubular NO production inhibits thick ascending limb chloride absorption. Am J Physiol Renal Physiol 281: F679–F686, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Plato CF, Pollock DM, Garvin JL. Endothelin inhibits thick ascending limb chloride flux via ETB receptor-mediated NO release. Am J Physiol Renal Physiol 279: F326–F333, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Plato CF, Shesely EG, Garvin JL. eNOS mediates l-arginine-induced inhibition of thick ascending limb chloride flux. Hypertension 35: 319–323, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol Renal Physiol 276: F159–F163, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Ramseyer VD, Garvin JL. Angiotensin II decreases nitric oxide synthase 3 expression via nitric oxide and superoxide in the thick ascending limb. Hypertension 53: 313–318, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol Regul Integr Comp Physiol 284: R893–R912, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Sakai T, Craig DA, Wexler AS, Marsh DJ. Fluid waves in renal tubules. Biophys J 50: 805–813, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt-Nielsen B The renal concentrating mechanism in insects and mammals: a new hypothesis involving hydrostatic pressures. Am J Physiol Regul Integr Comp Physiol 268: R1087–R1100, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Selemidis S, Dusting GJ, Peshavariya H, Kemp-Harper BK, Drummond GR. Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc Res 75: 349–358, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Shiose A, Sumimoto H. Arachidonic acid and phosphorylation synergistically induce a conformational change of p47phox to activate the phagocyte NADPH oxidase. J Biol Chem 275: 13793–13801, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Silva GB, Ortiz PA, Hong NJ, Garvin JL. Superoxide stimulates NaCl absorption in the thick ascending limb via activation of protein kinase C. Hypertension 48: 467–472, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Stoos BA, Carretero OA, Farhy RD, Scicli G, Garvin JL. Endothelium-derived relaxing factor inhibits transport and increases cGMP content in cultured mouse cortical collecting duct cells. J Clin Invest 89: 761–765, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoos BA, Garvin JL. Vectorial efflux of cGMP and its dependence on sodium in the cortical collecting duct. Am J Physiol Regul Integr Comp Physiol 271: R1676–R1681, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 34: 1359–1368, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Zou AP, Cowley AW Jr. α2-Adrenergic receptor-mediated increase in NO production buffers renal medullary vasoconstriction. Am J Physiol Regul Integr Comp Physiol 279: R769–R777, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Zou AP, Li N, Cowley AW Jr. Production and actions of superoxide in the renal medulla. Hypertension 37: 547–553, 2001. [DOI] [PubMed] [Google Scholar]