Abstract
The transient receptor potential cation channel, subfamily V, member 5 (TRPV5) gene, which encodes the Ca2+ channel in the apical membrane of distal convoluted tubule and connecting tubule of the kidney, exhibits an unusually high frequency of nonsynonymous single nucleotide polymorphisms (SNPs) among African Americans. To assess the functional impacts of the nonsynonymous SNP variations in TRPV5, these variants were analyzed with radiotracer 45Ca2+ influx assay and the voltage-clamp technique using Xenopus laevis oocytes. Among the variations tested, including A8V, R154H, A563T, and L712F, the latter two significantly increased TRPV5-mediated Ca2+ influx. The A563T variant, which exists in African Americans with relative high frequency, exhibited increased Ca2+ influx at extracellular Ca2+ from 0.01 to 2 mM despite a lower expression level at the plasma membrane. This variant also exhibited a reduction in Na+ current as a result of increased sensitivity to extracellular Mg2+. By substituting threonine-563 (Thr563) with serine or valine residue, the bulky side chain of Thr563 was shown to facilitate Ca2+ transport, whereas the hydroxyl group of Thr563 is likely related to Mg2+ sensitivity. The A563T variant was capable of increasing TRPV5-mediated Ca2+ influx, even when it was expressed under conditions mimicking heterozygous or compound state with other variants. In conclusion, the A563T variant of TRPV5 significantly increased Ca2+ influx by affecting the Ca2+ permeation pathway. Thus the A563T variation in TRPV5 may contribute to the superior ability of renal Ca2+ conservation in African Americans.
Keywords: single nucleotide polymorphisms; transient receptor potential cation channel, subfamily V, member 5; calcium reabsorption; African Americans
ca2+ balance of the body is maintained by intestinal absorption, renal reabsorption and excretion, as well as disposition of Ca2+ in and mobilization from the bone. The distal convoluted tubule (DCT) and connecting tubule (CNT) are critical segments of the nephron for regulating Ca2+ reabsorption (21, 30). Ca2+ is actively reabsorbed in this portion of the nephron by a vitamin D-regulated transcellular pathway. The renal epithelial Ca2+ channel transient receptor potential cation channel, subfamily V, member 5 (TRPV5) is expressed in the apical membrane of DCT and CNT and plays a gatekeeper role for active Ca2+ reabsorption (15, 16, 27). Mice lacking Trpv5 resulted in a 6- to 10-fold increase in urinary Ca2+ excretion, and ultimately in defects in bone mineralization (16).
Urinary Ca2+ excretion is an important factor for kidney stone formation and bone health. For instance, urine Ca2+ excretion correlates with bone loss in calcium-stone-forming patients with idiopathic hypercalciuria (3). Interestingly, African Americans exhibit lower urinary Ca2+ excretion than whites (8, 25, 29, 34, 35, 40, 45), and the risk of kidney stones in African American is lower than that in whites (32, 36, 38). Furthermore, African Americans have higher bone mass (5, 41) and lower incidence of osteoporosis-related fractures than whites (4, 6, 7). The mechanism underlying the lowered urinary Ca2+ in African Americans is not well understood. A lower 25-hydroxyvitamin D concentration in African Americans could result in an increased parathyroid hormone level and in turn renal Ca2+ conservation. However, Braun and colleagues (8) observed significantly lower urinary Ca2+ excretion in adolescent African American girls than white girls in a wide range of controlled Ca2+ intake, whereas the 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and parathyroid hormone values were not significantly different between the two groups. The difference in bone mass and urinary Ca2+ excretion between blacks and whites is not restricted to Americans; a similar finding was reported in South Africa (10). Thus genetic rather than social and environmental factors may play a major role in the superior renal Ca2+ conservation mechanism in African descendents.
Because TRPV5 is a key protein that regulates Ca2+ reabsorption, genetic variations of TRPV5 may influence urinary Ca2+ excretion. Interestingly, TRPV5 gene is one of the four contiguous genes, including EPHB6, TRPV6, TRPV5, and KEL, in chromosome 7q34-35 with striking evidence of a recent selective sweep in European Americans based on the analysis on the single nucleotide polymorphisms (SNPs) of over 100 genes from 24 African Americans and 23 European Americans provided by SeattleSNPs program (1, 37). Akey and colleagues (2) further showed that the TRPV6 haplotype, defined by three nonsynonymous SNPs, is nearly fixed in populations outside Africa, suggesting that these variations may confer a selective advantage, e.g., efficiency in Ca2+ absorption from dairy products or resistance to a pathogen, after early humans migrated out of Africa. The TRPV6 variant with the three nonsynonymous SNPs exhibited increased Ca2+ transport ability and may play a role in absorptive hypercalciuria and kidney stone formation (39). However, TRPV5 variations were not investigated in these studies. According to the data from SeatleSNPs, there are four nonsynonymous SNPs in TRPV5 gene; three of them are only present in African Americans. Eleven of 24 African Americans (45.8%) surveyed carry one or more of these African American specific nonsynonymous SNPs. Although further population studies are needed to confirm these observations, it is likely that these nonsynonymous SNPs in TRPV5 are common in African Americans. A large portion of African descents may be affected if these nonsynonymous SNPs have functional consequences. Thus functional characterization of these African American specific nonsynonymous SNPs in TRPV5 gene may help in understanding their potential impacts on Ca2+ reabsorption among African descents.
In the present study, we analyzed the functional significance of the four nonsynonymous SNPs of TRPV5 gene using the Xenopus laevis oocyte expression system. Two of them, A563T and L712F, exhibited significant increases in Ca2+ transport ability that may contribute to the lower level of urinary Ca2+ excretion in African Americans.
MATERIALS AND METHODS
cDNA constructs.
Human TRPV5 cDNA was prepared as described previously (26). For experiments using X. laevis oocytes, the cDNA was subcloned into the X. laevis oocyte expression vector pNWP as previously described (19). TRPV5 SNP variants and other mutations at amino acid 563 of TRPV5 were generated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) following the manufacturer's instruction. Constructs containing double variations (A8V-R154H, R154H-A563T, R154H-L712F) were constructed by substitution of the EcoR V-Nde I fragment containing R154H, with the same fragment from constructs with A8V, A563T, or L712F variation. All of the constructs containing variations generated with the QuikChange site-directed mutagenesis kit were confirmed by sequencing.
Ca2+ uptake in X. laevis oocytes and determination of the concentration dependence of Ca2+ uptake and Mg2+ sensitivity.
In vitro transcription, injection of the capped synthetic RNAs (cRNAs) into oocytes, and Ca2+ uptake assay in oocytes were performed as described previously (19, 28). The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. cRNAs for TRPV5 and its different mutations were injected at 12.5 ng/oocyte. When a combination of two cRNAs was required, the cRNAs were mixed, and the total concentration of the cRNAs was maintained.
For measuring the concentration dependence of Ca2+ uptake, groups of control oocytes and oocytes expressing TRPV5 (A563) or A563T (T563) were cultured at 18°C in 0.5× L-15 medium (Invitrogen, Carlsbad, CA) supplemented with 10 mM HEPES (pH 7.6), 5% heat-inactivated horse serum, penicillin (10,000 U/l), streptomycin (10 mg/l), and amphotericin B (25 μg/l) for 2 days after cRNA injection. Each group of seven to nine oocytes was washed three times with nonradioactive standard uptake solution with different Ca2+ concentrations ([Ca2+]) before uptake experiments. Ca2+ uptake was initiated by immersion of the oocytes in different [Ca2+] (0.01, 0.02, 0.05, 0.1, 0.2, 0.5, 1, and 2 mM) with 45Ca2+ tracer in standard uptake solution that contained (in mM) 100 NaCl, 2 KCl, 1 MgCl2, and 10 mM HEPES (pH 7.5). After a 30-min incubation at room temperature (∼24°C), oocytes were washed six times with ice-cold standard uptake solution without 45Ca2+ and were dissolved by 10% SDS solution, and the incorporated 45Ca2+ was determined using a scintillation counter. Data of 14 oocytes in each group from two frogs were expressed as means ± SE. The kinetic parameters of concentration dependence of Ca2+ uptake were determined by fitting the Ca2+ uptake data to the equation V = VmaxS/(Km + S) + NS, where V is the Ca2+ transport, Vmax is the derived saturable Ca2+ transport maximum, S is the [Ca2+], Km is the [Ca2+] at which saturable Ca2+ transport is half-maximal, and N is the slope of the nonsaturable Ca2+ transport. Parameter values (Vmax, Km, and N) were obtained using Graphpad Prism 5.0 software (La Jolla, CA). For measuring Mg2+ sensitivity, the standard uptake solution with [Ca2+] at 1 mM (including 45Ca2+ tracer) was used. Oocytes were washed with standard uptake solution without 45Ca2+ tracer and Mg2+ three times before adding uptake solution with varied Mg2+ concentration (0, 0.5, 1.0, 2.0, 5.0, or 10 mM). Data of three independent experiments with a total of 21 oocytes in each group were expressed as means ± SE.
Two-microelectrode voltage-clamp technique.
The two-microelectrode voltage-clamp experiments were performed as described previously (19, 28) with a GeneClamp 500 amplifier and pCLAMP software (version 9) (Axon Instruments, Foster City, CA). The resistance of microelectrodes filled with 3 M KCl was 0.5–2 MΩ. In experiments involving voltage jumps, the oocyte was clamped at the holding potential of −50 mV. Voltage pulses (100 ms) between −160 and +60 mV, in increments of 20 mV, were then applied, and steady-state currents were obtained as the average values in the interval from 60 to 95 ms after the initiation of the voltage pulses. The standard perfusion solution used contained (in mM) 100 choline chloride, 2 KCl, 1 MgCl2, and 10 HEPES, pH 7.5 (adjusted using Tris base and HCl). Choline chloride was substituted with NaCl when Na+ current was tested. CaCl2 (1 mM) was added to the standard perfusion solution when Ca2+-evoked current was recorded. For determining Mg2+ dose-dependent effect on Na+ current, oocytes need to be exposed to divalent cation free standard perfusion solution. Under this condition, the endogenous Ca2+-inactivated Cl− current (44), which is likely formed by connexin38 hemi-gap-junctional channels (13), was induced. To completely block the Ca2+-inactivated Cl− current in X. leavis oocyte, flufenamic acid at 200 μM was included in solutions (44). Flufenamic acid at this concentration has negligible effects on TRPV5-mediated Na+ current and Ca2+ uptake. Na+ currents were recorded in the presence of varied concentrations of Mg2+ (0, 0.1, 0.2, 0.5, 1, 2, and 5 mM) in response to voltage pulses between −160 and +60 mV, in increments of 20 mV. Net Na+ currents were obtained by subtracting adjacent recordings in the absence of Na+ (choline substitution).
Western blotting analysis.
For detection of TRPV5, oocytes were lysed in extraction buffer (100 mM NaCl, 1% Triton X-100, 20 mM Tris·HCl, pH 7.6) plus protease inhibitor cocktail and centrifuged at 5,000 g for 15 min to remove the cellular debris and yolk proteins. In general, extract supernatant corresponding to one oocyte was loaded per lane. After SDS-PAGE, the proteins were transferred to polyvinylidene difluoride membranes. The membrane was blocked with Tris-buffered saline (TBS) containing 5% nonfat milk for 1∼2 h at room temperature. Primary antibodies (TRPV5; Alpha Diagnostic Intl, CAT21-A) were incubated overnight at 4°C (1:3,000), followed by multiple washes in TBS-Tween 20. The appropriate horseradish peroxidase-conjugated secondary antibodies (1:3,000; Pierce Biotechnology, Rockford, IL) were incubated in the blocking solution for 1 h at room temperature, followed by multiple washes with TBS-Tween 20. Chemiluminescence was detected using a SuperSignal West Femto Maximum Sensitivity Substrate kit (34095; Pierce) in accordance with the manufacturer's protocol.
Oocyte surface biotinylation.
After cRNA injection (2 days), oocytes were washed with cold PBS three times and incubated for 15 min at room temperature in PBS with 0.005% subtilisin A (Sigma-Aldrich, St. Louis, MO) under mild end-to-end shaking to partially digest the vitelline membranes of oocytes. The oocytes were then incubated with Sulfo-NHS-SS-Biotin (1 mg/ml; Pierce) in PBS for 1 h at 4°C with end-to-end shaking. After incubation, oocytes were washed five times with cold PBS and then quenched with 100 mM glycine for 1 h at 4°C with end-to-end shaking. Oocytes were lysed, and the yolk was removed by centrifugation at 5,000 g for 15 min at 4°C. The supernatant was incubated with immobilized NeutrAvidin beads (Pierce) for 2 h at 4°C. The beads were centrifuged, and the supernatants were removed. The beads were then washed three times with TBS. Biotinylated proteins were eluted from the beads by 1× SDS-PAGE loading buffer with 50 mM dithiothreitol at 65°C for 10 min. Input cell lysates and biotinylated sample were subjected to SDS-PAGE and Western blot analysis as described above.
RESULTS
Nonsynonymous SNPs in TRPV5 gene prevalently exist in African Americans.
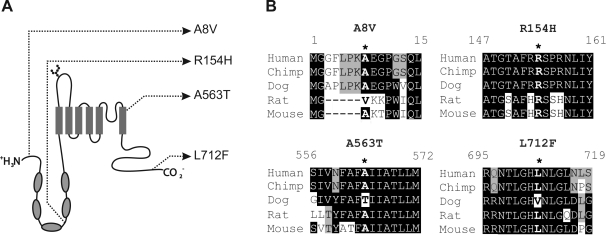
TRPV5 gene is one of the four genes in a contiguous region of chromosome 7 that exhibits evidence of selective sweep in recent human history by Akey and colleagues (1). Similar findings were reported by Stajich and Hahn (37). These studies utilized SNP genotype data for 132 (1) or 151 (37) genes sequenced in 24 African Americans and 23 European Americans from the SeattleSNPs program (9). Akey and colleagues further demonstrated the allele frequencies of the 3 nonsynonymous SNP variations of TRPV6 in 1,064 individuals from 52 populations, suggesting that the derived TRPV6 allele, which is nearly fixed in populations outside Africa, conferred a selective advantage after early humans migrated out of Africa (2). Although detailed TRPV5 nonsynonymous SNP data were not provided in these publications, they are available to public from the SeattleSNPs website (http://pga.mbt.washington.edu). Four nonsynonymous SNPs were identified by SeattleSNPs; they are summarized in Table 1. Interestingly, out of the four nonsynonymous SNPs, three of them (A8V, A563T, and L712F) were only present in African Americans, not in European Americans. A8V, R154H, and L712F are localized in the intracellular terminal regions; A563T, however, is in the last transmembrane domain (Fig. 1A).
Table 1.
Nonsynonymous SNPs in TRPV5 identified by SeattleSNPs
| dbSNP rs No. | dbSNP Allele | Amino Acid Residue | Amino Acid Position | AA Freq | EA Freq |
|---|---|---|---|---|---|
| rs4252372 | T | Val (V) | 8 | 0.12 | 0.00 |
| C | Ala (A) | 0.88 | 1.00 | ||
| rs4236480 | A | His (H) | 154 | 0.44 | 0.28 |
| G | Arg (R) | 0.56 | 0.72 | ||
| rs4252499 | A | Thr (T) | 563 | 0.17 | 0.00 |
| G | Ala (A) | 0.83 | 1.00 | ||
| rs4252509 | C | Phe (F) | 712 | 0.02 | 0.00 |
| G | Leu (L) | 0.98 | 1.00 |
SNP, single nucleotide polymorphism; dbSNP, Single Nucleotide Polymorphism database; rs, Reference SNP ID; TRPV5, transient receptor potential cation channel, subfamily V, member 5; AA and EA Freq, allele frequency in African Americans (European Americans).
Fig. 1.
Transient receptor potential cation channel, subfamily V, member 5 (TRPV5) nonsynonymous single nucleotide polymorphisms (SNPs) revealed by SeatleSNPs Project. A: position of nonsynonymous SNP variations in TRPV5 model. The cylinders represent transmembrane domains; the ellipses represent ankyrin repeats in the intracellularly localized NH2-terminal region of TRPV5. B: alignment of amino acid sequences surrounding the nonsynonymous SNPs of TRPV5 from various species.
The variations in TRPV5 are unique, since they are not commonly present in other species surveyed, including chimpanzee, canine, rat, and mouse, with the exceptions of Thr563 in dog and Val8 in rat (Fig. 1B). This is in striking contrast to the nonsynonymous SNP variations in TRPV6, which are conserved among these species (not shown).
The A563T variation significantly increases TRPV5-mediated Ca2+ transport activity.
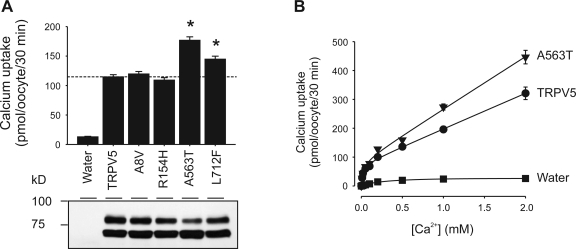
We next evaluated the functional impacts of these newly derived variations in TRPV5, including A8V, R154H, A563T, and L712F, using the X. laevis oocyte expression system. Among the TRPV5 variants evaluated, A563T and L712F exhibited a 54.1 ± 5.1% (n = 9) and 26.2 ± 4.5% (n = 9) increase in Ca2+ uptake, respectively, over the reference form of TRPV5 (Fig. 2, top). No significant changes were observed for the A8V or R154H variant, although the latter exhibited a slightly lower activity. With the exception of A563T, which exhibited a decrease in the fully glycosylated band (also see Fig. 3), the total protein expression levels of the other three variants were not significantly different from the reference TRPV5 as determined by Western blotting analysis using antibody against TRPV5 (Fig. 2A, bottom). Because the L712F variant only promoted a moderate increase in TRPV5-mediated Ca2+ uptake, and it existed only in one allele among 23 European and 24 African Americans examined in the SeatleSNPs program, we focused mainly on the difference between A563T variant and the reference form of TRPV5 in the following studies. For simplicity, when only these two forms of TRPV5 were compared, they were referred to as T563 and A563, respectively.
Fig. 2.
The A563T variant of TRPV5 increased Ca2+ influx. A: Ca2+ influx mediated by TRPV5 nonsynonymous SNP variants. Xenopus laevis oocytes were injected with 12.5 ng of cRNA of TRPV5 and its nonsynonymous SNP variants (A8V, R154H, A563T, and L712F), and radiotracer 45Ca2+ uptake and protein expression level were determined 2 days later. A representative Western blotting result using antibody against TRPV5 is shown at bottom. Ca2+ uptake data from 63 oocytes in each group from 9 independent experiments were pooled and are shown as means ± SE. *P <0.01 vs. TRPV5 group by Student's t-test. B: concentration dependence of Ca2+ influx mediated by TRPV5 and the A563T variant. Data at each point are shown as means ± SE of 14 oocytes from 2 frogs.
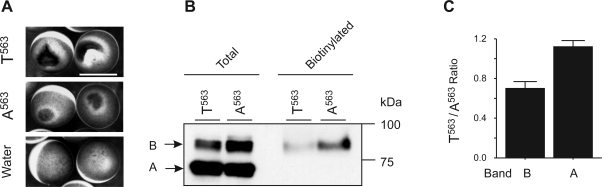
Fig. 3.
The T563 variant exhibited a decreased plasma membrane expression level relative to the A563 variant. A: the scar-like mark was more prominent in X. laevis oocytes expressing the T563 variant than those expressing the A563 variant. Shown are representative X. laevis oocytes 18 h after injection with cRNA of T563, A563, or water (as a control). B: biotinylated TRPV5 protein level was decreased in oocytes expressing T563 than those expressing A563, as shown in a representative Western blot analysis. X. laevis oocytes were injected with T563 or A563 cRNA at 12.5 ng/oocyte, and biotinylation experiments were performed 2 days later. Biotinylated proteins corresponding to 5 oocytes and total proteins corresponding to 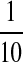 of an oocyte were loaded. C: intensity ratio between T563 and A563 variant in core-glycosylated and fully glycosylated bands (Fig. 3B, bands A and B, respectively). Data from 8 independent experiments are shown as means ± SE.
of an oocyte were loaded. C: intensity ratio between T563 and A563 variant in core-glycosylated and fully glycosylated bands (Fig. 3B, bands A and B, respectively). Data from 8 independent experiments are shown as means ± SE.
Because [Ca2+] gradually decreases along the distal tubule (43), we tested the ability of T563 to increase Ca2+ uptake at various extracellular [Ca2+]. T563 appeared to be able to increase Ca2+ uptake at all [Ca2+] tested from 0.01 to 2 mM. TRPV5-mediated Ca2+ uptake exhibited a Michaelis-Menten type of saturation kinetics at extracellular [Ca2+] between 0 to 0.1 mM; at higher concentrations (0.2–2.0 mM), TRPV5-mediated Ca2+ uptake exhibited a more linear relationship to extracellular [Ca2+].
The saturation kinetics may be a result of the Ca2+-dependent feedback inhibition mechanism of TRPV5. Once the intracellular [Ca2+] exceeds the limit of Ca2+-dependent feedback inhibition, a linear relationship becomes dominant. The overall Ca2+ transport kinetics fit well with the equation V = VmaxS/(Km + S) + NS. T563 exhibited a higher apparent Km, a higher Vmax, and a higher N value than A563 (Km 0.024 ± 0.010 vs. 0.019 ± 0.009 mM; Vmax 84.6 ± 11.6 vs. 75.4 ± 10.4 pmol·oocyte−1·30 min−1; N 182.2 ± 7.3 and 122.8 ± 6.3 pmol·oocyte−1·30 min−1·mM−1). When the three parameters of the fitted curves were compared with GraphPad Prism 5.0 software, there was a significant difference between the two sets (P < 0.0001).
The increased Ca2+ influx mediated by T563 variant is not a result of increased expression at the plasma membrane.
Because the overall activity of TRPV5 is largely determined by its abundance at the plasma membrane, we next evaluated the level of T563 at the plasma membrane. Oocytes expressing TRPV5 exhibited a scar-like mark, which is likely formed by redistribution of pigments in the oocyte due to Ca2+-induced aggregation of cytoskeleton components under the pigments (46). Oocytes expressing T563 exhibited a more prominent mark at the surface than those expressing A563, consistent with the increased Ca2+ uptake mediated by T563 (Fig. 3A). The increase in the scale of the mark correlates with the increased Ca2+ influx in TRPV5-expressing oocytes, and often the increased Ca2+ influx is a result of increased surface expression of TRPV5 (46). However, this is not the case for the T563 variant. In fact, a 30.0 ± 6.9% (n = 8) reduction of fully glycosylated T563 protein (band B), which corresponds to the mature TRPV5 at the plasma membrane (19), was observed (Fig. 3, B and C). The biotinylated T563 protein at the oocyte surface was also significantly decreased (Fig. 3B). Thus the increased scale of the scar-like mark in T563-expressing oocytes reflects the increased level of Ca2+ influx, not the level of protein expression at the oocyte surface.
The T563 variant exhibits a reduction in Na+ current.
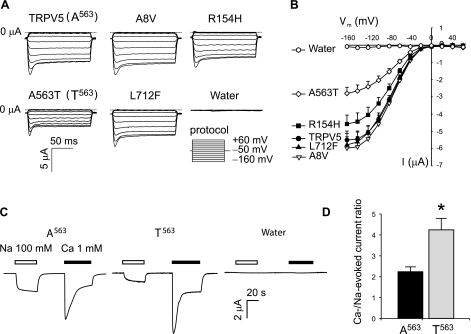
T563 exhibited increased Ca2+ influx without increased surface expression. This suggested that the channel properties are altered by the A563T substitution, which is in the last transmembrane domain of TRPV5. We next used the voltage-clamp approach to examine other alterations in TRPV5 properties. In the absence of Ca2+ in the extracellular solution, TRPV5 allows Na+ to pass through the channel (23). The amplitude of TRPV5-mediated Na+ current correlates with the amplitude of Ca2+ uptake and is often used as a measure of the channel activity of TRPV5 (19). Most of the SNP variants tested exhibited similar levels of Na+ current. However, in contrast to the increased Ca2+ influx, T563 exhibited a significant reduction in Na+ current (Fig. 4).
Fig. 4.
The A563T variant exhibited a reduction in Na+ current. A: representative current traces for TRPV5 and its nonsynonymous SNP variants at membrane potentials varied from −160 to +60 mV at 20-mV intervals. Na+-evoked currents were obtained by subtracting currents recorded in the choline solution (0 mM of Na+) from those recorded from Na+ solution (100 mM of Na+). Water-injected control oocytes (Water) exhibited negligible currents. B: current-voltage (I-V) plots of TRPV5 and its nonsynonymous SNP variants. Data are expressed as means ± SE from 3 experiments with a total of 17 oocytes in each group. C: external application of 100 mM Na+ or 1 mM Ca2+ evoked inward current when the oocyte expressing A563 or T563 variant held at −50 mV was perfused with standard solution without Na+ and Ca2+. The peak current amplitudes of Ca2+-evoked currents in oocytes expressing A563 and T563 were comparable; in contrast, the amplitude of Na+-evoked current in the oocyte expressing A563 was much higher than that in the oocyte expressing T563. Water-injected control oocytes exhibited negligible Na+- or Ca2+-evoked current. D: the ratio between the peaks of Ca2+- and the Na+-evoked currents was significantly increased for T563. Data from 14 oocytes of two batches of oocytes are expressed as means ± SE. *P <0.05 vs. A563 group by Student's t-test.
To confirm that the A563T variation has different effects on Na+ current and Ca2+ influx, we next compared the Na+- and Ca2+-evoked currents in the same oocytes (Fig. 4C). When an oocyte expressing A563 was held at −50 mV in Na+- and Ca2+-free solution, external application of Na+ at 100 mM induced an inward current (Fig. 4C). After removal of Na+ solution, subsequent application of Ca2+ at 1 mM induced an inward current (Fig. 4C) that was due to the activation of Ca2+-activated Cl− channels endogenously expressed in X. laevis oocytes (11, 22) likely mediated by TMEM16A (33). The amplitude of the Ca2+-activated Cl− current reflected the increase in intracellular Ca2+ resulting from TRPV5-mediated Ca2+ influx. In Fig. 4C, the Ca2+-evoked currents in an oocyte expressing T563 was slightly larger than that in an oocyte expressing A563; in contrast, the Na+ current of T563 was approximately one-half that of A563. As a result, the ratio between the peak levels of Ca2+- and Na+-evoked currents in the same oocytes expressing T563 was significantly increased (Fig. 4D).
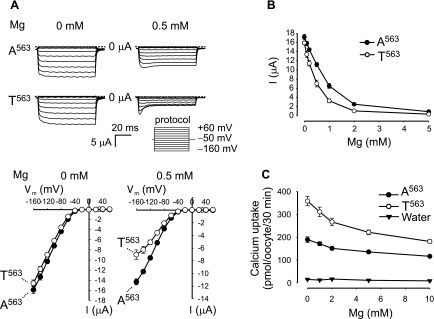
Increased sensitivity to extracellular Mg2+ in T563 variant leads to the reduction in Na+ current.
The increased Ca2+ influx with a reduction of Na+ current suggested that the pore of TRPV5 is altered by the A563T substitution. Because Ca2+ permeation and Mg2+ blockade of TRPV5 are linked to a single Asp542 residue at the pore (18, 24), we examined whether the sensitivity to extracellular Mg2+ is altered in T563. When measured without extracellular Mg2+, Na+ current of T563 was only slightly lower than that of A563 (Fig. 5A). In contrast, in the presence of 0.5 mM extracellular Mg2+, the Na+ current of T563 was significantly reduced compared with that of A563 (Fig. 5A). T563 exhibited a lower IC50 of Mg2+ blockade than A563 (0.42 vs. 0.72 mM), as deduced from the dose-response curve of extracellular Mg2+ concentration on Na+ current amplitude (Fig. 5B). Therefore, the reduction in Na+ current of T563 regularly recorded in the presence of 1 mM Mg2+ was the result of the increased sensitivity to extracellular Mg2+. The sensitivity of Ca2+ influx to extracellular Mg2+ was also increased in T563, albeit to a much lesser extent (Fig. 5C). Extracellular Mg2+ is expected to affect Na+ permeation more than Ca2+ permeation, because although Mg2+ affinity is much higher than Na+ affinity, it is ∼100 times lower than Ca2+ affinity of TRPV5 (18).
Fig. 5.
Decreased Na+ current of T563 variant was due to increased sensitivity to extracellular Mg2+. A: representative Na+ current traces of A563 and T563 variants in the presence of 0 and 0.5 mM extracellular Mg2+ are shown in top. The I-V curves of Na+ current for A563 and T563 were parallel in the absence of extracellular Mg2+ and departed in the presence of 0.5 mM extracellular Mg2+ (bottom). B: Na+ current of the T563 variant was more sensitive to extracellular Mg2+ concentration than that of the A563 variant. Currents at −160 mV from two independent experiments of 18 oocytes/group are expressed as means ± SE. Flufenamic acid at 200 μM was included in the solutions to block the Ca2+-inactivated Cl− current induced by the removal of both Ca2+ and Mg2+ in the extracellular solution. C: effect of extracellular Mg2+ on 45Ca2+ uptake mediated by oocytes expressing A563, T563, or by water-injected control oocytes (water). Mg2+ (10 mM) in the extracellular medium led to a stronger reduction in Ca2+ uptake in oocytes expressing T563 than those expressing A563 (49.4 ± 2.2 vs. 38.5 ± 2.5%). Data from 3 independent experiments are expressed as means ± SE.
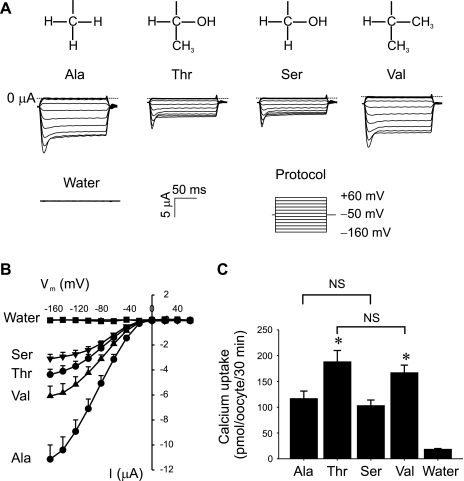
Roles of the hydroxyl and methyl group in the side chain of Thr563 in TRPV5-mediated cation transport.
The above observation indicated that the A563T substitution in TRPV5 significantly altered the cation transport properties of TRPV5. The side chain of threonine has an additional hydroxyl (·OH) and a methyl (·CH3) group compared with that of alanine (Fig. 6A, top); we thus examined the roles of the hydroxyl and the methyl group of Thr563 by serine or valine substitution (Fig. 6). When Thr563 was replaced by serine, which lacks a methyl group compared with threonine, the Na+ current remained low at the level similar to T563 (Fig. 6, A and B); however, the increase in Ca2+ uptake was abolished to the level comparable to A563 (Fig. 6C). When Thr563 was replaced by valine, which has a methyl group in the place of the hydroxyl group in threonine, the Na+ current was restored to some extent toward A563 (Fig. 6, A and B) while the Ca2+ uptake remained elevated to the level not significantly different from that of T563 (Fig. 6C). These results indicate that the methyl group of Thr563 is responsible for the increase in Ca2+ transport, whereas the hydroxyl group may contribute to the increased Mg2+ sensitivity that causes the reduction of Na+ transport.
Fig. 6.
Roles of the side chain in Thr563 in the transport property of A563T variant. A: Na+-evoked current of TRPV5 (Ala), A563T (Thr), A563S (Ser), and A563V (Val). Shown are representative Na+ currents in response to different membrane potentials from −160 to +60 mV. The side chains of Ala, Thr, Ser, and Val are also shown on the top of the respective groups. B: I-V relationships of Na+ current mediated by TRPV5 variants with different amino acid residues at position 563. C: Ca2+ uptake mediated by TRPV5 variants carrying different amino acid residues at position 563. Data from 3 independent experiments are expressed as means ± SE. *P <0.01 vs. Ala group by Student's t-test. NS, not statistically significant.
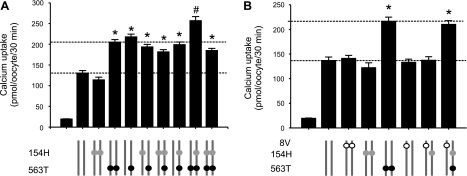
The A563T variation is capable of increasing TRPV5-mediated Ca2+ uptake when present as one of two alleles.
Among 24 African American subjects studied in the SeattleSNPs, six of them contained A563T variation, but only two of the six were homozygous, whereas four of them had A563T as one of two alleles. The nonsynonymous SNP R154H existed in both African Americans and European Americans at high allele frequencies (44 and 28%, respectively); thus, R154H variant should be considered as another reference form of TRPV5. Four of the African American individuals carrying A563T were in compound status with R154H. Because TRPV5 forms tetramers as a functional unit (14, 17), we mimicked transcripts from the two alleles in the cases found in African American subjects by coinjection of an equal amount of two cRNAs containing A563T and/or R154H in different combinations to assess whether the A563T variation is capable of increasing TRPV5-mediated Ca2+ transport in these situations (Fig. 7A). In each group, we combined an equal amount (6.25 ng/oocyte) of each of the two cRNA species encoding identical or different TRPV5 variants. We assumed this is equivalent to the mRNA species derived from the two alleles carrying identical or different variations. It was evident that A563T derived from only one allele was sufficient to increase Ca2+ uptake mediated by TRPV5 tetramer that contained half reference TRPV5 and half A563T. No significant difference was observed between the group that consisted of all A563T and that consisted of half A563T and half TRPV5. When coexisting with R154H, either in cis or in trans, the A563T variation increased TRPV5-mediated Ca2+ uptake to a similar level as that in the situation without R154H (Fig. 7A). An exceptional case observed, when the A563T variation was in homozygous and the R154H variation was in heterozygous condition, Ca2+ uptake was increased significantly over other combinations tested (Fig. 7A). It requires further investigation to understand the nature of this apparent synergistic action between the two variations.
Fig. 7.
The A563T variation was capable of increasing TRPV5-mediated uptake when present in a single allele and/or with other nonsynonymous SNP variations. A: the A563T variation increased TRPV5-mediated Ca2+ uptake in the presence of the R154H variation. The TRPV5 proteins encoded by 2 cRNA species mimicking the mRNAs derived from 2 identical or different alleles are shown using 2 adjacent vertical lines under the x-axis. Open, gray, and filled circles represent A8V, R154H, and A563T variation, respectively. The 2 cRNA species indicated in different groups were coinjected at an equal amount (6.25 ng/oocyte) to mimic the TRPV5 mRNA transcripts derived from the 2 alleles with different forms of variations in TRPV5. Data from 5 independent experiments are expressed as means ± SE. B: the A563T variation was capable of increasing TRPV5-mediated Ca2+ influx in the presence of A8V variation. Data from 3 independent experiments are expressed as means ± SE. *P <0.01 vs. control group (TRPV5). #P <0.01 vs. * groups by Student's t-test.
There was one case studied in the SeattleSNPs program that in which three nonsynonymous SNPs (A8V, R154H, and A563T) coexisted in one subject. We mimicked the situation and found the presence of the A8V variation did not significantly alter the ability of A563T to increase TRPV5-mediated Ca2+ uptake (Fig. 7B). The combinations tested in Fig. 7 include all cases of TRPV5 variation found in the SeattleSNPs.
DISCUSSION
Although the frequencies of the TRPV5 variations identified in the SeatleSNPs project need to be tested in larger population studies, the chance that these genetic variations in TRPV5 present at relative high prevalence among African Americans is high. Two of the three African-specific TRPV5 variants identified from the SeattleSNPs project, A563T and L712F, significantly increase TRPV5-mediated Ca2+ influx. This suggests that these variations may contribute to African Americans' superior ability in Ca2+ reabsorption. Among the two variants, A563T exhibits a significantly higher frequency. In addition, A563T is capable of increasing Ca2+ influx in the heterozygous state; thus, it is expected that the percentage of African Americans affected by this variation would be higher than its allele frequency.
The ∼50 or 25% increase in TRPV5-mediated Ca2+ influx caused by the A563T or L712F variation should have a significant impact on Ca2+ reabsorption, taking into account that the Ca2+ reabsorption is a continuing process. The DCT and CNT, where TRPV5 is expressed, are important nephron segments for regulating the level of Ca2+ excretion in the urine. In the rat, luminal [Ca2+] was 0.9 mM in the early distal tubule and dropped to 0.04 mM in the late distal tubule as measured by free-flow micropuncture experiments (43). TRPV5 plays a key role as a gatekeeper of this active Ca2+ reabsorption process in these nephron segments, as demonstrated by a 6- to 10-fold increase in urinary Ca2+ excretion in mice lacking Trpv5 (16). Thus it is reasonable to predict a significant reduction of urinary Ca2+ excretion in individuals carrying the A563T or L712F variation.
During the review of this manuscript, three nonsynonymous SNPs (A8V, R154H, and A561T) in TRPV5 were reported in a study of 20 French patients with renal hypercalciuria (31). All of these variations exhibited no significant difference in TRPV5 activity relative to the reference TRPV5 (31). The lack of significant functional changes in A8V and R154H is consistent with our results in this study. Interestingly, the A561T variation is just two amino acids apart from the A563T variation in our study, yet it has no significant effect on TRPV5 function, suggesting a crucial role of position 563 in TRPV5 function.
The location of A563T variation in the last transmembrane domain of TRPV5 implicates the possible alteration of cation transport properties of the channel. Indeed, increased Ca2+ influx with the reduction of surface expression in the A563T variant indicates an increased Ca2+ permeation in the A563T variant. This increase in Ca2+ permeation is accompanied by an increased apparent Km for Ca2+ and an increased Mg2+ affinity. The consequence of the increased Mg2+ affinity is a reduced permeation to Na+ in the presence of extracellular Mg2+. The increased Mg2+ affinity does not affect Ca2+ influx very much, likely because the Mg2+ affinity of TRPV5 is much higher than Na+ affinity but much lower than the Ca2+ affinity (18). TRPV5 shares many permeation properties with the classical voltage-gated Ca2+ channels that choose Ca2+ over Na+ at a ratio of over 100:1 (24, 42). Four negatively charged aspartate residues (Asp542) in tetrameric TRPV5 form the main selective filter for Ca2+ (24). Because the 563 position in the last transmembrane domain of TRPV5 is close to Asp542, it is likely that A563T substitution may affect the architecture of the pore and make it more accommodating for Ca2+ ion permeation, although the lack of an accurate three-dimensional structure of TRPV5 precludes a precise prediction how this happens. It has been recently demonstrated that the substitution of a hydrophobic amino acid residue with a positively charged lysine in the last transmembrane domain of TRPM2 and TRPM8 converts the channels from cation selective to anion selective (20). This suggests that amino acid residues in the last transmembrane domain may participate in defining ion permeation properties directly or indirectly by affecting the conformation of the amino acid residues in the cation filter at the adjacent pore region. Because both Ca2+ affinity and Mg2+ affinity are determined by the Asp542 residue in the pore region of TRPV5 (18), it is not surprising that both Ca2+ permeation and Mg2+ affinity of the A563T variant are affected. The Mg2+ affinity of TRPV5 is ∼100 times lower than the Ca2+ affinity (18); therefore, the relatively small increase in Mg2+ affinity of T563 only has robust effects on Na+ influx but not on Ca2+ influx. Ca2+ permeation is inversely related to Ca2+ affinity in cyclic nucleotide-gated channels, likely because Ca2+ influx is retarded by stronger binding (12). If this is applicable to TRPV5 channel, the increased Ca2+ permeation of T563 could be a result of a decreased Ca2+ affinity as implied by the increased apparent Km value of Ca2+ uptake, although we were unable to determine the apparent Ca2+ affinity due to the Ca2+-induced Cl− current in X. laevis oocytes under our experimental condition.
By substitution of Thr563 with serine or valine, we pinpointed that the methyl group in the side chain of Thr563 is responsible for the increase in Ca2+ uptake. It is likely that the bulky side chain of threonine relative to alanine supports a favorable translocation pathway for Ca2+. The hydroxyl group of Thr563, on the other hand, is likely responsible for the increase in Mg2+ affinity and does not appear to affect Ca2+ transport. These substitutions further confirm the importance of amino acid residue 563 in the cation permeation properties of TRPV5.
If the urinary calcium excretion data were compared between adolescent African American and white girls in a well-controlled Ca2+ balance study (8), it is noticeable that ∼¼ to ⅓ of African Americans showed a very low value of urinary calcium excretion while other individuals of African Americans had values not much different from the white subjects. This small portion of African Americans contributed significantly to the lowered urinary Ca2+ excretion in African Americans. It is possible that these African Americans may carry gene variations that provide superior ability to conserve Ca2+ over other African Americans and whites. It is of great interest to examine if the A563T variation in TRPV5 is among those genetic factors that contribute to the superior ability to conserve Ca2+ in these African Americans. Our result also warrants future studies on the potential association between A563T variation in TRPV5 gene and the lower prevalence of osteoporosis and kidney stones among African Americans.
GRANTS
Research in our laboratory is supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK-072154.
Acknowledgments
Part of this work was presented in the form of abstract at Experimental Biology 2008, San Diego, CA, April 5–9, 2008 and the Southern Salt, Water and Kidney Club, Longboat Key, Florida, December 6, 2008.
REFERENCES
- 1.Akey JM, Eberle MA, Rieder MJ, Carlson CS, Shriver MD, Nickerson DA, Kruglyak L. Population history and natural selection shape patterns of genetic variation in 132 genes. PLoS Biol 2: e286, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akey JM, Swanson WJ, Madeoy J, Eberle M, Shriver MD. TRPV6 exhibits unusual patterns of polymorphism and divergence in worldwide populations. Hum Mol Genet 15: 2106–2113, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Asplin JR, Donahue S, Kinder J, Coe FL. Urine calcium excretion predicts bone loss in idiopathic hypercalciuria. Kidney Int 70: 1463–1467, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol 52: 243–249, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Bell NH, Shary J, Stevens J, Garza M, Gordon L, Edwards J. Demonstration that bone mass is greater in black than in white children. J Bone Miner Res 6: 719–723, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Bohannon AD Osteoporosis and African American women. J Womens Health Gend Based Med 8: 609–615, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Bohannon AD, Hanlon JT, Landerman R, Gold DT. Association of race and other potential risk factors with nonvertebral fractures in community-dwelling elderly women. Am J Epidemiol 149: 1002–1009, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Braun M, Palacios C, Wigertz K, Jackman LA, Bryant RJ, McCabe LD, Martin BR, McCabe GP, Peacock M, Weaver CM. Racial differences in skeletal calcium retention in adolescent girls with varied controlled calcium intakes. Am J Clin Nutr 85: 1657–1663, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Crawford DC, Carlson CS, Rieder MJ, Carrington DP, Yi Q, Smith JD, Eberle MA, Kruglyak L, Nickerson DA. Haplotype diversity across 100 candidate genes for inflammation, lipid metabolism, and blood pressure regulation in two populations. Am J Hum Genet 74: 610–622, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniels ED, Pettifor JM, Schnitzler CM, Moodley GP, Zachen D. Differences in mineral homeostasis, volumetric bone mass and femoral neck axis length in black and white South African women. Osteoporos Int 7: 105–112, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Dascal N The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem 22: 317–387, 1987. [DOI] [PubMed] [Google Scholar]
- 12.Dzeja C, Hagen V, Kaupp UB, Frings S. Ca2+ permeation in cyclic nucleotide-gated channels. EMBO J 18: 131–144, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebihara L Xenopus connexin38 forms hemi-gap-junctional channels in the nonjunctional plasma membrane of Xenopus oocytes. Biophys J 71: 742–748, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci 118: 917–928, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Hoenderop JG, van der Kemp AW, Hartog A, van de Graaf SF, Van Os CH, Willems PH, Bindels RJ. Molecular Identification of the Apical Ca2+ Channel in 1,25- Dihydroxyvitamin D3-responsive Epithelia. J Biol Chem 274: 8375–8378, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Merillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest 112: 1906–1914, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J 22: 776–785, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jean K, Bernatchez G, Klein H, Garneau L, Sauve R, Parent L. Role of aspartate residues in Ca2+ affinity and permeation of the distal ECaC1. Am J Physiol Cell Physiol 282: C665–C672, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y, Ferguson WB, Peng JB. WNK4 enhances TRPV5-mediated calcium transport: potential role in hypercalciuria of familial hyperkalemic hypertension caused by gene mutation of WNK4. Am J Physiol Renal Physiol 292: F545–F554, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn FJ, Knop G, Luckhoff A. The transmembrane segment S6 determines cation versus anion selectivity of TRPM2 and TRPM8. J Biol Chem 282: 27598–27609, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Loffing J, Kaissling B. Sodium and calcium transport pathways along the mammalian distal nephron: from rabbit to human. Am J Physiol Renal Physiol 284: F628–F643, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Miledi R, Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol 357: 173–183, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilius B, Vennekens R, Prenen J, Hoenderop JG, Bindels RJ, Droogmans G. Whole-cell and single channel monovalent cation currents through the novel rabbit epithelial Ca2+ channel ECaC. J Physiol 527: 239–248, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilius B, Vennekens R, Prenen J, Hoenderop JG, Droogmans G, Bindels RJ. The single pore residue Asp542 determines Ca2+ permeation and Mg2+ block of the epithelial Ca2+ channel. J Biol Chem 276: 1020–1025, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Osorio AV, Alon US. The relationship between urinary calcium, sodium, and potassium excretion and the role of potassium in treating idiopathic hypercalciuria. Pediatrics 100: 675–681, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Peng JB, Brown EM, Hediger MA. Structural conservation of the genes encoding CaT1, CaT2, and related cation channels. Genomics 76: 99–109, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Peng JB, Chen XZ, Berger UV, Vassilev PM, Brown EM, Hediger MA. A rat kidney-specific calcium transporter in the distal nephron. J Biol Chem 275: 28186–28194, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem 274: 22739–22746, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Pratt JH, Manatunga AK, Peacock M. A comparison of the urinary excretion of bone resorptive products in white and black children. J Lab Clin Med 127: 67–70, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev 80: 277–313, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Renkema KY, Lee K, Topala CN, Goossens M, Houillier P, Bindels RJ, Hoenderop JG. TRPV5 gene polymorphisms in renal hypercalciuria. Nephrol Dial Transplant 2009. PMID: 19131347. [DOI] [PubMed]
- 32.Sarmina I, Spirnak JP, Resnick MI. Urinary lithiasis in the black population: an epidemiological study and review of the literature. J Urol 138: 14–17, 1987. [DOI] [PubMed] [Google Scholar]
- 33.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seifert-McLean CM, Cromer BA, Mosher G, Mahan JD. Urinary calcium excretion in healthy adolescents. J Adolesc Health Care 10: 300–304, 1989. [DOI] [PubMed] [Google Scholar]
- 35.So NP, Osorio AV, Simon SD, Alon US. Normal urinary calcium/creatinine ratios in African-American and Caucasian children. Pediatr Nephrol 16: 133–139, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin H. Demographic and geographic variability of kidney stones in the United States. Kidney Int 46: 893–899, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Stajich JE, Hahn MW. Disentangling the effects of demography and selection in human history. Mol Biol Evol 22: 63–73, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 63: 1817–1823, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki Y, Pasch A, Bonny O, Mohaupt MG, Hediger MA, Frey FJ. Gain-of-function haplotype in the epithelial calcium channel TRPV6 is a risk factor for renal calcium stone formation. Hum Mol Genet 17: 1613–1618, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Taylor EN, Curhan GC. Differences in 24-hour urine composition between black and white women. J Am Soc Nephrol 18: 654–659, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Trotter M, Broman GE, Peterson RR. Densites of bones of white and Negro skeletons. J Bone Joint Surg Am 42-A: 50–58, 1960. [PubMed] [Google Scholar]
- 42.Vennekens R, Hoenderop JG, Prenen J, Stuiver M, Willems PH, Droogmans G, Nilius B, Bindels RJ. Permeation and gating properties of the novel epithelial Ca2+ channel. J Biol Chem 275: 3963–3969, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Vick RS, Costanzo LS. In situ measurement of ionized Ca concentration ([Ca2+]i) in rat distal fluid. Kidney Int 33, 351. 1988. Abstract.
- 44.Weber WM, Liebold KM, Reifarth FW, Uhr U, Clauss W. Influence of extracellular Ca2+ on endogenous Cl− channels in Xenopus oocytes. Pflugers Arch 429: 820–824, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Wigertz K, Palacios C, Jackman LA, Martin BR, McCabe LD, McCabe GP, Peacock M, Pratt JH, Weaver CM. Racial differences in calcium retention in response to dietary salt in adolescent girls. Am J Clin Nutr 81: 845–850, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Na T, Peng JB. WNK3 positively regulates epithelial calcium channels TRPV5 and TRPV6 via a kinase-dependent pathway. Am J Physiol Renal Physiol 295: F1472–F1484, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]