the molecular mechanisms of the numerous cellular actions of thyroid hormone have been widely studied. The classical mechanism of thyroid hormone action occurs by uptake of l-thyroxine (T4) or 3,5,3′ triiodo-l-thyronine (T3) into cells, transport into the cell nucleus, binding with a thyroid receptor (TR), recruitment of coactivators, and regulation of gene transcription via thyroid response elements (TRE). T3 is more potent in these actions than T4. These genomic actions require access of the hormone to the cell interior, translocation to the nucleus, alteration of the rate of gene transcription, and translation of the specific gene product; thus, the overall response generally requires several hours to become manifest. Over the past decade, many actions of thyroid hormone have been described that do not involve initial nuclear action of thyroid receptors and/or gene transcription; therefore they are considered “nongenomic” (6). Davis and colleagues (6, 7) have described both TR-dependent and TR-independent novel nongenomic actions that involve cell surface receptors and signal transduction pathways. Some actions that begin nongenomically at the cell surface may ultimately become nuclear and cellular events. One example is the phosphorylation of the TRβ by T4 that results in derepression of the transcriptional activity of SMRT (silencing mediator of retinoid and thyroid hormone receptor) by dissociation of TR and SMRT (7). Another example is that thyroid hormone promotes cell proliferation via nongenomic actions in the chick chorioallantoic membrane model (4) and in glioma cells (5).
The nongenomic actions of thyroid hormone include generation of second messengers directly involved in signaling pathways that include the phosphatidylinositol 3-kinase (PI3K) (2, 12, 15, 16) or mitogen-activated protein kinase (MAPK) (11, 13, 17, 20) pathways. In a number of studies, T3 acted by stimulating the PI3K pathway, although this has not been demonstrated for T4. In human skin fibroblasts, Cao et al. (2) elucidated a T3-dependent signaling cascade leading to ZAKI-4α expression via mammalian target of rapamycin (mTOR) activation. The mTOR activation was mediated by a PI3K-Akt/PKB signaling cascade, because T3 induced phosphorylation of Akt/PKB more rapidly than that of mTOR. The T3-dependent phosphorylations were blocked both by PI3K inhibitors and by expression of a dominant-negative PI3K. The regulation of PI3K pathway by T3 was altered in gastric cancer, raising the possibility that changes in nongenomic signaling by T3 play a potential role in disease states (15). Lei and colleagues (12) demonstrated that T3 stimulated the PI3K/PKB pathway via the Src family of tyrosine kinases. In this system, activation of both Src kinase and PI3K was required for the T3-induced stimulation of Na-K-ATPase activity and its cell surface expression in adult rat alveolar epithelial cells (12).
Both T4 and T3 thyroid hormones nongenomically regulate signal transduction pathways other than the PI3K pathway, including MAP kinases. For example, T3 activates MAPK/ERK1/2 in alveolar epithelial cells, stimulating the sodium pump in a dose- and time-dependent manner (11). In prior work, Lin et al. (13) showed that thyroid hormone-enhanced IFN-γ induced antiviral activity in HeLa cells, which lack thyroid hormone receptors. This effect was activated by T4, T4-agarose, and, to a lesser extent, T3. This effect also required activation of the MAPK cascade and an interaction with the STAT1α pathway that is activated by the IFN-γ (13). Proangiogenic effects of thyroid hormone and its analogs also depend on ERK1/2 signaling in a chick chorioallantoic membrane model (17). T4, T4-agarose, and the thyroid hormone analog 3,5-diiodothyropropionic acid (DITPA) stimulated angiogenesis in this model, and the magnitude of the angiogenic effect was similar to that of VEGF and basic FGF. Either tetraiodothyroacetic acid (tetrac), a known inhibitor of binding of T4 to plasma membrane integrins, or a MAPK pathway inhibitor inhibited DITPA-induced angiogenesis. In human osteoblast-like cells, both T3 and T4 activated ERK, which resulted in DNA synthesis and cell proliferation (20). Thus there is accumulating evidence that thyroid hormones and their analogs can have rapid nongenomic effects and stimulate more than one signal transduction pathway. It is uncertain whether there is cross talk between different pathways that are stimulated by thyroid hormones.
T4 and T3 are able to activate other intracellular signal transduction cascades beyond PI3K and MAPK. Acting independently of TR, thyroid hormone modulates the activity of the plasma membrane Na+/H+ exchanger (10), Ca2+-dependent stimulation of adenosine triphosphatase (23), and other ion pumps or channels [inward potassium channel (19) and sodium current (9) in cardiac myocytes]. They also stimulate the guanosine triphosphatase activity of synaptosomes (8).
Studies of thyroid hormone action on cell surface events, such as calcium efflux (3, 18) or glucose uptake (21, 22), several decades ago implied the existence of one or more plasma membrane receptors for T3 or T4. Recently, integrin-αvβ3 has been reported as a cell surface receptor for T4 in CV-1 cells, a monkey fibroblast cell line that lacks functional thyroid hormone receptors (1). Inhibition of the proangiogenic effects of thyroid hormone in chick chorioallantoic membrane model by LM609, a monoclonal antibody directed against αvβ3-integrin, suggests the involvement of αvβ3 as a surface receptor (4).
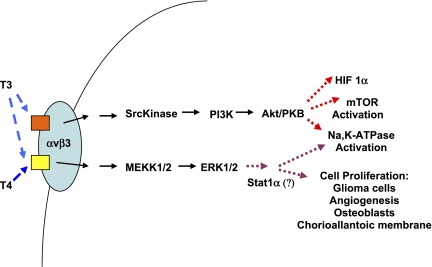
Lin et al. (14) studied the role of l-thyroxine and 3,5,3′ triiodo-l-thyronine in cell proliferation of human glioma cells and the contributions of MAPK (ERK1/2) and PI3K pathways in the actions of T3 and T4 (Fig. 1). They demonstrate that T3 and T4 activate ERK1/2 and proliferating cell nuclear antigen (PCNA) accumulation in a concentration-dependent manner. Although ERK activation occurred within 30 min, PCNA accumulation was seen at 24 h. In contrast, activation of PI3K with phosphorylation of its p85 subunit occurred in the U-87 MG cells treated with T3, but not with T4. The T3-induced activation of PI3K was blocked by Arg-Gly-Asp, indicating a role of integrin receptors. While the activation of the ERK1/2 pathway was necessary for thyroid hormone-induced cell proliferation in these glioma cells, the T3-induced PI3K activation caused nuclear accumulation of TRα, but not TRβ1. PI3K activation by T3 was required for T3-induced expression of hypoxia-inducible factor-1α. Taken in combination, their results suggest that there are two different receptor sites for thyroid hormones on one integrin molecule that cause downstream activation of ERK and/or PI3K. T3 binds to both sites and activates both the ERK and PI3K pathways, whereas T4 only activates ERK1/2 after binding to only one of the two surface integrin sites.
Fig. 1.
Specificity of thyroid hormone signaling through plasma membrane integrins. The plasma membrane αvβ3-integrin has distinct binding sites for 3,5,3′-triiodo-l-thyronine (T3) and l-thyroxine (T4). One binding site binds only T3 and activates the phosphatidylinositol 3-kinase (PI3K) pathway. The other binding site binds both T3 and T4 and activates the ERK1/2 MAP kinase pathway. HIF-1α, hypoxia-inducible factor-1α; mTOR, mammalian target of rapamycin.
This work reveals the novel finding of specificity of T3 and T4 acting on a single cell surface receptor at apparently distinct sites within the molecule that regulate activation of separate downstream signaling pathways. Like the glioma cells, alveolar epithelial cells also demonstrate T3 activation of both the MAPK and PI3K pathways, but the ligand to which T3 binds in that system has not been determined yet. It will be interesting to determine whether a single integrin molecule can differentially activate nongenomic signaling pathways depending on the specific ligands. The studies of Lin et al. (13, 14) add significantly to our knowledge of the rapid nongenomic actions of thyroid hormones, demonstrating a sophisticated specificity exerted at the cell surface binding of hormone to plasma membrane integrin that affects TR activity and signaling pathway activation. Another unresolved question is whether there are intracellular interactions between the PI3K and MAPK signaling pathways after they are triggered by thyroid hormones at the plasma membrane. These findings are important biologically and also offer the opportunity to target specific pathways that may be manipulated in treating pathological states.
GRANTS
The authors' work is supported by National Institutes of Health Grant 5T32HL007741 and philanthropic funding from the Cargill Pulmonary Research Fund of the Minnesota Medical Foundation.
REFERENCES
- 1.Bergh JJ, Lin HY, Lansing L, Mohamed SN, Davis FB, Mousa S, Davis PJ. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 146: 2864–2871, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Cao X, Kambe F, Moeller LC, Refetoff S, Seo H. Thyroid hormone induces rapid activation of Akt/protein kinase B-mammalian target of rapamycin-p70S6K cascade through phosphatidylinositol 3-kinase in human fibroblasts. Mol Endocrinol 19: 102–112, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Davis FB, Cody V, Davis PJ, Borzynski LJ, Blas SD. Stimulation by thyroid hormone analogues of red blood cell Ca2+-ATPase activity in vitro. Correlations between hormone structure and biological activity in a human cell system. J Biol Chem 258: 12373–12377, 1983. [PubMed] [Google Scholar]
- 4.Davis FB, Mousa SA, O'Connor L, Mohamed S, Lin HY, Cao HJ, Davis PJ. Proangiogenic action of thyroid hormone is fibroblast growth factor-dependent and is initiated at the cell surface. Circ Res 94: 1500–1506, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Davis FB, Tang HY, Shih A, Keating T, Lansing L, Hercbergs A, Fenstermaker RA, Mousa A, Mousa SA, Davis PJ, Lin HY. Acting via a cell surface receptor, thyroid hormone is a growth factor for glioma cells. Cancer Res 66: 7270–7275, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Davis PJ, Leonard JL, Davis FB. Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol 29: 211–218, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Davis PJ, Shih A, Lin HY, Martino LJ, Davis FB. Thyroxine promotes association of mitogen-activated protein kinase and nuclear thyroid hormone receptor (TR) and causes serine phosphorylation of TR. J Biol Chem 275: 38032–38039, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Giguere A, Fortier S, Beaudry C, Gallo-Payet N, Bellabarba D. Effect of thyroid hormones on G proteins in synaptosomes of chick embryo. Endocrinology 137: 2558–2564, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Huang CJ, Geller HM, Green WL, Craelius W. Acute effects of thyroid hormone analogs on sodium currents in neonatal rat myocytes. J Mol Cell Cardiol 31: 881–893, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Incerpi S, Luly P, De Vito P, Farias RN. Short-term effects of thyroid hormones on the Na/H antiport in L-6 myoblasts: high molecular specificity for 3,3′,5-triiodo-l-thyronine. Endocrinology 140: 683–689, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Lei J, Mariash CN, Bhargava M, Wattenberg EV, Ingbar DH. T3 increases Na-K-ATPase activity via a MAPK/ERK1/2-dependent pathway in rat adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 294: L749–L754, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Lei J, Mariash CN, Ingbar DH. 3,3′,5-Triiodo-l-thyronine up-regulation of Na,K-ATPase activity and cell surface expression in alveolar epithelial cells is Src kinase- and phosphoinositide 3-kinase-dependent. J Biol Chem 279: 47589–47600, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Lin HY, Davis FB, Gordinier JK, Martino LJ, Davis PJ. Thyroid hormone induces activation of mitogen-activated protein kinase in cultured cells. Am J Physiol Cell Physiol 276: C1014–C1024, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Lin H, Sun M, Tang H, Lin C, Luidens MK, Mousa SA, Incerpi S, Drusano GL, Davis FB, Davis PJ. l-Thyroxine vs. 3,5,3′-triiodo-l-thyronine and cell proliferation: activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am J Physiol Cell Physiol (January 21, 2009). doi: 10.1152/ajpcell.00305.2008. [DOI] [PubMed]
- 15.Liu R, Li Z, Bai S, Zhang H, Tang M, Lei Y, Chen L, Liang S, Zhao YL, Wei Y, Huang C. Mechanism of cancer cell adaptation to metabolic stress: proteomics identification of a novel thyroid hormone-mediated gastric carcinogenic signaling pathway. Mol Cell Proteomics 8: 70–85, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Moeller LC, Cao X, Dumitrescu AM, Seo H, Refetoff S. Thyroid hormone mediated changes in gene expression can be initiated by cytosolic action of the thyroid hormone receptor beta through the phosphatidylinositol 3-kinase pathway. Nucl Recept Signal 4: e020, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mousa SA, O'Connor L, Davis FB, Davis PJ. Proangiogenesis action of the thyroid hormone analog 3,5-diiodothyropropionic acid (DITPA) is initiated at the cell surface and is integrin mediated. Endocrinology 147: 1602–1607, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Nieman LK, Davis FB, Davis PJ, Cunningham EE, Gutman S, Blas SD, Schoenl M. Effect of end-stage renal disease on responsiveness to calmodulin and thyroid hormone of calcium-ATPase in human red blood cells. Kidney Int Suppl 16: S167–S170, 1983. [PubMed] [Google Scholar]
- 19.Sakaguchi Y, Cui G, Sen L. Acute effects of thyroid hormone on inward rectifier potassium channel currents in guinea pig ventricular myocytes. Endocrinology 137: 4744–4751, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Scarlett A, Parsons MP, Hanson PL, Sidhu KK, Milligan TP, Burrin JM. Thyroid hormone stimulation of extracellular signal-regulated kinase and cell proliferation in human osteoblast-like cells is initiated at integrin alphaVbeta3. J Endocrinol 196: 509–517, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Segal J, Ingbar SH. 3,5,3′-Tri-iodothyronine enhances sugar transport in rat thymocytes by increasing the intrinsic activity of the plasma membrane sugar transporter. J Endocrinol 124: 133–140, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Segal J, Ingbar SH. Evidence that an increase in cytoplasmic calcium is the initiating event in certain plasma membrane-mediated responses to 3,5,3′-triiodothyronine in rat thymocytes. Endocrinology 124: 1949–1955, 1989. [DOI] [PubMed] [Google Scholar]
- 23.Smith TJ, Davis FB, Davis PJ. Stereochemical requirements for the modulation by retinoic acid of thyroid hormone activation of Ca(2+)-ATPase and binding at the human erythrocyte membrane. Biochem J 284: 583–587, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]