Abstract
The σ-receptor, a broadly distributed integral membrane protein with a novel structure, is known to modulate various voltage-gated K+ and Ca2+ channels through a mechanism that involves neither G proteins nor phosphorylation. The present study investigated the modulation of the heart voltage-gated Na+ channel (Nav1.5) by σ-receptors. The σ1-receptor ligands [SKF-10047 and (+)-pentazocine] and σ1/σ2-receptor ligands (haloperidol and ditolylguanidine) all reversibly inhibited Nav1.5 channels to varying degrees in human embryonic kidney 293 (HEK-293) cells and COS-7 cells, but the σ1-receptor ligands were less effective in COS-7 cells. The same four ligands also inhibited Na+ current in neonatal mouse cardiac myocytes. In σ1-receptor knockout myocytes, the σ1-receptor-specific ligands were far less effective in modulating Na+ current, but the σ1/σ2-receptor ligands modulated Na+ channels as well as in wild type. Photolabeling with the σ1-receptor photoprobe [125I]-iodoazidococaine demonstrated that σ1-receptors were abundant in heart and HEK-293 cells, but scarce in COS-7 cells. This difference was consistent with the greater efficacy of σ1-receptor-specific ligands in HEK-293 cells than in COS-7 cells. σ-Receptors modulated Na+ channels despite the omission of GTP and ATP from the patch pipette solution. σ-Receptor-mediated inhibition of Na+ current had little if any voltage dependence and produced no change in channel kinetics. Na+ channels represent a new addition to the large number of voltage-gated ion channels modulated by σ-receptors. The modulation of Nav1.5 channels by σ-receptors in the heart suggests an important pathway by which drugs can alter cardiac excitability and rhythmicity.
Keywords: heart muscle, haloperidol
σ-receptors were originally identified by their ability to bind opioid receptor ligands (22), but they were subsequently shown not to bind opioid peptides (40). In addition to the natural hallucinogen, N,N-dimethyltryptamine (9), σ-receptors are sensitive to an impressive array of ligands, including opioid receptor ligands such as pentazocine; dopamine receptor ligands such as haloperidol; drugs of abuse such as cocaine and amphetamines; fungicides such as fenpropimorph; class III antiarrhythmics such as amiodarone; vasodilators such as infenprodil; and completely novel compounds such as ditolylguanidine (DTG) (24, 26, 32, 40, 44). Many mammalian tissues express σ-receptors, including heart, brain, liver, kidney, and placenta, as well as numerous forms of cancer (1, 38, 44).
σ-Receptors have been subdivided into two subtypes, σ1 and σ2. The σ1-receptor has been much better characterized: the encoding gene predicts a 25.3-kDa protein (11, 17, 38), with a deduced amino acid sequence that is highly conserved in mammals. It has no vertebrate homologues. When expressed in Xenopus oocytes, the σ1-receptor has two transmembrane segments, with both the COOH- and NH2 termini located in the cytoplasm (2). The σ2-receptor has a distinct pharmacological signature and a lower molecular mass of 19–21 kDa (14, 32), but its molecular structure is unknown.
It is well established that σ-receptors modulate membrane excitability. In rat sympathetic and parasympathetic neurons, σ-receptor ligands modulate N-, L-, P/Q- and R-type Ca2+ channels (48). In rat neurohypophysis, σ-receptors modulate two types of voltage-activated K+ channels (45). Additional studies in rat hippocampal slices, intracardiac neurons, and tumor cells have demonstrated modulation of various K+ channels (18, 27, 47). Association of the σ1-receptor and the Kv1.4 K+ channel has been shown in posterior pituitary nerve terminals and Xenopus oocytes (2), and these proteins colocalize in the focal adhesions of CHO-K1 cells (23). In general, the modulation of ion channels by σ-receptors does not depend on G proteins or phosphorylation (21, 33, 47) but instead results from a direct protein-protein interaction between the receptor and channel (2). σ-Receptors have also been observed in the endoplasmic reticulum, and their association with nascent plasma membrane ion channels may be relevant to their proposed function as a molecular chaperone (12).
A number of σ-receptor ligands influence cardiovascular function, and the heart has binding sites for σ-receptor ligands (6, 7). σ-Receptor ligands alter contractility, Ca2+ influx, and rhythmic activity in cultured cardiac myocytes, but these actions are complex and controversial. σ-Receptor ligands can either increase or decrease the contractile force, with a variable time course that depends on concentration and animal age (7, 8, 28, 29). In myocytes from newborn rats, σ-receptor ligands enhance Ca2+ entry (7), but in adult myocytes the elevation of cellular Ca2+ depends on Ca2+ from sarcoplasmic reticulum (28, 29). Some σ-receptor ligands have proarrhythmic effects, whereas others have antiarrhythmic effects (26). Various σ-receptor ligands inhibit K+ currents and increase the duration of the QT period (15, 27). On the other hand, the σ-receptor ligands haloperidol and chlorpromazine have been shown to block heart Na+ channels in a manner consistent with an antiarrhythmic action (31).
To clarify the effects of σ-receptor ligands on the heart, we have used patch-clamp techniques to study the modulation of cardiac Na+ channels by σ-receptors. Our results in both myocytes and heterologous expression systems indicate that σ-receptor ligands inhibit the cardiac voltage-gated Na+ channel Nav1.5. These results extend the already diverse range of voltage-gated ion channels that are modulated by σ-receptors, and may account for some of the actions of σ-receptor ligands on the cardiovascular system.
METHODS
Ethical approval.
All protocols were approved by the Animal Care and Use Committee of the University of Wisconsin, in accordance with the guidelines of the National Institutes of Health.
Cell culture and transfection.
Human embryonic kidney 293 (HEK-293) cells stably expressing Nav1.5 were provided by Dr. J. C. Makielski at the University of Wisconsin-Madison (43). COS-7 cells were transiently transfected with recombinant cDNA encoding Nav1.5 using Lipofectamine (Invitrogen, San Diego, CA) according to the manufacturer's instructions. Both cell types were cultured on glass coverslips at 37°C in 5% CO2-air atmosphere and were used for electrophysiological recordings within 3–5 days. HEK-293 cells were cultured in Eagle's minimum essential medium (Fisher Scientific, Pittsburg, PA) with 10% fetal bovine serum, 1% penicillin/streptomycin, 1% l-glutamine, 1% sodium pyruvate, and 400 μg/ml gentamicin to select for human cardiac Nav1.5 expressing cells. COS-7 cells were cultured in Dulbecco's modified Eagle's medium (Fisher Scientific) with 10% cosmic calf serum and 1% penicillin/streptomycin.
A σ1-receptor small interfering RNA (siRNA) construct was designed using the pRNAT-U6.1/Neo plasmid (GenScript, Piscataway, NJ). An siRNA sequence corresponding to nucleotides 500–519 of the human σ1-receptor open reading frame (PubMed nucleotide ID: NM005866) was inserted into the above plasmid for transfection into mammalian cells. HEK-293 cells stably expressing Nav1.5 were transiently transfected with this construct using TransIT-293 Transfection Reagent (Mirus Bio, Madison, WI) according to the manufacturer's instructions. Cells were cultured and used as described above.
Neonatal myocyte isolation and culture.
Neonatal mouse cardiac myocytes were enzymatically isolated and cultured on laminin-coated glass coverslips as previously described (3, 30). Briefly, hearts were aseptically removed from 1- to 3-day-old mouse pups 129/SvEvBrd × C57BL6/J, σ1-receptor knockout (20), and wild type following rapid decapitation. Hearts were minced in digestion enzyme solution consisting of Ca2+/Mg2+-free Hanks' balanced salt solution with collagenase type II (0.1 mg/ml) (Invitrogen), and pancreatin (1 mg/ml) (Sigma-Aldrich, St. Louis, MO), gently agitated repeatedly, and centrifuged to isolate single myocytes. Cells were plated on laminin-coated glass coverslips, cultured at 37°C in 5% CO2-air atmosphere, and used for electrophysiological recording within 5–8 days. Myocytes were cultured in Dulbecco's modified Eagle's medium (Fisher Scientific) with 25% medium 199, 1% penicillin/streptomycin, 1% l-glutamine, and either 10% horse serum plus 5% fetal bovine serum or 0.5% horse serum plus 0.5% fetal bovine serum. Spontaneously contracting cells appeared within 1–2 days.
Electrophysiology.
Na+ current (INa) was recorded from all cell types using whole cell patch-clamp techniques. Cultured cells were superfused with external recording solutions (compositions stated below) by gravity feed at approximately 1–2 ml/min. All experiments were conducted at room temperature (22–24°C). Individual cells were located with an upright microscope equipped with a Zeiss ×40 water immersion objective (Carl Zeiss MicroImaging, Thornwood, NY). Patch pipettes were fabricated from borosilicate or aluminosilicate glass (Garner Glass, Claremont, CA), and pipette shanks were coated with Sylgard to reduce electrode capacitance (10). Before contact with the cell membrane, resistances ranged from 1 MΩ to 3 MΩ. Immediately after breaking in, cell capacitance and series resistance were determined by transient cancellation. Series resistance was compensated 85–95% to reduce the effective series resistance to ∼1 MΩ. When the product of peak INa times the series resistance indicated a series resistance voltage error of >5 mV, the data from that recording were discarded. Recordings were made using an Axopatch-200B patch-clamp amplifier (Axon Instruments/Molecular Devices, Foster City, CA), interfaced to a PC. Data acquisition, voltage control, and analysis were carried out with pCLAMP7 software (Axon Instruments/Molecular Devices).
External solution for recordings in HEK-293 cells (based on Ref. 43) and COS-7 cells (based on Ref. 25) consisted of (in mM) 140 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 glucose, and 10 HEPES (pH 7.4 adjusted with NaOH). The pipette solution contained (in mM) 140 KCl, 2 MgCl2, 1 CaCl2, 5 EGTA, 10 glucose, and 10 HEPES (pH 7.2 KOH). Extracellular solution for recordings from neonatal cardiac myocytes (based on Ref. 30) consisted of (in mM) 100 tetraethylammonium chloride, 40 NaCl, 10 glucose, 1 MgCl2, 5 CsCl, 0.1 CaCl2, 1 NiCl2, and 10 HEPES (pH 7.3 CsOH). Intracellular solution contained (in mM) 135 CsCl, 5 NaCl, 10 EGTA, and 10 HEPES (pH 7.3 CsOH). Note that ATP and GTP were not included. INa was typically elicited with pulses from −80 mV to −10 mV for 25 ms. For current-voltage analysis, cells were held at −80 mV and were depolarized with 25-ms pulses from −70 to +70 mV in 10-mV increments.
Membrane preparation and photoaffinity labeling.
Photoaffinity labeling was performed using the σ1-receptor photolabel [125I]-iodoazidococaine (IACOC), as previously described (16, 32). HEK-293 and COS-7 cell homogenates were prepared by passing cells 50 times through a custom-built cell cracker. Mouse heart membranes were prepared as previously described with minor modifications (34). Three to five mouse hearts were homogenized at 4°C in 10 ml of TE buffer (10 mM Tris·HCl and 1 mM EDTA pH 7.4). The homogenate was centrifuged at 1,000 g for 10 min, and the pellet was resuspended in the same volume for another spin. The supernatants from both the spins were pooled and centrifuged at 100,000 g for 1 h. The resulting pellets were suspended in 50 mM Tris·HCl, pH 7.4, assayed for protein using a Bradford protein assay (Bio-Rad, Hercules, CA) aliquoted, and frozen at −80°C until use.
For photolabeling experiments, equivalent amounts (500 μg) of cell homogenate (HEK-293 cells, COS-7 cells, or mouse heart membranes) were incubated in the presence (when indicated) and absence of 5 μM haloperidol in 50 mM Tris, pH 7.4, for 25 min on ice. IACOC (1 nM) was then added, and the reaction was continued on ice for 15 min, after which samples were illuminated for 6 s with a high-pressure AH6-mercury lamp. Following photolabeling, the membranes were solubilized with 1% Triton X-100 or 0.2% CHAPS (in some cases), and centrifuged at 14,000 g for 30 min to separate the extract. σ1-Receptor polyclonal antibody (36) (2 μg) was added to the solubilized extract and incubated at 4°C for at least 4 h. Immune complexes were captured using protein A Sepharose (GE Healthcare). Proteins were eluted with sample buffer and separated on 12% SDS-PAGE. The gel was placed on a PhosphorImager (445 SI, Molecular Dynamics) exposure cassette for at least 8 h, after which the cassette was scanned to develop the autoradiogram.
Drug application.
SKF-10047, (+)-pentazocine, haloperidol, and DTG were obtained from Sigma-Aldrich. DTG and occasionally (+)-pentazocine were first dissolved in DMSO, and then diluted in external solution to obtain the desired drug concentration. Final DMSO never exceeded 0.1% (by volume), and control experiments verified that this level of DMSO had no effect on sodium currents. Drugs were applied in recording solution by gravity feed at approximately 1–2 ml/min. In general, currents were recorded at 15-s intervals for ∼5 min to obtain a stable baseline, after which the drug was applied. Drug effects typically appeared within 2–4 min of solution change and were recorded until a stable inhibition level was achieved. Reversal of response following drug removal was checked routinely.
Data analysis.
Current recordings were analyzed on a PC with pCLAMP7 software. Simple statistical analyses were performed on exported data using Microsoft Excel. Concentration dependence of INa inhibition was analyzed using Prism (Graphpad Software, San Diego, CA), by fitting to a single-site saturation equation of the form I = Icontrol/(IC50 + [X]), where I is peak current for a voltage step to −10 mV, [X] is drug concentration, and IC50 is the concentration producing 50% block. Arithmetic means were computed and presented with the standard error of the mean. Statistical significance was calculated using one-way analysis of variance followed by the post hoc Newman-Keuls test, also using Prism.
RESULTS
HEK-293 cells.
Patch-clamp recordings from HEK-293 cells stably expressing the human cardiac Na+ channel Nav1.5 produce large Na+ currents (INa) on the order of several nanoamps in response to voltage steps from −80 mV to −10 mV (Fig. 1, A and B). σ-Receptor ligands reversibly inhibited this INa, as illustrated in Fig. 1A for four different cells, each tested with one of the four ligands employed in this study (all at 100 μM). Two of these ligands, SKF-10047 and (+)-pentazocine, are σ1-receptor specific (13, 14), and the other two, haloperidol and DTG, bind to both types of σ-receptor and are considered σ1/σ2-receptor-specific ligands. SKF-10047, (+)-pentazocine, haloperidol, and DTG inhibited INa by 54 ± 10, 52 ± 6, 93 ± 1, and 92 ± 2%, respectively (n = 4–6). Each panel of Fig. 1 shows the control current prior to drug application, the reduced current in the presence of the drug, and the current following drug removal. Inhibition by all of these ligands was reversible; currents recovered to >75% of control levels within 10–15 min of the start of superfusion with drug-free solution. The effectiveness of this diverse group of ligands suggests that σ-receptors can modulate Nav1.5 and that HEK-293 cells express σ-receptors.
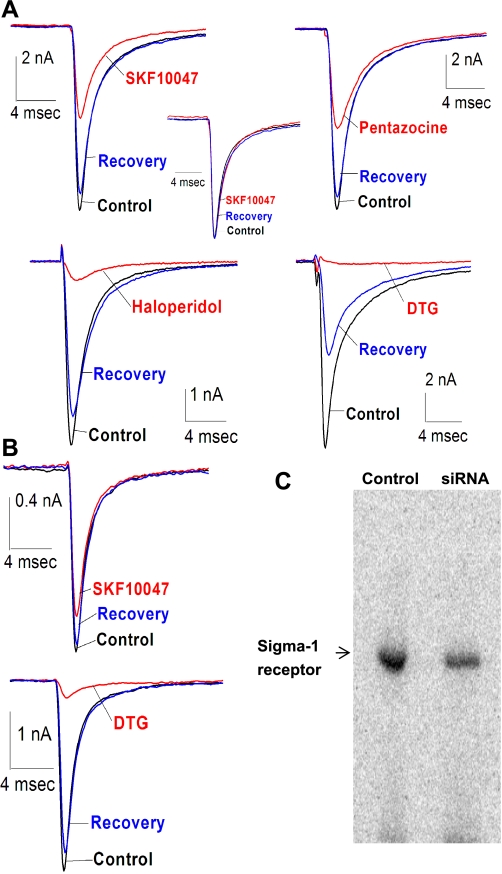
Fig. 1.
Sodium channel inhibition by σ-receptor ligands in human embryonic kidney 293 (HEK-293) cells. A: sodium currents evoked by voltage steps from −80 mV to −10 mV recorded from HEK-293 cells stably expressing human cardiac voltage-gated Na+ channel (hNav1.5) in the absence (control, black), presence (drug, red), and after washout (recovery, blue) of 100 μM SKF-10047, (+)-pentazocine, haloperidol, and ditolylguanidine (DTG). Inset: normalization of traces revealed that whole cell channel kinetics were not changed by ligand application (SKF-10047 in this case). B: similar currents were recorded from HEK-293 cells stably expressing hNav1.5 channel and transfected with σ1-receptor small interfering RNA (siRNA) in the absence, presence, and after washout of 100 μM SKF-10047 and DTG. C: immunoprecipitation-photolabeling experiments with [125I]-iodoazidococaine demonstrated that the σ1-receptor siRNA construct (siRNA) was able to knock down the level of σ1-receptor protein expression in HEK-293 cells. Average knockdown was 33 ± 9% of nontransfected (control) HEK-293 cells.
A σ1-receptor siRNA construct was designed and generated on the basis of the σ1-receptor gene sequence (the σ2-receptor has not been cloned so there is no sequence on which to base a σ2-receptor siRNA). Transfection of HEK-293 cells with this siRNA construct did not alter control INa but reduced the inhibition of INa by SKF-10047 to 26 ± 4% (compared with 54 ± 10%). The siRNA construct had no significant effect on INa inhibition by DTG (88 ± 1% vs. 92 ± 2%) (Fig. 1B; for averages with statistical significance, see Fig. 4). These results are consistent with a selective reduction of σ1-receptor protein levels by siRNA. We assayed σ1-receptor protein levels biochemically by photoaffinity labeling cell homogenates with IACOC, followed by immunoprecipitation enrichment with a σ1-receptor antibody. Resolution with SDS-PAGE and detection by autoradiography revealed strong photolabeling of a 26-kDa protein, the σ1-receptor. Expression of the σ1-receptor siRNA construct reduced this signal by an average of 33 ± 9% (Fig. 1C). This reduction observed biochemically parallels the reduction of channel modulation by the σ1-receptor-specific ligand SKF-10047.
All four ligands inhibited current in a concentration-dependent manner (Fig. 2A). Plots of peak INa versus concentration were well fitted by a single-site saturation equation (see methods), yielding IC50 values of 70, 53, 4, and 10 μM for SKF-10047, (+)-pentazocine, haloperidol, and DTG, respectively. As shown in Fig. 2A, at saturating concentrations, all four ligands reduced INa to <5% of control (data not shown).
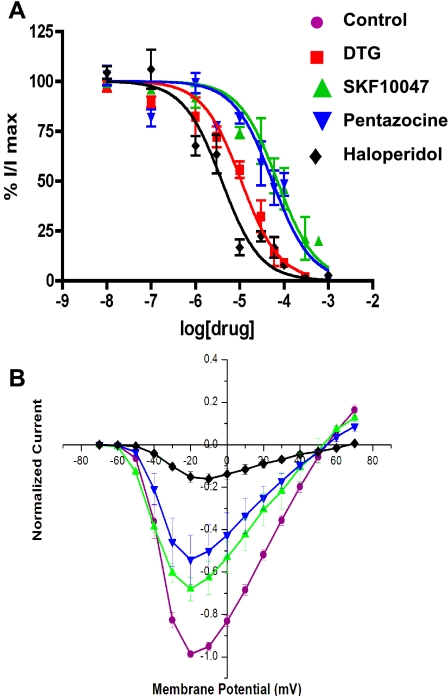
Fig. 2.
Concentration and voltage dependence of sodium channel modulation in HEK-293 cells. A: peak Na+ current (INa) amplitude, evoked by steps from −80 mV to −10 mV, was normalized to control and plotted versus drug concentration. Data points represent means ± SE for n = 4–7 cells. Curves represent best fits to the data using the single-site saturation equation given in methods. Half-maximal inhibition (IC50) values were 70, 53, 4, and 10 μM for SKF-10047(▴), pentazocine (▾), haloperidol (⧫), and DTG (▪), respectively. B: INa amplitude was determined for steps to different voltages, in the absence (control) and presence of 100 μM SKF-10047, (+)-pentazocine, and haloperidol. INa recorded in the presence of drugs was normalized to controls. Data points represent means ± SE for n = 3 cells.
These drugs inhibited INa despite the omission of ATP and GTP from the patch pipette filling solution. In recordings from HEK-293 cells lasting over an hour, modulation of INa continued unabated. Since G protein and protein kinase-mediated responses generally depend on GTP and ATP, respectively, and small molecules generally exchange in a few minutes in whole cell patch-clamp experiments (35, 42), the absence of rundown suggests that these responses to σ-receptor activation do not depend on G proteins or protein kinases.
Normalized currents recorded from HEK-293 cells before, during application, and after removal of 100 μM SKF-10047 were essentially superimposable (Fig. 1, inset). This suggests that the INa modulation simply prevents channels from opening rather than permits activity with altered kinetics. To explore this further, we examined the voltage dependence of INa modulation. Plots of current versus voltage (Fig. 2B) showed that σ-receptor ligands reduced INa at all voltages where current could be measured. The voltage threshold for INa was ∼−50 mV, with peak current between −10 and −20 mV and reversal at approximately +55 mV. Haloperidol increased the reversal potential by roughly 10 mV, but this small increase is probably insignificant and reflects the difficulty of quantitative measurements of reduced INa near its reversal potential. Fig. 2B indicates that alterations in the voltage dependence of Na+ channel gating do not play an important role in the modulation by σ-receptor ligands.
COS-7 cells.
Patch-clamp recordings from COS-7 cells transiently expressing Nav1.5 showed that voltage steps elicited INa (Fig. 3). The same four σ-receptor ligands tested in HEK-293 cells also inhibited INa in COS-7 cells. Again, haloperidol (100 μM) inhibited with the greatest potency, reducing INa by 92 ± 1%. SKF-10047, (+)-pentazocine, and DTG inhibited INa by 18 ± 5%, 28 ± 7%, and 89 ± 4%, respectively (n = 4–6). Figure 3 shows INa traces from four different COS-7 cells before, during application, and after removal, of SKF-10047, (+)-pentazocine, haloperidol, and DTG (all 100 μM). As in HEK-293 cells, INa inhibition by all of these ligands was reversible, recovering to >75% of control levels within 10–15 min (Fig. 3).
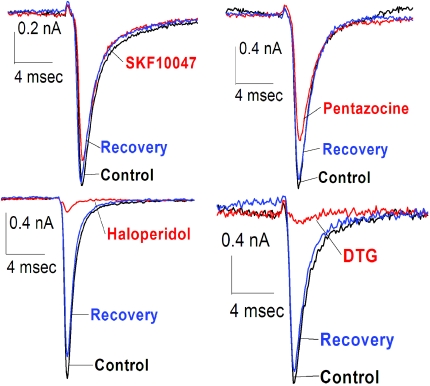
Fig. 3.
Sodium channel inhibition by σ-receptor ligands in COS-7 cells. INa was evoked by steps from −80 to −10 mV in COS-7 cells transiently expressing Nav1.5 channels in the absence (control, black), presence (drug, red), and after washout (recovery, blue) of 100 μM SKF-10047, (+)-pentazocine, haloperidol, and DTG.
As seen with HEK-293 cells (Fig. 2B), INa current-voltage plots from COS-7 cells revealed no significant shift in voltage dependence following ligand application (data not shown). Furthermore, current traces before, during, and after drug application were superimposable (data not shown), indicating that channel kinetics are not altered. Finally, the modulation continued unabated for more than half an hour despite the omission of ATP and GTP from the patch pipette filling solution.
HEK-293 cells versus COS-7 cells.
Patch-clamp recordings and photolabeling experiments did not yield identical results in COS-7 cells and HEK-293 cells. SKF-10047 and (+)-pentazocine inhibited INa much less in COS-7 cells than in HEK-293 cells. By contrast, haloperidol and DTG inhibited INa very effectively in both cell types. Figure 4A presents the averages for INa modulation in HEK-293 cells and COS-7 cells, including the siRNA-treated HEK-293 cells (from experiments as in Fig. 1B). Figure 4A shows that in control HEK-293 cells, all of the tested ligands strongly inhibited INa. By contrast, in COS-7 cells, SKF-10047 and (+)-pentazocine were much less effective than DTG and haloperidol. The difference between the σ1-receptor ligand actions in HEK-293 cells versus COS-7 cells paralleled the difference between control and siRNA-treated HEK-293 cells.
Fig. 4.
Comparison of INa inhibition in HEK-293 and COS-7 cells. A: average inhibition by each ligand at 100 μM was determined from INa traces in Figs. 1 and 3. Bars represent means ± SE for n = 4–6. The values for SKF-10047 and (+)-pentazocine differ significantly between the HEK-293 and COS-7 cells and in HEK-293 cells with and without σ1-receptor receptor siRNA (*P < 0.05). B: immunoprecipitation-photolabeling experiment with [125I]-iodoazidococaine demonstrated lower σ1-receptor expression in COS-7 cells compared with HEK-293 cells. Haloperidol (5 μM) reduced photolabeling in all cases.
Immunoprecipitation-photolabeling with IACOC provided an assessment of the differences in σ1-protein levels between HEK-293 and COS-7 cell homogenates. These experiments again demonstrated strong photolabeling of a 26-kDa protein in HEK-293 cells, and this labeling was blocked by haloperidol (Fig. 4B). By contrast, the photolabeling signal in COS-7 cells, while still haloperidol sensitive, was much weaker. Likewise, note that the low abundance of σ1-receptors in COS-7 cells, as demonstrated in σ1-receptor siRNA-transfected HEK-293 cells, parallels the very weak effects of the two σ1-receptor-specific ligands, tested in patch-clamp recordings [SKF-10047 and (+)-pentazocine]. The results of these photolabeling and patch-clamp experiments indicate that COS-7 cells have much lower levels of σ1-receptors than HEK-293 cells.
Neonatal cardiac myocytes.
The above studies showed that σ-receptor ligands modulate Nav1.5 in heterologous expression systems. To evaluate this modulation in native tissue, we tested σ-receptor ligands on INa in neonatal cardiac myocytes. Figure 5 shows INa recordings from wild-type and σ1-receptor knockout myocytes before, during, and after application of SKF-10047, (+)-pentazocine, haloperidol, and DTG (all 100 μM). These ligands inhibited INa by 46 ± 4, 49 ± 5, 90 ± 3, and 89 ± 3%, respectively (n = 3–4). In knockout myocytes, these drugs inhibited INa by 19 ± 5, 9 ± 2, 83 ± 1, and 90 ± 3%, respectively (n = 3–4). Figure 6A summarizes the percent inhibition of these drugs in myocytes. SKF-10047 and (+)-pentazocine produced much less inhibition in knockout compared with wild-type myocytes (P < 0.05). In fact, the comparison of the profiles of inhibition for the various drugs between wild-type and knockout myocytes shows a striking parallel to the comparison of profiles between HEK-293 cells and COS-7 cells (Fig. 4A). Immunoprecipitation photolabeling experiments further demonstrated this parallel. Identical experiments to those carried out with HEK-293 and COS-7 cell homogenates were performed on wild-type and σ1-receptor knockout mouse heart membranes. These experiments demonstrated the presence of σ1-receptors in wild-type mouse heart membranes and verified their absence in the knockout (Fig. 6B). Again these profiles indicate that σ1-receptors play a significant role in the inhibition of INa by the σ1-receptor-selective compounds SKF-10047 and (+)-pentazocine, but that in the absence of σ1-receptors, σ2-receptors, for which binding sites have been reported in the heart (6), can still mediate responses to haloperidol and DTG.
Fig. 5.
Sodium channel inhibition by σ-receptor ligands in cardiac myocytes. INa was evoked by steps from −80 mV to −10 mV in neonatal cardiac myocytes from wild-type (A) and σ1-receptor knockout (B) mice in the absence (control, black), presence (drug, red), and after washout (recovery, blue) of 100 μM SKF-10047, (+)-pentazocine, haloperidol, and DTG. Insets: normalization revealed that inhibition occurred without a change in channel kinetics [pentazocine (A) and DTG (B)]. Note that SKF-10047 and (+)-pentazocine, two σ1-receptor-specific ligands, inhibit INa by ∼50% in wild-type mice (A) and 20% or less in knockout (B) mice. By contrast, haloperidol and DTG, two nonspecific σ-receptor ligands, inhibited INa by ∼90 ± 3% in both wild-type and knockout mice (see Fig. 6).
Fig. 6.
Comparison of INa inhibition in wild-type (WT) and σ1-receptor knockout (KO) myocytes. A: average inhibition by each ligand at 100 μM was determined from INa traces in Fig. 5. Bars represent means ± SE for n = 3–4. There is a significant difference between current inhibition by SKF-10047 and (+)-pentazocine in the two systems (*P < 0.05). B: immunoprecipitation-photolabeling experiment with [125I]-iodoazidococaine demonstrated σ1-receptor expression in wild-type mouse heart membranes and confirmed the elimination of σ1-receptors in knockout mouse heart membranes. Haloperidol (5 μM) reduced photolabeling in wild-type mice; however, in knockout mice, there was no photolabeling either with or without haloperidol.
As in HEK-293 cells (Fig. 2B), INa peaked between −10 and −20 mV in the absence or presence of various σ-receptor ligands, in both wild-type and σ1-receptor knockout myocytes (data not shown). Channel kinetics also remained unchanged (insets of Fig. 5, A and B), as noted above for HEK-293 and COS-7 cells (inset of Fig. 1A and data not shown). In a further parallel with HEK-293 and COS-7 cells, the modulation of INa in myocytes does not require the inclusion of ATP or GTP in the patch pipette filling solution. Responses were seen in recordings lasting more than half an hour, and there was no decline in the magnitude of the response over time.
DISCUSSION
This study has shown that σ-receptor ligands modulate the voltage-gated Na+ channel Nav1.5. Experiments demonstrated this modulation in two different heterologous expression systems, as well as a native preparation (mouse cardiac myocytes). The myocyte experiments demonstrate this modulation in mice and the HEK-293 cell experiments demonstrate this modulation in humans. Thus, the present study makes the case for σ-receptor modulation of Nav1.5 channels in two different species and shows that, in each, the pharmacological profiles are similar (Figs. 4 and 6).
The four ligands tested here, SKF-10047, (+)-pentazocine, haloperidol, and DTG, all have well-documented actions on σ-receptors (40, 44, 45). Haloperidol also interacts with dopamine receptors, but as an antagonist. The minus stereoenanteomers of SKF-10047 and pentazocine interact with opioid receptors, but this interaction is much weaker for the plus stereoenanteomers used in the present study. Finally, DTG is not known to interact with receptors other than σ-receptors. We noted that similar concentrations of haloperidol were more effective at blocking photolabeling (Fig. 4B) than the inhibition of Na+ channels (Fig. 2A). A similar trend had been observed previously for SKF-10047 in DMS-114 cells, but this discrepancy was not statistically significant (46). These differences may reflect higher binding affinity of σ-receptors when not in a complex with channels. Thus, a larger excess of σ-receptors over Na+ channels in HEK cells could allow the binding properties of uncomplexed receptors to dominate in the photolabeling experiments. Although the quantitative discrepancy between photolabeling and channel modulation remains to be resolved definitively, the actions of all four of these ligands make a strong case that σ-receptors serve as the primary drug target in the modulation of INa. The present findings additionally suggest that σ-receptor activation accounts for the previously reported inhibition of cardiac Na+ channels by haloperidol (31).
The question of receptor specificity was addressed by comparing responses in cells containing high levels of σ1-receptors (HEK-293 cells and wild-type mouse cardiac myocytes), cells containing low levels of σ1-receptors (COS-7 cells and HEK-293 cells expressing σ1-receptor siRNA), and cells containing no σ1-receptors (myocytes from knockout mice). The different levels of σ1-receptor expression were established by photolabeling with IACOC (Figs. 1C, 4B, and 6B). In light of the demonstrated differences in levels of σ1-receptor, it is significant that SKF-10047 and (+)-pentazocine only slightly inhibited INa in cells with little or no σ1-receptor (COS-7 cells, HEK-293 cells expressing σ1-receptor siRNA, and knockout myocytes). These two drugs both exhibit a strong preference for σ1-receptors (13, 14), so the near elimination of their actions in three separate cellular systems provides strong evidence for σ1-receptors in the modulation of INa. By contrast, DTG and haloperidol interact strongly with both σ1- and σ2-receptors. It would thus appear that these compounds inhibit INa by binding to both σ1- and σ2-receptors. However, a more definitive demonstration of a role for σ2-receptors will have to await the development of a cell system in which this receptor species can be abolished. Until such experiments can be performed, the possibility that some inhibition of INa by direct effects of haloperidol and DTG on Na+ channels or by activation of some other type of receptor cannot be completely ruled out.
In general, σ-receptor modulation of diverse ion channels does not depend on G proteins or protein phosphorylation (21, 33, 46, 47). The present results conform to this general trend in demonstrating that modulation was maintained without attenuation for recordings lasting over an hour in HEK-293 cells and over half an hour in COS-7 cells and myocytes with patch pipette solutions containing neither ATP nor GTP. Since such solutions generally produce washout of responses that depend on protein kinases or G proteins (42), the absence of washout indicates that σ-receptor-mediated modulation of INa also does not depend on these signal transduction molecules.
σ-Receptor ligands have been shown to inhibit a large number of voltage-gated ion channels. Among K+ channels, targets include M-current, A-current, and Ca2+-activated K+ channels in sympathetic neurons (18), inward rectifying K+ channels in the heart (27); M-current, delayed rectifier, and Ca2+-activated K+ channels in intracardiac neurons (47); and A-current and Ca2+-activated K+ channels in neurohypophysis (45). σ-Receptors also inhibit a variety of Ca2+ channels, as demonstrated in hippocampal (5) and parasympathetic (48) neurons. The present study extends the σ-receptor ion channel interface by demonstrating the modulation of voltage-gated Na+ channels. If σ-receptors modulate Na+ channels by a direct association, as has been suggested for the modulation of the K+ channel Kv1.4 (2) and the L-type Ca2+ channel (41), then these receptors have a remarkable ability to associate with channel proteins from very different families. As the number of different ion channels targeted by σ-receptors increases, the question of the structural basis for the implied association assumes greater prominence. Elucidating the basis for this association may also shed light on how this modulation can be achieved either with (48) or without (2, 45) (present work) alterations in channel kinetics and shifts in voltage dependence.
The modulation of cardiac INa by σ-receptors is clearly relevant to the actions of drugs on the heart. σ-Receptors regulate intracellular Ca2+ and modulate cardiac contractility (26), with different effects in neonatal and adult rat cardiac myocytes (7, 8, 28, 29). σ-Receptor ligands have been shown to inhibit both inwardly rectifying K+ channels and human ether-a-go-go-related gene K+ channels in the heart (19, 27). Given the modulation of Ca2+ channels in neurons (5, 48), it will clearly be important to assess the effect of σ-receptor activation on cardiac Ca2+ current. The inhibition of INa described in the present study would be expected to inhibit contractility and rhythmicity, while the inhibition of various K+ channels could have the opposite effects. How these two opposing actions are resolved will be of critical importance in understanding how σ-receptor ligands alter the cardiac action potential to act either as antiarrhythmics, or to produce acquired arrhythmias such as acquired long QT, Brugada, and Timothy syndromes (4, 37, 39).
GRANTS
This work was funded by National Institutes of Health Grants MH-065503 and NS-30016.
Acknowledgments
We thank Dr. J. C. Makielski for making the HEK-293 Nav1.5 cell line available to us and for comments on this manuscript.
REFERENCES
- 1.Aydar E, Palmer CP, Djamgoz MB. Sigma receptors and cancer: possible involvement of ion channels. Cancer Res 64: 5029–5035, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron 34: 399–410, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci USA 103: 7500–7505, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang CE Congenital and acquired long QT syndrome. Current concepts and management. Cardiol Rev 12: 222–234, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Church J, Fletcher EJ. Blockade by sigma site ligands of high voltage-activated Ca2+ channels in rat and mouse cultured hippocampal pyramidal neurones. Br J Pharmacol 116: 2801–2810, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumont M, Lemaire S. Interaction of 1,3-di(2-[5-3H]tolyl) guanidine with sigma 2 binding sites in rat heart membrane preparations. Eur J Pharmacol 209: 245–248, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Ela C, Barg J, Vogel Z, Hasin Y, Eilam Y. Sigma receptor ligands modulate contractility, Ca++ influx and beating rate in cultured cardiac myocytes. J Pharmacol Exp Ther 269: 1300–1309, 1994. [PubMed] [Google Scholar]
- 8.Ela C, Hasin Y, Eilam Y. Apparent desensitization of a sigma receptor sub-population in neonatal rat cardiac myocytes by pre-treatment with sigma receptor ligands. Eur J Pharmacol 295: 275–280, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 323: 934–937, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981. [DOI] [PubMed] [Google Scholar]
- 11.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci USA 93: 8072–8077, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131: 596–610, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Hellewell SB, Bowen WD. A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res 527: 244–253, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol 268: 9–18, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Jeanjean AP, Mestre M, Maloteaux JM, Laduron PM. Is the sigma 2 receptor in rat brain related to the K+ channel of class III antiarrhythmic drugs? Eur J Pharmacol 241: 111–116, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Kahoun JR, Ruoho AE. (125I)iodoazidococaine, a photoaffinity label for the haloperidol-sensitive sigma receptor. Proc Natl Acad Sci USA 89: 1393–1397, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kekuda R, Prasad PD, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1). Biochem Biophys Res Commun 229: 553–558, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy C, Henderson G. Inhibition of potassium currents by the sigma receptor ligand (+)-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine in sympathetic neurons of the mouse isolated hypogastric ganglion. Neuroscience 35: 725–733, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein-activated inwardly rectifying K+ channels by ifenprodil. Neuropsychopharmacology 31: 516–524, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Langa F, Codony X, Tovar V, Lavado A, Gimenez E, Cozar P, Cantero M, Dordal A, Hernandez E, Perez R, Monroy X, Zamanillo D, Guitart X, Montoliu L. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur J Neurosci 18: 2188–2196, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Lupardus PJ, Wilke RA, Aydar E, Palmer CP, Chen Y, Ruoho AE, Jackson MB. Membrane-delimited coupling between sigma receptors and K+ channels in rat neurohypophysial terminals requires neither G-protein nor ATP. J Physiol 526: 527–539, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther 197: 517–532, 1976. [PubMed] [Google Scholar]
- 23.Mavlyutov TA, Ruoho AE. Ligand-dependent localization and intracellular stability of sigma-1 receptors in CHO-K1 cells. J Mol Signal 2: 8, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moebius FF, Reiter RJ, Hanner M, Glossmann H. High affinity of sigma 1-binding sites for sterol isomerization inhibitors: evidence for a pharmacological relationship with the yeast sterol C8–C7 isomerase. Br J Pharmacol 121: 1–6, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohapatra DP, Trimmer JS. The Kv2.1 C terminus can autonomously transfer Kv2.1-like phosphorylation-dependent localization, voltage-dependent gating, and muscarinic modulation to diverse Kv channels. J Neurosci 26: 685–695, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monassier L, Bousquet P. Sigma receptors: from discovery to highlights of their implications in the cardiovascular system. Fundam Clin Pharmacol 16: 1–8, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Monassier L, Manoury B, Bellocq C, Weissenburger J, Greney H, Zimmermann D, Ehrhardt JD, Jaillon P, Baro I, Bousquet P. Sigma(2)-receptor ligand-mediated inhibition of inwardly rectifying K(+) channels in the heart. J Pharmacol Exp Ther 322: 341–350, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Novakova M, Ela C, Barg J, Vogel Z, Hasin Y, Eilam Y. Inotropic action of sigma receptor ligands in isolated cardiac myocytes from adult rats. Eur J Pharmacol 286: 19–30, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Novakova M, Ela C, Bowen WD, Hasin Y, Eilam Y. Highly selective sigma receptor ligands elevate inositol 1,4,5-trisphosphate production in rat cardiac myocytes. Eur J Pharmacol 353: 315–327, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Nuss HB, Marban E. Electrophysiological properties of neonatal mouse cardiac myocytes in primary culture. J Physiol 479: 265–279, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogata N, Narahashi T. Block of sodium channels by psychotropic drugs in single guinea-pig cardiac myocytes. Br J Pharmacol 97: 905–913, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pal A, Hajipour AR, Fontanilla D, Ramachandran S, Chu UB, Mavlyutov T, Ruoho AE. Identification of regions of the sigma-1 receptor ligand binding site using a novel photoprobe. Mol Pharmacol 72: 921–933, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Palmer CP, Aydar E, Jackson MB. σ Receptor modulation of ion channels. In: Sigma Receptors: Chemistry, Cell Biology, and Clinical Implications, edited by Matsumoto R, Bowen WD, and Su TP. New York, NY: Kluwer Acad., 2007, p. 127–149.
- 34.Pond AL, Scheve BK, Benedict AT, Petrecca K, Van Wagoner DR, Shrier A, Nerbonne JM. Expression of distinct ERG proteins in rat, mouse, and human heart. Relation to functional I(Kr) channels. J Biol Chem 275: 5997–6006, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Pusch M, Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflügers Arch 411: 204–211, 1988. [DOI] [PubMed] [Google Scholar]
- 36.Ramachandran S, Lu H, Prabhu U, Ruoho AE. Purification and characterization of the guinea pig sigma-1 receptor functionally expressed in Escherichia coli. Protein Expr Purif 51: 283–292, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roden DM Drug-induced prolongation of the QT interval. N Engl J Med 350: 1013–1022, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Seth P, Leibach FH, Ganapathy V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem Biophys Res Commun 241: 535–540, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu W Acquired forms of the Brugada syndrome. J Electrocardiol 38: 22–25, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Su TP Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J Pharmacol Exp Ther 223: 284–290, 1982. [PubMed] [Google Scholar]
- 41.Tchedre KT, Huang RQ, Dibas A, Krishnamoorthy RR, Dillon GH, Yorio T. Sigma-1 receptor regulation of voltage-gated calcium channels involves a direct interaction. Invest Ophthalmol Vis Sci 49: 4993–5002, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Trussell LO, Jackson MB. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci 7: 3306–3316, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valdivia CR, Nagatomo T, Makielski JC. Late Na currents affected by alpha subunit isoform and beta1 subunit co-expression in HEK293 cells. J Mol Cell Cardiol 34: 1029–1039, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Walker JM, Bowen WD, Walker FO, Matsumoto RR, De Costa B, Rice KC. Sigma receptors: biology and function. Pharmacol Rev 42: 355–402, 1990. [PubMed] [Google Scholar]
- 45.Wilke RA, Lupardus PJ, Grandy DK, Rubinstein M, Low MJ, Jackson MB. K+ channel modulation in rodent neurohypophysial nerve terminals by sigma receptors and not by dopamine receptors. J Physiol 517: 391–406, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilke RA, Mehta RP, Lupardus PJ, Chen Y, Ruoho AE, Jackson MB. Sigma receptor photolabeling and sigma receptor-mediated modulation of potassium channels in tumor cells. J Biol Chem 274: 18387–18392, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Cuevas J. Sigma receptor activation blocks potassium channels and depresses neuroexcitability in rat intracardiac neurons. J Pharmacol Exp Ther 313: 1387–1396, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Cuevas J. Sigma receptors inhibit high-voltage-activated calcium channels in rat sympathetic and parasympathetic neurons. J Neurophysiol 87: 2867–2879, 2002. [DOI] [PubMed] [Google Scholar]