Abstract
Nebulin (NEB) is a large, rod-like protein believed to dictate actin thin filament length in skeletal muscle. NEB gene defects are associated with congenital nemaline myopathy. The functional role of NEB was investigated in gastrocnemius muscles from neonatal wild-type (WT) and NEB knockout (NEB-KO) mice, whose thin filaments have uniformly shorter lengths compared with WT mice. Isometric stress production in NEB-KO skeletal muscle was reduced by 27% compared with WT skeletal muscle on postnatal day 1 and by 92% on postnatal day 7, consistent with functionally severe myopathy. NEB-KO muscle was also more susceptible to a decline in stress production during a bout of 10 cyclic isometric tetani. Length-tension properties in NEB-KO muscle were altered in a manner consistent with reduced thin filament length, with length-tension curves from NEB-KO muscle demonstrating a 7.4% narrower functional range and an optimal length reduced by 0.13 muscle lengths. Expression patterns of myosin heavy chain isoforms and total myosin content did not account for the functional differences between WT and NEB-KO muscle. These data indicate that NEB is essential for active stress production, maintenance of functional integrity during cyclic activation, and length-tension properties consistent with a role in specifying normal thin filament length. Continued analysis of NEB's functional properties will strengthen the understanding of force transmission and thin filament length regulation in skeletal muscle and may provide insights into the molecular processes that give rise to nemaline myopathy.
Keywords: neonatal mouse, isometric stress, myosin heavy chain, length-tension curve
force generation in skeletal muscle results from the interdigitation of actin (thin) filaments and myosin (thick) filaments in the sarcomeres of the myofibrillar lattice. The magnitude of active force production is predicted by the sliding filament model, which states that active muscle force is proportional to the degree of thin and thick filament overlap (15, 16). The length-tension relationship quantifies the relationship between myofilament overlap and force production and is determined by eliciting isometric tetani at a discrete series of lengths (7, 9, 10, 14). Myofilaments are polymeric, but their lengths are controlled precisely during sarcomere assembly and are highly uniform within a fiber (6, 24). Myofilament length varies across muscle type and species (11, 13, 43, 44, 47), with resultant functional consequences on the shape of the length-tension curve (11). Therefore, the molecular basis of myofilament length regulation has substantial physiological implications.
Previous studies have demonstrated that thin filament length is partly controlled by the action of “capping” proteins. At the thin filament barbed end (anchored in the Z-disk), α-actinin nucleates thin filament polymerization, and the thin filament binds to CapZ (33, 41, 42). At the pointed end (pointed toward the A-band), tropomodulin is the cap (12, 23, 28, 53). CapZ and tropomodulin are clearly involved in thin filament length regulation, but current theories do not account for the precise number of actin monomers that define filament length. It is now generally accepted that other proteins participate with CapZ and tropomodulin to specify thin filament length, although there is disagreement about which mechanistic model of thin filament length regulation is the best representation of the molecular process (24).
Nebulin (NEB) is a giant, rod-like, and modular sarcomeric protein (500–900 kDa) that comprises ∼3% of myofibrillar protein in skeletal muscle (51, 52). NEB is thought to be a thin filament “ruler” that serves as an actin-binding template and specifies thin filament length during sarcomere assembly (17, 18, 27). This model arose based on the observation that thin filament length, which is variable across muscle type and species, roughly correlates with NEB isoform size (19, 20). Furthermore, disruption of NEB protein expression results in dysregulation of thin filament length (3, 29, 55). NEB also binds to CapZ and tropomodulin at the thin filament ends, suggesting a synergistic CapZ/NEB/tropomodulin-based capping system (28, 34, 55). NEB interacts with various other sarcomere constituents in the Z-disk, including the intermediate filament desmin (2), the Z-disk scaffolds myopalladin (4, 26) and α-actinin (31), and the giant elastic protein titin (26), suggesting a role for NEB in the maintenance of sarcomere mechanical integrity and force transmission. In addition, NEB is required for normal muscle contractility, which has been demonstrated by altered Ca2+ sensitivity (55) and inferior isometric stress production (3) in NEB-deficient muscle. NEB is of clinical interest because 50% or more of nemaline myopathy cases are caused by mutations in its gene (21, 35, 48, 49).
To assess the role of NEB, NEB knockout (NEB-KO) mice were compared with wild-type (WT) mice. NEB deficiency is a neonatal lethal mutation; NEB-KO mice have abnormally short thin filaments, exhibit rapid postnatal myofibrillar disorganization and muscle degeneration, and die after ∼1–2 postnatal weeks (3, 55). Therefore, it was necessary to develop physiological testing methods for immature neonatal skeletal muscle, allowing experiments at postnatal days 1 and 7 (P1 and P7, respectively). The purpose of this study was to test the hypothesis that the length-tension relationship of NEB-KO muscle is altered in a manner consistent with reduced thin filament length. These analyses also revealed a critical role for NEB in isometric stress production and the maintenance of muscle function during repetitive isometric activation.
METHODS
Animals and experimental model.
Male and female NEB-KO mice obtained at P1 and P7 were used for all physiological experiments, and additional tissue was sampled at postnatal days 5 and 11 (P5 and P11, respectively) for biochemistry. The genetic engineering of NEB-KO mice has been previously described in detail (3). Control animals consisted of age-matched WT (NEB+/+) littermates. Genotypes were confirmed by PCR analysis of tail-snip biopsies using primers targeting the following alleles: NEB WT (sense 5′-ATGGCATATGGAAAGTTTGTAGGT-3′ and antisense 5′-AACATGAAACATGCCTTCTTTGTA-3′) and NEB mutant (sense 5′-GTTCGCAAGAACCTGATGCACA-3′ and antisense 5′-CTAGAGCCTGTTTTGCACGTTC-3′). All mice were killed by decapitation. Both hindlimbs from each mouse were used for functional experiments to minimize the number of mice required. Procedures were performed in accordance with ethical guidelines and approved by the San Diego Veterans Affairs Institutional Animal Care and Use Committee.
The experimental model for physiological testing was the gastrocnemius muscle (GAS). The GAS was chosen because, of all the hindlimb muscles previously examined, the NEB-KO GAS had the greatest thin filament length decrease compared with WT (0.95 vs. 1.27 μm, respectively; see Ref. 3 for a complete list of thin filament lengths). Therefore, the NEB-KO GAS was expected to exhibit the most severe alteration in length-tension properties. A secondary benefit of using the GAS was that its comparatively large size in 1-day-old hindlimbs facilitated its dissection. However, the fragility of 1-day-old muscle and tendons precluded the surgical isolation of the GAS muscle-tendon unit, and, therefore, the entire bone-tendon-muscle-tendon complex associated with the GAS was used for physiological testing.
Muscle architecture and isometric stress measurement.
Hindlimbs were transected at the proximal femur, carefully skinned, and immersed in ice-cold mammalian Ringer solution (137 mM NaCl, 5 mM KCl, 1 mM NaH2PO4, 24 mM NaHCO3, 2 mM CaCl2, 1 mM MgSO4, 11 mM glucose, and 10 mg/l curare). Bone and soft tissue were cleared proximal to the GAS femoral origin, and the Achilles tendon was released at its calcaneal insertion. The tibia and anterior muscle compartment containing the dorsiflexors (tibalis anterior, extensor digitorum longus, and extensor hallucis longus) were carefully resected to isolate the GAS. The GAS was transferred to a custom muscle-testing chamber filled with Ringer solution (see Supplementary Fig. 1), which has been previously described (40). To secure the muscle in the chamber, the Achilles tendon was tied to a rigid post interfaced with a force transducer (Series 300B, Aurora Scientific, Aurora, ON, Canada) using silk sutures. The femur was secured to a rigid frame located on a micrometer-controlled horizontally adjustable platform that could impose precise displacements. Care was taken to tie sutures as close as possible to the myotendinous junctions to minimize extramuscular sites that could introduce confounding series compliance effects. The GAS muscle length (Lm) was adjusted such that the GAS was taut, and passive muscle tension was barely detectable by the force transducer (“slack length”). Lm was measured through a dissecting microscope fitted with an eyepiece crosshair reticule using a digital micrometer to translate the chamber under the field of view from the proximal GAS origin to the distal myotendinous junction. Muscle activation was provided by an electrical stimulator (Pulsar 6bp, FHC, Bowdoinham, ME) via platinum plate electrodes that extended across the entire GAS length (see Supplementary Fig. 1). Muscle twitches were elicited at successively higher stimulation voltages, beginning with 5 V, until maximum twitch force was achieved (typically ∼20 V). Voltage was then doubled to guarantee the recruitment of all fibers. Maximum isometric force was elicited by applying a 400-ms train of 0.3-ms pulses delivered at 100 Hz while maintaining constant Lm. Signal-to-noise ratios during isometric activation were ∼5–6 (see Supplementary Fig. 2). A computer algorithm in LabView (National Instruments, Austin, TX) was used to trigger the stimulator, acquire signals from the force transducer via a data-acquisition board sampling at 4,000 Hz (PCI-6040m National Instruments), and analyze force-time records. After being tested, the GAS was removed from the chamber, the Achilles tendon and femoral origin were cut off, and the GAS was dabbed dry and weighed.
To determine the maximum isometric stress generated, isometric force was normalized to physiological cross-sectional area (PCSA), an anatomic parameter that is directly proportional to force-generating capacity (36). For computations, previously published density and architectural values for the mouse GAS were used (5, 30). Fiber length (Lf; in mm) was determined for each muscle by multiplying Lm by the characteristic GAS Lf-to-Lm ratio of 0.46, which was determined in a pilot experiment. Muscle mass (M; in mg), muscle density (ρ = 1.056 g/cm3), fiber pennation angle (θ = 26.2°), and Lf were used to compute PCSA (in mm2) using the following formula:
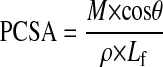 |
(1) |
Measurement of isometric stress instead of force allowed the comparison of intrinsic muscle contractility independent of muscle size.
Muscle functional assessments.
While muscle architecture and isometric stress were measured at both P1 and P7, the following assessments were performed only at P1 to avoid the confounding effects of postnatal muscle degeneration that is known to occur in NEB-KO mice with age (3, 55).
To measure the muscle response to cyclic activation as a measure of myofibrillar mechanical strength, the GAS was secured in the muscle testing chamber, and a series of 10 isometric tetani (Iso1–Iso10, 2 min apart) was elicited at slack length. Stimulation parameters and duration were identical to those described above. The response to cyclic activation was defined as a reduction in isometric stress across the isometric exercise bout. This value was computed both as an absolute reduction in stress and as a percentage of the stress produced by the first isometric tetanus. This control experiment was required because no data were available stating whether or not cyclic isometric activation has a deleterious effect on neonatal WT or NEB-KO muscle that could affect the determination of their length-tension properties.
To generate length-tension curves, isometric tetani (2 min apart) were elicited at slack length and then at a series of muscle lengths ±0.2 Lf, ±0.4 Lf, ±0.6 Lf, ±0.8 Lf, and ±1 Lf from slack length. At positive stretch lengths, passive tensile stresses were also recorded to assess passive whole muscle mechanical properties. (At negative lengths, passive tensile stress was always zero.) Tetani were elicited in a random sequence, such that tetani performed at any particular length would not be confounded by artifacts arising from repeated cyclic activation that occurred before the activation at that length. Stimulation parameters and duration were identical to those described above. Isometric stresses were normalized to the maximum isometric stress (to compute the fraction of peak tension), and Lm was normalized to slack length (to compute relative Lm). The fraction of peak tension was then plotted as a function of relative Lm. Parabolic regression was applied to quantify the width of the length-tension curve. The regression curve was required to have a coefficient of determination R2 >0.9, or else the muscle was excluded from this study. An applet written in MATLAB (The MathWorks, Natick, MA) computed the x-coordinate of the vertex [the optimal relative Lm (Lopt)] and full-width at half-maximum (FWHM) of each parabola. Lopt and FWHM were metrics of the center and breadth, respectively, of the length-tension curve.
Sarcomere length measurements.
Due to the relatively low myofibrillar packing in neonatal mouse muscle (8), laser transillumination did not provide a clear diffraction pattern for measuring average GAS sarcomere length (Ls) when muscles were in the testing chamber. Instead, a separate set of measurements was performed using 1-day-old WT and NEB-KO hindlimbs that had been immersion fixed overnight in 3.7% formaldehyde with knee and ankle angles set at 90° using anodized steel minutien pins through the foot and femur, respectively, corresponding to a “neutral” joint configuration. Muscle fiber bundles were carefully teased out of each GAS, laid onto glass slides, encased in Permount mounting medium, coverslipped, and photographed at ×40 magnification under phase-contrast illumination (Leica Microsystems, Bannockburn, IL). For calibrating distances under the microscope, diffraction gratings with known intergroove distances (1.66 and 2.50 μm) were also photographed. Images were analyzed in ImageJ (http://rsb.info.nih.gov/ij/, National Institutes of Health, Bethesda, MD). Ls was defined as the distance between successive Z-disks and averaged from three measurements taken at three different locations within each fiber bundle.
Additional Ls measurements were performed on 1-day-old GAS to determine the Ls that corresponded to slack Lm during isometric testing as well as to examine the effect of stretch on Ls. The GAS was dissected at the proximal femoral origins and secured to cork using minutien pins. On half of the muscles, the Achilles tendons were then gently elongated until visual inspection indicated that slack was eliminated from the muscle-tendon unit, and the Achilles tendons were then pinned to secure the muscle in position. On the other half, the GAS was stretched by +1 Lf before being pinned. Muscles were then immersion fixed overnight in 3.7% formaldehyde, and Ls values were measured as described above. It is important to note that measurement of “neutral” Ls, as determined by whole hindlimb fixation, yielded the same Ls value as measurement of slack Ls, as determined by lone muscle fixation, indicating that in situ GAS Lm when knee and ankle angles are set at 90° almost exactly corresponds to slack GAS Lm.
Sarcolipin RNA expression analysis.
The sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) inhibitor sarcolipin has previously been shown to be upregulated in NEB-KO muscle (32, 55), resulting in depression of sarcoplasmatic reticulum Ca2+ uptake and speed of relaxation, although without affecting muscle contractility (32). To determine whether upregulation of sarcolipin correlates with postnatal sarcomere structure abnormalities in NEB-KO muscle, sarcolipin RNA transcript levels were measured at different stages in NEB-KO and WT tibialis anterior muscle by quantitative real-time PCR. Total RNA was extracted from tibialis anterior muscle tissue isolated from WT and NEB-KO mice at P1, P5, P7, and P11 using TRIzol reagent (Invitrogen, Carlsbad, CA). To assess sarcolipin mRNA levels, quantitative real-time PCR was performed in triplicate on an ABI Prism7900 HT cycler using the QuantiFast Probe RT-PCR kit (Qiagen, Valencia, CA) and gene expression assays for mouse sarcolipin, β-actin, and GAPDH (Applied Biosystems, Foster City, CA). Threshold cycle (Ct) values were normalized to both β-actin and GAPDH, and relative gene expression was obtained using the 2−ΔΔCt method (25), as calculated using SDS version 2.2.2 (Applied Biosystems). Normalization to β-actin or GAPDH produced identical results, so only normalization to β-actin is presented.
Myosin heavy chain analysis.
Myosin heavy chain isoform distribution was measured using a previously described gel electrophoresis technique (45) and used to indicate tissue developmental maturity. Muscles were homogenized and centrifuged, and myofibril-rich pellets were washed and resuspended in buffers supplemented with protease inhibitor cocktail (5 μl each of 100 mM PMSF, 10 μg/μl leupeptin, and 10 μg/μl pepstatin A). Protein was then diluted in sample buffer [100 mM DTT, 2% SDS, 80 mM Tris base, 10% glycerol, and 1.2% (wt/vol) bromophenol blue] to a concentration of 0.125 mg/ml and boiled for 2 min. Separation of isoforms was performed by SDS-PAGE (16 × 22 cm, thickness: 0.75 mm) with 22 h of migration at 275 V at 4°C. Stacking and resolving gels were 4% and 8% polyacrylamide, respectively. After migration, gels were silver stained according to the manufacturer's protocol (Bio-Rad, Hercules, CA). Isoforms were identified by their relative electrophoretic mobilities, which have been previously characterized (1). Densitometry was performed to measure band intensities and compute isoform distributions (Quantity One, Bio-Rad). Total myosin heavy chain content was computed by summing all the myosin heavy chain band intensities in each sample.
Transmission electron microscopy.
To determine the ultrastructural properties of NEB-KO muscle at the time of functional measurements, transmission electron microscopy was performed on the tibialis anterior muscle essentially as previously described (3). Hindlimbs were pinned to cork with the knee joint fixed at 90° and the ankle joint at 180° (full plantarflexion) to stretch the tibialis anterior muscle. After being fixed overnight in 2% paraformaldehyde and 2% gluteraldehyde in 0.1 M sodium cacodylate buffer, muscles were then dissected out, cut into smaller pieces, postfixed, and stained for 2 h in 1% OsO4 and 1% K4Fe(CN)6 followed by 1 h in 1% uranyl acetate. Tissue was dehydrated in a series of ethanol and acetone baths and then embedded in Durcupan resin (EMD, San Diego, CA). Ultrathin (60–70 nm) sections were generated, stained with lead citrate, and imaged at 80 kV with an electron microscope (model 1200EX, JEOL, Tokyo, Japan).
Statistics.
Data are presented as means ± SE. When simultaneously determining the effects of genotype (WT vs. NEB-KO) and another experimental parameter, comparisons were made using two-way ANOVA with post hoc Fisher's protected least-significant difference analysis. When determining the effect of genotype alone, the unpaired Student's t-test was used. The relationship between Lopt and FWHM was examined using linear regression. Significance was defined as P < 0.05. Statistical analysis was performed in StatView (SAS, Cary, NC).
RESULTS
Evidence for functional and structural muscle degeneration.
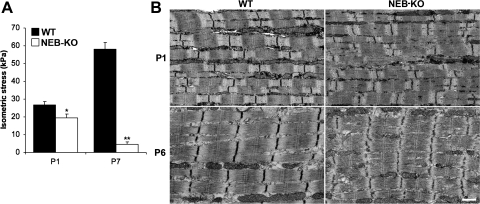
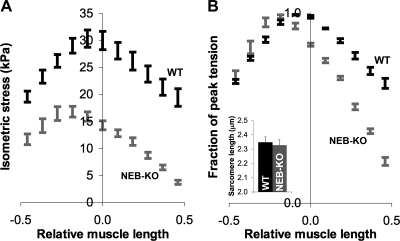
Measurements of isometric stress production demonstrated that the WT GAS exhibited an improvement of mechanical function during postnatal development, whereas the NEB-KO GAS exhibited postnatal degeneration (Fig. 1A). The isometric stress produced by the WT GAS increased from 26.8 ± 2.0 kPa at P1 to 58.1 ± 3.9 kPa at P7, consistent with a previous report (8) of intrinsic enhancement of skeletal muscle function during the postnatal development of mouse muscle. The NEB-KO GAS generated only 19.5 ± 2.0 kPa at P1, which was 27% less than the WT GAS (P < 0.05), and this value deteriorated to just 4.5 ± 1.3 kPa at P7, which was 92% less than the age-matched WT GAS (P < 0.0001). Therefore, the skeletal muscle phenotype of NEB-KO mice was predominantly due to a severe and rapidly progressive decline of mechanical performance. Ultrastructural analysis using transmission electron microscopy confirmed that the reduction in isometric stress production in NEB-KO muscles was accompanied by structural deterioration. Whereas WT muscle did not exhibit postnatal changes in sarcomere structure, NEB-KO muscle acquired wavy, fragmented, and disrupted Z-disks during postnatal development (Fig. 1B). Although the observed abnormalities were not as severe as previously found in the continuously contracting diaphragm, this is consistent with our previous finding (3) that NEB is important for the maintenance of sarcomeric structure during muscle contraction.
Fig. 1.
Characterization of isometric stress production in wild-type (WT) and nebulin (NEB) knockout (KO) mice at postnatal days 1 and 7 (P1 and P7, respectively; n = 8 WT at P1, 8 NEB-KO at P1, 10 WT at P7, and 4 NEB-KO at P7) and its relationship to muscle ultrastructure. A: Fisher's protected least-significant difference (PLSD) test showed that the NEB-KO gastrocnemius muscle (GAS) generated significantly less stress than the WT GAS at P1. The differences were even more pronounced by P7, indicating that the WT GAS exhibited both extrinsic and intrinsic postnatal enhancement of contractile function, whereas the NEB-KO GAS exhibited postnatal deterioration, indicating rapidly progressive myopathy. *P < 0.05 compared with WT; **P < 0.0001 compared with WT. B: transmission electron microscopy on tibialis anterior muscle confirmed that the WT muscle maintains normal sarcomere structure during postnatal development, whereas the NEB-KO muscle exhibits progressive deterioration. Deterioration in NEB-KO muscle is distinguished by wavy, fragmented, and disrupted Z-disks. P6, postnatal day 6. Scale bar = 500 nm.
Sarcolipin RNA expression analysis.
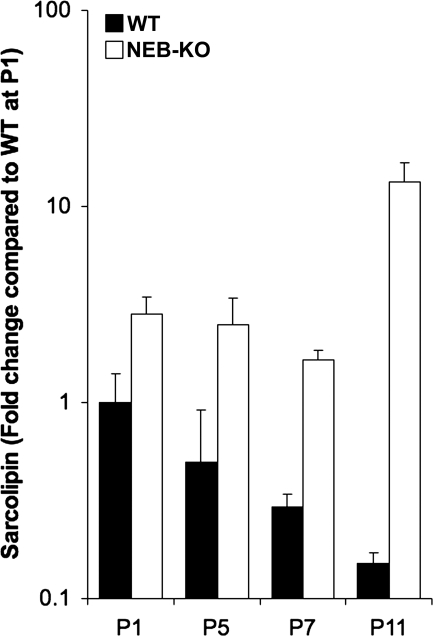
As expected, sarcolipin transcript levels were elevated in NEB-KO mice. However, unlike previously reported (32), sarcolipin upregulation in NEB-KO muscle increased with age (∼2.8 fold at P1, ∼5.0 fold at P5, ∼5.6 fold at P7, and ∼88.4 fold at P11), suggesting that increased sarcolipin transcript levels and consequent reduced rate of sarcoplasmic reticulum Ca2+ uptake occur secondarily to NEB ablation and synchronously with the development of progressive sarcomere structural abnormalities. Interestingly, sarcolipin transcript levels decreased in a monotonic fashion during postnatal development of both WT and NEB-KO muscle until P7. However, at P11, sarcolipin transcript levels continued to decrease in WT muscle (∼6.6-fold decrease from P1 to P11; Fig. 2), whereas sarcolipin transcript levels dramatically increased in NEB-KO muscle (∼4.7-fold increase from P1 to P11; Fig. 2). Thus, increased sarcolipin transcript levels are correlated with sarcomere structural abnormalities and stress reduction in NEB-KO mice.
Fig. 2.
Relative quantification of sarcolipin RNA transcript levels in WT and NEB-KO tibialis anterior muscle tissue from P1 to P11 (n = 3 WT and 3 NEB-KO at each time point). All data are shown as the fold expression of sarcolipin compared with WT muscle at P1. Sarcolipin upregulation in NEB-KO muscle increased with age, whereas sarcolipin transcript levels decreased monotonically in WT muscle. The y-axis uses a logarithmic scale for clarity.
Myosin heavy chain analysis.
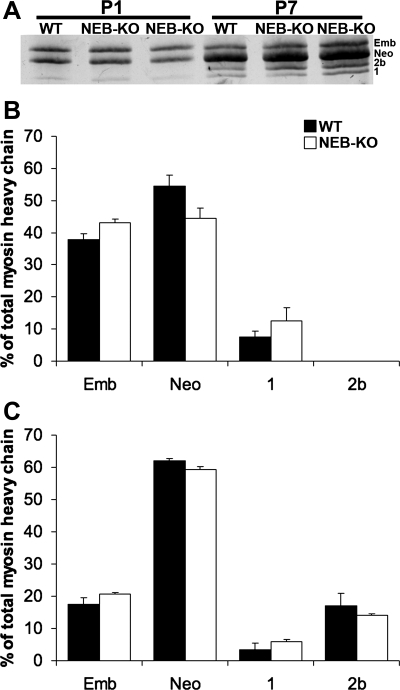
Electrophoretic separation of myosin heavy chain isoforms revealed similar isoform expression profiles in WT and NEB-KO GAS. Embryonic, neonatal, and low levels of type 1 myosin were expressed at both P1 and P7, and type 2b myosin was expressed at P7 (Fig. 3A). Isoforms 2a and 2x were not expressed at either postnatal day. Densitometric analysis of myosin heavy chain isoform distributions showed no significant differences in the percentages of embryonic, neonatal, type 1, and type 2b myosin at either postnatal day in NEB-KO compared with WT GAS, although the NEB-KO GAS exhibited a slight but statistically insignificant trend toward elevated expression of embryonic myosin at the expense of neonatal myosin (P = 0.06–0.50; Fig. 3, B and C). Thus, it is unlikely that the inferior functional quality of NEB-KO skeletal muscle is due to altered developmental maturity. Total myosin heavy chain content in the NEB-KO GAS was 1.02 ± 0.02-fold elevated compared with the WT GAS, indicating that total myosin heavy chain content was unchanged (P = 0.98). Therefore, functional properties in the NEB-KO GAS are unlikely to be compromised by impaired biosynthesis of myofibrillar matrix material.
Fig. 3.
Myosin heavy chain isoform distributions in the WT and NEB-KO GAS at P1 and P7 (n = 4 WT at P1, 4 NEB-KO at P1, 4 WT at P7, and 4 NEB-KO at P7). A: sample silver-stained SDS-PAGE gel with bands ordered by increasing electrophoretic mobilities. After band intensities were quantified using densitometry, Student's t-test found no significant differences in embryonic (Emb), neonatal (Neo), type 1, and type 2b myosin isoform levels at both P1 (B) and P7 (C). Type 2a and 2x isoforms were not detectable in either genotype at either postnatal day.
Cyclic contractile testing.
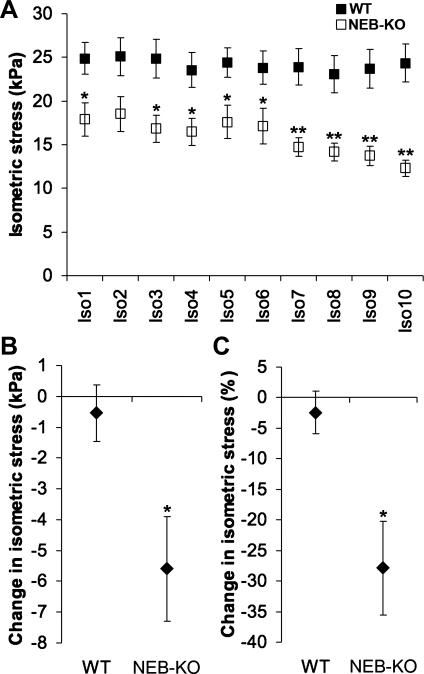
When the WT GAS and NEB-KO GAS were subjected to a series of 10 cyclic isometric tetani, the NEB-KO GAS consistently generated less stress (P < 0.05 at Iso1, P = 0.051 at Iso2, P < 0.05 at Iso3–Iso6, and P < 0.01 at Iso7–Iso10; Fig. 4A), as expected. Interestingly, the isometric stress produced by the NEB-KO GAS began to decline beyond the sixth isometric tetanus. No comparable decline was observed in the WT GAS.
Fig. 4.
Responses of the WT and NEB-KO GAS at P1 to cyclic isometric activation (n = 6 WT and 6 NEB-KO). A: isometric stress achieved during a series of 10 isometric tetani (Iso1–Iso10) spaced 2 min apart. The NEB-KO GAS generated less stress than the WT GAS throughout the isometric exercise bout, although the difference became most pronounced beyond Iso6. B: change in isometric stress production across the isometric exercise bout expressed as an absolute change in stress. C: change in isometric stress production across the isometric exercise bout expressed as a percentage of Iso1 stress. Student's t-test indicated that the NEB-KO GAS was more vulnerable than the WT GAS to stress decline during cyclic activation. *P < 0.05 compared with WT; **P < 0.01 compared with WT.
Across the entire bout of isometric exercise, the WT GAS exhibited a near-zero change in isometric stress production (−0.5 ± 0.9 kPa), whereas the NEB-KO GAS exhibited a decline of significantly greater magnitude (−5.6 ± 1.7 kPa, P < 0.05; Fig. 4B). Likewise, when the change in isometric stress production was considered as a percentage of the stress produced by the first isometric tetanus, it was again confirmed that the WT GAS exhibited a near-zero change (−2.4 ± 3.5%), whereas the NEB-KO GAS exhibited a decline of significantly greater magnitude (−27.9 ± 7.7%, P < 0.05; Fig. 4C). Regardless of whether the response to cyclic activation was defined as an absolute or percent drop in stress across the isometric exercise bout, the data indicate that NEB-KO muscle was more severely compromised than WT muscle.
Length-tension curves and Ls measurements.
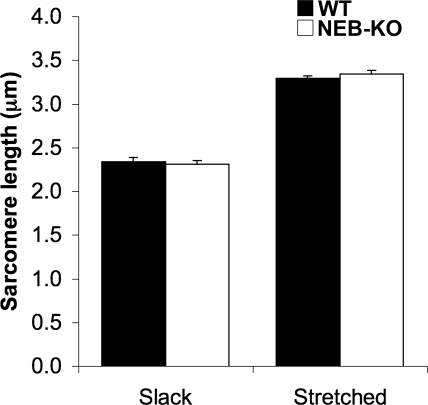
Examination of length-tension curves showed that the NEB-KO GAS generated less stress than the WT GAS at all testing lengths (P < 0.001 at all lengths; Fig. 5A). Normalization of isometric stresses to peak stress revealed that the descending limb of the length-tension curve was steeper in the NEB-KO GAS, although the steepness of the ascending limb was unchanged. The fraction of peak tension produced by the NEB-KO GAS was significantly less than the WT GAS at muscle lengths ranging from −0.2 Lf to +1 Lf (P < 0.05 at a length of −0.2 Lf, P < 0.001 at lengths above −0.2 Lf; Fig. 5B). Qualitatively, the length-tension curves of the NEB-KO GAS had a lower breadth, indicating a narrower functional range of Lm. The curves were also shifted leftward, suggesting a shorter optimum Lm for stress production (i.e., the length where myofilament overlap was maximized). These observations are consistent with shorter thin filament length in NEB-KO muscle. In addition, phase-contrast microscopy found no difference in “neutral” Ls between WT and NEB-KO GAS after hindlimbs had been fixed at neutral knee and ankle angles of 90°. Ls values were 2.35 ± 0.04 and 2.32 ± 0.4 μm in WT and NEB-KO GAS, respectively (P = 0.70; Fig. 5B, inset). This observation is consistent with a previous report (3) showing that deletion of NEB does not affect the gross cytoarchitectural organization of the sarcomere. Furthermore, it demonstrates that the NEB-KO GAS does not alter the resting state of its sarcomeres in response to shorter thin filaments.
Fig. 5.
Length-tension curves of the WT and NEB-KO GAS at P1 (n = 8 WT and 8 NEB-KO). Mechanical function was expressed as maximum isometric stress (A) or a fraction of peak tension (B). Note the leftward shift and narrowing of the length-tension curve of the NEB-KO GAS relative to the WT GAS, which are consistent with shorter thin filament length in NEB-KO muscle. Despite the difference in thin filament length, “neutral” sarcomere lengths in both the WT GAS (n = 4) and NEB-KO GAS (n = 4) were identical (inset).
Ls measurement before and after passive muscle stretch showed that the WT and NEB-KO GAS had identical responses to stretch (P = 0.39–0.63), indicating that NEB does not create an altered capacity for distributing externally imposed tensile strains among its sarcomeres and that resting Ls is not altered in the absence of NEB. The WT and NEB-KO GAS exhibited 41% and 45% increases in Ls in response to a stretch of +1 Lf, respectively (Fig. 6). These values are consistent with the previously published GAS Lf-to-Lm value of 0.46 (5). Sarcomere structural integrity was maintained during stretch in both genotypes. Any systematic discrepancy would most likely be due to the mismatching series compliance and/or stress relaxation of the Achilles tendon and intramuscular connective tissue.
Fig. 6.
Sarcomere length in the WT and NEB-KO GAS at P1 either at slack length or stretched by +1 fiber length (n = 4 WT slack, 4 WT stretched, 4 NEB-KO slack, and 4 NEB-KO stretched). Fisher's PLSD test revealed no significant difference in sarcomere length at either slack or stretched conditions, indicating that deletion of NEB does not interfere with the ability of the GAS to distribute tensile strains among its sarcomeres.
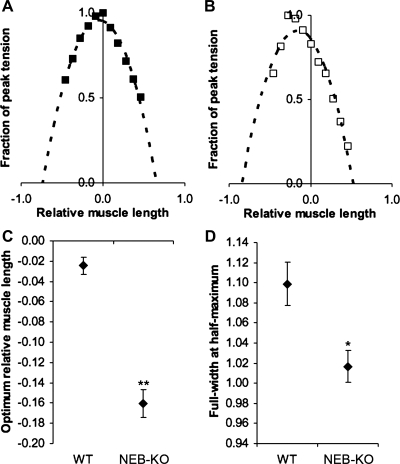
Parabolic regression analysis was applied to quantitatively validate the alterations in the shape of the length-tension curve in the NEB-KO GAS (Fig. 7, A and B). Of a total of 18 completed length-tension curves, parabolic regression analysis was performed on the 16 curves that met the preestablished inclusion criterion of the parabolic regression having R2 > 0.9 (n = 8 WT and 8 NEB-KO). The value of Lopt was significantly reduced in the NEB-KO GAS by 0.13 Lm (P < 0.01; Fig. 7C), thereby verifying that the length-tension curve was indeed shifted to the left. Similarly, FWHM in the NEB-KO GAS was reduced by 7.4% compared with the WT GAS (P < 0.05; Fig. 7D), verifying that the length-tension curve was significantly narrower. Both of these observations are qualitatively consistent with the fact that deletion of NEB reduces thin filament length (3). Furthermore, after removing the Lopt and FWHM of individual muscles from their genotype-specific bins and plotting their values against one another, a significant correlation between Lopt and FWHM was found (see Supplementary Fig. 3). The correlation between Lopt and FWHM was stronger for the subset of ordered pairs corresponding to the NEB-KO GAS; this was predominantly due to a wider domain of Lopt values observed in the NEB-KO muscle tests. These results were reasonable because a rightward shift and widening of the length-tension curve are expected to occur jointly with increasing thin filament length (11).
Fig. 7.
Parabolic regression analysis of the length-tension properties of the WT and NEB-KO GAS at P1 (n = 8 WT and 8 NEB-KO). A and B: representative length-tension curves from tests of an individual WT GAS (A) and NEB-KO GAS (B) with parabolic regression applied to the data. The regression equations in A and B were y = −1.98x2 − 0.16x + 0.95 (R2 = 0.97) and y = −1.28x2 − 0.80x + 0.84 (R2 = 0.99), respectively. All regression curves were required to meet the goodness-of-fit criterion of R2 > 0.9, or else they were excluded from this study and further analysis. C: optimum relative muscle length (Lopt) of the GAS. D: Full-width at half-maximum (FWHM) of the length-tension curves generated for the GAS. Student's t-test revealed lower Lopt and reduced FWHM in the NEB-KO GAS, both of which are qualitatively consistent with shorter thin filament length in NEB-KO muscle. *P < 0.05 compared with WT; **P < 0.01 compared with WT.
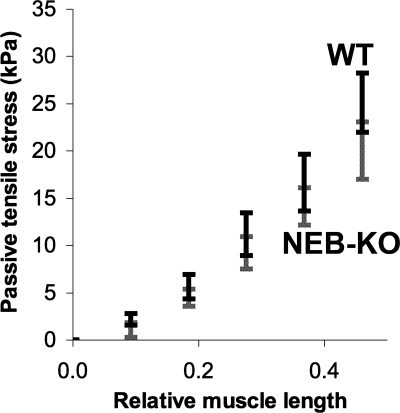
No differences were found in the passive load-bearing capacity of the WT and NEB-KO GAS (Fig. 8). While there was a trend toward lower passive tensile stresses in the NEB-KO GAS, the trend did not achieve statistical significance (P > 0.05 at all stretch lengths).
Fig. 8.
Passive length-tension curves of the WT and NEB-KO GAS at P1 (n = 8 WT and 8 NEB-KO). Student's t-test found no significant differences in passive tensile stress at any muscle length, although there was a trend toward lower passive stresses in the NEB-KO GAS.
DISCUSSION
This study compared the length-tension properties of normal and NEB-deficient skeletal muscles, whose thin filaments have distinctly different lengths. Based on distributed deconvolution of fluorescence images, Bang et al. (3) demonstrated that thin filaments in the NEB-KO GAS are only 0.95 μm compared with 1.27 μm in the WT GAS. Granzier et al. (11) previously used electron microscopy to measure thin filament lengths in fast- and slow-twitch fibers in the perch (0.94 vs. 1.24 μm, respectively) and assessed the effect of thin filament length on length-tension properties. The difference in filament length was similar in both studies; therefore, it is not surprising that the results of this comparison of WT versus NEB-KO muscles parallel the results of the comparison of fast- versus slow-twitch fibers. The leftward shift of the length-tension curve of the NEB-KO GAS is consistent with shorter thin filaments, lending support to a model where a NEB-independent mechanism allows thin filaments to achieve subphysiological lengths, whereas NEB permits thin filaments to elongate further and reach their final physiological lengths (see Supplementary Fig. 4). Short thin filaments alter the length-tension relationship of NEB-KO muscle through a mechanism that can be predicted by the sliding filament theory (11): the length-tension curve narrows and shifts leftward due to a smaller domain of Ls values in which thin filament interference (i.e., “double overlapping” of thin filaments and interaction of thick filaments with thin filaments of incorrect polarity) manifests as the shallow subregion of the ascending limb (46). The remainder of the length-tension curve then scales appropriately to compensate for the attenuation of the shallow ascending limb.
Length-tension properties in the WT and NEB-KO GAS showed a 7.4% decline in FWHM and a reduction in Lopt of 0.13 Lm, corresponding to a 0.32 μm (25.2%) shortening of the thin filament due to NEB deletion. However, construction and analysis of idealized length-tension curves based on thin filament length measurements and the sliding thin filament theory indicate that a 25.2% shortening of the thin filament is expected to result in a 32.9% decline in FWHM and a reduction in Lopt of 0.22 Lm (see Supplementary Fig. 5). Therefore, while the data are qualitatively consistent with the filament length change measured, other factors cause the length-tension curve of the NEB-KO GAS to be wider than the sliding filament theory predicts. Possibilities include increased Ls and Lf heterogeneity (54) and/or altered frequency-dependent activation in the NEB-KO GAS (37). For example, additional modeling demonstrates that increasing Ls heterogeneity from 0 to 0.1 μm induces a 3.5% increase in FWHM, which is modest compared with the ∼30% FWHM difference predicted by the sliding filament theory. Ls heterogeneity would be sufficient to explain length-tension differences between the WT and NEB-KO GAS only if the difference in Ls heterogeneity were extremely high (closer to 0.8 μm). Such heterogeneity is unlikely. Modeling also shows that changing Lf heterogeneity from 0 to 0.1 mm induces a corresponding 7% increase in FWHM, which is also relatively modest. Lf heterogeneity would have to be as high as 0.4 mm to correspond to the FWHM difference predicted by the sliding filament theory. Such large Lf heterogeneity was not corroborated by the architectural data of the NEB-KO GAS.
A potential cause for altered FHWM in the NEB-KO GAS is altered frequency-dependent activation. It is known that a muscle's length-tension curve widens and shifts rightward at increasing stimulation rates (37), which was observed here. Increasing stimulation frequency from 10 to 35 Hz can widen a length-tension curve up to 33%. This is well within the discrepancy of the FWHM difference between the WT and NEB-KO GAS that was predicted by the sliding filament theory. Given that NEB may play a role in excitation-contraction coupling (38, 39), altered frequency-dependent activation cannot be ruled out as a basis for altered length-tension properties in the NEB-KO GAS.
The descending limb of the length-tension curve of the NEB-KO GAS was slightly steeper than the corresponding limb in the WT GAS. NEB-mediated enhancements of cross-bridge formation or force transmission do not adequately explain this observation. However, Lieber et al. (22) demonstrated that the shape of a whole muscle length-tension curve is sensitive to the series passive mechanical properties of the tendon. In particular, the slope of the descending limb increases with tendon compliance. In this study, care was taken to secure the GAS at its myotendinous junction to minimize series compliance.
While the length-tension curve in the NEB-KO GAS shifted leftward, microstructural investigation using phase-contrast microscopy showed that “neutral” and “stretched” Ls values were unchanged. Thus, systematic differences in Ls cannot explain the alteration in the descending limb of the length-tension curve. Furthermore, short thin filaments reduce the intrinsic ability for NEB-KO muscle to produce stress by reducing the effective number of cross-bridges during muscle activation (10). Reduced isometric stress capacity is not immediately apparent from length-tension curves that depict the fraction of peak tension rather than absolute tension. Both isometric stress production and length-tension properties should be considered together for complete understanding of intrinsic muscle properties.
The reduced isometric stress production in NEB-KO muscle was too great to be explained by the shorter thin filaments alone, so it is likely that other muscle-specific processes were compromised as well. For example, NEB may play a role in excitation-contraction coupling, based on its Ca2+/calmodulin dependence (38). In addition, short thin filaments in NEB-KO muscle presumably have fewer troponin/tropomyosin regulatory complexes, since NEB is thought to dictate their periodic placement along the filament (50). The exact role of NEB in excitation-contraction coupling is likely very subtle and difficult to discern without the application of specialized assays. Previous work has shown that thin filament-associated proteins, including actin, troponin, tropomyosin, and tropomodulin, are correctly assembled and localized in NEB-KO muscle, despite reduced thin filament length and progressive loss of sarcomere structure (3, 55). Identifying any subtle changes in the dynamics of these proteins in NEB-KO muscle was beyond the scope of this study.
Sarcolipin has previously been shown to be dramatically upregulated in NEB-KO muscle at P5 and P15 (32). Sarcolipin transcript levels were measured at different postnatal ages in WT and NEB-KO muscle, and, in agreement with previously published data, sarcolipin levels were increased in NEB-KO muscle compared with WT muscle. However, unlike previously reported, sarcolipin upregulation in NEB-KO muscle increased with age from ∼2.8-fold at P1 to ∼88.4-fold at P11, whereas sarcolipin transcript levels in WT muscle decreased ∼6.6-fold during postnatal development from P1 to P11. Thus, increased sarcolipin expression is associated with postnatal stress reduction and increased sarcomere structural abnormalities reflecting deterioration of NEB-KO muscle.
NEB is also thought to be involved in mechanocoupling and force transmission at the Z-disk, based on its interactions with other force-transmitting proteins (see Supplementary Fig. 4). NEB is known to bind to the Z-disk scaffold myopalladin, a protein that is believed to have an important structural role through interactions with its sarcomeric binding partners, including α-actinin and cardiac ankyrin repeat protein (4). Independently of myopalladin, NEB also binds directly to α-actinin (31). Furthermore, NEB may transmit force laterally and align Z-disks via desmin (2). Finally, ultrastructural investigation of NEB-KO muscle has revealed progressive thickening and dissolution of Z-disks (3, 55). These observations indicate an additional role for NEB as a structural scaffold or force coupler, likely through a mechanism involving Z-disk stabilization. When considering the development of isometric muscle stress, NEB's role in Z-disk stabilization likely acts in combination with its role in thin filament length specification.
During activation, the isometric stress produced by the NEB-KO GAS was consistently lower than the stress produced by the WT GAS. NEB may account for the difference by improving both cross-bridge formation and lateral force transmission through the mechanisms described above. However, the relative importance of cross-bridge formation and force transmission depends on Ls. When Ls is low (below twice the thin filament length), the short thin filaments in NEB-KO muscle are actually beneficial to cross-bridge formation, due to less double overlap between thin filaments. Therefore, one would conclude that the reduced stress production is predominantly due to impaired force transmission. On the other hand, when Ls is high (above the sum of twice the thin filament length and the width of the thick filament bare zone), the short thin filaments in NEB-KO muscle are detrimental to cross-bridge formation, since the extent of myofilament overlap is reduced. Therefore, one would conclude that the reduced stress production is predominantly due to impaired cross-bridge formation. Slack Ls was ∼2.3 μm during basal isometric and cyclic contractile testing for both genotypes, indicating that isometric tests were performed on the ascending limb of the length-tension curve of the WT GAS (optimum Ls = 2.54–2.74 μm) but on the descending limb of the NEB-KO GAS (optimum Ls = 1.90–2.10 μm). Since the WT GAS was generating submaximal stress due to thin filament double overlap, it follows that the force-transmitting function of NEB likely predominated.
Interestingly, NEB-KO muscle was more susceptible to the effects of repetitive activation compared with WT muscle. This observation is consistent with a report (3) showing that NEB-KO muscle exhibits the most severe structural deterioration in chronically active muscles, such as the diaphragm, whereas deterioration is relatively mild and mostly consists of Z-disk misalignment in infrequently active muscles, such as the tibialis anterior muscle. Taken together, these results indicate that NEB confers a protective effect on activated muscle and maintains the integrity and stability of muscle during activation. Our measurements were functional, and no attempt was made to differentiate between metabolic factors, damage to the myofibrillar lattice, or a combination of both as the cause for stress reduction. There is currently no evidence in the literature implicating NEB in skeletal muscle metabolism, so myofibrillar damage is likely the most important factor. Intriguingly, the increased response to cyclic activation in NEB-KO muscle is distinct from the results of similar experiments on desmin knockout muscle that showed cyclic isometric tetani have no damaging effects on muscle (40). While desmin and NEB are known to interact and likely comprise a sarcomeric force-coupling system (2), their mechanical functions are apparently distinct.
In summary, these data demonstrate changes in the isometric length-tension properties of the NEB-KO mouse that are consistent with, but not quantitatively explained by, a decrease in thin filament length. Future studies are required to determine the precise molecular basis for altered stress production and the reasons why such alterations lead to muscle pathology.
GRANTS
The authors gratefully acknowledge support from National Institutes of Health Grants AR-40050, HL-46345, and HL-66100, the Department of Veterans Affairs, Telethon-Italy Grant TCP07006, and Fondazione Cariplo. Transmission electron microscopy was carried out at the National Center for Microscopy and Imaging Research, which is supported by NIH Grant RR-040500.
Supplementary Material
Acknowledgments
The authors thank Shannon Bremner, Taylor Winters, Alan Kwan, and Andrea Thor for technical assistance. The authors also thank Dr. Samuel Ward for generously providing research equipment as well as helpful discussions.
Supplemental material for this article is available at the American Journal of Physiology-Cell Physiology website.
REFERENCES
- 1.Agbulut O, Noirez P, Beaumont F, Butler-Browne G. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol Cell 95: 399–406, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Bang ML, Gregorio C, Labeit S. Molecular dissection of the interaction of desmin with the C-terminal region of nebulin. J Struct Biol 137: 119–127, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bang ML, Li X, Littlefield R, Bremner S, Thor A, Knowlton KU, Lieber RL, Chen J. Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J Cell Biol 173: 905–916, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bang ML, Mudry RE, McElhinny AS, Trombitas K, Geach AJ, Yamasaki R, Sorimachi H, Granzier H, Gregorio CC, Labeit S. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol 153: 413–427, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol 221: 177–190, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol 18: 637–706, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Edman KA The relation between sarcomere length and active tension in isolated semitendinosus fibres of the frog. J Physiol 183: 407–417, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gokhin DS, Ward SR, Bremner SN, Lieber RL. Quantitative analysis of neonatal skeletal muscle functional improvement in the mouse. J Exp Biol 211: 837–843, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Gordon AM, Huxley AF, Julian FJ. Tension development in highly stretched vertebrate muscle fibres. J Physiol 184: 143–169, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184: 170–192, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granzier HL, Akster HA, ter Keurs HE. Effect of thin filament length on the force-sarcomere length relation of skeletal muscle. Am J Physiol Cell Physiol 260: C1060–C1070, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Gregorio CC, Weber A, Bondad M, Pennise CR, Fowler VM. Requirement of pointed-end capping by tropomodulin to maintain actin filament length in embryonic chick cardiac myocytes. Nature 377: 83–86, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Herzog W, Kamal S, Clarke HD. Myofilament lengths of cat skeletal muscle: theoretical considerations and functional implications. J Biomech 25: 945–948, 1992. [DOI] [PubMed] [Google Scholar]
- 14.Huxley AF Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7: 255–318, 1957. [PubMed] [Google Scholar]
- 15.Huxley AF, Niedergerke R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature 173: 971–973, 1954. [DOI] [PubMed] [Google Scholar]
- 16.Huxley H, Hanson J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature 173: 973–976, 1954. [DOI] [PubMed] [Google Scholar]
- 17.Jin JP, Wang K. Cloning, expression, and protein interaction of human nebulin fragments composed of varying numbers of sequence modules. J Biol Chem 266: 21215–21223, 1991. [PubMed] [Google Scholar]
- 18.Jin JP, Wang K. Nebulin as a giant actin-binding template protein in skeletal muscle sarcomere. Interaction of actin and cloned human nebulin fragments. FEBS Lett 281: 93–96, 1991. [DOI] [PubMed] [Google Scholar]
- 19.Kruger M, Wright J, Wang K. Nebulin as a length regulator of thin filaments of vertebrate skeletal muscles: correlation of thin filament length, nebulin size, and epitope profile. J Cell Biol 115: 97–107, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labeit S, Gibson T, Lakey A, Leonard K, Zeviani M, Knight P, Wardale J, Trinick J. Evidence that nebulin is a protein-ruler in muscle thin filaments. FEBS Lett 282: 313–316, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Lehtokari VL, Pelin K, Sandbacka M, Ranta S, Donner K, Muntoni F, Sewry C, Angelini C, Bushby K, Van den Bergh P, Iannaccone S, Laing NG, Wallgren-Pettersson C. Identification of 45 novel mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Hum Mutat 27: 946–956, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Lieber RL, Brown CG, Trestik CL. Model of muscle-tendon interaction during frog semitendinosis fixed-end contractions. J Biomech 25: 421–428, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Littlefield R, Almenar-Queralt A, Fowler VM. Actin dynamics at pointed ends regulates thin filament length in striated muscle. Nat Cell Biol 3: 544–551, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Littlefield R, Fowler VM. Defining actin filament length in striated muscle: rulers and caps or dynamic stability? Annu Rev Cell Dev Biol 14: 487–525, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Ma K, Wang K. Interaction of nebulin SH3 domain with titin PEVK and myopalladin: implications for the signaling and assembly role of titin and nebulin. FEBS Lett 532: 273–278, 2002. [DOI] [PubMed] [Google Scholar]
- 27.McElhinny AS, Kazmierski ST, Labeit S, Gregorio CC. Nebulin: the nebulous, multifunctional giant of striated muscle. Trends Cardiovasc Med 13: 195–201, 2003. [DOI] [PubMed] [Google Scholar]
- 28.McElhinny AS, Kolmerer B, Fowler VM, Labeit S, Gregorio CC. The N-terminal end of nebulin interacts with tropomodulin at the pointed ends of the thin filaments. J Biol Chem 276: 583–592, 2001. [DOI] [PubMed] [Google Scholar]
- 29.McElhinny AS, Schwach C, Valichnac M, Mount-Patrick S, Gregorio CC. Nebulin regulates the assembly and lengths of the thin filaments in striated muscle. J Cell Biol 170: 947–957, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism 9: 184–188, 1960. [Google Scholar]
- 31.Nave R, Furst DO, Weber K. Interaction of alpha-actinin and nebulin in vitro. Support for the existence of a fourth filament system in skeletal muscle. FEBS Lett 269: 163–166, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Ottenheijm CA, Fong C, Vangheluwe P, Wuytack F, Babu GJ, Periasamy M, Witt CC, Labeit S, Granzier H. Sarcoplasmic reticulum calcium uptake and speed of relaxation are depressed in nebulin-free skeletal muscle. FASEB J 22: 2912–2919, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papa I, Astier C, Kwiatek O, Raynaud F, Bonnal C, Lebart MC, Roustan C, Benyamin Y. Alpha actinin-CapZ, an anchoring complex for thin filaments in Z-line. J Muscle Res Cell Motil 20: 187–197, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Pappas CT, Bhattacharya N, Cooper JA, Gregorio CC. Nebulin interacts with CapZ and regulates thin filament architecture within the Z-disc. Mol Biol Cell 19: 1837–1847, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelin K, Hilpela P, Donner K, Sewry C, Akkari PA, Wilton SD, Wattanasirichaigoon D, Bang ML, Centner T, Hanefeld F, Odent S, Fardeau M, Urtizberea JA, Muntoni F, Dubowitz V, Beggs AH, Laing NG, Labeit S, de la Chapelle A, Wallgren-Pettersson C. Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci USA 96: 2305–2310, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell PL, Roy RR, Kanim P, Bello MA, Edgerton VR. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol 57: 1715–1721, 1984. [DOI] [PubMed] [Google Scholar]
- 37.Rack PM, Westbury DR. The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J Physiol 204: 443–460, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Root DD, Wang K. Calmodulin-sensitive interaction of human nebulin fragments with actin and myosin. Biochemistry 33: 12581–12591, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Root DD, Wang K. High-affinity actin-binding nebulin fragments influence the actoS1 complex. Biochemistry 40: 1171–1186, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Sam M, Shah S, Friden J, Milner DJ, Capetanaki Y, Lieber RL. Desmin knockout muscles generate lower stress and are less vulnerable to injury compared with wild-type muscles. Am J Physiol Cell Physiol 279: C1116–C1122, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Schafer DA, Cooper JA. Control of actin assembly at filament ends. Annu Rev Cell Dev Biol 11: 497–518, 1995. [DOI] [PubMed] [Google Scholar]
- 42.Schafer DA, Hug C, Cooper JA. Inhibition of CapZ during myofibrillogenesis alters assembly of actin filaments. J Cell Biol 128: 61–70, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sosnicki AA, Loesser KE, Rome LC. Myofilament overlap in swimming carp. I. Myofilament lengths of red and white muscle. Am J Physiol Cell Physiol 260: C283–C288, 1991. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki S, Pollack GH. Bridgelike interconnections between thick filaments in stretched skeletal muscle fibers observed by the freeze-fracture method. J Cell Biol 102: 1093–1098, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol 75: 2337–2340, 1993. [DOI] [PubMed] [Google Scholar]
- 46.Trombitas K, Tigyi-Sebes A. Cross-bridge interaction with oppositely polarized actin filaments in double-overlap zones of insect flight muscle. Nature 309: 168–170, 1984. [DOI] [PubMed] [Google Scholar]
- 47.Walker SM, Schrodt GR. I segment lengths and thin filament periods in skeletal muscle fibers of the Rhesus monkey and the human. Anat Rec 178: 63–81, 1974. [DOI] [PubMed] [Google Scholar]
- 48.Wallgren-Pettersson C, Donner K, Sewry C, Bijlsma E, Lammens M, Bushby K, Giovannucci Uzielli ML, Lapi E, Odent S, Akcoren Z, Topaloglu H, Pelin K. Mutations in the nebulin gene can cause severe congenital nemaline myopathy. Neuromuscul Disord 12: 674–679, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Wallgren-Pettersson C, Pelin K, Nowak KJ, Muntoni F, Romero NB, Goebel HH, North KN, Beggs AH, Laing NG. Genotype-phenotype correlations in nemaline myopathy caused by mutations in the genes for nebulin and skeletal muscle alpha-actin. Neuromuscul Disord 14: 461–470, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Wang K, Knipfer M, Huang QQ, van Heerden A, Hsu LC, Gutierrez G, Quian XL, Stedman H. Human skeletal muscle nebulin sequence encodes a blueprint for thin filament architecture. Sequence motifs and affinity profiles of tandem repeats and terminal SH3. J Biol Chem 271: 4304–4314, 1996. [DOI] [PubMed] [Google Scholar]
- 51.Wang K, Williamson CL. Identification of an N2 line protein of striated muscle. Proc Natl Acad Sci USA 77: 3254–3258, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang K, Wright J. Architecture of the sarcomere matrix of skeletal muscle: immunoelectron microscopic evidence that suggests a set of parallel inextensible nebulin filaments anchored at the Z line. J Cell Biol 107: 2199–2212, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber A, Pennise CR, Babcock GG, Fowler VM. Tropomodulin caps the pointed ends of actin filaments. J Cell Biol 127: 1627–1635, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willems ME, Huijing PA. Heterogeneity of mean sarcomere length in different fibres: effects on length range of active force production in rat muscle. Eur J Appl Physiol Occup Physiol 68: 489–496, 1994. [DOI] [PubMed] [Google Scholar]
- 55.Witt CC, Burkart C, Labeit D, McNabb M, Wu Y, Granzier H, Labeit S. Nebulin regulates thin filament length, contractility, and Z-disk structure in vivo. EMBO J 25: 3843–3855, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.