Abstract
Stress granules (SGs) arise as a consequence of cellular stress, contain stalled translation preinitiation complexes, and are associated with cell survival during environmental insults. SGs are dynamic entities with proteins relocating into and out of them during stress. Among the repertoire of proteins present in SGs is eukaryotic initiation factor 4E (eIF4E), a translation factor required for cap-dependent translation and that regulates a rate-limiting step for protein synthesis. Herein, we demonstrate that localization of eIF4E to SGs is dependent on the presence of a family of repressor proteins, eIF4E-binding proteins (4E-BPs). Our results demonstrate that 4E-BPs regulate the SG localization of eIF4E.
Keywords: eukaryotic initiation factor 4E, stress granules
in response to environmental stress, eukaryotic cells reprogram their gene expression profile by evoking transcriptional and posttranscriptional regulatory mechanisms. An early phase of this response involves a change in mRNA metabolism that is associated with the formation of cellular aggregates, known as stress granules (SGs) (for a review, see Refs. 1 and 30). SG formation occurs upon exposure of cells to a number of stresses, including heat shock, ultraviolet irradiation, endoplasmic reticulum stress, oxidative stress, viral infection, and osmotic stress. Nontranslating mRNAs, eukaryotic translation factors, and other RNA-binding proteins become deposited in SGs, and these appear to act as temporary storage sites until the elimination of the stress-inducing stimulus (for a review, see Ref. 30). Inhibitors of translation that block initiation or cause disassembly of polysomes (e.g., puromycin) stimulate SG formation, whereas those that stabilize polysomes induce SG disassembly. Fluorescence recovery after photobleaching (FRAP) has demonstrated the shuttling of several proteins (GFP-TIA1, GFP-PABP, YFP-eIF4E, EGFP-Ago2) in and out of SGs (15, 23, 30). Labile mRNAs are also stabilized during stress (3, 20). Taken together, these results have led to the suggestion that SGs constitute dynamic entities where mRNAs assemble for quality control before being redirected for translation, degradation, or storage (16).
Most eukaryotic cellular mRNA translation is thought to occur by a cap-dependent process, which is effected by the eukaryotic initiation factor (eIF) 4F complex (for a review, see Ref. 12). eIF4F is composed of three subunits: 1) eIF4E, the cap-binding protein that binds to the cap structure; 2) eIF4A, a DEAD-box RNA helicase thought to unwind local RNA structure and facilitate access of the 43S ribosomal complex to the mRNA; and 3) eIF4G, a modular scaffold that binds eIF4E and eIF4A and recruits ribosomes to mRNA via its interactions with eIF3. All eIF4F subunits localize to SGs under stress (5, 14, 18, 19, 25). The activity of eIF4E is regulated by a family of translation initiation repressors referred to as eIF4E-binding proteins (4E-BPs). eIF4E shuttles between the active eIF4F complex and the inhibitory 4E-BP/eIF4E heterodimer with 4E-BPs and eIF4G competing for a common binding site on eIF4E (12). In mammals, there are three 4E-BP members, of which 4E-BP1 and 4E-BP2 are expressed in most tissues, whereas 4E-BP3 expression is more restricted (31, 34). The binding of eIF4E to 4E-BP1 (the best characterized 4E-BP) is regulated by phosphorylation of 4E-BP1 by mammalian target of rapamycin (mTOR; for a review, see Ref. 13). Increased phosphorylation of 4E-BP1 decreases its affinity for eIF4E, eventually leading to its dissociation and allowing binding of eIF4G.
Although the majority of eIF4E is cytoplasmic, a significant fraction (12–33%) is also present in the nucleus and localizes with splicing factors into speckles (7, 22). The nuclear import of eIF4E is mediated by 4E-T, a transporter that binds eIF4E through a conserved motif that is also shared by 4E-BP and eIF4G (6). In addition, a sizeable portion of 4E-BP1 (∼30%) is present in the nucleus and regulates eIF4E nuclear localization under stress and in response to mTOR activity (32). In the current study, we report that 4E-BPs regulate the localization of eIF4E to SGs. We find that localization of eIF4E to SGs is dependent on the presence of 4E-BPs, although 4E-BPs do not localize to SGs. In addition, we demonstrate a stimulus-dependent regulation of eIF4E localization to SGs that is 4E-BP dependent. These results highlight 4E-BPs as key regulators of eIF4E localization to SGs.
MATERIALS AND METHODS
General methods and cell maintenance.
Immortalized WT and 4E-BP1/2+/+4E-BP1/2−/− MEFs were established by passing primary cells for more than 16 generations, and experiments were performed using cells between passage 30 and 40. Cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. Plasmids MSCV-GFP-eIF4E-BP(5A) and MSCV-GFP-eIF4E-BP(5A)Δ4EBS were transiently transfected using Lipofectamine2000 according to the manufacturer's instructions (Invitrogen). Heat shock treatments were conducted by floating cell chambers in a water bath at the indicated temperatures and time. Cells were treated with 100 nM rapamycin (Calbiochem) or 0.5 mM sodium arsenite (Sigma-Aldrich) for 1 h in a 37°C/5% CO2 incubator.
Plasmid constructs.
MSCV constructs [eIF4E-BP1, eIF4E-BP(5A), and eIF4E-BP(5A)Δ4E] were made by subcloning XhoI/EcoRI fragments harboring 4E-BPs into the same restriction sites in MSCV-GFP. eIF4E-BP(5A) and eIF4E-BP(5A)Δ4E were described previously (11). To generate cell lines that stably express 4E-BP1 protein, MSCV constructs were transiently transfected into Phoenix-293T packaging cell line. After 48 h, virus-containing medium was filtered (0.45 μm), collected, and used to infect MEFs in the presence of 5 μg/ml polybrene (Sigma-Aldrich). The infection was repeated the next day. Forty-eight hours after the second infection, cells were FACS sorted for green fluorescent protein-positive (GFP+) cells.
Antibodies.
Anti-eIF4E antibody (monoclonal) was a gift from S. Kimball (19). Rabbit polyclonal anti-G3BP and monoclonal ant-HuR antibodies have been previously described (9, 10). Anti-eIF4GI and anti-4E-BP1 antibodies were obtained from Cell Signaling Technology (Danvers, MA).
Fluorescence microscopy.
Cells used for immunofluorescence were seeded in four-well slide chambers at 20,000 cells per well and grown overnight. After treatment, cells were fixed using 4% formaldehyde in PBS, permeabilized with 4% formaldehyde/0.1% Triton X-100 in PBS, and blocked with 50% FBS-6% milk-3% BSA-0.1% Triton X-100–0.05% NaN3 in PBS [modified from Dostie et al. (6)]. Cells were then interrogated with anti-eIF4E (1:100), anti-G3BP (1:1,000), anti-HuR (1:2,000), anti-4E-BP1 (1:100), or anti-eIF4GI antibodies (1:100) for 16 h at 4°C. Cells were washed with PBS and incubated with secondary anti-rabbit and anti-mouse antibodies conjugated to Alexa Fluor 488 or 594. Nuclei were stained either with Hoechst 33324 (Molecular Probes) or 4′,6-diamidino-2-phenylindole (DAPI; D-1306, Molecular Probes) diluted at 1:2,000. The immunofluorescence acquisition was performed more than five times, and the most representative results are shown. Images were taken from a ×63 objective of a Zeiss LSM 510 confocal microscope.
The cell scoring module from MetaMorph (Molecular Devices) was used to quantify the amount of SGs. For this purpose, we used the DAPI-stained images to mask for the nuclear area. Then the software created an overlay with the images obtained with the anti-eIF4E and the anti-G3BP antibodies. This overlay image was used to quantitate the eIF4E-positive SGs. In addition, we use the G3BP images to quantitate the total number of SGs. The SG diameter range was set between 0.8 and 5 μm; the software is capable of limiting the appropriate particles that fit within this range. By thresholding the background to 500 in a gray scale, the nonspecific background particles will be cancelled. The outcome will be the total number of SGs or the total number of eIF4E-positive SGs.
RESULTS
4E-BPs affect eIF4E localization to SGs.
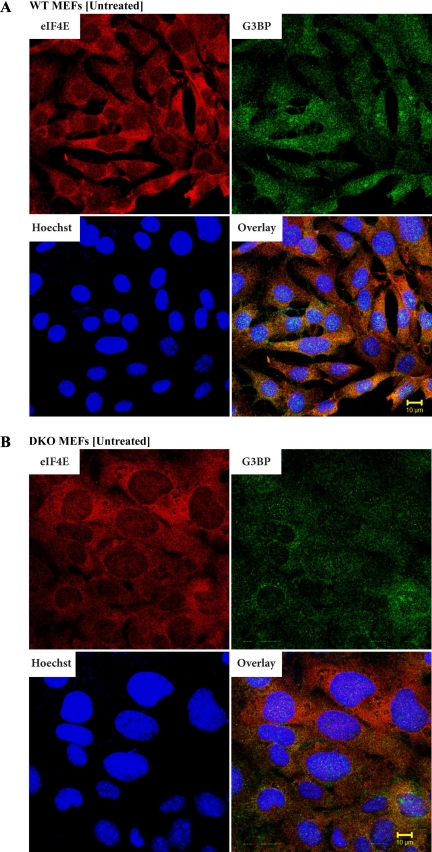
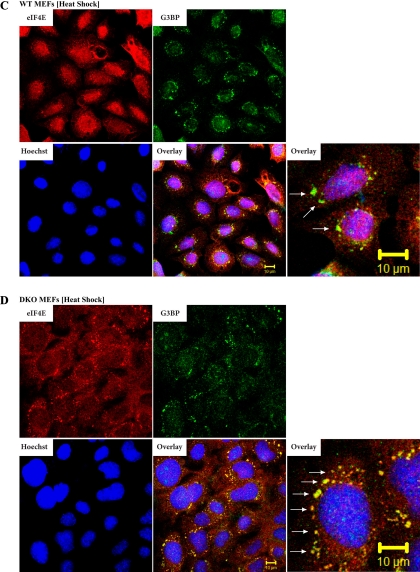
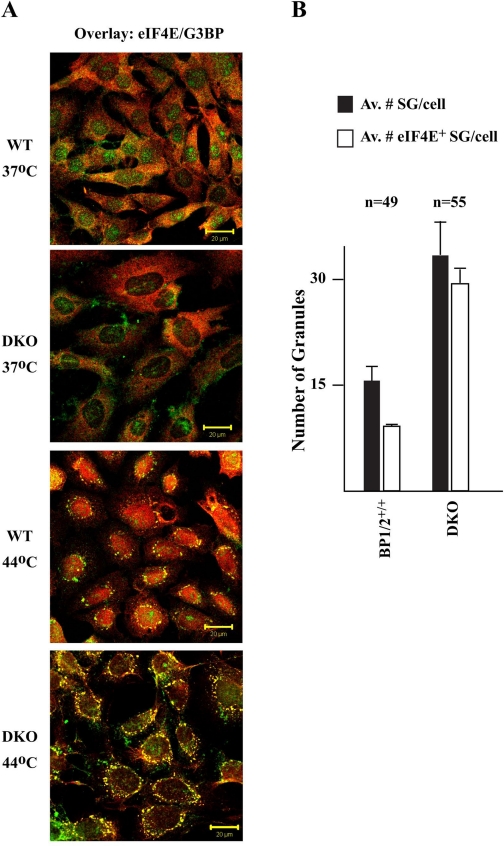
We have previously reported that a significant fraction of 4E-BP1 resides in the nucleus and regulates eIF4E nuclear localization (32). This prompted us to assess whether 4E-BP could impact other events affecting subcellular targeting of eIF4E, notably the relocalization of eIF4E to SGs during stress. To this end, wild-type (WT) and 4E-BP1−/−/4E-BP2−/− mouse embryonic fibroblasts (MEFs) (referred to as DKO for double-knockout henceforth) were used. Although MEFs do not generally yield SGs that are as clear and distinct as in other cell types (16), we were limited in the choice of our cells due to our desire to use defined isogenic lines. MEFs were heat stressed and analyzed by double-label immunofluorescence microscopy to score for eIF4E and G3BP or HuR (as markers for SG formation) subcellular localization (16). Under standard growth conditions, endogenous eIF4E is localized primarily in the cytoplasm of both WT and DKO MEFs, with a minor amount (10–15%) of eIF4E present in the nuclei of WT MEFs (Fig. 1, A and B). G3BP localization is diffuse and present in the cytoplasm and nuclei of both WT and DKO MEFs, as previously reported (16) (Fig. 1, A and B). When WT and DKO MEFs were subjected to heat stress, SG formation was readily apparent (Fig. 1, C and D). In WT MEFs, relocalization of eIF4E to the nucleus is evident (Fig. 1C). [The difficulty in observing eIF4E in SGs of WT MEFs appears to be a cell-type phenomenon since in heat-shocked HeLa cells, relocalization of eIF4E to SGs (and nuclei) was readily apparent (Supplemental Fig. 1; the online version of this article contains supplemental data)]. When DKO cells were exposed to 44°C, there appeared to be an increase in the number of eIF4E-staining SGs (compare Fig. 1D to Fig. 1C and Fig. 2A). The levels of eIF4E in the nuclei of DKO cells was also reduced compared with heat-shocked WT MEFs (compare Fig. 1D to Fig. 1C and Fig. 2A). A significant difference in the total amount of SGs as well as in eIF4E-positive SGs per cell between WT and DKO MEFs was observed (Fig. 2B). The number of SGs was smaller (∼2-fold) in WT MEFs relative to DKO MEFs (Fig. 2B), whereas the number of eIF4E-positive SGs was ∼3-fold smaller.
Fig. 1.
Distribution of eukaryotic initiation factor 4E (eIF4E) in wild-type (WT) and eIF4E-binding protein 1 (4E-BP1)−/−/4E-BP2−/− [double-knockout (DKO)] mouse embryonic fibroblasts (MEFs) upon heat stress. Untreated (A and B) or heat stressed (C and D) WT (A and C) and DKO (B and D) MEFs were fixed and stained for eIF4E and G3BP using a mouse monoclonal antibody and Alexa Fluor 594-conjugated secondary antibody (red) and a rabbit polyclonal antibody and Alexa Fluor 488-conjugated secondary antibody (green), respectively. White arrows highlight eIF4E-positive stress granules (SG). Hoechst was used as nuclear marker. Images were taken from a ×63 objective of a Zeiss LSM 510 confocal microscope.
Fig. 2.
DKO MEFs contain more SGs than WT MEFs. A: double overlay exposure of untreated or heat-shocked WT and DKO cells fixed and stained for eIF4E (red) and G3BP (green). B: quantitation of the number of SGs and eIF4E+ SGs in heat-shocked (44°C/30 min) WT and DKO cells. Av, average; n, number of cells that were inspected in this experiment.
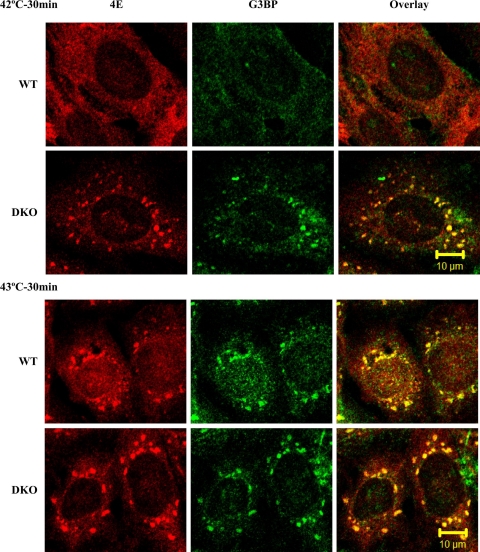
We next examined the conditions used to induce SGs. SG formation was apparent in WT MEFs and DKOs as early as 10 min after application of heat shock and did not increase in size 20 min after heat shock (Supplemental Fig. 2, A and B). We also monitored the effect of varying the temperature of the heat shock on SG formation (Fig. 3). In this experiment, cells were exposed for 30 min to 42°C or 43°C and the presence of SGs scored for. We note that both WT MEFs and DKOs showed the presence of SGs at 43°C, but only DKOs showed SG formation at 42°C (Fig. 3). Reintroduction of 4E-BP1 into DKO cells prevented the formation of SGs at 42°C (Supplemental Fig. 3, A and B). These results indicate that DKO MEFs are more sensitive than WT MEFs at forming SGs. There was no difference in cell viability, as judged by the percentage of cells staining for Annexin-PI after 4 h exposure to 42°C, between WT and DKO MEFs (R. Sukarieh, unpublished observations).
Fig. 3.
SGs form at a lower temperature in DKO MEFs. WT and DKO MEFs were exposed to 42°C or 43°C for 30 min, fixed, and stained for the presence of eIF4E (4E) and G3BP.
We also evaluated the reversibility of SG formation in WT and DKO MEFs that had been exposed to 44°C for 30 min (Supplemental Fig. 4, A and B). For both WT and DKO MEFs, SGs disappeared by 4 h (Supplemental Fig. 4B), although they appeared to resolve from DKO cells slightly faster than from MEFs (compare the 1–2 h post-heat shock treatment between DKO and WT MEFs). We observed no differences in cell viability between WT and DKO MEFs 6 h following exposure of cells to 44°C for 30 min (Supplemental Fig. 5).
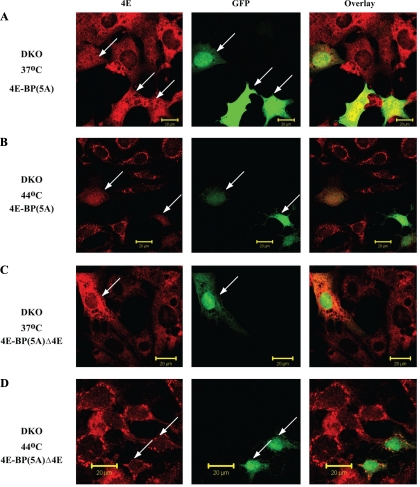
To determine whether the increased SG localization of eIF4E in DKO cells was due to the absence of 4E-BPs, we reintroduced GFP-4E-BP1 into DKO cells by transient transfection. For these studies, we used a mutant of 4E-BP1, 4E-BP(5A), in which five phosphorylation sites (Thr37, Thr46, Ser65, Thr70, and Ser83) are converted to alanines and which is no longer regulated by mTOR. Introducing GFP-4E-BP(5A) into DKO MEFs increased the amount of eIF4E localizing to the nucleus (Fig. 4A; compare the eIF4E nuclear staining pattern of GFP+ cells to GFP− staining cells). The presence of 4E-BP(5A) in DKO cells exposed to heat shock caused an enrichment of nuclear eIF4E and a reduction in the number of eIF4E-positive SGs (Fig. 4B). The SGs also stained positive for G3BP (data not shown). This altered localization of eIF4E is dependent on binding of eIF4E and 4E-BP since removal of the eIF4E binding site on 4E-BP1, 4E-BP(5A)Δ4E, prevented detectable relocalization of endogenous eIF4E from the cytoplasm to the nucleus (Fig. 4, C and D). These results suggest that localization of eIF4E to SGs is dependent on its ability to exit the inhibitory 4E-BP:eIF4E complex.
Fig. 4.
Relocalization of eIF4E in MEF cells in the presence of 4E-BP(5A) or 4E-BP(5A)Δ4E mutants. WT and DKO MEF cells were transiently transfected either with MSCV-GFP-4E-BP(5A) (A and B) or with MSCV-GFP-4E-BP(5A)Δ4E (C and D). Cells were fixed and stained for eIF4E (red). GFP fluorescence was visualized as a marker of positively transfected cells. Arrows represent cells expressing either 4E-BP(5A) or 4E-BP(5A)Δ4E.
Rapamycin is a chemical suppressor of eIF4E localization to SGs.
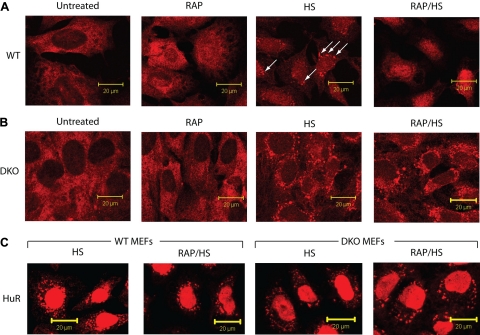
We have previously shown that inhibition of mTOR activity with rapamycin increases nuclear retention of eIF4E (32). We wished to examine whether rapamycin would affect eIF4E localization to SGs (Fig. 5). As previously reported, rapamycin causes an enrichment of eIF4E in the nuclei of WT MEFs (Fig. 5A). Heat stress caused eIF4E to relocalize into SGs in WT MEFs, whereas preexposure to rapamycin dampened this response by causing retention of eIF4E in the nucleus and reducing the formation of eIF4E-containing SGs (Fig. 5A). Rapamycin did not affect the formation of heat-induced SGs, as judged by the ability of HuR (Fig. 5C) or G3BP (Supplemental Fig. 6) to localize to SGs in WT or DKO cells. Exposure of DKO cells to rapamycin did not visibly alter the subcellular distribution of eIF4E in cells at 37°C or heat shocked at 44°C (Fig. 5B). 4E-BP phosphorylation was inhibited by rapamycin (Supplemental Fig. 7, compare lane 2 to lane 1) but not by heat shock treatment (compare lane 3 to lane 1), as previously reported (33). Combined treatment of rapamycin and heat shock caused an even higher level of hypophosphorylated 4E-BP (compare lane 4 to lanes 1–3).
Fig. 5.
Rapamycin (Rap) alters the subcellular localization of eIF4E to SGs in WT, but not DKO, MEFs. WT (A) or DKO (B) MEF cells were exposed to vehicle (untreated) or to Rap (100 nM for 1 h) or heat shock (HS; 44°C/30 min) or to Rap treatment followed by a heat shock (Rap/HS). Cells were fixed and stained for eIF4E. C: WT and DKO cells were heat shocked or pretreated with Rap and then heat shocked, fixed, and stained for HuR. Arrows represent eIF4E-positive stress ganules.
4E-BP1 does not localize to SGs.
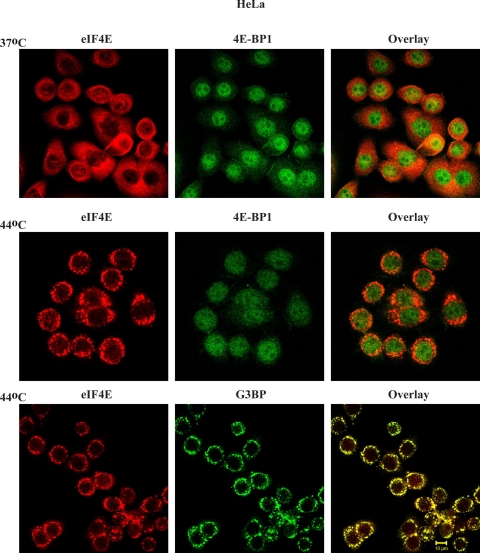
To investigate whether 4E-BP1 localizes to SGs under stress conditions, we analyzed the subcellular localization of 4E-BP1 under heat shock conditions. For these experiments, we used HeLa cells since they exhibit more distinct SGs. At 37°C, eIF4E is predominantly cytoplasmic, whereas there is 4E-BP1 in the nucleus (Fig. 6, top). Exposure of HeLa cells to 44°C resulted in the formation of SGs containing eIF4E and G3BP, but not 4E-BP1 (Fig. 6).
Fig. 6.
4E-BP1 is not recruited to SGs upon heat shock. HeLa cells were either maintained at 37°C or heat shocked (44°C/30 min), fixed, and stained for eIF4E, 4EBP1, or G3BP.
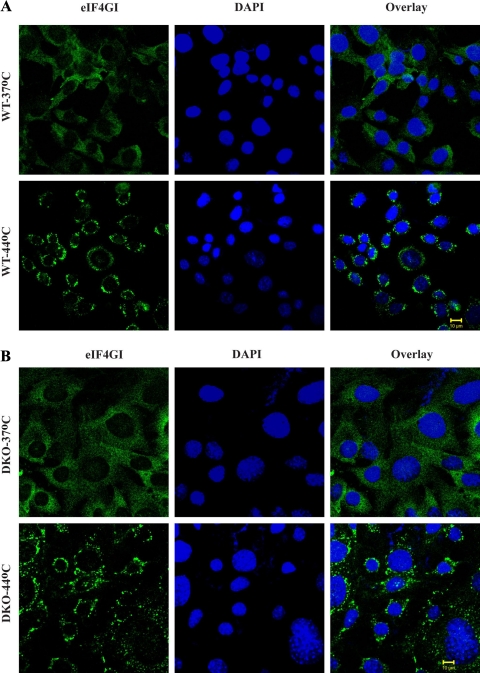
Because both 4E-BP1 and eIF4GI compete for a common binding site on eIF4E, we examined whether the localization of eIF4G to SGs is affected by the presence of 4E-BP1. To investigate this, WT and DKO MEF cells were heat shocked and stained for eIF4G (Fig. 7, A and B). As previously reported, eIF4GI localized to SGs upon heat shock (18). The absence of 4E-BP did not impact on eIF4GI distribution to SGs upon heat shock (Fig. 7, A and B). Thus, although 4E-BP1 plays a crucial role in the localization of eIF4E in the nucleus, it is not present in SGs and does not affect eIF4G localization.
Fig. 7.
eIF4GI recruitment to SGs is not affected by the presence or absence of 4E-BP1/2. WT (A) or DKO (B) MEFs were maintained at 37°C or exposed to 44°C/30 min, fixed, and stained for eIF4GI and 4′,6-diamidino-2-phenylindole (DAPI; nuclear marker).
Oxidative stress causes eIF4E localization to SGs regardless of the presence or absence of 4E-BP1/2.
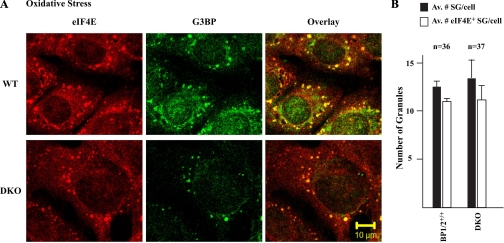
Exposure of cells to oxidative stress (arsenite) has previously been shown to induce the localization of eIF4E to SGs (16). We then asked whether the absence of 4E-BPs would similarly allow an increase in the localization of eIF4E to SGs under oxidative stress. WT and DKO MEFs were exposed to arsenite and analyzed by double-label immunofluorescence microscopy using anti-eIF4E and anti-G3BP. Inducing oxidative stress caused eIF4E to relocalize to SGs in both WT and DKO MEFs to similar extents (Fig. 8, A and B). These results suggest that, unlike heat shock, the presence or absence of 4E-BP1/2 does not impact on the ability of eIF4E to localize to SGs, when these are induced to form by oxidative stress. Unlike for heat shock, there was no change in 4E-BP1 phosphorylation status in WT MEFs exposed to arsenite (R. Sukarieh, unpublished observations). These findings suggest that 4E-BPs do not play a role in the localization of eIF4E to SGs upon arsenite treatment.
Fig. 8.
Relocalization of eIF4E in MEF cells upon arsenite treatment. A: WT or DKO MEFs were exposed to arsenite (0.5 mM for 1 h) after which time cells were fixed and stained for eIF4E and G3BP. B: quantitation of the number of SGs and eIF4E+ SGs in arsenite-treated WT and DKO cells; n, number of cells that were inspected in this experiment.
DISCUSSION
Nuclear sequestration of eIF4E has been previously documented to be regulated by 4E-BPs (32). Attenuation of mTOR by serum starvation or rapamycin treatment causes increased nuclear sequestration of eIF4E. In addition, loss of nuclear 4E-BP1 in c-Ha-Ras expressing MEFs ablates starvation-induced nuclear accumulation of eIF4E (32). As well, ectopic expression of eIF4E-transporter causes mRNP remodeling by triggering the movement of eIF4E into P-bodies, an event associated with inhibition of cap-dependent protein synthesis (21). Our data extend these results by providing evidence that 4E-BP1 influences the cytoplasmic routing of eIF4E to SGs during heat shock stress. This is suggested by the increased presence of eIF4E in SGs of heat-shocked DKO MEFs, relative to WT MEFs (Fig. 1) and reintroduction of a nonphosphorylatable 4E-BP1 mutant into DKO cells that diminished the amount of eIF4E localizing to SGs (Fig. 4). We interpret the ability of rapamycin to alter eIF4E localization upon heat shock to be a consequence of reduced 4E-BP phosphorylation, which prevents release of eIF4E from the nucleus to the cytoplasm (Fig. 5). Our results do not distinguish whether 4E-BPs directly or indirectly contribute to eIF4E accumulation in SGs, as could occur if SG accumulation were mediated by other competing factors in the absence of 4E-BPs. However, there does appear to be a strong correlation between 4E-BP phosphorylation status and eIF4E re-localization.
Our data are consistent with previous suggestions that 4E-BPs regulate the localization of eIF4E (32). Nuclear import of eIF4E is mediated by 4E-T via the importin-α/β pathway (6). In the nucleus, eIF4E colocalizes with splicing factors (7). Binding of eIF4E in the nucleus to 4E-BP could regulate the amount of eIF4E present in the cytoplasm and hence the amount of eIF4E available for shuttling to SGs upon heat shock stress. The idea that cytoplasmic availability of eIF4E is regulated by 4E-BP is supported by data demonstrating that 4E-BP(5A), which is constitutively bound to eIF4E, decreases the number of eIF4E-positive SGs in heat shock-treated DKO cells (Fig. 4). A 4E-BP1(5A) mutant lacking the eIF4E-binding site failed to alter the amount of eIF4E-positive SGs in DKO cells (Fig. 4). Consistent with this, inhibition of mTOR by rapamycin retained eIF4E into the nucleus and decreased the number of eIF4E+ SGs in WT MEFs (Fig. 5).
The formation of SGs is considered to be one of the cellular hallmarks that ensures protection of the cell against stress-induced damage that might otherwise preclude recovery. SGs occur in cells exposed to a number of other environmental stresses, including oxidative stress, ultraviolet irradiation, endoplasmic reticulum stress, viral infection, and osmotic shock (2, 4, 9, 17, 27, 28). Since the pathways leading to the formation of SGs remain undefined, it is possible that different stimuli use different pathways, and, indeed, this is reinforced by our findings that eIF4E localization to SGs appears more dependent on 4E-BP phosphorylation status in MEFs that are heat stressed, compared with MEFs that have been exposed to oxidative stress (Fig. 8) and where 4E-BP1 phosphorylation status is unchanged. Both heat stress and arsenite treatment were shown to induce the phosphorylation of eIF2α through the kinase activity of the heme-regulated inhibitor HRI (24, 26). Although both heat stress and arsenite treatment were shown to enhance the eIF4E:4E-BP1 interaction (8, 29, 35), we did not detect hypophosphorylation of 4E-BP1 upon heat shock or arsenite treatment (data not shown). Patel et al. (29) used Chinese hamster ovary cells in their study to document increased association between eIF4E and 4E-BP; we used MEFs in our current study.
Taken together, our data have provided evidence that 4E-BP1 regulates eIF4E localization to SGs. Hence, not only is eIF4E nuclear localization affected by 4E-BP1, but the amount of cytoplasmic eIF4E impacts on how much eIF4E is available to shuttle to SGs. Our results do not rule out the possibility that cytoplasmic sequestration of eIF4E by 4E-BPs also contributes to the differences observed in eIF4E SG localization between WT and DKO MEFs. As first noted by Nover and coworkers (27), the heat shock response is associated with a shift in the pattern and intracellular distribution of mRNAs. They reported that mRNAs encoding heat shock proteins were predominantly present in polysomes of heat-shocked cells, whereas nontranslating mRNAs were associated with SGs (27). This led to the proposal that SGs act as storage sites for untranslated mRNAs that are retrieved upon elimination of the stress-inducing stimulus. If eIF4E is involved in trafficking mRNA to SGs via the cap structure, then altering eIF4E levels could impact on how quickly SG formation occurs or how quickly protection is afforded.
GRANTS
This work was supported by grants from National Institutes of Health (1-01-CA-114475) and National Cancer Institute of Canada (17099) to J. Pelletier.
Supplementary Material
Acknowledgments
We thank Mark Livingstone for helpful comments on the manuscript. All images were taken at the McGill University Life Sciences Complex Imaging Facility.
REFERENCES
- 1.Anderson P, Kedersha N. RNA granules. J Cell Biol 172: 803–808, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrigo AP, Suhan JP, Welch WJ. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol Cell Biol 8: 5059–5071, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bollig F, Winzen R, Kracht M, Ghebremedhin B, Ritter B, Wilhelm A, Resch K, Holtmann H. Evidence for general stabilization of mRNAs in response to UV light. Eur J Biochem 269: 5830–5839, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Collier NC, Heuser J, Levy MA, Schlesinger MJ. Ultrastructural and biochemical analysis of the stress granule in chicken embryo fibroblasts. J Cell Biol 106: 1131–1139, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dang Y, Kedersha N, Low WK, Romo D, Gorospe M, Kaufman R, Anderson P, Liu JO. Eukaryotic initiation factor 2alpha-independent pathway of stress granule induction by the natural product pateamine A. J Biol Chem 281: 32870–32878, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Dostie J, Ferraiuolo M, Pause A, Adam SA, Sonenberg N. A novel shuttling protein, 4E-T, mediates the nuclear import of the mRNA 5′ cap-binding protein, eIF4E. EMBO J 19: 3142–3156, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dostie J, Lejbkowicz F, Sonenberg N. Nuclear eukaryotic initiation factor 4E (eIF4E) colocalizes with splicing factors in speckles. J Cell Biol 148: 239–247, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feigenblum D, Schneider RJ. Cap-binding protein (eukaryotic initiation factor 4E) and 4E-inactivating protein BP-1 independently regulate cap-dependent translation. Mol Cell Biol 16: 5450–5457, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallouzi IE, Brennan CM, Stenberg MG, Swanson MS, Eversole A, Maizels N, Steitz JA. HuR binding to cytoplasmic mRNA is perturbed by heat shock. Proc Natl Acad Sci USA 97: 3073–3078, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallouzi IE, Parker F, Chebli K, Maurier F, Labourier E, Barlat I, Capony JP, Tocque B, Tazi J. A novel phosphorylation-dependent RNase activity of GAP-SH3 binding protein: a potential link between signal transduction and RNA stability. Mol Cell Biol 18: 3956–3965, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev 13: 1422–1437, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem 68: 913–963, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 18: 1926–1945, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Kedersha N, Chen S, Gilks N, Li W, Miller IJ, Stahl J, Anderson P. Evidence that ternary complex (eIF2-GTP-tRNAiMet)-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol Biol Cell 13: 195–210, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, Golan DE, Anderson P. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol 151: 1257–1268, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fitzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol 169: 871–884, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol 147: 1431–1442, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim WJ, Back SH, Kim V, Ryu I, Jang SK. Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions. Mol Cell Biol 25: 2450–2462, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimball SR, Horetsky RL, Ron D, Jefferson LS, Harding HP. Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am J Physiol Cell Physiol 284: C273–C284, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Laroia G, Cuesta R, Brewer G, Schneider RJ. Control of mRNA decay by heat shock-ubiquitin-proteasome pathway. Science 284: 499–502, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Lee HC, Cho H, Kim YK. Ectopic expression of eIF4E-transporter triggers the movement of eIF4E into P-bodies, inhibiting steady-state translation but not the pioneer round of translation. Biochem Biophys Res Commun 369: 1160–1165, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Lejbkowicz F, Goyer C, Darveau A, Neron S, Lemieux R, Sonenberg N. A fraction of the mRNA 5′ cap-binding protein, eukaryotic initiation factor 4E, localizes to the nucleus. Proc Natl Acad Sci USA 89: 9612–9616, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung AK, Calabrese JM, Sharp PA. Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc Natl Acad Sci USA 103: 18125–18130, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu L, Han AP, Chen JJ. Translation initiation control by heme-regulated eukaryotic initiation factor 2alpha kinase in erythroid cells under cytoplasmic stresses. Mol Cell Biol 21: 7971–7980, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazroui R, Sukarieh R, Bordeleau ME, Kaufman RJ, Northcote P, Tanaka J, Gallouzi I, Pelletier J. Inhibition of ribosome recruitment induces stress granule formation independently of eukaryotic initiation factor 2alpha phosphorylation. Mol Biol Cell 17: 4212–4219, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McEwen E, Kedersha N, Song B, Scheuner D, Gilks N, Han A, Chen JJ, Anderson P, Kaufman RJ. Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J Biol Chem 280: 16925–16933, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Nover L, Scharf KD, Neumann D. Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol Cell Biol 9: 1298–1308, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nover L, Scharf KD, Neumann D. Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol Cell Biol 3: 1648–1655, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel J, McLeod LE, Vries RG, Flynn A, Wang X, Proud CG. Cellular stresses profoundly inhibit protein synthesis and modulate the states of phosphorylation of multiple translation factors. Eur J Biochem 269: 3076–3085, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Pelletier J, Mazroui R, Sukarieh R, Harvey I, Eddine Gallouzi I. Stress Granules in Cell Survival. Kerala, India: Research Signpost, 2008.
- 31.Poulin F, Gingras AC, Olsen H, Chevalier S, Sonenberg N. 4E-BP3, a new member of the eukaryotic initiation factor 4E-binding protein family. J Biol Chem 273: 14002–14007, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Rong L, Livingstone M, Sukarieh R, Petroulakis E, Gingras AC, Crosby K, Smith B, Polakiewicz RD, Pelletier J, Ferraiuolo MA, Sonenberg N. Control of eIF4E cellular localization by eIF4E-binding proteins, 4E-BPs. RNA 14: 1318–1327, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheper GC, Mulder J, Kleijn M, Voorma HO, Thomas AA, van Wijk R. Inactivation of eIF2B and phosphorylation of PHAS-1 in heat-shocked rat hepatoma cells. J Biol Chem 272: 26850–26856, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z, Gingras AC, Katsume A, Elchebly M, Spiegelman BM, Harper ME, Tremblay ML, Sonenberg N. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med 7: 1128–1132, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Vries RG, Flynn A, Patel JC, Wang X, Denton RM, Proud CG. Heat shock increases the association of binding protein-1 with initiation factor 4E. J Biol Chem 272: 32779–32784, 1997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.