Abstract
Impaired scavenger receptor class B type I (SR-BI)-mediated uptake of HDL-cholesterol esters (HDL-CE) induces adrenal insufficiency in mice. Humans contain an alternative route of HDL-CE clearance, namely through the transfer by cholesteryl ester transfer protein (CETP) to apolipoprotein B lipoproteins for subsequent uptake via the LDL receptor. In this study, we determined whether CETP can compensate for loss of adrenal SR-BI. Transgenic expression of human CETP (CETP Tg) in SR-BI knockout (KO) mice increased adrenal HDL-CE clearance from 33–58% of the control value. SR-BI KO/CETP Tg and SR-BI KO mice displayed adrenal hypertrophy due to equally high plasma adrenocorticotropic hormone levels. Adrenal cholesterol levels and plasma corticosterone levels were 38–52% decreased in SR-BI KO mice with and without CETP expression. SR-BI KO/CETP Tg mice also failed to increase their corticosterone level after lipopolysaccharide challenge, leading to an identical >4-fold increased tumor necrosis factor-α response compared with controls. These data indicate that uptake of CE via other routes than SR-BI is not sufficient to generate the cholesterol pool needed for optimal adrenal steroidogenesis. In conclusion, we have shown that CETP-mediated transfer of HDL-CE is not able to reverse adrenal insufficiency in SR-BI knockout mice. Thus, SR-BI-mediated uptake of serum cholesterol is essential for optimal adrenal function.
Keywords: adrenals, cholesteryl ester transfer protein, high density lipoprotein, low density lipoprotein, cholesteryl ester, corticosterone, inflammation
HDL is a small, dense protein-lipid complex that consists of a hydrophilic phospholipid monolayer and a hydrophobic core filled with cholesteryl esters. The HDL-mediated transport of cholesterol from peripheral tissues back to the liver for subsequent excretion from the body, often referred to as reverse cholesterol transport [reviewed in Van Eck et al. (1) and Lewis and Rader (2)], is considered to be an important physiological process to maintain total body cholesterol homeostasis. Scavenger receptor class B type I (SR-BI) is a multiligand cell surface receptor predominantly expressed in the liver and steroidogenic tissues (i.e., testis, ovary, and adrenals) (3). In mice, SR-BI is a prominent factor in the reverse cholesterol transport process, as it is the sole molecule involved in the selective uptake of cholesteryl esters from HDL by the liver (4). In addition to its established function in liver cholesterol metabolism, we (5) and others (6) have recently shown that SR-BI also plays a major role in adrenal cholesterol metabolism and steroid hormone production in mice. As a result, SR-BI deficiency in mice is associated with a depletion of adrenal cholesterol stores, resulting in a diminished steroid hormone (i.e., glucocorticoid) production in response to physiological stress.
Importantly, the metabolism of HDL differs significantly between humans and mice as humans, in contrast to rodents, naturally express cholesteryl ester transfer protein (CETP). CETP is synthesized by macrophage-rich tissues, such as the spleen and liver, after which it is secreted into the blood circulation (7–10). In plasma, CETP is able to transfer cholesteryl esters from HDL to apolipoprotein B (ApoB)-containing particles, such as VLDLs and LDLs. LDL-associated cholesteryl esters can subsequently be cleared from the blood circulation via whole particle uptake by the LDL receptor (ApoB receptor) (11, 12). In addition to SR-BI, the CETP→LDL→LDL receptor pathway can thus serve as an alternative route for the delivery of HDL-cholesteryl esters to cells in humans. However, the relative contribution of the SR-BI and the CETP→LDL→LDL receptor routes for the uptake of HDL-cholesteryl esters in humans is still unclear because polymorphisms in the human SR-BI gene leading to functional SR-BI deficiency so far have not been detected.
In vitro studies using adrenocortical cells have suggested that LDL receptor-mediated uptake of LDL is coupled to steroid hormone synthesis (13). In addition, Kita et al. (14) and Fong et al. (15) have shown that the adrenals express a functional LDL receptor in vivo. It is thus likely that the CETP→LDL→LDL receptor route is relevant for adrenal cholesterol homeostasis in the human situation. To gain insight in the possible role for the CETP→LDL→LDL receptor route in adrenal cholesterol metabolism, we have determined whether transgenic expression of human CETP can rescue the adrenal glucocorticoid insufficiency in SR-BI knockout (KO) mice.
MATERIALS AND METHODS
Animals
SR-BI KO mice were kindly provided by Dr. M. Krieger (Massachusetts Institute of Technology, Boston, MA) (16). CETP transgenic mice (CETP Tg; strain 5203) expressing human CETP under the control of its own promoter and other major regulatory elements were obtained from The Jackson Laboratory (Bar Harbor, ME) (17). SR-BI KO mice were bred with CETP Tg mice to generate heterozygous SR-BI KO mice expressing CETP on one allele. These mice were subsequently cross-bred to generate SR-BI KO mice expressing human CETP on one allele (SR-BI KO/CETP Tg) or control SR-BI KO mice and wild-type (WT) nontransgenic littermates. The presence of the targeted and wild-type SR-BI alleles as well as the CETP transgene was assessed by PCR amplification of DNA extracted from tail biopsies (primers: 5′-GATGGGACATGGGACACGAAGCCATTCT-3′ and 5′-TCTGTCTCCGTCTCCTTCAGGTCCTGA-3′ for SR-BI and 5′-CTAGGCCACAGAATTGAAAGATCT-3′, 5′-GTAGGTGGAAATTCTAGCATCATCC-3′, 5′-GAATGTCTCAGAGGACCTCCC-3′, and 5′-CTTGAACTCGTCTCCCATYCAG-3′ for CETP). Mice were fed a sterilized regular chow powder diet (RM3; Special Diet Services, Witham, UK). Animal experiments were performed at the Gorlaeus Laboratories of the Leiden/Amsterdam Center for Drug Research in accordance with the national laws. All experimental protocols were approved by the Ethics Committee for Animal Experiments of the Leiden University.
Analysis of gene expression by real-time quantitative PCR
Quantitative gene expression analysis on snap-frozen organs was performed as described (18). In short, total RNA was isolated according to Chomczynski and Sacchi (19) and reverse transcribed using RevertAid™ reverse transcriptase. Gene expression analysis was performed using real-time SYBR Green technology (Eurogentec) with the primers displayed in Table 1, which were validated for identical efficiencies (slope = −3.3 for a plot of threshold cycle number (Ct) versus log ng cDNA). Hypoxanthine guanine phosphoribosyl transferase (HPRT), GAPDH, and acidic ribosomal phosphoprotein P0 (36B4) were used as the standard housekeeping genes. Relative gene expression numbers were calculated by subtracting the Ct of the target gene from the average Ct of HPRT, GAPDH, and 36B4 (Ct housekeeping) and raising 2 to the power of this difference. Genes that exhibited a Ct value of >35 were considered not detectable. The average Ct of three housekeeping genes was used to exclude that changes in the relative expression were caused by variations in the expression of the separate housekeeping genes.
TABLE 1.
Primers used for real-time PCR analysis
| GenBank Accession Number | Forward Primer | Reverse Primer | |
|---|---|---|---|
| 36B4 | X15267 | GGACCCGAGAAGACCTCCTT | GCACATCACTCAGAATTTCAATGG |
| HPRT | J00423 | TTGCTCGAGATGTCATGAAGGA | AGCAGGTCAGCAAAGAACTTATAG |
| GAPDH | NM008084 | TCCATGACAACTTTGGCATTG | TCACGCCACAGCTTTCCA |
| CETP | NM000078 | CAGATCAGCCACTTGTCCAT | CAGCTGTGTGTTGATCTGGA |
| LDL receptor | Z19521 | CTGTGGGCTCCATAGGCTATCT | GCGGTCCAGGGTCATCTTC |
| SR-BI | NM016741 | GGCTGCTGTTTGCTGCG | GCTGCTTGATGAGGGAGGG |
Isolation and labeling of HDL
Human HDL was isolated from blood of healthy subjects by differential ultracentrifugation as described by Redgrave, Roberts, and West (20) and dialyzed against PBS with 1 mM EDTA. HDL (1.063 < d < 1.21) was labeled with [3H]cholesteryl ether (CEt) via exchange from donor particles as reported previously (21).
Adrenal uptake of 3H-Cholesteryl ether HDL
A dose of 200 μg apolipoprotein (±1.2 × 106 dpm) of [3H]CEt-HDL (total volume 100 μl) was injected into the tail vein. At 5 min after injection, a blood sample was drawn to verify the injected dose. For analysis of adrenal cholesteryl ether uptake, 24 h after tracer injection adrenals were excised, weighed, solubilized, and counted for 3H-radioactivity in a Packard liquid scintillation unit. A correction was made for the radioactivity in the blood present in the adrenals at the time of sampling (135.2 μl/g tissue).
Lipoprotein distribution analysis
The distribution of cholesterol or 3H-label over the different lipoproteins in plasma was analyzed by fractionation of pooled plasma using a Superose 6 column (3.2 × 300 mm, Smart-system; Pharmacia). Total cholesterol content of the effluent was determined using enzymatic colorimetric assays (Roche Diagnostics). Counting for 3H-radioactivity was performed in a Packard liquid scintillation unit.
Adrenal histology and immunohistochemical analysis for CETP
Formalin-fixed cryosections (8–10 μM) were prepared on a Leica CM3050-S cryostat. Cryosections were routinely stained with hematoxylin and Oil red O for nuclei and neutral lipids, respectively. For immunohistochemical staining of CETP, cryostat sections were incubated for 5 min with prewarmed (37°C) 0.025% trypsin at room temperature, blocked with 1% BSA in TBS, and incubated with a primary CETP antibody TP-2 (Ottawa Heart Institute, Ontario, Canada) and a secondary antibody conjugated to peroxidase (Jackson ImmunoResearch Labs, Suffolk, UK). Images were obtained with a Leica image analysis system, consisting of a Leica DMRE microscope coupled to a camera and Leica Qwin Imaging software (Cambridge, UK).
Plasma hormone analysis
Blood was drawn via the tail vein between 0900 and 1200 h for subsequent hormone analyses. Corticosterone and adrenocorticotropic hormone (ACTH) levels in plasma were determined using the CORTICOSTERONE and ACTH Double Antibody 125I Radioimmunoassay (RIA) kits from MP Biomedicals (Irvine, CA) according to the protocols from the supplier.
Tumor necrosis factor-α response upon lipopolysaccharide challenge
Mice were intravenously injected at 0900 h with 50 μg/kg LPS from Salmonella minnesota R595 (List Biological Laboratories, Hornby, Canada) into the tail vein. Blood samples were collected after 30, 60, 90, 120, and 180 min, and plasma tumor necrosis factor-α (TNF-α) protein levels were determined by ELISA (OptEIA kit; BD Biosciences Pharmingen, San Diego, CA).
Data analysis
Data were presented as means ± SEM. Statistical analyses were performed using one- and two-way ANOVA using Graphpad Prism Software (Graphpad Software, San Diego, CA). The level of statistical significance was set at P < 0.05.
RESULTS
In this study, we determined whether the CETP→LDL→LDL receptor route may be physiologically relevant for adrenal cholesterol metabolism and steroid hormone production. For this purpose, we cross-bred mice carrying the human CETP transgene linked to its natural flanking sequences (CETP Tg) with SR-BI KO mice to generate “human-like” SR-BI KO mice carrying the CETP transgene (SR-BI KO/CETP Tg mice). SR-BI KO/CETP Tg mice, like control SR-BI KO mice, lack a functional SR-BI gene, as evident from the undetectable mRNA expression of SR-BI in liver, spleen, adrenals, and white adipose tissue (Table 2). However, in contrast with control SR-BI KO mice, SR-BI KO/CETP Tg mice express relatively high levels of human CETP mRNA in macrophage-rich tissues, such as the liver and spleen, similarly as found in the human situation (8).
TABLE 2.
Relative mRNA expression levels of SR-BI and CETP in different organs isolated from WT mice, SR-BI KO mice, and SR-BI KO mice expressing human CETP (SR-BI KO/CETP Tg)
| Liver |
Spleen |
Adrenal |
White Adipose Tissue |
|||||
|---|---|---|---|---|---|---|---|---|
| SR-BI | CETP | SR-BI | CETP | SR-BI | CETP | SR-BI | CETP | |
| WT | 0.12 ± 0.02 | n.d. | 0.018 ± 0.001 | n.d. | 0.42 ± 0.12 | n.d. | 0.029 ± 0.015 | n.d. |
| SR-BI KO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| SR-BI KO/CETP Tg | n.d. | 0.14 ± 0.13 | n.d. | 0.10 ± 0.03 | n.d. | 0.012 ± 0.002 | n.d. | 0.025 ± 0.004 |
Values are relative to the housekeeping gene expression and represent means ± SEM of three mice per group. n.d., not detectable.
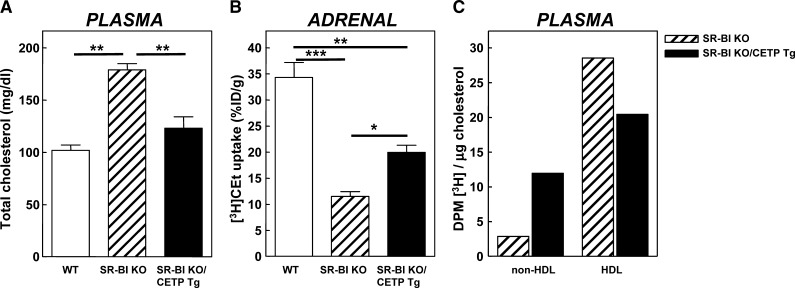
To investigate whether CETP expression in SR-BI KO mice induces transfer of cholesteryl esters from HDL to ApoB-containing lipoproteins for subsequent uptake by the LDL receptor in the adrenals, we determined the effect of transgenic expression of human CETP on plasma total cholesterol levels and the adrenal HDL-cholesteryl ester uptake in SR-BI KO mice. As shown in Fig. 1A, transgenic expression of human CETP induced an almost complete normalization of plasma total cholesterol levels in SR-BI KO mice, indicating that the CETP-mediated transfer of cholesteryl esters from HDL to VLDL/LDL fraction can also act as an effective alternative pathway to remove cholesteryl esters from the blood circulation in SR-BI KO mice. In parallel to the observed change in plasma cholesterol levels, the uptake of [3H]CEt-HDL by the adrenals was also partially restored in SR-BI KO/CETP Tg mice compared with SR-BI KO mice [20 ± 1% vs. 11 ± 1% of the injected dose/g tissue (P < 0.05), respectively, compared with 34 ± 3% for wild-type mice; Fig. 1B]. CETP-mediated transfer of cholesteryl esters from HDL to ApoB-containing lipoproteins thus does significantly increase the adrenal uptake of HDL-cholesteryl esters in SR-BI KO mice probably via the action of the LDL receptor. Evaluation of the [3H]CEt distribution over the different lipoprotein fractions at 8 h after [3H]CEt-HDL injection showed that 4.2-fold more of the [3H]label resided in the nonHDL fraction per μg cholesterol in SR-BI KO/CETP Tg mice compared with SR-BI KO mice, while a 30% lower amount of [3H]label per μg cholesterol was detected in the HDL fraction (Fig. 1C), resulting in an overall 6-fold higher nonHDL/HDL label distribution ratio in SR-BI KO/CETP Tg mice compared with SR-BI KO mice. This suggests that human CETP, as anticipated, efficiently transports cholesteryl esters from HDL to ApoB-containing (nonHDL) lipoproteins in SR-BI KO mice.
Fig. 1.
A: Plasma total cholesterol levels in WT (white bars), control SR-BI KO mice (hatched bars), and SR-BI KO mice expressing human CETP (SR-BI KO/CETP Tg; black bars). B) The adrenal uptake of HDL-cholesteryl ether (CEt) in WT (white bars), control SR-BI KO mice (hatched bars), and SR-BI KO mice expressing human CETP (SR-BI KO/CETP Tg; black bars). Mice were injected intravenously with [3H]CEt-HDL, and after 24 h the amount of radioactivity in the adrenals was determined. Values are expressed as percentage of the injected dose per gram tissue (%ID/g) and are means ± SEM (n = 3). C: Distribution of [3H]CEt over the different lipoprotein fractions generated from pooled plasma of three mice at 8 h after injection of the [3H]CEt-HDL in control SR-BI KO mice (hatched bars) and SR-BI KO mice expressing human CETP (SR-BI KO/CETP Tg; black bars). * P < 0.05, ** P < 0.01, and *** P < 0.001 (one-way ANOVA).
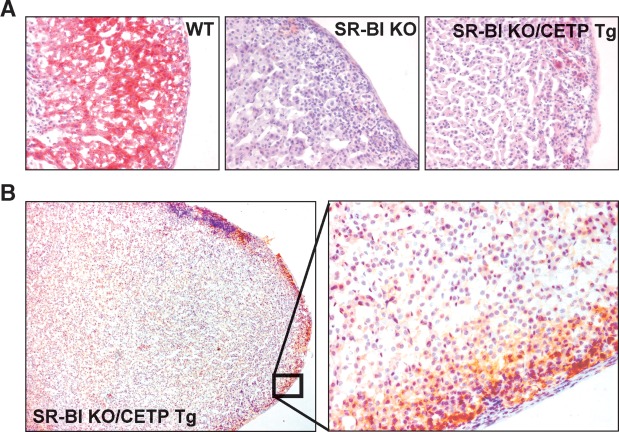
Compared with wild-type mice, adrenals from SR-BI KO mice are darker due to an almost complete depletion of lipid in the adrenal cortex as a result of the impaired adrenal uptake of HDL-cholesteryl esters (5). Strikingly, macroscopically the adrenals of SR-BI KO/CETP Tg mice and SR-BI KO mice are essentially identical and are significantly different from those of wild-type mice. As can be appreciated from the photograph depicted in Fig. 2A, both the adrenals of SR-BI KO/CETP Tg and SR-BI KO are visually darker (red color instead of white color) and have a larger volume compared with those of wild-type mice. In agreement, an overall higher weight of the adrenals was observed (5.2 ± 0.4 mg and 4.8 ± 0.3 mg for SR-BI KO/CETP and SR-BI KO mice vs. 3.4 ± 0.2 mg for WT mice; P < 0.05 for both; Fig. 2B). As the external phenotype of the adrenals in SR-BI KO mice was not affected by the expression of CETP, we determined the extent of lipid loading in the adrenal cortex of the three different types of mice (Fig. 3). In accordance with our previous findings (5), Oil red O neutral lipid staining in the adrenal cortex was almost completely absent in SR-BI KO mice compared with wild-type mice (Fig. 3A). Furthermore, no remarkable change in Oil red O staining was observed upon expression of CETP in SR-BI KO mice (Fig. 3A) as only limited Oil red O staining could be seen specifically in the zona glomerulosa (the mineralocorticoid producing cells) but not the zona fasciculata (the glucocorticoid producing cells) or zona reticularis (production of androgens) of the adrenal cortex of both SR-BI KO/CETP and control SR-BI KO mice. In contrast, all three zones of the cortex stained positive for neutral lipid in the adrenals of WT mice (Fig. 3A). Lipid analyses indicated that the adrenal cholesteryl ester content was not significantly different between SR-BI KO (7.3 ± 1.1 μg/mg protein) and SR-BI KO/CETP Tg mice (8.5 ± 0.6 μg/mg protein) but was significantly lower (P < 0.001 for both) compared with wild-type controls (16.5 ± 1.4 μg/mg protein). Immunohistochemical staining for CETP showed that the CETP protein is specifically expressed in the zona glomerulosa within the cortex of adrenals from SR-BI KO/CETP Tg mice (Fig. 3B). Combined, these findings suggest that the transfer of HDL-cholesteryl esters to ApoB-containing lipoproteins leading to subsequent uptake via the LDL receptor does not significantly contribute to cholesteryl ester storage in the adrenal cortex, while the adrenal expression of human CETP is insufficient to normalize cholesteryl ester storage.
Fig. 2.
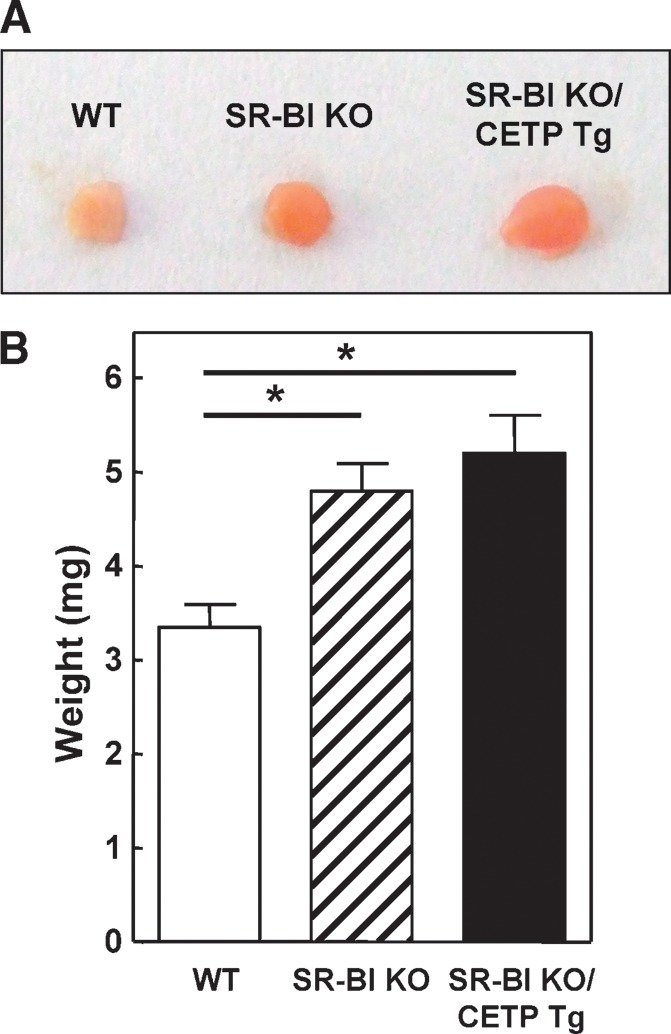
A: Photograph of adrenals from WT, control SR-BI KO mice, and SR-BI KO mice expressing human CETP (SR-BI KO/CETP Tg). B: Weight of the adrenals from WT (white bars), SR-BI KO mice (hatched bars), and SR-BI KO/CETP Tg mice (black bars). Values are means ± SEM (n = 3). * P < 0.05 (one-way ANOVA).
Fig. 3.
A: Oil red O neutral lipid staining on cryosections of adrenals from WT mice, SR-BI KO mice, and SR-BI KO mice expressing human CETP (SR-BI KO/CETP Tg). Sections were counterstained with hematoxylin for nuclei. Note the clearly diminished lipid staining in the adrenal cortex of SR-BI KO mice, which is not reversed by expression of human CETP. B: Immunohistochemical staining for CETP on adrenal sections from SR-BI KO/CETP Tg mice. CETP protein expression is localized specifically in the zona glomerulosa of the adrenal cortex.
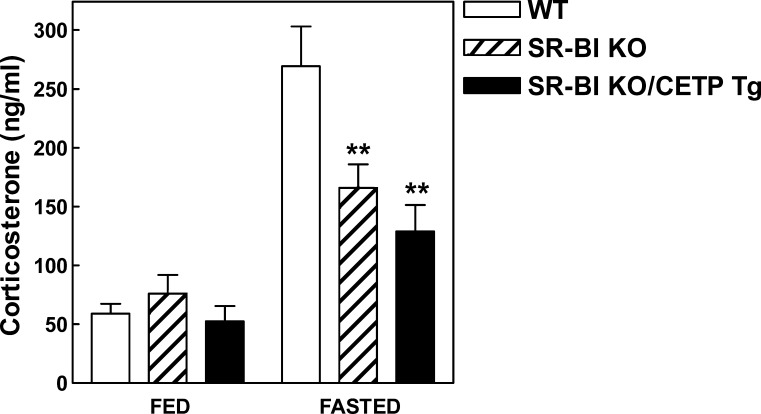
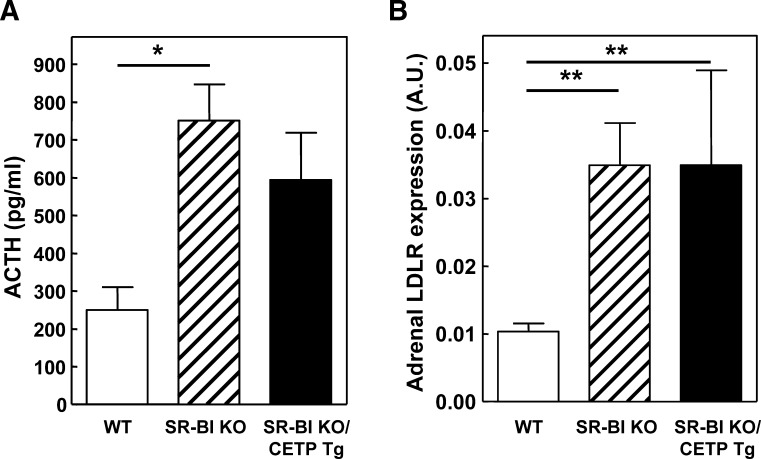
Importantly, adrenocortical cells within the zona fasciculata need cholesterol to produce glucocorticoid hormones, such as cortisol in humans and corticosterone in rodents. As the zona fasciculata of SR-BI KO mice with or without transgenic expression of CETP is equally depleted of lipid, we determined the effect of CETP expression on plasma corticosterone levels. Under fed (nonstressed) conditions, no significant change in the plasma corticosterone levels was observed between the three different genotypes (Fig. 4). In contrast, plasma corticosterone levels in SR-BI KO and SR-BI KO/CETP Tg mice were significantly lower (P < 0.01 for both) compared with those of WT mice under conditions of physiological stress induced by overnight fasting (Fig. 4). Measurements of ACTH, a potent activator of adrenal cortex growth and steroidogenesis (22), indicated that the impaired corticosterone response to fasting was not due to a decrease in ACTH levels as SR-BI KO and SR-BI KO/CETP Tg mice displayed a 2- to 3-fold increase (P < 0.05) in plasma ACTH levels compared with WT controls under fasting conditions (Fig. 5A). In addition, the mRNA expression of the LDL receptor, which is rapidly induced by ACTH in adrenocortical cells both in vitro (13, 23) and in vivo (24), was stimulated >3-fold (P < 0.01) in the adrenals of SR-BI KO mice and SR-BI KO/CETP Tg mice, indicative for optimal ACTH signaling in the adrenals of these animals (Fig. 5B). As CETP expression does not normalize the corticosterone response to fasting at a background of relatively high adrenal LDL receptor expression in SR-BI KO mice, it is suggested that SR-BI KO/CETP Tg mice similarly to control SR-BI KO mice also suffer from adrenal glucocorticoid insufficiency.
Fig. 4.
Plasma corticosterone levels in WT (white bars), control SR-BI KO mice (hatched bars) mice, and SR-BI KO mice expressing human CETP (SR-BI KO/CETP Tg; black bars) that were nonfasted (FED) or subjected to 16 h of fasting (FASTED). Values are means ± SEM (n = 4–15). Fasting induced a significant rise in plasma corticosterone levels (P < 0.001). ** P < 0.01 compared with fasted WT mice (two-way ANOVA).
Fig. 5.
Plasma ACTH levels (A) and adrenal relative LDL receptor (LDLR) mRNA expression levels (B) in WT (white bars), control SR-BI KO mice (hatched bars), and SR-BI KO mice expressing human CETP (SR-BI KO/CETP Tg; black bars) that were subjected to 16 h of fasting. Values are means ± SEM (n = 3–14). * P < 0.05 and ** P < 0.01 (one-way ANOVA).
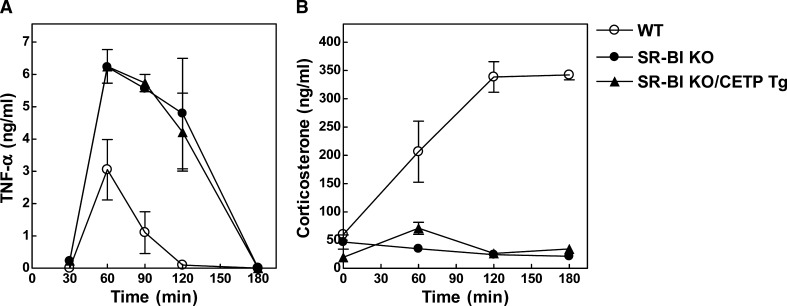
Recent studies by Cai et al. (6) have indicated that SR-BI KO mice are more susceptible to LPS-induced septic shock and death as a result of the adrenal glucocorticoid insufficiency. We did also observe a significantly (P < 0.001) enhanced response of the pro-inflammatory cytokine TNF-α in SR-BI KO mice compared with wild-type mice upon injection of a sublethal dose of LPS (50 μg/kg) (Fig. 6A). Interestingly, the peak value of the plasma TNF-α level at 60 min after LPS injection (6.25 ± 0.52 ng/ml for SR-BI KO/CETP Tg and 6.23 ± 0.03 ng/ml for SR-BI KO mice) and the total TNF-α response in time (area-under-curve 553 vs. 573 ng/ml·min, respectively) were identical between SR-BI KO/CETP Tg and control SR-BI KO mice upon the challenge with LPS (Fig. 6A). In addition, both SR-BI KO and SR-BI KO/CETP Tg mice failed to induce plasma corticosterone levels in response to LPS (Fig. 6B), which further establishes that transgenic expression of CETP is not able to overcome the adrenal glucocorticoid insufficiency in SR-BI KO mice.
Fig. 6.
LPS-induced TNF-α response (A) and corticosterone response (B) in WT (open circles), control SR-BI KO mice (closed circles), and SR-BI KO mice expressing human CETP (SR-BI KO/CETP Tg; closed triangles). Mice were injected intravenously with 50 μg/kg LPS. Values are means ± SEM (n = 4–6). The TNF-α and corticosterone responses in SR-BI KO/CETP and SR-BI KO mice were essentially the same and significantly different from those of WT mice (P < 0.001 for both; two-way ANOVA).
DISCUSSION
SR-BI deficiency in mice leads to glucocorticoid insufficiency as a result of an impaired adrenal uptake of cholesteryl esters from HDL (4, 5). Within the adrenals, a constant supply of cholesterol is required to serve as precursor for the synthesis of mineralocorticoids and glucocorticoids (25). The cholesterol needed for optimal steroid synthesis in the adrenals can be acquired from 1) intracellular de novo synthesis of free cholesterol by the enzyme HMG-CoA reductase, 2) intracellular catabolism of stored cholesteryl esters to free cholesterol by neutral cholesteryl ester hydrolase, or 3) receptor-mediated uptake and subsequent intracellular catabolism of cholesteryl esters from circulating VLDL, LDL, and HDL particles [reviewed in Kraemer (25)]. In addition to the “HDL receptor” SR-BI, adrenals express the LDL receptor, which is involved in the whole particle endocytosis of the ApoB-containing lipoproteins VLDL and LDL (12). Importantly, the expression of SR-BI and the LDL receptor and steroidogenesis are coordinately regulated by activators of both glucocorticoid (dibutyryl cAMP and ACTH) and mineralocorticoid (PMA) synthesis in adrenocortical cells both in vitro and in vivo (24, 26–31), leading to the generally accepted suggestion that the LDL receptor may also contribute to adrenal steroid hormone synthesis in vivo. However, it has never been experimentally defined to what extent the LDL receptor indeed allows optimal adrenal hormone production, i.e., corticosterone production. There are no patients with a deficiency of the human ortholog of SR-BI called CLA-1 (CD36 and lysosomal integral membrane protein-II analogous-1) (31). In the human situation, an improper functioning of CLA-1 is expected to be compensated for by the presence of CETP, a protein that redistributes cholesteryl esters from HDL to ApoB-containing lipoproteins, including LDL, leading to an alternative uptake route of cholesteryl esters by the adrenals. In our experiments, we confirm that expression of human CETP in SR-BI KO mice indeed leads to the transfer of cholesteryl esters from the relatively large HDL particles to ApoB-containing lipoproteins accessible for LDL receptor-mediated internalization (32). As already anticipated, the presence of CETP in SR-BI KO mice resulted in an increased delivery of cholesteryl esters from HDL via ApoB-containing lipoproteins to the adrenals. The enhanced delivery of cholesteryl esters upon CETP expression, however, was not associated with a reversal of the changes in adrenal morphology or plasma corticosterone and ACTH levels associated with SR-BI deficiency. Furthermore, no difference was observed in the LPS-induced TNF-α response between SR-BI KO mice with and without CETP expression. These data show for the first time that the uptake of cholesteryl esters via other routes than SR-BI (i.e., via the LDL receptor) is not sufficiently effective to generate the cholesterol pool needed for optimal adrenal steroid hormone production. In accordance with this, recent studies by Kraemer et al. (33) using LDL receptor KO mice have indicated that endocytic adrenal cholesterol uptake via the LDL receptor is not necessary for ACTH- and dibutyryl cAMP-induced maximal corticosterone production in vivo. Furthermore, inhibition of SR-BI function in murine adrenocortical cells in vitro induced a marked 70–80% decrease in selective cholesteryl ester uptake and corticosterone secretion (34). We now firmly establish that SR-BI-mediated uptake of cholesteryl esters is the primary route for the delivery of HDL-cholesterol to the steroidogenic pathway in vivo and that LDL receptor-mediated uptake cannot compensate for the absence of the SR-BI-mediated uptake route.
In conclusion, we have shown that under human-like conditions, that is, in the presence of human CETP, the transfer of cholesteryl esters from HDL to ApoB-containing lipoproteins by CETP is not able to reverse the adrenal insufficiency in SR-BI KO mice. Our findings suggest that the adrenal uptake of HDL-cholesteryl esters via SR-BI probably also contributes significantly to adrenal steroidogenesis in humans. However, it should be acknowledged that the relative acceptor (i.e., LDL) availability for CETP-mediated transfer of cholesteryl esters from HDL is much higher in the human situation (high LDL and HDL levels) than in our current SR-BI KO mouse model (high HDL and very low LDL levels). An additional key point is that the higher LDL levels in humans provide more substrate for the LDL receptor-mediated uptake of LDL cholesterol by the adrenals. This is also why the results with LDL-poor mice should be interpreted cautiously when extrapolating to humans. It will therefore be important to study whether carriers of polymorphisms in the human SR-BI (CLA-1) gene that are associated with changes in HDL-cholesterol levels (35–38) are indeed more susceptible to adrenal insufficiency, stress-related diseases (i.e., anxiety and depression), and/or inflammatory diseases (i.e., sepsis, rheumatoid arthritis, and atherosclerosis).
Abbreviations
ACTH, adrenocorticotropic hormone
ApoB, apolipoprotein B
CEt, [3H]cholesteryl ether
CETP, cholesteryl ester transfer protein
Ct, threshold cycle number
HPRT, hypoxanthine guanine phosphoribosyl transferase
KO, knockout
SR-BI, scavenger receptor class B type I
TNF-a, tumor necrosis factor-α
WT, wild-type
This research was supported by Top Institute Pharma (TIPharma project T2-110; M.H. and T.J.C.V.B.), by Grants 2001T41 (M.V.E.), 2006B107 (B.L), and 2008T070 (M.H.) from the Netherlands Heart Foundation, and by VIDI Grant 917.66.301 from the Netherlands Organization for Scientific Research (M.V.E.). M.V.E. is an Established Investigator of the Netherlands Heart Foundation (Grant 2007T056).
Published, JLR Papers in Press, January 28, 2009.
References
- 1.Van Eck M., M. Pennings, M. Hoekstra, R. Out, and T. J. Van Berkel. 2005. Scavenger receptor BI and ATP-binding cassette transporter A1 in reverse cholesterol transport and atherosclerosis. Curr. Opin. Lipidol. 16 307–315. [DOI] [PubMed] [Google Scholar]
- 2.Lewis G. F., and D. J. Rader. 2005. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 96 1221–1232. [DOI] [PubMed] [Google Scholar]
- 3.Acton S., A. Rigotti, K. T. Landschulz, S. Xu, H. H. Hobbs, and M. Krieger. 1996. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 271 518–520. [DOI] [PubMed] [Google Scholar]
- 4.Out R., M. Hoekstra, J. A. Spijkers, J. K. Kruijt, M. Van Eck, I. S. Bos, J. Twisk, and T. J. Van Berkel. 2004. Scavenger receptor class B type I is solely responsible for the selective uptake of cholesteryl esters from HDL by the liver and the adrenals in mice. J. Lipid Res. 45 2088–2095. [DOI] [PubMed] [Google Scholar]
- 5.Hoekstra M., I. Meurs, M. Koenders, R. Out, R. B. Hildebrand, J. K. Kruijt, M. Van Eck, and T. J. Van Berkel. 2008. Absence of HDL cholesterol ester uptake in mice via SR-BI impairs an adequate adrenal glucocorticoid-mediated stress response to fasting. J. Lipid Res. 49 738–745. [DOI] [PubMed] [Google Scholar]
- 6.Cai L., A. Ji, F. C. de Beer, L. R. Tannock, and D. R. van der Westhuyzen. 2008. SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J. Clin. Invest. 118 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagashima M., J. W. McLean, and R. M. Lawn. 1988. Cloning and mRNA tissue distribution of rabbit cholesteryl ester transfer protein. J. Lipid Res. 29 1643–1649. [PubMed] [Google Scholar]
- 8.Drayna D., A. S. Jarnagin, J. McLean, W. Henzel, W. Kohr, C. Fielding, and R. Lawn. 1987. Cloning and sequencing of human cholesteryl ester transfer protein cDNA. Nature. 327 632–634. [DOI] [PubMed] [Google Scholar]
- 9.Pape M. E., E. F. Rehberg, K. R. Marotti, and G. W. Melchior. 1991. Molecular cloning, sequence, and expression of cynomolgus monkey cholesteryl ester transfer protein. Inverse correlation between hepatic cholesteryl ester transfer protein mRNA levels and plasma high density lipoprotein levels. Arterioscler. Thromb. 11 1759–1771. [DOI] [PubMed] [Google Scholar]
- 10.Van Eck M., D. Ye, R. B. Hildebrand, J. Kar Kruijt, W. de Haan, M. Hoekstra, P. C. Rensen, C. Ehnholm, M. Jauhiainen, and T. J. Van Berkel. 2007. Important role for bone marrow-derived cholesteryl ester transfer protein in lipoprotein cholesterol redistribution and atherosclerotic lesion development in LDL receptor knockout mice. Circ. Res. 100 678–685. [DOI] [PubMed] [Google Scholar]
- 11.Tall A. R. 1993. Plasma cholesteryl ester transfer protein. J. Lipid Res. 34 1255–1274. [PubMed] [Google Scholar]
- 12.Windler E. E., P. T. Kovanen, Y. S. Chao, M. S. Brown, R. J. Havel, and J. L. Goldstein. 1980. The estradiol-stimulated lipoprotein receptor of rat liver. A binding site that membrane mediates the uptake of rat lipoproteins containing apoproteins B and E. J. Biol. Chem. 255 10464–10471. [PubMed] [Google Scholar]
- 13.Kovanen P. T., J. R. Faust, M. S. Brown, and J. L. Goldstein. 1979. Low density lipoprotein receptors in bovine adrenal cortex. I. Receptor-mediated uptake of low density lipoprotein and utilization of its cholesterol for steroid synthesis in cultured adrenocortical cells. Endocrinology. 104 599–609. [DOI] [PubMed] [Google Scholar]
- 14.Kita T., U. Beisiegel, J. L. Goldstein, W. J. Schneider, and M. S. Brown. 1981. Antibody against low density lipoprotein receptor blocks uptake of low density lipoprotein (but not high density lipoprotein) by the adrenal gland of the mouse in vivo. J. Biol. Chem. 256 4701–4703. [PubMed] [Google Scholar]
- 15.Fong L. G., E. Bonney, J. C. Kosek, and A. D. Cooper. 1989. Immunohistochemical localization of low density lipoprotein receptors in adrenal gland, liver, and intestine. J. Clin. Invest. 84 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rigotti A., B. L. Trigatti, M. Penman, H. Rayburn, J. Herz, and M. Krieger. 1997. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc. Natl. Acad. Sci. USA. 94 12610–12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang X. C., L. B. Agellon, A. Walsh, J. L. Breslow, and A. Tall. 1992. Dietary cholesterol increases transcription of the human cholesteryl ester transfer protein gene in transgenic mice. Dependence on natural flanking sequences. J. Clin. Invest. 90 1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoekstra M., J. K. Kruijt, M. Van Eck, and T. J. Van Berkel. 2003. Specific gene expression of ATP-binding cassette transporters and nuclear hormone receptors in rat liver parenchymal, endothelial, and Kupffer cells. J. Biol. Chem. 278 25448–25453. [DOI] [PubMed] [Google Scholar]
- 19.Chomczynski P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162 156–159. [DOI] [PubMed] [Google Scholar]
- 20.Redgrave T. G., D. C. Roberts, and C. E. West. 1975. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal. Biochem. 65 42–49. [DOI] [PubMed] [Google Scholar]
- 21.Fluiter K., H. Vietsch, E. A. Biessen, G. M. Kostner, T. J. van Berkel, and W. Sattler. 1996. Increased selective uptake in vivo and in vitro of oxidized cholesteryl esters from high-density lipoprotein by rat liver parenchymal cells. Biochem. J. 319 471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho M. M., and G. P. Vinson. 1997. Peptide growth factors and the adrenal cortex. Microsc. Res. Tech. 36 558–568. [DOI] [PubMed] [Google Scholar]
- 23.Heikkilä P., J. Arola, J. Liu, and A. I. Kahri. 1998. ACTH regulates LDL receptor and CLA-1 mRNA in the rat adrenal cortex. Endocr. Res. 24 591–593. [DOI] [PubMed] [Google Scholar]
- 24.Kovanen P. T., J. L. Goldstein, D. A. Chappell, and M. S. Brown. 1980. Regulation of low density lipoprotein receptors by adrenocorticotropin in the adrenal gland of mice and rats in vivo. J. Biol. Chem. 255 5591–5598. [PubMed] [Google Scholar]
- 25.Kraemer F. B. 2007. Adrenal cholesterol utilization. Mol. Cell. Endocrinol. 265–266 42–45. [DOI] [PubMed] [Google Scholar]
- 26.Martin G., A. Pilon, C. Albert, M. Vallé, D. W. Hum, J. C. Fruchart, J. Najib, V. Clavey, and B. Staels. 1999. Comparison of expression and regulation of the high-density lipoprotein receptor SR-BI and the low-density lipoprotein receptor in human adrenocortical carcinoma NCI-H295 cells. Eur. J. Biochem. 261 481–491. [DOI] [PubMed] [Google Scholar]
- 27.Pilon A., G. Martin, S. Bultel-Brienne, D. Junquero, A. Delhon, J. C. Fruchart, B. Staels, and V. Clavey. 2003. Regulation of the scavenger receptor BI and the LDL receptor by activators of aldosterone production, angiotensin II and PMA, in the human NCI-H295R adrenocortical cell line. Biochim. Biophys. Acta. 1631 218–228. [DOI] [PubMed] [Google Scholar]
- 28.Liu J., P. Heikkilä, Q. H. Meng, A. I. Kahri, M. J. Tikkanen, and R. Voutilainen. 2000. Expression of low and high density lipoprotein receptor genes in human adrenals. Eur. J. Endocrinol. 142 677–682. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y., N. Wang, and A. R. Tall. 1999. Regulation of adrenal scavenger receptor-BI expression by ACTH and cellular cholesterol pools. J. Lipid Res. 40 1799–1805. [PubMed] [Google Scholar]
- 30.Rigotti A., E. R. Edelman, P. Seifert, S. N. Iqbal, R. B. DeMattos, R. E. Temel, M. Krieger, and D. L. Williams. 1996. Regulation by adrenocorticotropic hormone of the in vivo expression of scavenger receptor class B type I (SR-BI), a high density lipoprotein receptor, in steroidogenic cells of the murine adrenal gland. J. Biol. Chem. 271 33545–33549. [DOI] [PubMed] [Google Scholar]
- 31.Murao K., V. Terpstra, S. R. Green, N. Kondratenko, D. Steinberg, and O. Quehenberger. 1997. Characterization of CLA-1, a human homologue of rodent scavenger receptor BI, as a receptor for high density lipoprotein and apoptotic thymocytes. J. Biol. Chem. 272 17551–17557. [DOI] [PubMed] [Google Scholar]
- 32.Harder C., P. Lau, A. Meng, S. C. Whitman, and R. McPherson. 2007. Cholesteryl ester transfer protein (CETP) expression protects against diet induced atherosclerosis in SR-BI deficient mice. Arterioscler. Thromb. Vasc. Biol. 27 858–864. [DOI] [PubMed] [Google Scholar]
- 33.Kraemer F. B., W. J. Shen, S. Patel, J. Osuga, S. Ishibashi, and S. Azhar. 2007. The LDL receptor is not necessary for acute adrenal steroidogenesis in mouse adrenocortical cells. Am. J. Physiol. Endocrinol. Metab. 292 E408–E412. [DOI] [PubMed] [Google Scholar]
- 34.Temel R. E., B. Trigatti, R. B. DeMattos, S. Azhar, M. Krieger, and D. L. Williams. 1997. Scavenger receptor class B, type I (SR-BI) is the major route for the delivery of high density lipoprotein cholesterol to the steroidogenic pathway in cultured mouse adrenocortical cells. Proc. Natl. Acad. Sci. USA. 94 13600–13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu L. A., Y. L. Ko, S. Wu, M. S. Teng, T. Y. Peng, C. F. Chen, C. F. Chen, and Y. S. Lee. 2003. Association between a novel 11-base pair deletion mutation in the promoter region of the scavenger receptor class B type I gene and plasma HDL cholesterol levels in Taiwanese Chinese. Arterioscler. Thromb. Vasc. Biol. 23 1869–1874. [DOI] [PubMed] [Google Scholar]
- 36.Morabia A., B. M. Ross, M. C. Costanza, E. Cayanis, M. S. Flaherty, G. B. Alvin, K. Das, R. James, A. S. Yang, O. Evagrafov, et al. 2004. Population-based study of SR-BI genetic variation and lipid profile. Atherosclerosis. 175 159–168. [DOI] [PubMed] [Google Scholar]
- 37.Osgood D., D. Corella, S. Demissie, L. A. Cupples, P. W. Wilson, J. B. Meigs, E. J. Schaefer, O. Coltell, and J. M. Ordovas. 2003. Genetic variation at the scavenger receptor class B type I gene locus determines plasma lipoprotein concentrations and particle size and interacts with type 2 diabetes: the framingham study. J. Clin. Endocrinol. Metab. 88 2869–2879. [DOI] [PubMed] [Google Scholar]
- 38.Acton S., D. Osgood, M. Donoghue, D. Corella, M. Pocovi, A. Cenarro, P. Mozas, J. Keilty, S. Squazzo, E. A. Woolf, et al. 1999. Association of polymorphisms at the SR-BI gene locus with plasma lipid levels and body mass index in a white population. Arterioscler. Thromb. Vasc. Biol. 19 1734–1743. [DOI] [PubMed] [Google Scholar]