Abstract
High levels of the inflammatory cytokine tumor necrosis factor-α (TNF-α) are present in atherosclerotic lesions. TNF-α regulates expression of multiple genes involved in various stages of atherosclerosis, and it exhibits proatherosclerotic and antiatherosclerotic properties. ACAT catalyzes the formation of cholesteryl esters (CE) in monocytes/macrophages, and it promotes the foam cell formation at the early stage of atherosclerosis. We hypothesize that TNF-α may be involved in regulating the ACAT gene expression in monocytes/macrophages. In this article, we show that in cultured, differentiating human monocytes, TNF-α enhances the expression of the ACAT1 but not ACAT2 gene, increases the cholesteryl ester accumulation, and promotes the lipid-laden cell formation. Several other proinflammatory cytokines tested do not affect the ACAT1 gene expression. The stimulation effect is consistent with a receptor-dependent process, and is blocked by using nuclear factor-kappa B (NF-kappa B) inhibitors. A functional and unique NF-kappa B element located within the human ACAT1 gene proximal promoter is required to mediate the action of TNF-α. Our data demonstrate that TNF-α, through the NF-kappa B pathway, specifically enhances the expression of human ACAT1 gene to promote the CE-laden cell formation from the differentiating monocytes, and our data support the hypothesis that TNF-α is proatherosclerotic during early phase of lesion development.
Keywords: atherosclerosis, cholesterol esterification, inflammatory cytokine, lipid metabolism, transcription control
In mammals, two members of the Acyl-CoA:cholesterol acyltransferase (ACAT) family have been identified: ACAT1 (1) and ACAT2 (2–4). Under normal conditions, based on mRNA, protein, and enzyme activity analyses, human ACAT1 is the major isoenzyme in almost all of the tissues or cells examined, and ACAT2 is most abundantly expressed in the epical epithelium of intestines (5–9). Relevant to our current study is the fact that ACAT1 is highly expressed in monocytes/macrophages forming the foam cells in atherosclerotic plaques, especially at the early stage of atherosclerosis development (8–11).
During the early stage of atherosclerosis, monocytes enter the intima of the artery and differentiate into macrophages. Through scavenger receptors, the macrophages continuously engorge large quantities of denatured lipoproteins, and the cholesterols derived from these lipoproteins are converted into cholesteryl esters (CE) by the enzyme ACAT (10). The accumulation of cholesteryl esters as abundant cytoplasmic lipid droplets causes the macrophages to be foamy in appearance. The foam cell formation is a hallmark of the early stage of atherosclerosis. In macrophages present in human atherosclerotic lesions, a close connection between the foam cell appearance and the ACAT1 protein expression has been demonstrated (10, 11). In addition to the ACAT1, detectable ACAT2 is also present in some but not all of the macrophages from the same specimens (12).
TNFα, an important inflammatory cytokine, is secreted by activated lymphocytes, macrophages, endothelial cells, and smooth muscle cells (13). TNFα participates in every step of the inflammation process (14–16). Most of the molecular actions of TNFα on gene expression depend on the NF-κB pathway (17). In mice models of atherosclerosis, inhibition of TNFα reduces the progression of atherosclerosis (18). In healthy males, plasma TNFα levels correlate with early carotid atherosclerosis (19). In patients with chronic inflammatory diseases, TNFα inhibitors may improve insulin resistance and lipid profiles (20).
Many effects of TNFα on cultured monocytes/macrophages have been demonstrated (16). These findings show that the effects of TNFα are mostly proatherosclerotic. However, there have been conflicting results in cultured cells. These differing responses may be a reflection of the heterogeneity of macrophages as well as the culture conditions employed (16, 21), which may lead to differences in the phenotype of the cultured cells. In this paper, we design experiments in cultured, differentiating human monocytes and in human monocytic cell lines to test the possibility that TNFα may be involved in regulating the gene expression of ACAT1 and/or ACAT2 in these cells.
MATERIALS AND METHODS
Reagents
Fetal bovine serum was obtained from GIBCO BRL (Grand Island, USA). Recombinant human TNFα was the product of Roche Applied Science (Indianapolis, USA). Anti-β-actin monoclonal antibodies, all-trans retinoic acid (ATRA) and recombinant products of human macrophage-colony stimulating factor (M-CSF), granulocyte/macrophage-colony stimulating factor (GM-CSF), monocyte chemotactic protein-1 (MCP-1), interleukin-1 β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10),interferon-γ (IFN-γ), and lipopolysaccharide (LPS) were from Sigma Aldrich (Milwaukee, USA). Prostaglandin A1 (PGA1) and 15-deox-Δ12, 14-prostaglandin J2 (PGJ2) were from Cayman Chemical (Ann Arbor, USA). Anti-human ACAT1, anti-p65 and anti-p50 polyclonal antibodies were from Santa Cruz Biotechnology (Santa Cruz, USA). Anti-human ACAT2 polyclonal antibodies were generated by immunizing rabbits followed by affinity purification with antigen (22). SYBR Green I and Trizol total RNA extraction kit was purchased from Invitrogen (Carlsbad, USA). Moloney murine leukemia virus reverse transcriptase was from Promega (Madison, USA). The Hot Start Taq or Pfu DNA polymerase and dNTPs were from TaKaRa (Dalian, China). The β-galactosidase detection kit II was from Clontech (Mountain, USA). The expression plasmid (pRC/β-actin-mIκBα) for the mutant of inhibitor of NF-κB α (IκBα) was a gift from generous Dr. Jian-Guo Geng (Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences). All the oligonucleotides were synthesized with an automated DNA synthesizer in Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
Cell culture
Human mononuclear cells were obtained from Shanghai Blood Service Center, and human monocytes were isolated according to the published procedure (23). THP-1, U937, HL60, HeLa, and HEK293 cells were from American Type Culture Collection (ATCC). These cells were cultured in a 37°C incubator with humid atmosphere, 5% CO2 and 95% air. THP-1, U937, and HL60 monocytes were grown in RPMI 1640 media supplemented with 100 μg/ml ampicillin, 100 μg/ml streptomycin, 2 g/l sodium bicarbonate, plus 10% fetal bovine serum (FBS). HeLa and HEK293 cells were grown in DMEM media supplemented with 100 μg/ml ampicillin, 100 μg/ml streptomycin, 2 g/l sodium bicarbonate, plus 10% fetal bovine serum (FBS).
Treatment of human monocytic cells, lipid droplet staining, and cholesterol assay
Immediately upon isolation, human blood monocytes were adhered on cover slips in a 12-well plate for 48 h and treated with or without TNFα for 40 h in the RPMI 1640 medium supplemented with 7% human AB serum. Human THP-1 monocytes were adhered on cover slips in a 12-well plate with treatment of 1 μM ATRA or ATRA plus TNFα in the RPMI 1640 medium supplemented with 10% FBS for 40 h; for the NF-κB inhibition assay, the inhibitor PGA1 was added to the medium prior to stimulation of TNFα. Then the cells were cultured with oxidized low-density lipoproteins (oxLDL; 40 μg/ml), which are prepared as described (24, 25) but without TNFα, for another 48 h in the fresh RPMI 1640 medium containing 10% lipoprotein-deficient serum (LPDS). For the ACAT inhibition assay, the ACAT inhibitor CP-113,818 was added to the fresh RPMI 1640 medium containing 10% LPDS and oxLDL (40 μg/ml) after stimulation of TNFα. The treated cells were used for analysis of lipid droplet staining and cellular cholesterol assay.
Lipid-laden cells (lipid droplet positive cells) that stained positively with oil red O as previously reported (25) were evaluated under a microscope Olympus BX51. For each condition, the percentages of the lipid-laden cells to total cells were calculated by collecting five different fields of cells (where each field contained approximately 150 cells). The relative lipid-laden cell was calculated from the percentage of the lipid-laden cells to total cells by setting the average percentage of cells without TNFα stimulation as 1.0.
Cellular cholesterol contents were measured by using Amplex Red Cholesterol Assay Kit (Molecular Probes/Invitrogen) as described (26) with slight modification. Briefly, for the determination of cellular total and free cholesterols, cells were extracted in chloroform/methanol (2:1; v/v); the chloroform phase was separated, dried, and resuspended in assay reaction buffer (100 mM potassium phosphate, pH 7.4, 50 mM NaCl, 5 mM cholic acid, 0.1% Triton X-100). The total and free cholesterol contents were measured and used to calculate the CE contents. The protein amounts were determined using protein assay kit (Bio-Rad Laboratories, USA).
Western blot analysis
Various cells with different treatments were harvested and lysed according to previous work (27). Protein concentrations of lysates were determined using the protein assay kit (Bio-Rad Laboratories, USA). Lysates (containing proteins 50 μg for ACAT1 and β-actin or 100 μg for ACAT2 per lane) were then subjected to 10% SDS-PAGE for western analysis according to a method described previously (27).
Reverse transcription-quantitative PCR (RT-qPCR) analysis
The total RNAs from various cells with different treatments were freshly prepared using the Trizol Regent (Life Technologies, Inc.) and reverse-transcribed using the primer oligo(dT)18 to obtain the relative cDNAs (28). By qPCR using Brilliant SYBR Green qPCR Master Mix and Mx3005PTM instrument (Stratagene), the quantification of cDNAs from transcripts were done and the content of human ACAT1 or ACAT2 mRNA was obtained by normalizing to that of the human GAPDH mRNA in each sample (29). Specific primer sets for different qPCR analysis were: 5′GATGAAGGAAGGCTGGTGC3′/5′GGAAGCTGGTGGCAGTGTAT3′ for human ACAT1 cDNA, 5′CATGCTGCTGCTCATCTTCT3′/5′ACTGCGGAGACCAGGAACA3′ for human ACAT2 cDNA, and 5′ACCCACTCCTCCACCTTTG3′/5′CTGTAGCCAAATTCGTTGTCAT3′ for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA.
Construction of plasmids
The luciferase reporter (Luc) plasmid pM50 containing the core region (–125/+34) of human ACAT1 gene proximal promoter has been constructed in previous work (28). The different promoter fragments with the sequential 5′-deletions (–100/+34, –78/+34, –66/+34 and –32/+34) were generated from the plasmid pM50 by the PCR amplification with individual forward primers (Table 1) and a common reverse primer (GLP2, 5′CTTTATGTTTTTGGCGTCTTCCA3′) that corresponds to the 3′-flanking sequence of multiple cloning sites. Those with the sequential 3′-deletions (–125/–27, –125/–67 and –125/–79) were generated from the plasmid pM50 by the PCR amplification with a common forward primer (GLP1, 5′TGTATCTTATGGTACTGTAACTG3′) that corresponds to the 5′-flanking sequence of multiple cloning sites and individual reverse primers (Table 2). A special promoter fragment with the 3′-deletion (–125/–101) was directly obtained by synthesizing and annealing of two complementary oligonucleotides, 5′CAAGGGGCGGGGAGGTGGGCGGAG3′ and 5′CTAGCTCCGCCCACCTCCCCGCCCCTTGGTAC3′, which contain complementary nucleotides (bold). All the obtained promoter fragments were digested by restriction enzymes and inserted into the Kpn I and Nhe I sites of Luc vector pGL2-E to generate the expression plasmids pD52-55 containing the sequential 5′-deletion promoter regions (–100/+34, –78/+34, –66/+34, –32/+34) and pD31-35 containing the sequential 3′-deletion promoter regions (–125/–27, –125/–67, –125/–79, –125/–101), respectively.
TABLE 1.
Forward primers for deletions in the 5′ region of human ACAT1 gene proximal promoter
| Primer Name | Primer Sequence | Plasmid |
|---|---|---|
| D52F | 5′AAAggtaccACTGGCAACCTG3′ | pD52 |
| D53F | 5′AAAggtaccATAGGATGCTCAGCC3′ | pD53 |
| D54F | 5′AAAggtaccGCCGGAGGTGGCCCTG3′ | pD54 |
| D55F | 5′AAAggtaccTCGGAGGCAGGGGGC3′ | pD55 |
The Kpn I site of each primer is indicated as lowercase letters.
TABLE 2.
Reverse primers for deletions in the 3′ region of human ACAT1 gene proximal promoter
| Primer Name | Primer Sequence | Plasmid |
|---|---|---|
| D31R | 5′AAAgctagcTCCGAGCACCGC3′ | pD31 |
| D33R | 5′AAAgctagcTGAGCATCCTATTG3′ | pD33 |
| D34R | 5′AAAgctagcTGGTGGTCCCCA3′ | pD34 |
The Nhe I site of each primer is indicated as lowercase letters.
The mutations were introduced into the 3′-deletion promoter region (–125/–79) of the expression plasmid pD34 by the amplification of PCR with the above common forward primer GLP1 and individual reverse primers with mutations (Table 3). These amplified fragments were digested by restriction enzymes and inserted into the Kpn I and Nhe I sites of pGL2-E to generate expression plasmids pD34m1-9, respectively.
TABLE 3.
Reverse primers for mutations in the TNFα responding region of human ACAT1 gene proximal promoter
| Primer Name | Primer Sequence | Plasmid |
|---|---|---|
| m1R | 5′AAAgctagcTGGTGGTCATAAGGTTGCC3′ | pD34m1 |
| m2R | 5′AAAgctagcTTTTGGTCATAAGGTTGCC3′ | pD34m2 |
| m3R | 5′AAAgctagcTGGGTTGAATAAGGTTGCC3′ | pD34m3 |
| m4R | 5′AAAgctagcTTTGTTGAATAAGGTTGCC3′ | pD34m4 |
| m5R | 5′AAAgctagcTGGTGGTCCCCAGGTGTAACGGCTCCGCCC3′ | pD34m5 |
| m6R | 5′AAAgctagcTGGTGGTCCCCCTTGTGCCAGG3′ | pD34m6 |
| m7R | 5′GAAgctagcTGGTGTGCCCCAGGTTGCCC3′ | pD34m7 |
| m8R | 5′AAAgctagcTGGAGGTCCCCAGGTTGCC3′ | pD34m8 |
| m9R | 5′AAAgctagcTGGCGGTCCCCAGGTTGCC3′ | pD34m9 |
The mutated nucleotides are underlined. The others are same as in Table 2.
Restriction enzyme digestion and DNA sequencing confirmed all the constructed plasmids.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts (NE) were prepared from THP-1 cells as described (28) except with or without stimulation of TNFα for 2 h. The synthesized oligonucleotides 5′CTTAACCTGGGGACCACCAATAG3′ and 5′CTTCTATTGGTGGTCCCCAGGTT3′ were annealed as the fragment (–95/–75) of human ACAT1 gene proximal promoter, which contains a NF-κB element (bold) and with overhang (italic) for labeling. This annealed fragment (–95/–75) was then labeled with the Klenow enzyme by fill-in with the dNTPs including [α-32P]dATP. The oligonucleotides annealed for mutated cold probes and competitors were synthesized: κBm1 (5′CTTAACCTTATGACCACCAATAG3′/5′CTTCTATTGGTGGTCATAAGGTT3′) and κBm8 (5′CTTAACCTGGGGACCTCCAATAG3′/5′CTTCTATTGGAGGTCCCCAGGTT3′) with mutations (underlined) of the NF-κB element in human ACAT1 gene proximal promoter; κBcon (5′CTTAGTTGAGGGGACTTTCCCAGGC3′/5′CTTGCCTGGGAAAGTCCCCTCAACT3′ for NF-κB consensus sequence as positive control and AP-1 (5′CTTCGCTGGATGAGTCAGCCGGAA 3′/5′CTTTTCCGGCTGACTCATCCAGCG3′) for activator protein-1 (AP-1) consensus sequence as negative control, which were designed according to the manual of Promega Gel Shift Assay Systems. The EMSAs were carried out as previous report (28). For competition assay, 100-fold molar excess cold probes or competitors were individually added to the reaction mixture. For supershift assay, 2 μg of anti-p65 and/or anti-p50 antibodies (Santa Cruz, USA) were added to the reaction mixture.
Transfection and luciferase activity assay
The plasmids were transfected into human THP-1, U937 and HL60 monocytes with the DEAE-dextran method as previous report (28). HeLa and HEK293 cells were transfected with lipofectamine 2000 (Invitrogen USA) as described in the manual. Luciferase and β-galactosidase activities were measured as previously described (28).
RESULTS
TNFα promotes the formation of lipid-laden cells from differentiating monocytes in part by enhancing the ACAT1 gene expression
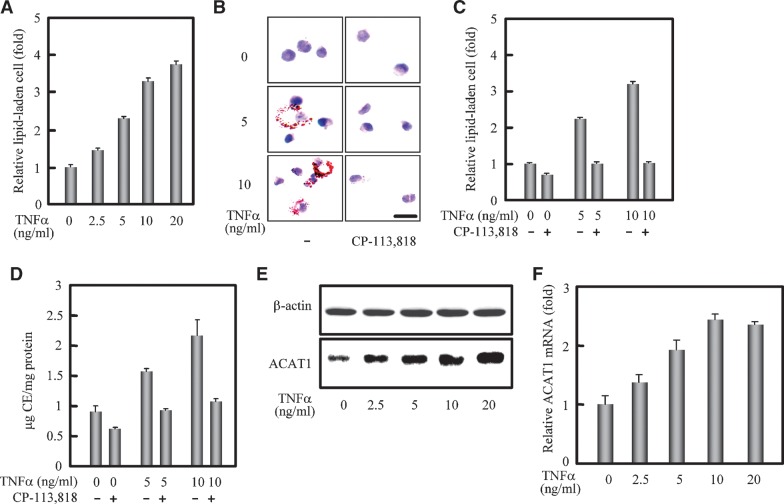
We first used human blood monocytes to test the effect of TNFα on the formation of lipid-laden cells. The human blood monocytes can slowly differentiate into macrophages when cultured on plastic with supplement of AB serum in vitro (30). In our experiments, human blood monocytes were cultured for 88 h. Under this condition, they are known to express phenotypes characteristic of both monocyte and macrophage, but not those characteristic of mature macrophages (31, 32). We therefore designate these cells as differentiating monocytes in our current manuscript. As shown in Fig. 1A, the lipid droplet staining assay displayed that the relative lipid-laden cell were increased to 1.5-, 2.3-, 3.3-, and 3.7-fold at the concentrations 2.5, 5, 10, and 20 ng/ml of TNFα, respectively. This result indicated that TNFα significantly promoted the formation of lipid-laden cells from human blood monocytes under the culture condition employed. To address whether ACAT is involved in the formation of lipid-laden cells promoted by TNFα, we performed the inhibition assay by adding the specific ACAT inhibitor CP-113,818, which inhibits both ACAT1 and ACAT2 with almost equal efficiency (6). The results showed that the ACAT inhibitor (8 μM) significantly blocked the lipid-laden cell formation promoted by TNFα at 5 and 10 ng/ml (Fig. 1B). Quantification revealed that the ACAT inhibitor could completely block the promoting effect of TNFα on the lipid-laden cell formation (Fig. 1C). Furthermore, the results of cellular cholesterol assay showed that the CE contents were increased with the treatment of TNFα, and reduced when the ACAT inhibitor was added (Fig. 1D). These results indicated that TNFα promoted the lipid-laden cell formation and increased the CE accumulation via ACAT. In human monocyte/macrophage cells, the major enzyme synthesizing CEs is ACAT1 but not ACAT2 (8). So we subsequently detected the effect of TNFα on the human ACAT1 gene expression. Results of western blot and RT-qPCR (Figs. 1E, 1F) showed that there was a TNFα-dose-dependent increase of the human ACAT1 gene expression at both protein and mRNA levels. These results suggest that human ACAT1 gene might be a downstream target of TNFα.
Fig. 1.
Formation of CE-laden cells promoted by TNFα correlates with enhancement of the ACAT1 expression. A: Formation of lipid-laden cells from human blood differentiating monocytes promoted by TNFα. Human blood monocytes were cultured for 48 h, stimulated with different concentrations of TNFα (0, 2.5, 5, 10, and 20 ng/ml) for 40 h, and then incubated with oxLDL (40 μg/ml) for another 48 h without TNFα. Lipid-laden cells (lipid droplet positive cells) were then evaluated. The detailed treatment and evaluation methods are described in “Materials and Methods.” The data represented the means ± SD from 5 fields of cells. B: Blockage of the TNFα-promoting lipid-laden cell formation by ACAT inhibitor. Human blood monocytes were cultured for 48 h, stimulated with different concentrations of TNFα (0, 5, and 10 ng/ml) for 40 h, and then incubated with oxLDL (40 μg/ml) plus the ACAT inhibitor CP-113,818 (8 μM) for another 48 h without TNFα. The detailed treatments and lipid droplet staining are described in “Materials and Methods.” Representative lipid droplet staining images are shown. Scale bar, 20 μm. C: Quantification for blockage of the TNFα-promoting lipid-laden cell formation by ACAT inhibitor. The lipid-laden cells in (B) were quantified and represented as (A). D: Cellular cholesterol assay. The cells were treated as (B) and the contents of total and free cholesterols as well as proteins were measured as described in “Materials and Methods.” The CE content in each condition was obtained from the difference of total and free cholesterol contents. The data shown as the ratio of CE and protein contents (μg CE /mg protein) represented the means ± SD from triplicate determinations. E: Immunoblotting of human ACAT1 protein. Human blood monocytes were cultured for 48 h and stimulated with different concentrations of TNFα for 40 h the same as in (A) but without the oxLDL treatment. Immunoblotting was performed as described in “Materials and Methods.” F: RT-qPCR analysis of human ACAT1 mRNA. Human blood monocytes were cultured and treated the same as in (A). RT-qPCR analysis with the prepared total RNAs and appropriate primers are described in “Materials and Methods.” The content of human ACAT1 mRNA was normalized to that of GAPDH mRNA in each condition. The data shown as the relative ACAT1 mRNA (fold) were calculated from the normalized contents by setting the average content of that without treatment to 1.0, and represented the means ± SD from triplicate determinations. All of the experiments were repeated three times with similar results.
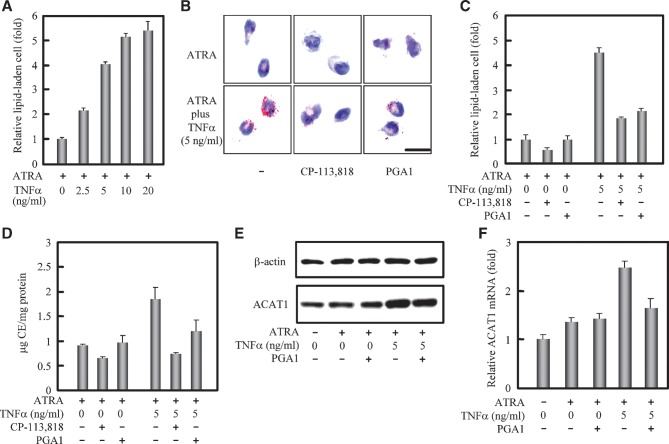
Next, we used human THP-1 monocytes to study the promoting effect of TNFα on the formation of lipid-laden cells. THP-1 cells can be induced to partly differentiate and adhere to dishes from suspension by ATRA in the culture condition (33). The results from the differentiating THP-1 monocytes induced by ATRA showed that, similar to those results by using human blood monocytes, the formation of lipid-laden cells was significantly promoted (Fig. 2A) and the CE accumulation was increased (Fig. 2D) with the treatment of TNFα. These effects were effectively blocked by adding the ACAT inhibitor (Figs. 2B, 2C, 2D). In THP-1 cells, the expression of human ACAT1 gene was also enhanced in a TNFα-dose-dependent manner (data not shown). Since TNFα can upregulate gene expression via NF-κB pathway, we used the NF-κB inhibitor prostaglandin A1 (PGA1) (34) to test whether NF-κB was involved in the promoting effect of TNFα. The results showed that PGA1 could significantly block the TNFα-effects in promoting the lipid-laden cell formation (Figs. 2B, 2C), in increasing the cellular CE contents (Fig. 2D), and in enhancing the expression of human ACAT1 gene at protein and mRNA levels, respectively [Figs. 2E, 2F (right two tests)]. The effect of PGA1 was not observed in cells without TNFα stimulation [Figs. 2E, 2F (left three controls)]. These results show that NF-κB is required for enhancing the expression of human ACAT1 gene by TNFα in the differentiating THP-1 monocytes. Taken together, the above data indicate that human blood- and cell line-derived differentiating monocytes can be stimulated to form lipid-laden cells by TNFα, and TNFα acts in part by enhancing the expression of human ACAT1 gene through the NF-κB pathway.
Fig. 2.
TNFα promotes the CE-laden cell formation and enhances the human ACAT1 gene expression via the NF-κB pathway. A: TNFα-promoting effect on the lipid-laden cell formation from human THP-1 monocytes adhered by the ATRA treatment. Human THP-1 monocytes were cultured with ATRA (1 μM) or ATRA plus different concentrations of TNFα (0, 2.5, 5, 10, and 20 ng/ml) for 40 h, and then incubated with oxLDL (40 μg/ml) for another 48 h without TNFα. The detailed treatments and lipid droplet staining are described in “Materials and Methods.” The data were obtained and represented the same as in Fig. 1A. B: Blockage of the TNFα-promoting lipid-laden cell formation by the NF-κB and ACAT inhibitors. THP-1 cells were treated with ATRA (1 μM) and TNFα (0 or 5 ng/ml) for 40 h. The NF-κB inhibitor PGA1 (15 μM) was added 30 min prior to stimulation of TNFα, and after stimulation of TNFα, the cells were incubated with the oxLDL (40 μg/ml) plus ACAT inhibitor CP-113,818 (8 μM) for another 48 h without TNFα. The detailed treatments and lipid droplet staining are described in “Materials and Methods.” Representative lipid droplet staining images are shown. Scale bar, 20 μm. C: Quantification for blockage of the TNFα-promoting lipid-laden cell formation by the NF-κB and ACAT inhibitors. The lipid-laden cells in (B) were quantified and represented the same as in Fig. 1A. D: Cellular cholesterol assay. The cells were treated as (B) and the contents of total and free cholesterols as well as proteins were measured as described in “Materials and Methods.” The data is represented the same as in Fig. 1D. E: Immunoblotting of human ACAT1 protein. THP-1 cells were untreated (left lane) or treated with ATRA (1 μM), TNFα (5 ng/ml), PGA1 (15 μM) as indicated for 40 h. The NF-κB inhibitor PGA1 was added 30 min prior to stimulation of TNFα. Immunoblotting was performed as described in “Materials and Methods.” F: RT-qPCR analysis of human ACAT1 mRNA. THP-1 cells were treated as same as in (E). The content of human ACAT1 mRNA was normalized to that of GAPDH mRNA in each condition. The data were obtained and represented the same as in Fig. 1F. All of the experiments were repeated three times with similar results.
The expression of human ACAT1 gene is specifically enhanced in monocyte by TNFα
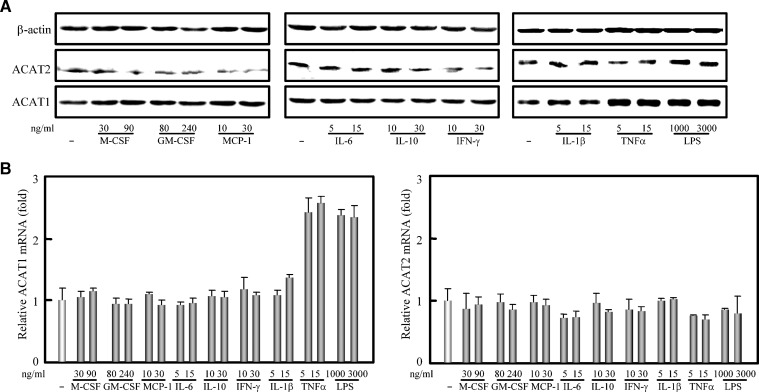
Many cytokines such as M-CSF, GM-CSF, MCP-1, IL-1β, IL-6, IL-10, IFN-γ, and TNFα are present in atherosclerosis plaques (35). To investigate whether the enhancing effect of TNFα on the human ACAT1 expression is specific to a certain cytokine(s), human THP-1 monocytes without any treatment were directly treated with the cytokines described above individually to detect their possible effects on the expression of both ACAT1 and ACTA2 genes. As shown in Fig. 3, TNFα could also dramatically upregulate the human ACAT1 but not ACAT2 gene expression at both protein and mRNA levels; IL-1β could slightly increase the expression of human ACAT1 gene, while all the other cytokines tested had no obvious effect on the expression of both ACAT genes. These results suggest that although many kinds of cytokines are present in the atherosclerosis plaques, TNFα may be the major one that directly upregulates the expression of human ACAT1 gene to promote the formation of foam cells. Additional results showed that the expression of human ACAT1 gene [Fig. 3A (right panel) and Fig. 3B (left panel)] can also be enhanced by lipopolysaccharide (LPS), an agent known to induce the production of TNFα in various cell types (36).
Fig. 3.
Cytokine specificity of TNFα in upregulating the expression of human ACAT1 gene. A: Immunoblotting of human ACAT1 and ACAT2 proteins. THP-1 cells were treated without or with M-CSF, GM-CSF, MCP-1, IL-6, IL-10, IFN-γ, IL-1β, TNFα, and LPS individually at the indicated concentrations for 40 h. Immunoblotting was performed as described in “Materials and Methods.” B: RT-qPCR analysis of human ACAT1 and ACAT2 mRNAs. THP-1 cells were treated as same as in (A). The content of human ACAT1 or ACAT2 mRNA was normalized to that of GAPDH mRNA in each condition. The data were obtained and represented the same as in Fig. 1F. All of the experiments were repeated three times with similar results.
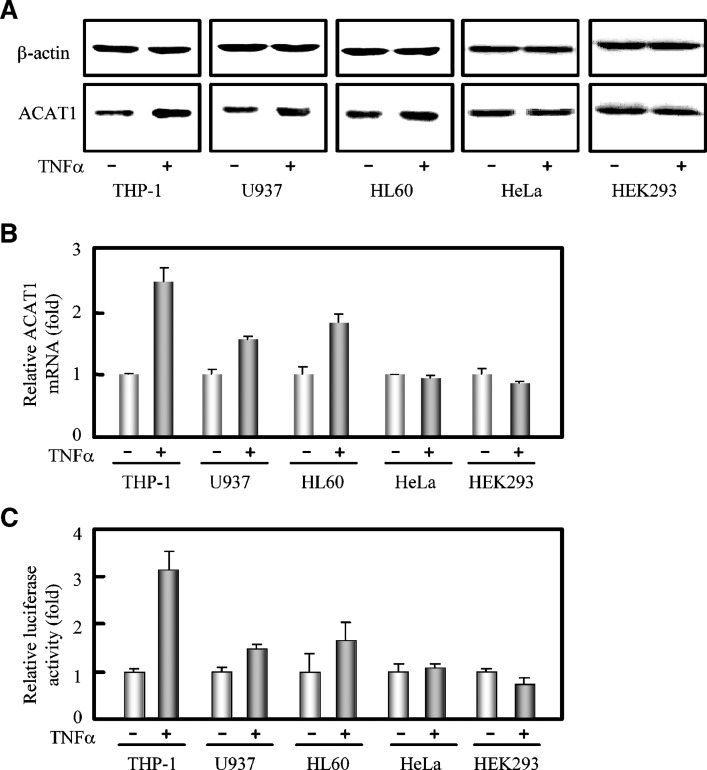
To assess the cell type specificity, we then treated other human cells, including monocytic (U937 and HL60) and nonmonocytic (HeLa and HEK293) cells with TNFα and examined the ACAT1 gene expression. Results (Figs. 4A, 4B) showed that TNFα could also enhance the expression of human ACAT1 gene by about 2-fold at protein and mRNA levels in the other monocytic cell lines U937 and HL60, but it had no effect in the HeLa and HEK293 cells. We next investigated the effect of TNFα on the ACAT1 gene promoter activity. We have previously reported that the maximal transcription activity was located within the 159 base pairs from –125 to +34 in the ACAT1 gene proximal promoter (28). Therefore, the Luc construct containing this core region was used to transfect into THP-1, U937, HL60, HeLa, and HEK239 cells. The luciferase assay showed that the reporter gene expression driven by human ACAT1 gene promoter was significantly increased by TNFα in the THP-1, U937, and HL60 monocytes, but not in nonmonocytic cells HeLa and HEK239 (Fig. 4C). This result demonstrates that TNFα can upregulate the expression of human ACAT1 gene by enhancing its promoter activity specifically in monocytes.
Fig. 4.
Cell type specificity of TNFα in upregulating the expression of human ACAT1 gene. A: Immunoblotting of human ACAT1 protein. Human monocytes (THP-1, U937, and HL60) and nonmonocytic cells (HeLa and HEK293) were directly treated without or with TNFα (5 ng/ml) for 40 h. Immunoblotting was performed as described in “Materials and Methods.” B: RT-qPCR analysis of human ACAT1 mRNA. All the cells were treated the same as in (A). The data were obtained and represented the same as in Fig. 1F. C: Effect of TNFα on the human ACAT1 gene promoter activity. The Luc plasmid pM50 containing the core region (–125/+34) of human ACAT1 gene promoter was cotransfected with pCH110 (for the expression of galactosidase) into human monocytes (THP-1, U937, and HL60) and nonmonocytic cells (HeLa and HEK293) as indicated in “Materials and Methods.” 7 h after transfection, the cells were treated without or with TNFα (5 ng/ml) for another 40 h and harvested for luciferase activity analysis as described in “Materials and Methods.” The luciferase activities were normalized to those of galactosidase in each condition. The data shown as the relative luciferase activities (fold) were calculated from the normalized luciferase activities by setting the average activities of that without TNFα stimulation to 1.0, and represented the means ± SD from triplicate determinations. All of the experiments were repeated three times with similar results.
A NF-κB element responsible for the TNFα stimulation is identified in the human ACAT1 gene proximal promoter
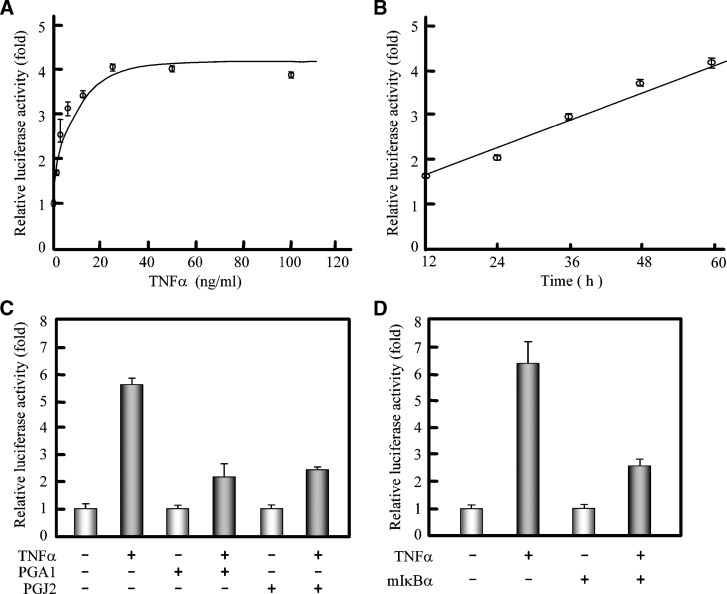
The above results showed that TNFα upregulated the expression of human ACAT1 gene by enhancing its promoter activity. We then explored if this enhancing effect of TNFα depended on the binding of TNFα to its membrane receptor. We used the Luc construct containing the core region of human ACAT1 gene promoter as the probing downstream target in intact cells. The results showed that the effect of TNFα on the activity of human ACAT1 gene promoter exhibited receptor-bound saturation effect, with maximal enhancement when the concentration of TNFα reached 25 ng/ml, and the enhancement increased with the incubation time (Figs. 5A and 5B). This result indicated that the TNFα-enhancing effect on the activity of the human ACAT1 gene promoter was consistent with the receptor dependent process and in a time-dependent manner. To further study whether the enhancement of the ACAT1 gene promoter activity by TNFα is through the downstream NF-κB pathway, we used two NF-κB inhibitors, PGA1 and PGJ2 (34), and an expression plasmid for a mutant of IκBα to examine their influence at the promoter level. The luciferase assays showed that, similar to PGA1 inhibiting the TNFα-enhancing expression of human ACAT1 gene, both PGA1 and PGJ2 could block this enhancing effect at promoter level (Fig. 5C); as expected, the expression of the exogenous IκBα with Ser32 and Ser36 mutations, which are resistant to phosphorylation and degradation (37), could diminish the TNFα-enhancing effect (Fig. 5D). In addition, we tested the effect of inhibitors for other signal pathways (such as JNK, p38, Akt, MEK, and PKC), and we found that these inhibitors did not significantly reduce the TNFα-enhancing effect (data not shown).
Fig. 5.
Involvement of the NF-κB pathway in enhancing the human ACAT1 gene promoter activity by TNFα. A: Receptor-bound saturation of the TNFα effect. The Luc plasmid pM50 was cotransfected with pCH110 into THP-1 cells. 7 h after transfection, the cells were treated with the indicated concentrations of TNFα for another 40 h and harvested for luciferase activity analysis. B: Time-course of the TNFα effect. The Luc plasmid pM50 was cotransfected with pCH110 into THP-1 cells. 7 h after transfection, the cells were treated with TNFα (5 ng/ml) for the indicated time and harvested for luciferase activity analysis. C: Blockage of TNFα effect by NF-κB inhibitors. The constructed Luc plasmid pD34 containing the –125 to –79 region of human ACAT1 gene proximal promoter was cotransfected with pCH110 into THP-1 cells. 7 h after transfection, the cells were treated without or with TNFα (5 ng/ml) for another 40 h and harvested for luciferase activity analysis. The NF-κB inhibitor PGA1 (15 μM) or PGJ2 (6 μM) was added 30 min prior to stimulation of TNFα as indicated. D: Blockage of TNFα effect by exogenous mutated IκBα (mIκBα). The constructed Luc plasmid pD34 was cotransfected with pCH110 into THP-1 cells in the absence or presence of plasmid pRcβ-mIκBα (for the expression of mIκBα). 24 h after transfection, the cells were treated without or with TNFα (5 ng/ml) for another 40 h and harvested for luciferase activity analysis. The data were expressed the same as in Fig. 4C. All of the experiments were repeated three times with similar results.
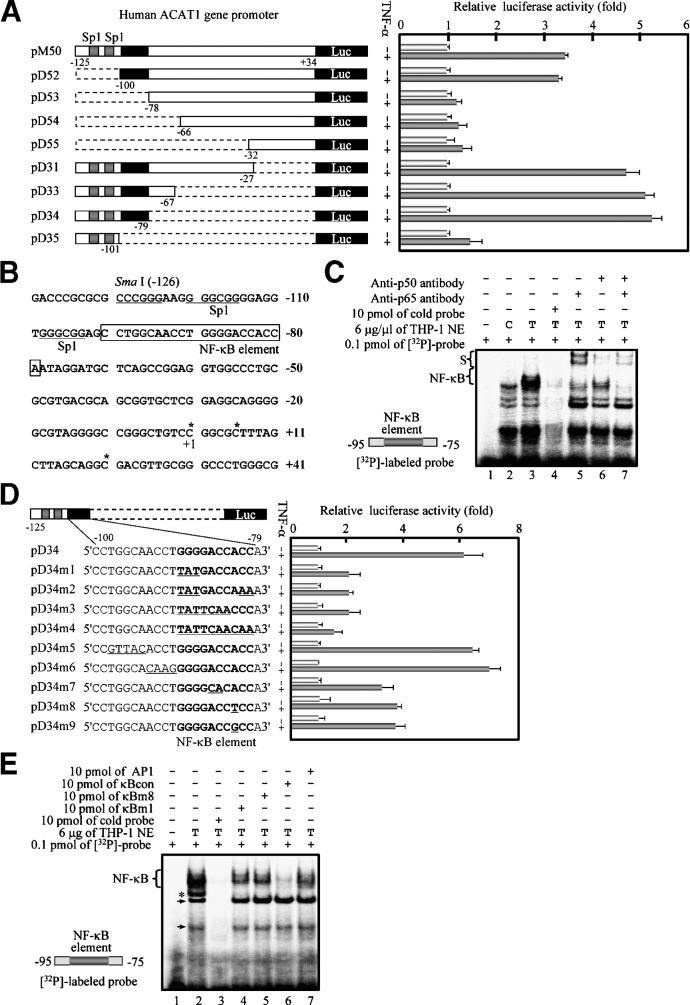
All these results described above indicated that the NF-κB pathway was required for the enhancement of human ACAT1 gene promoter activity by TNFα, implying that this promoter might contain a potential element responsible for the TNFα-enhancing effect. To identify this element, we first made a series of Luc constructs containing different promoter fragments with the sequential 5′- and 3′-deletions; the results demonstrated that a minimal 22-bp region (–100 to –79) was responsible for the TNFα-enhancing effect (Fig. 6A). In this TNFα-responding region [Fig. 6B (boxed)], there was a potential NF-κB site 5′GGGGACCACC3′ (–89 to –80), which is similar to the NF-κB consensus sequence 5′GGGRNNYYCC3′ (where R is purine and Y is pyrimidine) (38). The further EMSA results (Fig. 6C) illustrated that much more specific NF-κB-bound bands were formed upon the TNFα treatment (comparing the lanes 3 with 2) and supershifted by incubating with the anti-p65 and/or anti-p50 antibodies (lanes 5, 6, and 7). These results supported the interpretation that the NF-κB site in the human ACAT1 gene proximal promoter could function as an element that was bound by the transcription factors p50 and p65 upon the TNFα treatment. By site-specific mutation, this functional NF-κB element was further confirmed in both the luciferase assay (Fig. 6D) and the competition EMSA (Fig. 6E). Noticeably, the –82 nucleotide A (purine) was not in accord with that (pyrimidine) of NF-κB consensus sequence, but replacement of this nucleotide by other nucleotides led to much less effect of TNFα than that of wild-type [Figs. 6D (bottom two) and 6E (lane 5)], indicating that this A was the specific nucleotide of the unique NF-κB element in the human ACAT1 gene proximal promoter.
Fig. 6.
Identification of a unique NF-κB element responsible for the enhancement of the human ACAT1 gene promoter activity by TNFα. A: Luciferase activity assays for identification of the TNFα-responding region by stepwise deletions of human ACAT1 gene proximal promoter. The Luc pM50 and its derived plasmids (shown in the left panel) containing serial 5′- and 3′-deletions as indicated between –125 and +34 of the ACAT1 gene promoter core region were individually cotransfected with pCH110 into THP-1 cells. 7 h after transfection, the cells were treated without or with TNFα (5 ng/ml) for another 40 h and harvested for luciferase activity analysis. The data were expressed the same as in Fig. 4C. Gray bar, specificity protein 1 (Sp1) site; broken bar, deletion region; black bar, region responsible for TNFα effect (–100 to –79); Luc, open reading frame region of luciferase gene. B: TNFα-responding region located in the human ACAT1 gene proximal promoter. The 22-bp TNFα-responding region (boxed) contained the NF-κB site (bold). Asterisks indicate the three major transcription initiation sites. C: EMSA for binding of p50 and p65 to the NF-κB element. A synthesized DNA fragment (–95 to –75) containing the NF-κB element in human ACAT1 gene promoter was labeled as a probe (left bottom) and nuclear extracts (NE) were prepared as described in “Materials and Methods.” The [32P]labeled probe (0.1 pmol) was incubated in the absence or presence of NE (6 μg protein) of THP-1 cells treated without (C) or with TNFα (T). NE were preincubated with 100-fold excess cold probe (lane 4) or with 2 μg anti-p65 and/or anti-p50 antibodies (lanes 5, 6, and 7) for 10 min. The binding bands and supershift bands of NF-κB were indicated as NF-κB and S on left, respectively. D: Luciferase activity assays for identification of the NF-κB element by site-specific mutations. The Luc pD34 and its derived plasmids (shown on the left panel) containing various nucleotide mutations (underlined) in the TNFα-responding region were individually cotransfected with pCH110 into THP-1 cells. 7 h after transfection, the cells were treated without or with TNFα (5 ng/ml) for another 40 h and harvested for luciferase activity analysis. The data were expressed same as in Fig. 4C. All symbols are represented the same as in (A). E: Competition EMSA. A synthesized DNA fragment (–95 to –75) containing the NF-κB element in human ACAT1 gene promoter was labeled as a probe (left bottom) and nuclear extracts (NE) were prepared as described in “Materials and Methods.”.The [32P]labeled probe 0.1 pmol) was incubated in the absence or the presence of NE (6 μg protein) of THP-1 cells treated with TNFα (T). NE were preincubated individually with 100-fold excess wild-type (lane 3) or mutated (lanes 4 and 5) cold probe and positive (lane 6) or negative (lane 7) control competitor for 10 min. Sequences of the mutated cold probes (κBm1 and κBm8) and positive and negative control competitors (κBcon and AP-1) were described in “Materials and Methods.” The binding bands of NF-κB were indicated as NF-κB on left. Arrows, nonNF-κB-bound specific bands; asterisk, nonspecific band. All of the experiments were repeated three times with similar results.
DISCUSSION
In the current work, we have demonstrated that in both human blood- and cell line-derived differentiating monocytes, TNFα can promote the CE-laden cell formation, and it acts in part by enhancing the ACAT1 gene expression. The effect of TNFα was cytokine- and cell-type specific, and it affected ACAT1 but not ACAT2. To provide a molecular basis for the specificity observed, we then identified a functional and unique NF-κB element within the human ACAT1 gene proximal promoter, and showed that binding of the transcription factor NF-κB to this element was required to stimulate the human ACAT1 gene promoter activity in response to TNFα.
Earlier work has shown that in fibroblast and cholesterol-loaded human macrophages, high concentration of TNFα (60 ng/ml) can increase the CE contents; it acts in part by activating cell membrane-associated neutral sphingomyelinase (39, 40). These investigators did not examine the ACAT gene expression in these studies. The expression of human ACAT1 gene was shown to be increased during the monocyte differentiation process in vitro (11, 41). In addition, TNFα is shown to increase the ACAT1 activity in phorbol ester-induced THP-1 macrophages (42); however, the molecular mechanism involved was not reported. Whether TNFα regulates ACAT1 gene expression in differentiating monocytes has not been reported. In this work, we showed that TNF-α at low concentration (2.5-5 ng/ml) promotes the CE-laden cell formation in differentiating human blood monocytes and THP-1 monocytes without the phorbol ester treatment, and we showed that TNF-α acts in part by enhancing the ACAT1 expression (Figs. 1–3). Our current results may help to explain the various proatherogeneic effects of TNFα that have been observed in model animals and may also help to explain the earlier finding that in vivo, the ACAT1 protein content is highly expressed in resident macrophages of human atherosclerotic lesions (11).
In addition to the TNFα effect demonstrated here, we previously showed that in monocyte/macrophage, the expression of the human ACAT1 gene was synergistically upregulated by interferon-γ, a cytokine that exerts many proatherosclerotic effects in model animals (16, 28) with ATRA. Other laboratories have shown that the cytokine transforming growth factor-β1 (TGF-β1) also increases ACAT1 activity in human monocytes (43). In addition, urotensin II, the potent vasoconstrictor peptide that leads to hypertension and atherosclerosis in humans, was shown to upregulate the ACAT1 gene expression in human macrophages (44). In contrast, the human ACAT1 gene expression was downregulated by adiponectin, an adipocytokine that exerts many antiatherosclerotic effects in cell culture studies (45). Together, these results suggest that cytokine regulation of ACAT1 gene expression in monocytes/macrophages plays important roles in the initiation and progression of atherosclerosis. Our current work also supports the hypothesis that blocking ACAT1 activity is beneficial during the early stage of atherosclerosis. Experimentally, in a mouse model for atherosclerosis, partial inhibition of ACAT1 activity by an ACAT1-specific inhibitor K604 reduced the CE content at the atherosclerogenic lesions and caused regression of the atherosclerotic plaques without altering the overall serum cholesterol levels in the treated animals (46). However, complete inhibition of ACAT1 by gene inactivation in macrophages produced severe cytotoxicity and enlarged the atherosclerotic lesions (47, 48). In addition, when rodent macrophage cell lines were grown under conditions with minimal cellular cholesterol efflux (a situation that may occur in advanced atherosclerotic lesions), addition of isotype nonspecific ACAT inhibitors to the cells caused undesirable build up of cellular free (unesterified) cholesterol and led to the cells undergoing apoptosis (49). It is possible that ACAT, through its enzymatic product CE and/or substrate free cholesterol, plays different roles at different stages of atherosclerosis. Further experimentation is needed to evaluate the pros and cons of ACAT inhibitors in treating atherosclerosis.
TNFα is involved in the progression of atherosclerosis in various stages. Most of the TNFα effects reported are proatherosclerotic (18–20). Recently, in a model system for advanced lesions, TNFα was shown to induce ABCA1 mRNA and protein and promote cellular cholesterol efflux in macrophages, and it may help to limit plaque development (50). It is possible that TNFα may exhibit proatherosclerotic effects in early development of atherosclerosis and certain antiatherosclerotic effects in advanced lesions. Further experimentation is required to test this hypothesis.
Acknowledgments
The authors thank our colleagues Guang-Jing Hu, Ming Lu, and Jia-Jia Xu for helpful discussion during this study. The authors thank Dr. Jian-Guo Geng for the plasmid pRC/β-actin-mIκBα that is used to express the mutant of IκBα.
Abbreviations
ACAT, acyl-coenzyme A:cholesterol acyltransferase
AP-1, activator protein-1
ATRA, all-trans retinoic acid
CE, cholesteryl ester
GAPDH, glyceraldehyde-3-phosphate dehydrogenase
GM-CSF, granulocyte-macrophage colony stimulating factor
IFN-γ, interferon-gamma
IκBα, inhibitor of NF-κB-alpha
IL-1β, interleukin-1-beta
IL-6, interleukin-6
IL-10, interleukin-10
LPDS, lipoprotein-deficient serum
LPS, lipopolysaccharide
MCP-1, monocyte chemotactic protein-1
M-CSF, macrophage-colony stimulating factor
NE, nuclear extract
NF-κB, nuclear factor-kappa B
oxLDL, oxidized low density lipoprotein
PGA1, prostaglandin A1
PGJ2, 15-deox-Δ12, 14-prostaglandin J2
Sp1, specificity protein 1
TNFα, tumor necrosis factor-alpha
This work was supported by grants from the Ministry of Science and Technology of China (No. 2006CB910600, 2009CB919000), the National Natural Science Foundation of China (No. 30571057, 30623002), and Shanghai Science and Technology Committee (No. 07JC14061, 08431900500) (B-L.L., B-L.S., and Y.X.), and by National Institutes of Health Grant HL-60306 to T-Y.C.).
Published, JLR Papers in Press, February 2, 2009.
References
- 1.Chang C. C. Y., H. Y. Huh, K. M. Cadigan, and T. Y. Chang. 1993. Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J. Biol. Chem. 268 20747–20755. [PubMed] [Google Scholar]
- 2.Oelkers P., A. Behari, D. Cromley, J. T. Billheimer, and S. L. Sturley. 1998. Characterization of two human genes encoding acyl coenzyme A:cholesterol acyltransferase-related enzymes. J. Biol. Chem. 273 26765–26771. [DOI] [PubMed] [Google Scholar]
- 3.Cases S., S. Novak, Y. W. Zheng, H. Myers, S. R. Lear, E. Sande, C. B. Welch, A. J. Lusis, T. A. Spencer, B. R. Krause, et al. 1998. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J. Biol. Chem. 273 26755–26764. [DOI] [PubMed] [Google Scholar]
- 4.Anderson R. A., C. Joyce, M. Davis, J. W. Reagan, M. Clark, G. S. Shelness, and L. L. Rudel. 1998. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J. Biol. Chem. 273 26747–26754. [DOI] [PubMed] [Google Scholar]
- 5.Lee O., C. C. Y. Chang, W. Lee, and T. Y. Chang. 1998. Immunodepletion experiments suggest that acyl-coenzyme A:cholesterol acyltransferase-1(ACAT-1) protein plays a major catalytic role in adult human liver, adrenal gland, macrophages, and kidney, but not in intestines. J. Lipid Res. 39 1722–1727. [PubMed] [Google Scholar]
- 6.Chang C. C. Y., N. Sakashita, K. Ornvold, O. Lee, E. T. Chang, R. Dong, S. Lin, C. Y. Lee, S. C. Strom, R. Kashyap, et al. 2000. Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J. Biol. Chem. 275 28083–28092. [DOI] [PubMed] [Google Scholar]
- 7.Song B. L., C. H. Wang, X. M. Yao, L. Yang, W. J. Zhang, Z. Z. Wang, X. N. Zhao, J. B. Yang, W. Qi, X. Y. Yang, et al. 2006. Human acyl-CoA:cholesterol acyltransferase 2 gene expression in intestinal Caco-2 cells and in hepatocellular carcinoma. Biochem. J. 394 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang C., R. Dong, A. Miyazaki, N. Sakashita, Y. Zhang, J. Liu, M. Guo, B. L. Li, and T. Y. Chang. 2006. Human acyl-CoA:cholesterol acyltransferase (ACAT) and its potential as a target for pharmaceutical intervention against atherosclerosis. Acta Biochim. Biophys. Sin. (Shanghai). 38 151–156. [DOI] [PubMed] [Google Scholar]
- 9.Chang, T. Y., B. L. Li, C. C. Y. Chang, and Y. Urano. 2009. Acyl-coenzyme A:cholesterol acyltransferase. Am. J. Physiol. Endocrinol. Metab. In press. [DOI] [PMC free article] [PubMed]
- 10.Chang T. Y., C. C. Chang, and D. Cheng. 1997. Acyl-coenzyme A:cholesterol acyltransferase. Annu. Rev. Biochem. 66 613–638. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki A., N. Sakashita, O. Lee, K. Takahashi, S. Horiuchi, H. Hakamata, P. M. Morganelli, C. C. Chang, and T. Y. Chang. 1998. Expression of ACAT-1 protein in human atherosclerotic lesions and cultured human monocytes-macrophages. Arterioscler. Thromb. Vasc. Biol. 18 1568–1574. [DOI] [PubMed] [Google Scholar]
- 12.Sakashita N., A. Miyazaki, C. C. Chang, T. Y. Chang, E. Kiyota, M. Satoh, Y. Komohara, P. M. Morganelli, S. Horiuchi, and M. Takeya. 2003. Acyl-coenzyme A:cholesterol acyltransferase 2 (ACAT2) is induced in monocyte-derived macrophages: in vivo and in vitro studies. Lab. Invest. 83 1569–1581. [DOI] [PubMed] [Google Scholar]
- 13.Libby P., and G. K. Hansson. 1991. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab. Invest. 64 5–15. [PubMed] [Google Scholar]
- 14.Getz G. S. 2005. Thematic review series: The immune system and atherogenesis. Immune function in atherogenesis. J. Lipid Res. 46 1–10. [DOI] [PubMed] [Google Scholar]
- 15.Raines E. W., and N. Ferri. 2005. Thematic review series: The immune system and atherogenesis. Cytokines affecting endothelial and smooth muscle cells in vascular disease. J. Lipid Res. 46 1081–1092. [DOI] [PubMed] [Google Scholar]
- 16.Daugherty A., N. R. Webb, D. L. Rateri, and V. L. King. 2005. Thematic review series: The immune system and atherogenesis. Cytokine regulation of macrophage functions in atherogenesis. J. Lipid Res. 46 1812–1822. [DOI] [PubMed] [Google Scholar]
- 17.Baud V., and M. Karin. 2001. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 11 372–377. [DOI] [PubMed] [Google Scholar]
- 18.Branen L., L. Hovgaard, M. Nitulescu, E. Bengtsson, J. Nilsson, and S. Jovinge. 2004. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 24 2137–2142. [DOI] [PubMed] [Google Scholar]
- 19.Skoog T., W. Dichtl, S. Boquist, C. Skoglund-Andersson, F. Karpe, R. Tang, M. G. Bond, U. de Faire, J. Nilsson, P. Eriksson, et al. 2002. Plasma tumour necrosis factor-alpha and early carotid atherosclerosis in healthy middle-aged men. Eur. Heart J. 23 376–383. [DOI] [PubMed] [Google Scholar]
- 20.Popa C., M. G. Netea, P. L. van Riel, J. W. van der Meer, and A. F. Stalenhoef. 2007. The role of TNFalpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J. Lipid Res. 48 751–762. [DOI] [PubMed] [Google Scholar]
- 21.Waldo S. W., Y. Li, C. Buono, B. Zhao, E. M. Billings, J. Chang, and H. S. Kruth. 2008. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am. J. Pathol. 172 1112–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song B. L., W. Qi, X. Y. Yang, C. C. Y. Chang, J. Q. Zhu, T. Y. Chang, and B. L. Li. 2001. Genomic organization of human ACAT-2 gene and its cell type-specific promoter activity. Biochem. Biophys. Res. Commun. 282 580–588. [DOI] [PubMed] [Google Scholar]
- 23.Cheng W., K. V. Kvilekval, and N. A. Abumrad. 1995. Dexamethasone enhances accumulation of cholesteryl esters by human macrophages. Am. J. Physiol. 269 E642–E648. [DOI] [PubMed] [Google Scholar]
- 24.Havel R. J., H. A. Eder, and J. H. Bragdon. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L., J. B. Yang, J. Chen, G. Y. Yu, P. Zhou, L. Lei, Z. Z. Wang, C. Cy Chang, X. Y. Yang, T. Y. Chang, et al. 2004. Enhancement of human ACAT1 gene expression to promote the macrophage-derived foam cell formation by dexamethasone. Cell Res. 14 315–323. [DOI] [PubMed] [Google Scholar]
- 26.Huttunen H. J., C. Greco, and D. M. Kovacs. 2007. Knockdown of ACAT-1 reduces amyloidogenic processing of APP. FEBS Lett. 581 1688–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L., O. Lee, J. Chen, C. C. Chang, P. Zhou, Z. Z. Wang, H. H. Ma, H. F. Sha, J. X. Feng, Y. Wang, et al. 2004. Human acyl-coenzyme A:cholesterol acyltransferase 1 (acat1) sequences located in two different chromosomes (7 and 1) are required to produce a novel ACAT1 isoenzyme with additional sequence at the N terminus. J. Biol. Chem. 279 46253–46262. [DOI] [PubMed] [Google Scholar]
- 28.Yang J. B., Z. J. Duan, W. Yao, O. Lee, L. Yang, X. Y. Yang, X. Sun, C. C. Y. Chang, T. Y. Chang, and B. L. Li. 2001. Synergistic transcriptional activation of human ACAT1 gene by IFN-g and All-trans-retinoic acid in THP-1 cells. J. Biol. Chem. 276 20989–20998. [DOI] [PubMed] [Google Scholar]
- 29.Chen J., X. N. Zhao, L. Yang, G. J. Hu, M. Lu, Y. Xiong, X. Y. Yang, C. C. Chang, B. L. Song, T. Y. Chang, et al. 2008. RNA secondary structures located in the interchromosomal region of human ACAT1 chimeric mRNA are required to produce the 56-kDa isoform. Cell Res. 18 921–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreesen R., W. Brugger, C. Scheibenbogen, M. Kreutz, H. G. Leser, A. Rehm, and G. W. Lohr. 1990. Surface phenotype analysis of human monocyte to macrophage maturation. J. Leukoc. Biol. 47 490–497. [DOI] [PubMed] [Google Scholar]
- 31.Faivre V., A. C. Lukaszewicz, A. Alves, D. Charron, D. Payen, and A. Haziot. 2007. Accelerated in vitro differentiation of blood monocytes into dendritic cells in human sepsis. Clin. Exp. Immunol. 147 426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCullough K. C., S. Basta, S. Knotig, H. Gerber, R. Schaffner, Y. B. Kim, A. Saalmuller, and A. Summerfield. 1999. Intermediate stages in monocyte-macrophage differentiation modulate phenotype and susceptibility to virus infection. Immunology. 98 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemmi H., and T. R. Breitman. 1985. Induction of functional differentiation of a human monocytic leukemia cell line (THP-1) by retinoic acid and cholera toxin. Jpn. J. Cancer Res. 76 345–351. [PubMed] [Google Scholar]
- 34.Rossi A., P. Kapahi, G. Natoli, T. Takahashi, Y. Chen, M. Karin, and M. G. Santoro. 2000. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 403 103–108. [DOI] [PubMed] [Google Scholar]
- 35.Tedgui A., and Z. Mallat. 2006. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol. Rev. 86 515–581. [DOI] [PubMed] [Google Scholar]
- 36.Covert M. W., T. H. Leung, J. E. Gaston, and D. Baltimore. 2005. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 309 1854–1857. [DOI] [PubMed] [Google Scholar]
- 37.DiDonato J., F. Mercurio, C. Rosette, J. Wu-Li, H. Suyang, S. Ghosh, and M. Karin. 1996. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol. Cell. Biol. 16 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghosh S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16 225–260. [DOI] [PubMed] [Google Scholar]
- 39.Chatterjee S. 1994. Neutral sphingomyelinase action stimulates signal transduction of tumor necrosis factor-alpha in the synthesis of cholesteryl esters in human fibroblasts. J. Biol. Chem. 269 879–882. [PubMed] [Google Scholar]
- 40.Chinetti G., S. Lestavel, J. C. Fruchart, V. Clavey, and B. Staels. 2003. Peroxisome proliferator-activated receptor alpha reduces cholesterol esterification in macrophages. Circ. Res. 92 212–217. [DOI] [PubMed] [Google Scholar]
- 41.Wang H., S. J. Germain, P. P. Benfield, and P. J. Gillies. 1996. Gene expression of acyl-coenzyme-A:cholesterol-acyltransferase is upregulated in human monocytes during differentiation and foam cell formation. Arterioscler. Thromb. Vasc. Biol. 16 809–814. [DOI] [PubMed] [Google Scholar]
- 42.He P., B. Cheng, Y. Wang, and H. Wang. 2007. Effect of tumor necrosis factor-alpha on acyl coenzyme A: cholesteryl acyltransferase activity and ACAT1 gene expression in THP-1 macrophages. J. Huazhong Univ. Sci. Technolog. Med. Sci. 27 170–172. [DOI] [PubMed] [Google Scholar]
- 43.Hori M., A. Miyazaki, H. Tamagawa, M. Satoh, K. Furukawa, H. Hakamata, Y. Sasaki, and S. Horiuchi. 2004. Up-regulation of acyl-coenzyme A:cholesterol acyltransferase-1 by transforming growth factor-beta1 during differentiation of human monocytes into macrophages. Biochem. Biophys. Res. Commun. 320 501–505. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe T., T. Suguro, T. Kanome, Y. Sakamoto, S. Kodate, T. Hagiwara, S. Hongo, T. Hirano, M. Adachi, and A. Miyazaki. 2005. Human urotensin II accelerates foam cell formation in human monocyte-derived macrophages. Hypertension. 46 738–744. [DOI] [PubMed] [Google Scholar]
- 45.Furukawa K., M. Hori, N. Ouchi, S. Kihara, T. Funahashi, Y. Matsuzawa, A. Miyazaki, H. Nakayama, and S. Horiuchi. 2004. Adiponectin down-regulates acyl-coenzyme A:cholesterol acyltransferase-1 in cultured human monocyte-derived macrophages. Biochem. Biophys. Res. Commun. 317 831–836. [DOI] [PubMed] [Google Scholar]
- 46.Ikenoya M., Y. Yoshinaka, H. Kobayashi, K. Kawamine, K. Shibuya, F. Sato, K. Sawanobori, T. Watanabe, and A. Miyazaki. 2007. A selective ACAT-1 inhibitor, K-604, suppresses fatty streak lesions in fat-fed hamsters without affecting plasma cholesterol levels. Atherosclerosis. 191 290–297. [DOI] [PubMed] [Google Scholar]
- 47.Fazio S., A. S. Major, L. L. Swift, L. A. Gleaves, M. Accad, M. F. Linton, and R. V. Farese, Jr. 2001. Increased atherosclerosis in LDL receptor-null mice lacking ACAT1 in macrophages. J. Clin. Invest. 107 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su Y. R., D. E. Dove, A. S. Major, A. H. Hasty, B. Boone, M. F. Linton, and S. Fazio. 2005. Reduced ABCA1-mediated cholesterol efflux and accelerated atherosclerosis in apolipoprotein E-deficient mice lacking macrophage-derived ACAT1. Circulation. 111 2373–2381. [DOI] [PubMed] [Google Scholar]
- 49.Feng B., P. M. Yao, Y. Li, C. M. Devlin, D. Zhang, H. P. Harding, M. Sweeney, J. X. Rong, G. Kuriakose, E. A. Fisher, et al. 2003. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 5 781–792. [DOI] [PubMed] [Google Scholar]
- 50.Gerbod-Giannone M. C., Y. Li, A. Holleboom, S. Han, L. C. Hsu, I. Tabas, and A. R. Tall. 2006. TNFalpha induces ABCA1 through NF-kappaB in macrophages and in phagocytes ingesting apoptotic cells. Proc. Natl. Acad. Sci. USA. 103 3112–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]