Abstract
The role of farnesoid X receptor (FXR) in the development of atherosclerosis has been unclear. Here, LDL receptor (LDLR−/−) or apolipoprotein E (apoE−/−) female or male mice were fed a Western diet and treated with a potent synthetic FXR agonist, WAY-362450. Activation of FXR blocked diet-induced hypertriglyceridemia and elevations of non-HDL cholesterol and produced a near complete inhibition of aortic lesion formation. WAY-362450 also induced small heterodimer partner (SHP) expression and repressed cholesterol 7α-hydroxylase (CYP7A1) and sterol 12 α-hydroxylase (CYP8B1) expression. To determine if SHP was essential for these protective activities, LDLR−/−SHP−/− and apoE−/−SHP−/− mice were similarly treated with WAY-362450. Surprisingly, a notable sex difference was observed in these mice. In male LDLR−/−SHP−/− or apoE−/−SHP−/− mice, WAY-362450 still repressed CYP7A1 and CYP8B1 expression by 10-fold and still strongly reduced non-HDL cholesterol levels and aortic lesion area. In contrast, in the female LDLR−/−SHP−/− or apoE−/−SHP−/− mice, WAY-362450 only slightly repressed CYP7A1 and CYP8B1 expression and did not reduce non-HDL cholesterol or aortic lesion size. WAY-362450 inhibition of hypertriglyceridemia remained intact in LDLR−/− or apoE−/− mice lacking SHP of both sexes. These results suggest that activation of FXR protects against atherosclerosis in the mouse, and this protective effect correlates with repression of bile acid synthetic genes, with mechanistic differences between male and female mice.
Keywords: small heterodimer partner, cholesterol 7α-hydroxylase, sterol 12 α-hydroxylase, atherosclerosis
Farnesoid X receptor (FXR) is a nuclear hormone receptor critically involved in the regulation of bile acid homeostasis. It is now recognized that bile acids serve as the natural ligands for FXR (1). Once activated, FXR in turn induces the expression of another nuclear hormone receptor, small heterodimer partner (SHP). SHP does not contain a DNA binding domain, but functions as a transcriptional repressor by interacting with other transcription factors bound to DNA. In bile acid metabolism, SHP functions to repress activity of LRH-1 and HNF4 (2–6). These two factors drive expression of cholesterol 7α-hydroxylase (CYP7A1) and sterol 12 α-hydroxylase (CYP8B1), the two enzymes controlling the bile acid pool size and the hydrophobicity of the bile acid pool (7, 8). In this manner, a classical negative feedback pathway is established in which bile acids regulate their own synthesis.
In regard to lipid metabolism, the FXR activation by either bile acid ligands or potent synthetic ligands has been shown to consistently decrease plasma triglyceride (TG) levels. Multiple mechanisms are involved in this process, including control of lipoprotein lipase activity by FXR-induced stimulation of apoCII expression (9) and repression of apoCIII expression (10), as well as decreased production of VLDL due to diminished SREBP-1c activity and TG synthesis in the liver (11). In regard to cholesterol metabolism, a more complex picture has emerged. A major pathway for elimination of cholesterol from the body is conversion of cholesterol into bile acids. FXR repression of CYP7A1 expression might thus be predicted to increase dyslipidemia and atherosclerosis. Furthermore, FXR can repress apoAI expression and decrease HDL cholesterol levels (12), another potential detrimental effect. Conversely, bile acids are essential for cholesterol absorption (8), and inhibition of cholesterol absorption by ezetimibe strongly decreases plasma LDL cholesterol levels and atherosclerosis (13). Thus, activation of FXR could theoretically have either beneficial effects or negative effects on plasma cholesterol levels and atherosclerosis.
These two possibilities have been initially addressed with FXR−/− mice, which on standard chow diets were found to have a generally proatherogenic alteration in the lipid profile, with both TG and cholesterol levels elevated. To assess the role of FXR in atherosclerotic lesion development, FXR−/− mice have been crossed into both apoE−/− and LDL receptor (LDLR−/−) backgrounds (14–16). ApoE−/−FXR−/− mice fed a diet containing high (1.25%) cholesterol levels have increased plasma cholesterol levels and a doubling of the aortic lesion area. Conversely, in male LDLR−/−FXR−/− mice fed 1.25% cholesterol, plasma cholesterol levels and aortic lesion size were reduced by ∼50%. Surprisingly, in the same study, female LDLR−/−FXR−/− mice showed no difference in plasma cholesterol levels or lesion size. The basis for the differences between the apoE−/−FXR−/− and LDR−/−FXR−/− studies is not readily apparent nor is it evident whether similar results would be obtained with Western diets containing cholesterol levels more typically present in human diets.
Recently, a potent selective FXR synthetic agonist, WAY-362450, with high bioavailability has been demonstrated to reduce plasma LDL cholesterol levels in dyslipidemic mice in an FXR-dependent manner (17). Here, we demonstrate that WAY-362450 has the ability to abolish atherosclerotic lesion formation in both apolipoprotein E (apoE−/−) and LDRL−/− mice and demonstrate that this effect is dependent upon SHP in female but not in male mice.
MATERIALS AND METHODS
Chemicals
Taurocholic acid (TCA) and taurochenodeoxycholic acid (TCDCA) were obtained from Sigma-Aldrich (St. Louis, MO). Tauro-β-muricholic acid (TβMCA) was obtained from Steraloids (Newport, RI).
Mice
To generate LDLR−/−SHP−/− mice, SHP−/− mice in a mixed 129 background (18) were backcrossed to C57BL/6J LDLR−/− mice for four generations at Jackson Labs using speed congenics to maximize C57BL/6J content at each generation. The fourth generation heterozygous mice were interbred to generate LDLR−/−SHP−/− mice, which were then used to generate experimental mice. A similar strategy was used to generate apoE−/−SHP−/− mice except that six backcrosses were used without speed congenics.
In vivo studies
All procedures involving animals were reviewed and approved by the Wyeth Institutional Animal Care and Use Committee. Age- and sex-matched 8- to 12-week-old mice were fed standard chow (5001; Harlan Teklad, Madison, WI) and housed in temperature-controlled virus-free facility on a 12-h-light/dark cycle with free access to food and water. Where indicated, mice were fed either a Western diet containing 42% fat and 0.2% cholesterol by weight (TD88317; Harlan Teklad, Madison, WI) or this Western diet supplemented to contain 0.225 mg WAY-362450 per gram of diet. The mice consumed ∼4 g diet per day, resulting in the delivery of ∼30 mg of WAY-362450 per kg of body weight, assuming a 30 g body weight. Animals were maintained on these diets for 6 (apoE−/−) or 12 weeks (LDLR−/−). At the end of each study, animals were euthanized and blood samples collected via the orbital eye for lipid analysis. Liver and ileum tissue were removed for mRNA quantification, and aortas were removed and stored in 10% buffered formalin solution.
Bile Acid Analysis
The intestine, gall bladder, and a small portion of the liver were removed from mice and stored at −70°C until further use. Samples were homogenized in 1.4 ml of water in a 10 ml polypropylene tube and aliquoted into 10 fractions (∼100 mg each) in 10 × 160 mm glass tubes. Samples used for recovery experiments were spiked in individual samples at 10 μl each of 8 and 16 mM for TβMCA and TCA, and 0.4 and 0.8 mM for TCDCA. Samples were flash frozen and lyophilized overnight. To each sample, 2 ml of ethanol was added, and each sample was sonicated for 60 min and then refluxed for 15 min at 100°C. After centrifugation at 1,500 g for 10 min, the supernatant was transferred to a 50 ml polypropylene tube. The pellet was resuspended in 2 ml of 80% ethanol and refluxed for 15 min at 100°C, and the supernatants were combined after centrifugation at 1,500 g for 10 min. An additional extraction was performed twice by addition of 2 ml chloroform/methanol (1:1) by refluxing, centrifugation, and combining the supernatants as before. The pooled extracts were evaporated, and the residue was reconstituted in 0.6 ml of methanol and sonicated. Water (2.4 ml) was added to each reconstituted sample with vortexing. Samples were then applied to a Waters Sep-Pak C18 cartridge (Waters, Milford, MA) with sequential washes of 4 ml of water and 4 ml of hexane. Bile acids were eluted with 4 ml of methanol, evaporated under nitrogen, and reconstituted in 200 μl of 50% ethanol. After centrifugation for 3 min at 2,500 g, a 10 μl aliquot of each sample was diluted 100-fold in 50% ethanol, and 10 μl of this sample was injected onto the LC/MS/MS system. Calibration standards were prepared by serial dilution of an 800 μM stock solution of TβMCA, TCA, and TCDCA in 50% ethanol, resulting in concentrations of 0.01, 0.1, 1, 2.5, 6.25, 12.5, and 25 μM. Each calibration sample was run in triplicate.
Quantification of bile acids was performed on an Agilent 1200 HPLC interfaced to an Agilent triple quadrupole mass spectrometer (Agilent Technologies, Wilmington, DE). A Supelco Ascentis Express 2 × 150 mm, 2.7 mm C18 column (Supelco, Bellefonte, PA) held at 45°C was used to separate the bile acids via gradient elution. The linear gradient consisted of 50% 10 mM ammonium acetate/0.01% formic acid in water to 95% 50:50 acetonitrile/methanol over 3.2 min at a flow rate of 0.5 ml/min. The mass spectrometer was operated in the negative ion mode with electrospray ionization. The nitrogen gas temperature of the electrospray source was 350°C with a gas flow of 12 l/min and a nebulizer gas pressure of 50 psi. The capillary voltage was 4 kV, and the electron multiplier voltage was 1930 V. Quantification of the bile acids was accomplished by multiple reaction monitoring with the transitions of m/z 514.3 to 79.9 for TβMCA and TCA and m/z 498.3 to 79.9 for TCDCA. Dwell times, fragmentor voltages, and collision energies were 150 ms, 140 V, and 80 eV, respectively, for all of the bile acids. Data were processed with the quantitative software package of MassHunter software. Calibration curves (n = 3) were linear (1/x2 weighting) for each of the bile acids in the concentration range of 0.01 to 25 μM with correlation coefficients of 0.9950 or better and accuracies at each calibration level between 85 and 110% with coefficients of variation within 5%. Recoveries of bile acids (n = 12) with spiking levels of 20 and 40 μM for TCDCA, and 400 and 800 μM for TβMCA and TCA ranged from 85 to 99% with coefficients of variation ranging from 8 to 15%. The limit of quantitation (S/N = 10) for each of the bile acids was 0.01 μM and the limit of detection was 0.005 μM (S/N = 3).
RNA Analysis
RNA was isolated from frozen tissue as described previously (19). Gene expression was measured by real-time RT-PCR using an ABI PRISM 7900 sequence detection system (PE Applied Biosystems, Foster City, CA). Specific real-time PCR primers and probes were used for the following mouse genes: SHP, CYP7A1, CYP8B1, bile salt export protein (BSEP), ileal bile acid binding protein (IBABP), HMGS, and proprotein convertase subtilisin/kexin type 9 (PCSK9). All results were normalized to GAPDH (4308313; PE Applied Biosystems, Foster City, CA) and are the mean ± SEM. Statistical significance was determined by ANOVA.
Lipid Analysis
Total serum cholesterol (Roche kit# 12217295 001), TG (12146029 216), ALT (12217317 001), and AST (12217309 001) levels were quantified with a Roche 912 clinical chemistry analyzer (Roche Diagnostics, Indianapolis, IN). Serum VLDL, LDL, and HDL cholesterol were determined by fast performance liquid chromatography as previously described (18). For measurements of hepatic lipid levels, the method described by Folch, Lees, and Sloane Stanley (20) was used with the following modifications. Frozen liver sections weighing ∼250 mg were homogenized in 1.5 ml chloroform/methanol (2:1) for 9 min at 30 Hz using the TissueLyser II (Qiagen, Valencia, CA). Homogenate was transferred to 50 ml polypropyline tube and chloroform/methanol was added to a final volume of 10 ml. Samples were gassed with nitrogen and shaken overnight at room temperature. Following the addition of 4 ml of water, samples were centrifuged (20 min at 1500 rcf) and the lower layer was measured and transferred to glass vials. Then, 0.5 ml of each sample was dried under nitrogen and resuspended in 0.8 ml of isopropanol by sonication (Misonix, Farmingdale, NY). As recovery controls, 240 μg of glyceryl trioleate (Sigma-Aldrich; T7140) or 100 μg of cholesterol (Sigma-Aldrich; C3045) were added to control samples prior to drying the samples under nitrogen. Following resuspension in isopropanol, TG and cholesterol levels were measured using a Roche 912 clinical chemistry analyzer.
Aortic Lesion Measurements
For quantification of aortic lesions, aortas were isolated and stored in 10% buffered formalin solution. Fat surrounding the aortas was carefully removed prior to staining with oil red O. After destaining, aortic lesions were visualized using a Zeiss Stemi 2000c microscope, and images were captured using an Axio Cam MRc5 camera (Carl Zeiss Microimaging, Thornwood, NY). Lesions were quantified using the MetaVue imaging software (Molecular Devices, Downingtown, PA) and a variation of the methods described by Tangirala, Rubin, and Palinski (21). Briefly, the edge of the aorta (including the aortic arch and thoracic area) was first traced by the operator. The extent of the lesions was determined by setting the threshold range of three basic colors. This threshold range was initially determined by using representative aortas from each treatment group. The threshold range that most accurately captured the visualized lesions was used as the “default threshold” to analyze all the samples in a given study. The selection of the threshold range was verified by a second investigator. The extent of the lesions was expressed as the percentage surface area of the combined aortic arch and thoracic regions.
RESULTS
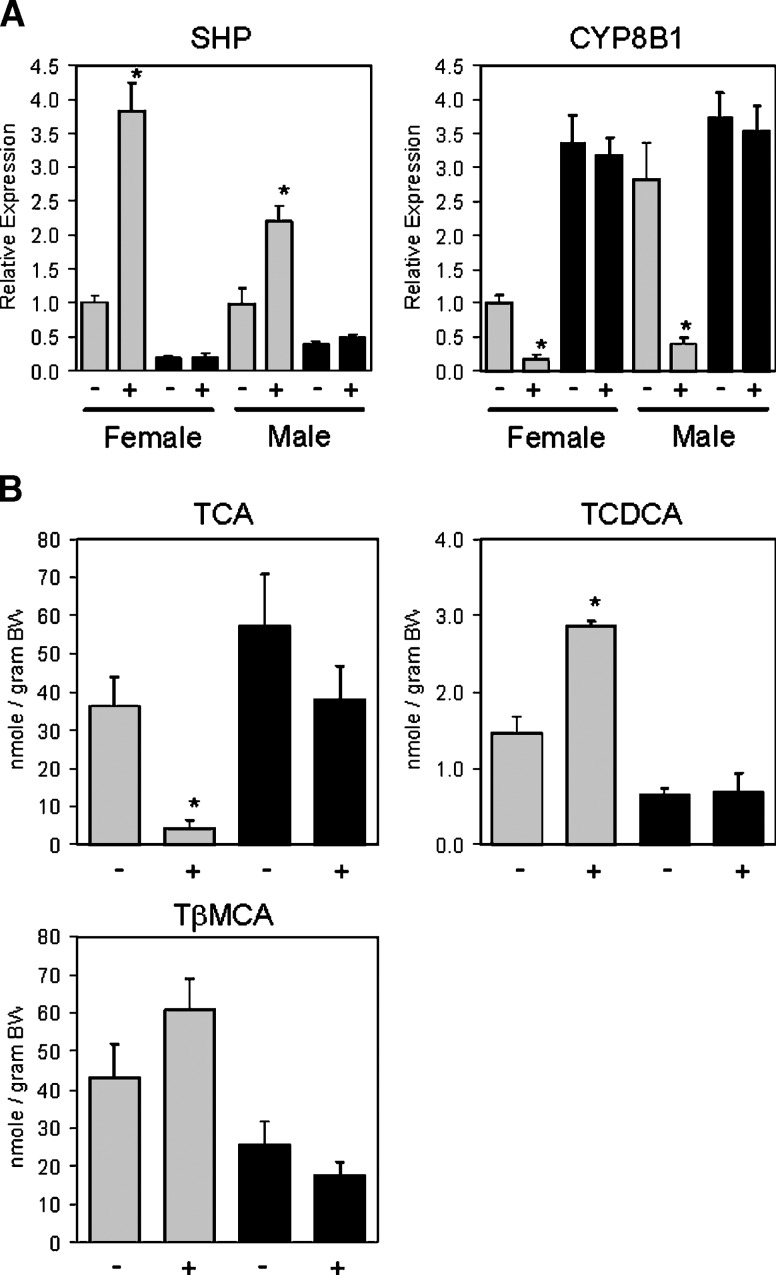
Treatment of wild-type mice with 30 mg/kg WAY-362450 resulted in induction of SHP expression in wild-type mice but not in FXR−/− mice. Consistent with the known effects of SHP induction on bile acid synthetic gene expression, WAY-362450 strongly repressed expression of the CYP8B1 bile acid synthetic gene in wild-type mice but had no effect on CYP8B1 gene expression in FXR−/− mice (Fig. 1A). These gene expression changes altered the primary bile acid composition of the total bile acid pool (Fig. 1B). The levels of TCA, the product of the classical biosynthetic pathway involving CYP8B1, were strongly reduced in wild-type mice treated with WAY-362450. In contrast, the levels of TCDCA and TβMCA, products of the acidic pathway, were not altered or slightly increased. Importantly, WAY-362450 had no effect on bile acid composition in FXR−/− mice.
Fig. 1.
WAY-362450 requires FXR for CYP8B1 repression and regulation of bile acid pool composition. A: C57BL/6J wild-type (gray bars) or FXR−/− mice (black bars) on a standard chow diet were treated by daily oral gavage with vehicle (−) or 30 mg/kg WAY-362450 (+) for 7 days. Two hours following the final dosing, mice were euthanized and livers removed for RNA analysis. SHP and CYP8B1 expression was quantified by real-time PCR and normalized for GAPDH expression. Expression levels in C57BL/6J wild-type mice females were defined as 1.0. Values are the mean ± SEM (n = 6–7 animals per group). * P < 0.01 for regulation by WAY-362450. B: Male C57BL/6J wild-type (gray bars) or FXR−/− mice (black bars) treated as in A were euthanized and a portion of the liver, the gall bladder, and the complete small intestine were removed. The contents of the taurine conjugates TCA, TCDCA, and TβMCA in these tissues were determined by LC/MS/MS and are reported as nmoles of conjugated bile acid per gram of body weight. Values are the mean ± SEM (n = 3 animals per group). * P < 0.05 for regulation by WAY-362450.
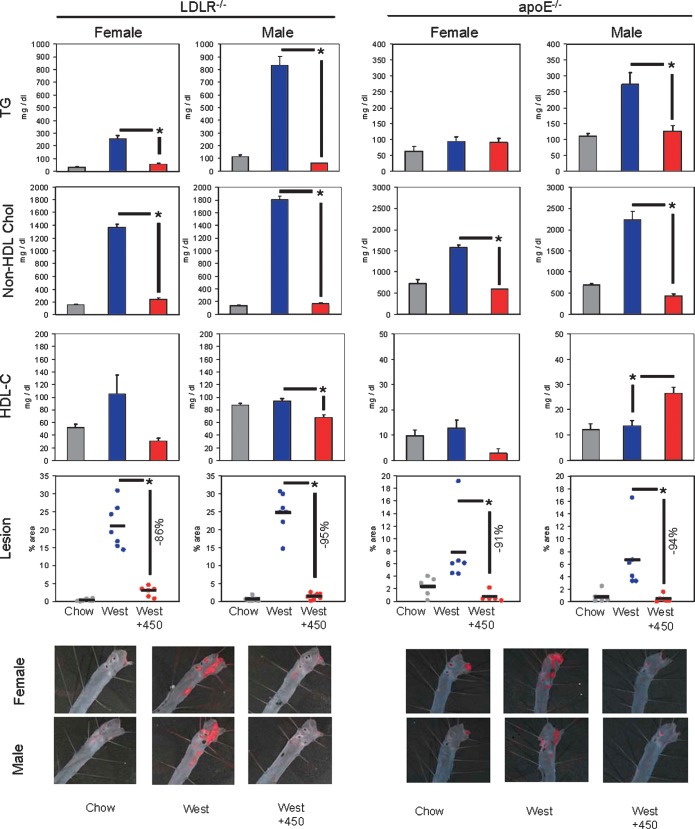
To determine whether activation of FXR would have protective effects against dyslipidemia and atherosclerotic lesion formation, apoE−/− or LDLR−/− mice of both sexes were fed a chow diet, Western diet, or Western diet supplemented to deliver 30 mg/kg WAY-362450. Treatment with WAY-362450 had no effect on body weight (data not shown). In both genetic dyslipidemia models, the Western diet significantly increased plasma TG levels and non-HDL cholesterol levels (Fig. 2). The one exception was female apoE−/− mice, in which plasma TG levels were not increased. In all instances, WAY-362450 treatment produced plasma TG and non-HDL cholesterol levels comparable to the levels in chow-fed mice. A trend toward WAY-362450 reduction of HDL cholesterol occurred in LDLR−/−, although achieving significance only in males. In apoE−/− mice, HDL cholesterol levels were very low, as previously reported (22), and WAY-362450 produced a small increase in males. The Western diet increased TG and total cholesterol content of the liver (Table 1). The increase in total cholesterol levels correlated with diminished expression of cholesterol-regulated genes, such as HMG-CoA synthase and PCSK9. WAY-362450 treatment strongly inhibited the increase in hepatic TG, maintained hepatic total cholesterol levels comparable to those seen in chow-fed mice, and blocked the repression of HMG-CoA synthase and PCSK9 expression. Finally, en face aortic lesion analysis demonstrated that WAY-362450 reduced aortic lesion size by 86 to 95% in female or male LDLR−/− or apoE−/− mice (Fig. 2).
Fig. 2.
WAY-362450 inhibition of dyslipidemia and aortic atherosclerosis. Female or male LDLR−/− or apoE−/− mice 8–10 weeks of age were fed a chow diet, a Western diet containing 0.2% cholesterol, or the Western diet supplemented to deliver ∼30 mg/kg body weight of WAY-362450. LDLR−/− mice were fed the diets for 12 weeks; apoE−/− mice were fed the diets for 6 weeks. At the completion of the study, mice were euthanized, with aortas harvested for lesion analysis, liver and ileums frozen in liquid nitrogen for RNA analysis, and plasma collected by retro-orbital puncture for lipid analysis. Plasma total TG and total cholesterol contents were quantified using a Roche 912 Clinical Chemistry analyzer. Non-HDL (summed VLDL + LDL cholesterol) and HDL cholesterol levels were determined by fast performance liquid chromatography as previously described (17). Aortas were stained by oil red O, and the percentage of area covered was determined. * P < 0.01 for WAY-362450 treatment versus Western diet alone; n = 5–7 mice per group.
TABLE 1.
WAY-362450 activity in the liver of LDLR−/− and apoE−/− mice
| TG (mg/g) |
Cholesterol (mg/g) |
HMG-CoA Synthase mRNA |
PCSK9 mRNA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chowa | Western | Western + 450 | Chow | Western | Western+450 | Chow | Western | Western+450 | Chow | Western | Western+450 | ||
| LDLR−/− | Female | 12.7 ± 3.0 | 102.0 ± 6.3 | 50.3 ± 15.2c | 3.8 ± 0.2 | 27.4 ± 1.8 | 5.4 ± 1.2c | 1.00 ± 0.11b | 0.14 ± 0.02 | 1.03 ± 0.09c | 1.00 ± 0.12 | 0.33 ± 0.05 | 1.34 ± 0.08c |
| Male | 7.5 ± 0.8 | 295.0 ± 14.6 | 21.2 ± 4.4c | 3.1 ± 0.3 | 21.1 ± 2.5 | 3.9 ± 0.3c | 0.44 ± 0.11 | 0.08 ± 0.01 | 0.95 ± 0.14c | 0.55 ± 0.12 | 0.10 ± 0.02 | 0.85 ± 0.07c | |
| apoE−/− | Female | 3.6 ± 0.3 | 12.1 ± 2.2 | 3.7 ± 0.4c | 9.7 ± 0.7 | 19.1 ± 1.7 | 12.5 ± 0.3c | 1.00 ± 0.09b | 0.21 ± 0.02 | 2.42 ± 0.27c | 1.00 ± 0.08 | 0.38 ± 0.05 | 1.78 ± 0.21c |
| Male | 1.2 ± 0.2 | 13.1 ± 6.1 | 4.5 ± 1.0c | 5.5 ± 0.6 | 19.2 ± 2.3 | 4.3 ± 0.2c | 0.57 ± 0.08 | 0.19 ± 0.03 | 3.23 ± 0.35c | 1.48 ± 0.23 | 0.23 ± 0.04 | 1.77 ± 0.25c | |
Mice were fed a standard chow diet, a Western diet, or a Western diet with WAY-362450 as described in Fig. 2. At the conclusion of the study, hepatic total TG and cholesterol levels were determined.
Expression levels in female mice fed the chow diet were defined as 1.00 for LDLR−/− and apoE−/−, respectively.
P < 0.05 for effect of WAY-362450.
A major FXR regulatory pathway is mediated via induction of SHP, which represses expression of CYP7A1, decreasing the bile acid pool size, and the expression of CYP8B1, decreasing the hydrophobicity of the bile acid pool. These bile acid pool alterations lead to decreased cholesterol absorption. To determine if the beneficial effects mediated by WAY-362450 required activation of the SHP pathway, SHP−/− mice were backcrossed to LDLR−/− or apoE−/− mice to generate LDLR−/−SHP−/− and apoE−/−SHP−/− mice. SHP expression was not detected in the liver or ileum of the LDLR−/−SHP−/− or apoE−/−SHP−/− mice (Table 2). SHP expression was induced in liver and ileum of LDLR−/− or apoE−/− mice treated with WAY-362450. Known FXR-mediated inductions of BSEP in the liver (23) and IBABP in the ileum (24) occurred to a similar magnitude with WAY-362450 treatment in LDLR−/−SHP−/− compared with LDLR−/− mice and in apoE−/−SHP−/− compared with apoE−/− mice (Table 2).
TABLE 2.
WAY-362450 regulation of gene expression
| Hepatic SHP mRNA |
Ileum SHP mRNA |
Hepatic BSEP mRNA |
Ileum IBABP mRN |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chowa | Western | Westerrn + 450 | Chow | Western | Western+450 | Chow | Western | Western+450 | Chow | Western | Western+450 | ||
| LDLR−/− | Female | 1.00 ± 0.14 | 0.49 ± 0.05 | 0.82 ± 0.06c | 1.00 ± 0.23 | 1.75 ± 0.58 | 5.68 ± 1.28c | 1.00 ± 0.14b | 0.84 ± 0.09 | 1.98 ± 0.19c | 1.00 ± 0.24 | 0.59 ± 0.09 | 1.72 ± 0.19c |
| Male | 0.57 ± 0.15 | 0.50 ± 0.07 | 0.89 ± 0.05c | 0.59 ± 0.17 | 0.66 ± 0.13 | 9.42 ± 1.21c | 0.71 ± 0.08 | 0.68 ± 0.09 | 1.40 ± 0.23c | 1.93 ± 0.32 | 1.51 ± 0.25 | 2.57 ± 0.41c | |
| apoE−/− | Female | 1.00 ± 0.24 | 0.99 ± 0.16 | 1.34 ± 0.11c | 1.00 ± 0.19 | 4.17 ± 0.53 | 16.7 ± 0.77c | 1.00 ± 0.17b | 0.54 ± 0.11 | 1.53 ± 0.12c | 1.00 ± 0.10 | 1.15 ± 0.18 | 2.75 ± 0.14c |
| Male | 0.37 ± 0.04 | 0.52 ± 0.07 | 1.45 ± 0.13c | 0.37 ± 0.16 | 2.58 ± 0.31 | 19.5 ± 3.92c | 0.52 ± 0.03 | 0.48 ± 0.04 | 2.48 ± 0.12c | 0.89 ± 0.10 | 1.26 ± 0.40 | 3.50 ± 0.20c | |
| LDLR−/− | Female | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.97 ± 0.09 | 0.48 ± 0.04 | 1.08 ± 0.10c | 1.23 ± 0.25 | 1.47 ± 0.32 | 2.19 ± 0.36c |
| SHP−/− | Male | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.44 ± 0.08 | 0.32 ± 0.05 | 1.20 ± 0.12c | 2.13 ± 0.47 | 1.79 ± 0.40 | 3.69 ± 0.32c |
| apoE−/− | Female | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1.01 ± 0.10 | 0.51 ± 0.11 | 1.20 ± 0.04c | 1.29 ± 0.04 | 1.25 ± 0.19 | 2.03 ± 0.45c |
| SHP−/− | Male | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1.17 ± 0.09 | 0.62 ± 0.13 | 1.58 ± 0.21c | 1.35 ± 0.14 | 1.19 ± 0.09 | 3.12 ± 0.13c |
Mice were fed a standard chow diet, a Western diet, or a Western diet with WAY-362450 as described in Fig. 2. At the conclusion of the study, hepatic total TG and cholesterol levels were determined.
Expression levels in female mice fed the chow diet were defined as 1.00 for LDLR−/− and apoE−/−, respectively.
P < 0.05 for effect of WAY-362450.
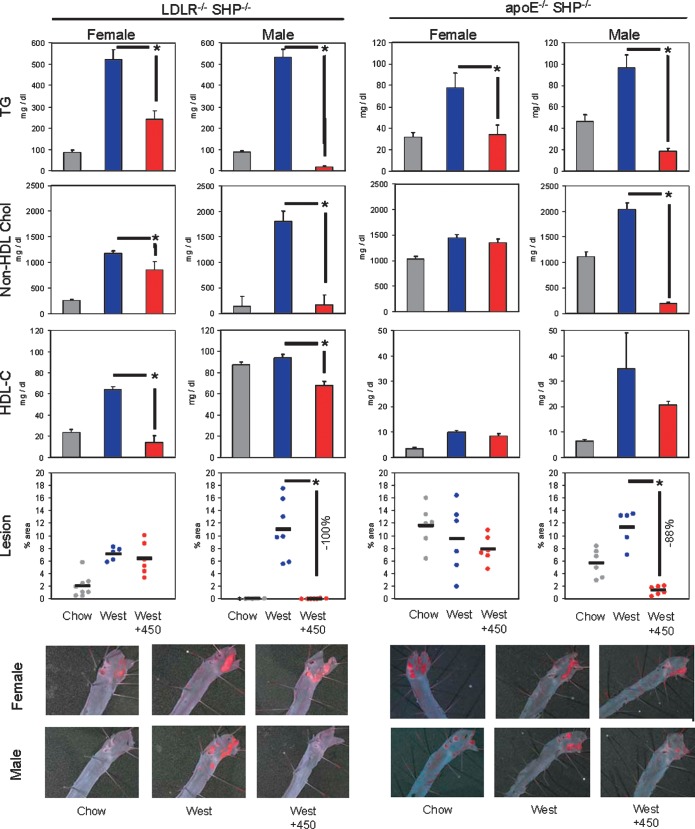
Feeding the Western diet increased plasma TG and non-HDL cholesterol levels in the LDLR−/−SHP−/− mice to a similar magnitude as observed in LDLR−/− mice (Fig. 3). The Western diet also induced aortic lesion formation in LDLR−/−SHP−/− mice, although the lesion size was reduced by >50% in LDLR−/−SHP−/− as compared with the LDLR−/− mice (21% aortic area in LDLR−/− vs. 7% aortic area in LDLR−/−SHP−/− for female mice and 25% aortic area in LDLR−/− vs. 11% aortic area in LDLR−/−SHP−/− for male mice). In apoE−/−SHP−/− mice, plasma levels of non-HDL cholesterol were slightly greater in chow-fed animals and were also increased by the Western diet. Surprisingly, when fed a chow diet, the absence of SHP resulted in increased lesion size (2.4% aortic area in apoE−/− vs. 12% aortic area in apoE−/−SHP−/− for female mice and 0.7% aortic area in apoE−/− vs. 5.6% aortic area in apoE−/−SHP−/− for male mice). When fed a Western diet, the loss of SHP did not affect lesion size in apoE−/− mice. Thus, in all cases significant dyslipidemia occurred and aortic lesions were still formed in the absence of SHP, allowing the role of SHP in WAY-362450 protection to be delineated.
Fig. 3.
Requirement of SHP for WAY-362450 inhibition of dyslipidemia and aortic atherosclerosis. SHP−/− mice in a 129 strain background (18) were backcrossed to C57BL/6J mice for four generations using speed congenics to maximize the C57BL/6J background at each cross. The resulting mice were then bred to C57BL/6J LDLR−/− mice to generate LDLR−/−SHP−/− mice. Similarly, SHP−/− mice were backcrossed to C57BL/6J apoE−/− mice for six generations to generate apoE−/−SHP−/− mice. The LDLR−/−SHP−/− and apoE−/−SHP−/− were analyzed as described in Fig. 2.
The Western diet increased plasma TG levels in LDLR−/−SHP−/− or apoE−/−SHP−/− mice, and WAY-362450 treatment still strongly decreased plasma TG levels in these mice (Fig. 3). Similarly, the Western diet-mediated increase in hepatic cholesterol content and repression of HMG-CoA synthase and PCSK9 gene expression was largely blocked by WAY-362450 treatment of LDLR−/−SHP−/− or apoE−/−SHP−/− mice (Table 3). Similar trends were observed for hepatic TG content, with the exception of male LDLR−/−SHP−/− mice, in which treatment with WAY-362450 did not decrease TG content. This set of WAY-362450 protective effects thus appeared to be largely independent of the SHP regulatory pathway.
TABLE 3.
WAY-362450 activity in the liver of LDLR−/−SHP−/− and apoE−/−SHP−/− mice
| TG (mg/g) |
Cholesterol (mg/g) |
HMG-CoA Synthase mRNA |
PCSK9 mRNA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chowa | Western | Western+450 | Chow | Western | Western+450 | Chow | Western | Western+450 | Chow | Western | Western+450 | ||
| LDLR−/− | Female | 5.2 ± 1.8 | 86.6 ± 18.6 | 30.6 ± 3.0c | 4.6 ± 0.5 | 25.6 ± 4.0 | 14.4 ± 1.5c | 0.89 ± 0.07b | 0.42 ± 0.09 | 1.24 ± 0.20c | 0.80 ± 0.10 | 0.98 ± 0.14 | 1.37 ± 0.21c |
| SHP−/− | Male | 4.3 ± 0.2 | 34.6 ± 18.5 | 53.7 ± 1.0 | 3.3 ± 0.2 | 16.3 ± 1.9 | 4.4 ± 1.0c | 0.29 ± 0.10 | 0.05 ± 0.01 | 1.51 ± 0.23c | 0.53 ± 0.05 | 0.31 ± 0.05 | 1.45 ± 0.20c |
| apoE−/− | Female | 3.7 ± 0.8 | 14.7 ± 2.0 | 9.5 ± 1.0c | 5.3 ± 0.5 | 31.3 ± 4.1 | 8.3 ± 0.9c | 1.65 ± 0.50b | 0.46 ± 0.11 | 3.11 ± 0.39c | 1.72 ± 0.15 | 1.60 ± 0.43 | 2.41 ± 0.3 |
| SHP−/− | Male | 1.4 ± 0.4 | 16.5 ± 2.0 | 5.1 ± 1.3c | 3.8 ± 0.1 | 17.7 ± 0.8 | 4.5 ± 0.3c | 1.87 ± 0.12 | 0.31 ± 0.11 | 3.35 ± 0.32c | 1.40 ± 0.21 | 0.61 ± 0.17 | 1.78 ± 0.18c |
Mice were fed a standard chow diet, a Western diet, or a Western diet with WAY-362450 as described in Fig. 2. At the conclusion of the study, hepatic total TG and cholesterol levels were determined.
Expression levels in female mice fed the chow diet were defined as 1.00 for LDLR−/− and apoE−/−, respectively.
P < 0.05 for effect of WAY-362450.
Surprisingly, the results for WAY-362450 regulation of non-HDL cholesterol levels depended upon the sex of the animals. Thus, in male LDLR−/−SHP−/− or apoE−/−SHP−/− mice, WAY-362450 still strongly reduced non-HDL cholesterol levels (Fig. 3). In contrast, in female apoE−/−SHP−/− mice, WAY-362450 had no significant effect on non-HDL cholesterol levels, and in LDLR−/−SHP−/−,WAY-362450 only partially reduced non-HDL cholesterol levels. En face aortic lesion analysis showed the same sex dependence, with WAY-362450 reducing lesion area by 100 and 88% in male LDLR−/−SHP−/− or apoE−/−SHP−/− mice, respectively, but having no effect on lesion size in female LDLR−/−SHP−/− or apoE−/−SHP−/− mice.
While bile acids can still repress CYP7A1 and CYP8B1 expression in SHP−/− mice, the synthetic FXR agonist GW4064 does not repress either gene in SHP−/− mice (3, 25). This raised the possibility that the dyslipidemia and aortic lesion protective activity of WAY-362450 in male LDLR−/−SHP−/− or apoE−/−SHP−/− mice could be independent of FXR repression of CYP7A1 and CYP8B1. In apoE−/− and LDLR−/− mice, WAY-362450 strongly repressed CYP7A1 and CYP8B1 expression in both female and male mice (Fig. 4A). In female LDLR−/−SHP−/− or apoE−/−SHP−/− mice, WAY-362450 did not significantly repress CYP7A1 expression and produced only a slight decrease in CYP8B1 expression. In contrast, in male LDLR−/−SHP−/− or apoE−/−SHP−/− mice, WAY-362450 repressed both CYP7A1 and CYP8B1 expression to nearly the same magnitude as seen in the male apoE−/− or LDLR−/− mice. In agreement with these results, expression of genes induced by cholesterol [via oxysterol activation of liver X receptor (LXR)] in the ileum, such as ABCG5 and ABCA1, was reduced in LDLR−/− and apoE−/− mice of both sexes by treatment with WAY-362450 (Fig. 4B). In LDLR−/−SHP−/− or apoE−/−SHP−/− mice, WAY-362450 treatment reduced ileum ABCG5 and ABCA1 expression in male mice but not in female mice. Although cholesterol content of the ileum could not be measured directly due to residual diet in the lumen, these gene expression results suggest a reduction of cholesterol content in enterocytes in all mice in which hepatic CYP7A1 and CYP8B1 expression was reduced. Thus, repression of CYP7A1 and CYP8B1 expression correlated precisely with protection from dyslipidemia and atherosclerotic lesion formation.
Fig. 4.
WAY-362450 repression of diet-induced gene expression. A: Total liver RNA prepared from apoE−/−, LDLR−/−, apoE−/−SHP−/−, and LDLR−/−SHP−/− mice was analyzed for mRNA levels of CYP7A1 and CYP8B1 by real-time PCR. Expression values for all samples were normalized for expression of GAPDH. The expression observed in female apoE−/− or LDLR−/− mice was defined as 1.0 for each panel. Values are the mean ± SEM. B: Total RNA was prepared from ileum and analyzed as above for expression of ABCG5 and ABCA1. * P < 0.01 for effect of WAY-362450 on gene expression.
DISCUSSION
Numerous lines of evidence have suggested that increased CYP7A1 expression leads to diminished plasma cholesterol levels and ultimately protection from atherosclerosis. For example, hamsters, which express low levels of cholesterol-7-α-hydroxylase activity, are susceptible to diet-induced dyslipidemia, while mice or rats, which express high levels of cholesterol-7-α-hydroxylase activity, are resistant to diet-induced dyslipidemia (26). Adenovirus-mediated overexpression of CYP7A1 in the hamster reduces plasma LDL cholesterol in direct proportion to the magnitude of increase in cholesterol-7-α-hydroxylase activity (27). Similarly, transgenic overexpression of CYP7A1 blocks diet-induced atherosclerosis in wild-type and LDLR−/− mice (28, 29). Generally in these studies, CYP7A1 has been expressed at ∼10-fold elevated levels over the already high levels present in the mice. Conversely, CYP7A1−/− mice have been reported to be hypercholesterolemic by some but not all groups (8, 30). In humans, CYP7A1 deficiency also results in a hypercholesterolemic phenotype in a single kindred (31). Conversely, bile acids are essential for absorption of cholesterol from the diet. Thus, CYP7A1−/− mice have almost no intestinal absorption of dietary cholesterol (8), and CYP8B1−/− mice also have reduced cholesterol absorption (32). Furthermore, humans with an absence of bile acids also have almost no cholesterol absorption (33, 34). In animal studies, until recently the major tool to repress CYP7A1 and CYP8B1 expression has been bile acid feeding, which while repressing expression of these genes will also promote increased cholesterol absorption.
Expression of CYP7A1 is controlled predominantly at the transcriptional level through multiple nuclear receptors including the LXR, which induces CYP7A1 transcription in rodents (35), and FXR, which represses transcription of CYP7A1 (36) via induction of SHP (3, 25). FXR−/− mice have both increased intestinal cholesterol absorption and a pro-atherogenic plasma lipid profile, including increased non-HDL cholesterol and TG levels (37). The initial potent synthetic activator of FXR, GW4064 (38), lowers plasma cholesterol levels in mice (39). Another recently identified FXR agonist, WAY-362450, has been shown to protect against dyslipidemia in wild-type mice but not in FXR−/− mice (17). Thus, studies on FXR activation generally suggest a beneficial effect in regard to the plasma lipid profile.
In regard to atherosclerosis, the role of FXR has been unclear. In apoE−/− mice, the loss of FXR either increases atherosclerotic lesions in mice fed a high 1.25% cholesterol diet (15) or reduces atherosclerotic lesions in mice fed a high fat diet with no added cholesterol (14). In LDLR−/− mice fed a diet with 1.25% cholesterol, loss of FXR reduced atherosclerotic lesion size in male mice but not in female mice (16). To ascertain the effects of FXR activation by a synthetic agonist on atherosclerotic lesion development, LDLR−/− and apoE−/− mice of both sexes were fed a Western diet containing 0.2% cholesterol and treated with WAY-362450. In all instances, WAY-362450 protected against diet-mediated increases in plasma non-HDL cholesterol (Fig. 2), hepatic cholesterol (Table 1), and aortic lesion formation (Fig. 2). As expected, WAY-362450 activation of FXR induced SHP expression (Table 2), repressed hepatic CYP7A1 and CYP8B1 expression (Fig. 4), and decreased the hydrophobicity of the bile acid pool.
To determine whether the repression of bile acid synthetic gene expression was essential to the observed protection, LDLR−/−SHP−/− and apoE−/−SHP−/− mice of both sexes were treated with WAY-362450. As predicted by the canonical model, in female LDLR−/−SHP−/− or apoE−/−SHP−/− mice, WAY-362450 no longer significantly repressed CYP7A1 or CYP8B1 expression (Fig. 3), reduced non-HDL cholesterol levels only slightly, and did not reduce aorta lesion size (Fig. 3). Surprisingly, in male LDLR−/−SHP−/− or apoE−/−SHP−/− mice, WAY-362450 still repressed CYP7A1 and CYP8B1 expression by 10-fold, strongly reduced non-HDL cholesterol levels, and nearly abolished aorta lesion formation. Other transcriptional pathways can lead to the repression of CYP7A1 or CYP8B1 gene expression. For example, the pregnane X receptor (PXR) agonist pregnenolone-16α-carbonitrile strongly reduces CYP7A1 expression (40). In rodents, FXR induces expression of PXR (41), although conversely SHP induction can inhibit the ability of PXR to activate gene expression (42). We observed WAY-362450 induction of the PXR target gene CYP3A11 in the livers of male and female mice to a similar extent in all genotypes (data not shown), suggesting this pathway may not be responsible for the repression of CYP7A1 and CYP8B1 observed in the male LDLR−/−SHP−/− and apoE−/−SHP−/− mice.
In addition to cholesterol regulation, activation of FXR reduces plasma TG levels via multiple proposed mechanisms. ApoCII expression is induced by FXR (9), while apoCIII expression is repressed by FXR, not through regulation of SHP expression but rather via a negative FXR response element in which FXR binding displaces HNF4α (10). ApoCII is an activator of lipoprotein lipase activity, while apoCIII inhibits lipoprotein lipase activity. The net effect of FXR activity is thus to enhance the activity of lipoprotein lipase, resulting in enhanced hydrolysis of TG in the plasma. Alternatively, it has been proposed that FXR reduces plasma TG levels via induction of SHP, which then represses LXR-mediated expression of SREBP-1c (11). Here, FXR activation by WAY-362450 greatly attenuated Western diet-mediated increases in plasma TG levels (Fig. 2), and this effect was largely maintained in LDLR−/−SHP−/− and apoE−/−SHP−/− mice (Fig. 3). In concordance with a SHP-independent mechanism, apoCII expression was induced by WAY-362450 treatment (data not shown). Recently, several clinical trials have pointed to the importance of plasma TG levels as a risk factor for development of cardiovascular disease (43–45), suggesting the potent lowering of elevated plasma TG levels by synthetic FXR agonists, such as WAY-362450, will provide further benefit beyond cholesterol lowering.
Numerous pharmacological paradigms have been tested for reduction of atherosclerosis in mice [reviewed in (46)]. Due to the use of various genetic models and diets, it is difficult to directly compare the magnitude of effects of various treatments. However, the near complete prevention of lesion formation seen here compares favorably with the magnitude of effects seen for ezetimibe, a potent inhibitor of cholesterol absorption (13) or for synthetic LXR agonists (47), thought to act largely through activation of cholesterol efflux from foam cells (48, 49). Human bile acid physiology and regulation have notable differences from mouse. Of particular relevance here, FXR represses CYP8B1 expression via induction of SHP in mouse, whereas FXR directly stimulates CYP8B1 expression through an FXR response element in humans (50). Whether these species differences will attenuate the protective effect of FXR agonists against dyslipidemia or atherosclerosis in humans will require further study.
Supplementary Material
Acknowledgments
The authors thank Paige Mahaney for providing WAY-362450, KehDih Lai for assistance with aorta dissections, Ted Simon for breeding of the apoE−/−SHP−/− mice, Wyeth Bioresources for animal husbandry, and Doug Harnish for helpful discussions.
Abbreviations
apoE, apolipoprotein E
BSEP, bile salt export protein
CYP7A1, cholesterol 7α-hydroxylase
CYP8B1, sterol 12 α-hydroxylase
FXR, farnesoid X receptor
IBABP, ileal bile acid binding protein
LDLR, low density lipoprotein receptor
LXR, liver X receptor
PCSK9, proprotein convertase subtilisin/kexin type 9
PXR, pregnane X receptor
SHP, small heterodimer partner
TβMCA, tauro-β-muricholic acid
TCA, taurocholic acid
TCDCA, taurochenodeoxycholic acid
TG, triglyceride
Published, JLR Papers in Press, January 27, 2009.
References
- 1.Wang H., J. Chen, K. Hollister, L. C. Sowers, and B. M. Forman. 1999. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 3 543–553. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin B., S. A. Jones, R. R. Price, M. A. Watson, D. D. McKee, L. B. Moore, C. Galardi, J. G. Wilson, M. C. Lewis, M. E. Roth, et al. 2000. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell. 6 517–526. [DOI] [PubMed] [Google Scholar]
- 3.Kerr T. A., S. Saeki, M. Schneider, K. Schaefer, S. Berdy, T. Redder, B. Shan, D. W. Russell, and M. Schwarz. 2002. Loss of nuclear receptor SHP impairs but does not eliminate negative feedback regulation of bile acid synthesis. Dev. Cell. 2 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu T. T., M. Makishima, J. J. Repa, K. Schoonjans, T. A. Kerr, J. Auwerx, and D. J. Mangelsdorf. 2000. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell. 6 507–515. [DOI] [PubMed] [Google Scholar]
- 5.Sinal C. J., M. Tohkin, M. Miyata, J. M. Ward, G. Lambert, and F. J. Gonzalez. 2000. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 102 731–744. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M., and J. Y. Chiang. 2001. Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4alpha in mediating bile acid repression. J. Biol. Chem. 276 41690–41699. [DOI] [PubMed] [Google Scholar]
- 7.Pandak W. M., P. Bohdan, C. Franklund, D. H. Mallonee, G. Eggertsen, I. Bjorkhem, G. Gil, Z. R. Vlahcevic, and P. B. Hylemon. 2001. Expression of sterol 12alpha-hydroxylase alters bile acid pool composition in primary rat hepatocytes and in vivo. Gastroenterology. 120 1801–1809. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz M., D. W. Russell, J. M. Dietschy, and S. D. Turley. 1998. Marked reduction in bile acid synthesis in cholesterol 7alpha-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J. Lipid Res. 39 1833–1843. [PubMed] [Google Scholar]
- 9.Kast H. R., C. M. Nguyen, C. J. Sinal, S. A. Jones, B. A. Laffitte, K. Reue, F. J. Gonzalez, T. M. Willson, and P. A. Edwards. 2001. Farnesoid X-activated receptor induces apolipoprotein C–II transcription: a molecular mechanism linking plasma triglyceride levels to bile acids. Mol. Endocrinol. 15 1720–1728. [DOI] [PubMed] [Google Scholar]
- 10.Claudel T., Y. Inoue, O. Barbier, D. Duran-Sandoval, V. Kosykh, J. Fruchart, J. C. Fruchart, F. J. Gonzalez, and B. Staels. 2003. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology. 125 544–555. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe M., S. M. Houten, L. Wang, A. Moschetta, D. J. Mangelsdorf, R. A. Heyman, D. D. Moore, and J. Auwerx. 2004. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest. 113 1408–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claudel T., E. Sturm, H. Duez, I. P. Torra, A. Sirvent, V. Kosykh, J. C. Fruchart, J. Dallongeville, D. W. Hum, F. Kuipers, et al. 2002. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J. Clin. Invest. 109 961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis H. R., Jr., D. S. Compton, L. Hoos, and G. Tetzloff. 2001. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler. Thromb. Vasc. Biol. 21 2032–2038. [DOI] [PubMed] [Google Scholar]
- 14.Guo G. L., S. Santamarina-Fojo, T. E. Akiyama, M. J. Amar, B. J. Paigen, B. Brewer, Jr., and F. J. Gonzalez. 2006. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim. Biophys. Acta. 1761 1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanniman E. A., G. Lambert, T. C. McCarthy, and C. J. Sinal. 2005. Loss of functional farnesoid X-receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J. Lipid Res. 46 2595–2604. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., X. Wang, C. Vales, F. Y. Lee, H. Lee, A. J. Lusis, and P. A. Edwards. 2006. FXR deficiency causes reduced atherosclerosis in Ldlr−/− mice. Arterioscler. Thromb. Vasc. Biol. 26 2316–2321. [DOI] [PubMed] [Google Scholar]
- 17.Evans M. J., P. E. Mahaney, L. Borges-Marcucci, K. Lai, S. Wang, J. A. Krueger, S. J. Gardell, C. Huard, R. Martinez, G. P. Vlasuk, et al. 2009. A synthetic farnesoid X receptor (FXR) agonist promotes cholesterol lowering in models of dyslipidemia. Am. J. Physiol. Gastrointest. Liver Physiol. 296 G543–G552. [DOI] [PubMed] [Google Scholar]
- 18.Hartman H. B., K. Lai, and M. J. Evans. 2009. Loss of small heterodimer partner (SHP) expression in the liver protects against dyslipidemia. J. Lipid Res. 50 193–203. [DOI] [PubMed] [Google Scholar]
- 19.Lai K., D. C. Harnish, and M. J. Evans. 2003. Estrogen receptor alpha regulates expression of the orphan receptor small heterodimer partner. J. Biol. Chem. 278 36418–36429. [DOI] [PubMed] [Google Scholar]
- 20.Folch J., M. Lees, and G. H. Sloane Stanley. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226 497–509. [PubMed] [Google Scholar]
- 21.Tangirala R. K., E. M. Rubin, and W. Palinski. 1995. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J. Lipid Res. 36 2320–2328. [PubMed] [Google Scholar]
- 22.Ray A., K. E. Prefontaine, and P. Ray. 1994. Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J. Biol. Chem. 269 12940–12946. [PubMed] [Google Scholar]
- 23.Ananthanarayanan M., N. Balasubramanian, M. Makishima, D. J. Mangelsdorf, and F. J. Suchy. 2001. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J. Biol. Chem. 276 28857–28865. [DOI] [PubMed] [Google Scholar]
- 24.Grober J., I. Zaghini, H. Fujii, S. A. Jones, S. A. Kliewer, T. M. Willson, T. Ono, and P. Besnard. 1999. Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer. J. Biol. Chem. 274 29749–29754. [DOI] [PubMed] [Google Scholar]
- 25.Wang L., Y. K. Lee, D. Bundman, Y. Han, S. Thevananther, C. S. Kim, S. S. Chua, P. Wei, R. A. Heyman, M. Karin, et al. 2002. Redundant pathways for negative feedback regulation of bile acid production. Dev. Cell. 2 721–731. [DOI] [PubMed] [Google Scholar]
- 26.Horton J. D., J. A. Cuthbert, and D. K. Spady. 1995. Regulation of hepatic 7 alpha-hydroxylase expression and response to dietary cholesterol in the rat and hamster. J. Biol. Chem. 270 5381–5387. [DOI] [PubMed] [Google Scholar]
- 27.Spady D. K., J. A. Cuthbert, M. N. Willard, and R. S. Meidell. 1995. Adenovirus-mediated transfer of a gene encoding cholesterol 7 alpha-hydroxylase into hamsters increases hepatic enzyme activity and reduces plasma total and low density lipoprotein cholesterol. J. Clin. Invest. 96 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyake J. H., X. T. Duong-Polk, J. M. Taylor, E. Z. Du, L. W. Castellani, A. J. Lusis, and R. A. Davis. 2002. Transgenic expression of cholesterol-7-alpha-hydroxylase prevents atherosclerosis in C57BL/6J mice. Arterioscler. Thromb. Vasc. Biol. 22 121–126. [DOI] [PubMed] [Google Scholar]
- 29.Ratliff E. P., A. Gutierrez, and R. A. Davis. 2006. Transgenic expression of CYP7A1 in LDL receptor-deficient mice blocks diet-induced hypercholesterolemia. J. Lipid Res. 47 1513–1520. [DOI] [PubMed] [Google Scholar]
- 30.Erickson S. K., S. R. Lear, S. Deane, S. Dubrac, S. L. Huling, L. Nguyen, J. S. Bollineni, S. Shefer, H. Hyogo, D. E. Cohen, et al. 2003. Hypercholesterolemia and changes in lipid and bile acid metabolism in male and female cyp7A1-deficient mice. J. Lipid Res. 44 1001–1009. [DOI] [PubMed] [Google Scholar]
- 31.Pullinger C. R., C. Eng, G. Salen, S. Shefer, A. K. Batta, S. K. Erickson, A. Verhagen, C. R. Rivera, S. J. Mulvihill, M. J. Malloy, et al. 2002. Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J. Clin. Invest. 110 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li-Hawkins J., M. Gafvels, M. Olin, E. G. Lund, U. Andersson, G. Schuster, I. Bjorkhem, D. W. Russell, and G. Eggertsen. 2002. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J. Clin. Invest. 110 1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson F. A., and J. M. Dietschy. 1971. Differential diagnostic approach to clinical problems of malabsorption. Gastroenterology. 61 911–931. [PubMed] [Google Scholar]
- 34.Wilson F. A., and J. M. Dietschy. 1972. Approach to the malabsorption syndromes associated with disordered bile acid metabolism. Arch. Intern. Med. 130 584–594. [PubMed] [Google Scholar]
- 35.Lehmann J. M., S. A. Kliewer, L. B. Moore, T. A. Smith-Oliver, B. B. Oliver, J. L. Su, S. S. Sundseth, D. A. Winegar, D. E. Blanchard, T. A. Spencer, et al. 1997. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 272 3137–3140. [DOI] [PubMed] [Google Scholar]
- 36.Chiang J. Y., R. Kimmel, C. Weinberger, and D. Stroup. 2000. Farnesoid X receptor responds to bile acids and represses cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J. Biol. Chem. 275 10918–10924. [DOI] [PubMed] [Google Scholar]
- 37.Lambert G., M. J. Amar, G. Guo, H. B. Brewer, Jr., F. J. Gonzalez, and C. J. Sinal. 2003. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J. Biol. Chem. 278 2563–2570. [DOI] [PubMed] [Google Scholar]
- 38.Maloney P. R., D. J. Parks, C. D. Haffner, A. M. Fivush, G. Chandra, K. D. Plunket, K. L. Creech, L. B. Moore, J. G. Wilson, M. C. Lewis, et al. 2000. Identification of a chemical tool for the orphan nuclear receptor FXR. J. Med. Chem. 43 2971–2974. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y., F. Y. Lee, G. Barrera, H. Lee, C. Vales, F. J. Gonzalez, T. M. Willson, and P. A. Edwards. 2006. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl. Acad. Sci. USA. 103 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiang J. Y., W. F. Miller, and G. M. Lin. 1990. Regulation of cholesterol 7 alpha-hydroxylase in the liver. Purification of cholesterol 7 alpha-hydroxylase and the immunochemical evidence for the induction of cholesterol 7 alpha-hydroxylase by cholestyramine and circadian rhythm. J. Biol. Chem. 265 3889–3897. [PubMed] [Google Scholar]
- 41.Jung D., D. J. Mangelsdorf, and U. A. Meyer. 2006. Pregnane X receptor is a target of farnesoid X receptor. J. Biol. Chem. 281 19081–19091. [DOI] [PubMed] [Google Scholar]
- 42.Ourlin J. C., F. Lasserre, T. Pineau, J. M. Fabre, A. Sa-Cunha, P. Maurel, M. J. Vilarem, and J. M. Pascussi. 2003. The small heterodimer partner interacts with the pregnane X receptor and represses its transcriptional activity. Mol. Endocrinol. 17 1693–1703. [DOI] [PubMed] [Google Scholar]
- 43.Bansal S., J. E. Buring, N. Rifai, S. Mora, F. M. Sacks, and P. M. Ridker. 2007. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 298 309–316. [DOI] [PubMed] [Google Scholar]
- 44.Miller M., C. P. Cannon, S. A. Murphy, J. Qin, K. K. Ray, and E. Braunwald. 2008. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J. Am. Coll. Cardiol. 51 724–730. [DOI] [PubMed] [Google Scholar]
- 45.Nordestgaard B. G., M. Benn, P. Schnohr, and A. Tybjaerg-Hansen. 2007. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 298 299–308. [DOI] [PubMed] [Google Scholar]
- 46.Zadelaar S., R. Kleemann, L. Verschuren, J. de Vries-Van der Weij, J. van der Hoorn, H. M. Princen, and T. Kooistra. 2007. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler. Thromb. Vasc. Biol. 27 1706–1721. [DOI] [PubMed] [Google Scholar]
- 47.Joseph S. B., E. McKilligin, L. Pei, M. A. Watson, A. R. Collins, B. A. Laffitte, M. Chen, G. Noh, J. Goodman, G. N. Hagger, et al. 2002. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. USA. 99 7604–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levin N., E. D. Bischoff, C. L. Daige, D. Thomas, C. T. Vu, R. A. Heyman, R. K. Tangirala, and I. G. Schulman. 2005. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler. Thromb. Vasc. Biol. 25 135–142. [DOI] [PubMed] [Google Scholar]
- 49.Tangirala R. K., E. D. Bischoff, S. B. Joseph, B. L. Wagner, R. Walczak, B. A. Laffitte, C. L. Daige, D. Thomas, R. A. Heyman, D. J. Mangelsdorf, et al. 2002. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc. Natl. Acad. Sci. USA. 99 11896–11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanyal S., A. Bavner, A. Haroniti, L. M. Nilsson, T. Lundasen, S. Rehnmark, M. R. Witt, C. Einarsson, I. Talianidis, J. A. Gustafsson, et al. 2007. Involvement of corepressor complex subunit GPS2 in transcriptional pathways governing human bile acid biosynthesis. Proc. Natl. Acad. Sci. USA. 104 15665–15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.