Abstract
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator (CFTR) that affect protein structure and channel function. CFTR, localized in the apical membrane within cholesterol and sphingomyelin rich regions, is an ABC transporter that functions as a chloride channel. Here, we report that expression of defective CFTR (ΔF508CFTR or decreased CFTR) in human lung epithelial cell lines increases sphingolipid synthesis and mass of sphinganine, sphingosine, four long-chain saturated ceramide species, C16 dihydroceramide, C22, C24, C26-ceramide, and sphingomyelin, and decreases mass of C18 and unsaturated C18:1 ceramide species. Decreased expression of CFTR is associated with increased expression of long-chain base subunit 1 of serine-palmitoyl CoA, the rate-limiting enzyme of de novo sphingolipid synthesis and increased sphingolipid synthesis. Overexpression of ΔF508CFTR in bronchoalveolar cells that do not express CFTR increases sphingolipid synthesis and mass, whereas overexpression of wild-type CFTR, but not of an unrelated ABC transporter, ABCA7, decreases sphingolipid synthesis and mass. The data are consistent with a model in which CFTR functions within a feedback system that affects sphingolipid synthesis and in which increased sphingolipid synthesis could reflect a physiological response to sequestration of sphingolipids or altered membrane structure.
Keywords: cystic fibrosis transmembrane conductance regulator, sphingomyelin, sphingosine, sphinganine, serine-palmitoyl transferase, long-chain base subunit 1
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator (CFTR), an ABC protein that functions as a chloride channel (1). CFTR is expressed in the apical membrane of epithelial cells and is connected through filamin with the actin cytoskeleton (2). Several studies suggested that CFTR localizes within cholesterol- and sphingomyelin-rich membrane regions (3, 4). It is well accepted that reduced airway surface liquid volume is the initiating event in CF airways disease pathogenesis; however, how defective CFTR causes the complex phenotype associated with CF that includes an increased inflammatory state, dyslipidemia and osteoporosis, has not been fully understood (5). Global gene expression and proteomic analyses have not identified a distinct pathway but revealed that defective CFTR results in upregulation of genes and proteins involved in protein ubiquitination, mitochondrial oxidoreductase activity, and lipid metabolism and implicated defects in the NFκB, lipid, and HSP70 systems (6–8). More recently, defective CFTR has been associated with altered sphingolipid patterns and metabolism (9–12).
Sphingolipids are a ubiquitous and highly diverse class of cellular lipids that are defined by the presence of a sphingosine backbone. Sphingolipids are key structural components of the cell membrane; they define, together with cholesterol, lipid rafts and constitute, partially independent of this structural function, a specific group of lipid signaling compounds (13). Elaborate feedback mechanisms regulate synthesis and catabolism to ensure homeostasis of pro-apoptotic ceramide, proliferative sphingosine, and synthesis of sphingomyelin, a major membrane lipid. The major pathway of sphingolipid synthesis occurs through recycling of long-chain sphingoid bases that are derived from the catabolism of complex sphingolipids. In contrast, de novo synthesis is not as ubiquitous and is often found to be increased in cells with a high proliferation rate (14, 15). Serine-palmitoyl transferase (SPT), found in the endoplasmic reticulum, is the rate-limiting enzyme of de novo synthesis. SPT is a multimeric enzyme that is composed of a long-chain base subunit 1 (LCB1) that heterodimerizes with subunits II and III (16–18).
Two different groups recently investigated ceramide mass in different CF mouse models, yet came to opposite conclusions whether absence of wild-type CFTR increases or decreases ceramide mass (11, 12). The group that found significantly increased plasma and tissue ceramide levels in mice older than 16 weeks had investigated several different CFTR−/− mouse models (12). One mouse model, Cftrtm1Unc-Tg(FABPCFTR), is deficient for the mouse equivalent of human CFTR but expresses human CFTR in the gut under control of a fatty acid binding protein promoter to prevent acute intestinal obstruction. Other mouse models produced low levels of CFTR or expressed the S489× mutation. Increased ceramide mass was shown to result from an imbalance of acid sphingomyelinase and ceramidase activity. Intraperitoneal treatment with amitryptiline, a cationic amphiphile that disrupts lysosomal function resulting in proteolytic degradation of acid ceramidase as well as sphingomyelinase, decreased pulmonary ceramide and prevented all pathological findings, including increased susceptibility to infection (12). The other group found decreased ceramide in plasma, lung, ileum, and pancreas in a complete CFTR knockout model and decreased levels in heterozygote animals (11). In contrast to the studies that found increased ceramide mass, mice found to have decreased ceramide were complete CFTR knockouts that necessitate a special diet, such as Peptamen, to avoid intestinal obstruction. It was observed that this diet can reduce ceramide levels and inhibit acid sphingomyelinase by inducing a 3-fold increase of cholesterol in the lungs of CFTR-deficient mice (12). Specific mechanisms for decreased ceramide mass were not evaluated in the study that found decreased ceramide mass, but analysis of plasma from eight CF patients that express the ΔF508CFTR mutation and two patients with a non-ΔF508CFTR genotype also showed a decrease in the average mass of total ceramide plasma and noted a decrease of select ceramide species (11). Administration of fenretinide increased ceramide levels and was associated with a significantly increased ability to combat infection and, in a consecutive study, to prevent the development of osteoporosis that characterizes CF (11, 19). Aside from the potential effect of the diet, other possible causes that could lead to the discrepant results in ceramide levels in CFTR knockout mice could be the different mouse strains, evaluation at different ages, and different methods used to determine ceramide mass.
In this study, we demonstrate that expression of defective CFTR in cultured cells increases sphingolipid synthesis through de novo and recycling pathways, resulting in increased mass of sphinganine, sphingosine, sphingomyelin, four long-chain saturated ceramides, and decreased mass of C18 and C18:1 ceramide species.
MATERIALS AND METHODS
Materials
3H-serine (0.1 mCi/mmol), 3H-choline (86 Ci/mmol), and choline 32P-ATP (6,000 μCi/mmol) were purchased from Perkin-Elmer (Boston, MA). 3H-sphinganine (60 Ci/mmol) and 14C-sphingomyelin (55 mCi/mmol) were purchased from American Radiolabeled Chemicals (St. Louis, MO). Fenretinide (N-4-hydroxyphenyl-retinamide), sphingomyelinase (staphylococcus aureus), 4-aminoantipyrine, fumonisin B1, and desipramine were obtained from Sigma-Aldrich (St. Louis, MO). C6-ceramide (d-erythro-hexanoylsphingosine), sphingosine, sphinganine, phosphatidylcholine, and phosphatidylserine standards were obtained from Avanti Polar Lipids (Alabaster, AL). LH-8 medium was obtained from Invitrogen (Carlsbad, CA). Neomycin (G418) and all other cell culture reagents were obtained from Life Technologies (Grand Island, NY). All organic solvents were purchased from Fisher Scientific (Springfield, NJ).
Cells
IB3, C38, and A549 cells were obtained from American Type Culture Collection (Rockville, MD). The IB3 cells express the ΔF508 mutation ((DF508/W1282×). The control C38 cells are IB3 cells that were stably transduced with AAV episomal copies of normal CFTR (20). A549 are human alveolar epithelial cells that do not express CFTR (21). 16HBE14o(−) are human bronchial epithelial cells that stably express episomal CFTR in the sense or antisense orientation. These cells were obtained from Dr. Alice Prince at Columbia University (22). C38 and IB3 cells were grown in LH-8 medium (Invitrogen), 16HBE14o(−) cells were grown in MEM/F12 medium (Gibco), and A549 cells were grown in DMEM (Gibco). All media were supplemented with 1% glutamine (v/v), 1% penicillin/streptomycin (v/v), and 10% FBS (v/v). All cells were grown at 37°C in humidified CO2 (5%).
Adenovirus vectors
All adenoviral vectors used in this study were purchased from the Penn Vector Core (www.uphs.upenn.edu/penngen/gtp). The AdNull vector contains no transgene, AdCFTR contains human wild-type CFTR, and AdΔF508CFTR contains the ΔF508 mutant. All adenovirus vectors are serotype 5 vectors.
Adenovirus-mediated transduction
Cells were plated in 24- or 96-well plates at 80% confluency and incubated with 1012 pfu/ml Ad-vector in serum-free medium. After 2 h, growth medium containing 10% FBS was added. Experiments were carried out 48 h after infection.
Protein determination
Total cellular protein concentration was determined by the Bio-Rad (Hercules, CA) method, and BSA was used as a standard.
Sphingolipid synthesis
Sphingolipid synthesis was assessed by radiotracer incorporation. Cells were plated at 80% confluence in 12-well plates and incubated for 2 h in 1% fatty acid-free BSA in serum-free medium containing either 3H-serine (1 μl/ml) to assess sphingolipid de novo synthesis or 3H-sphinganine (1 μl/ml) to assess sphingolipid synthesis through recycling pathways. To assess sphingomyelin synthesis, cells were incubated for 4 h in 1% fatty acid-free BSA in serum-free medium containing 3H-choline (1 μl/ml). At the end of the incubation period, medium was discarded and cells were washed twice, lysed with 300 μl of lysis buffer (250 mM Tris-HCl), and harvested by scraping. A fraction was used for protein quantification. Lipids were extracted using chloroform/methanol/water (1:1:0.9) (23, 24). The organic phase was evaporated, dissolved in 50 μl chloroform/methanol (1:1) spotted on thin-layer chromatography plates (Merck Silica gel 60; Darmstadt, Germany), and chromatographed for 70 min with chloroform-methanol-water (64:35:4, v/v) to separate all ceramides from sphingomyelin or chloroform-ammonium hydroxide–water (65:25:4) to separate sphingosine, sphinganine, and ceramide. Ceramides, sphingomyelin, phosphatidylserine, sphinganine, and sphingosine, all dissolved at 1 μg/μl, were run as standards. The lipids were identified according to their Rf values after visualization in an iodine vapor tank. The thin-layer chromatography plate was cut at the corresponding lipid spot, mixed with scintillation fluid (Ultima Gold; Packard Instrument, Meriden, CT), and analyzed in a scintillation counter (Perkin-Elmer Wallac, Gaithersburg, MD). Results are calculated as dpm/μg protein and, for comparison between different experiments, expressed as a percentage of control.
Sphingomyelin mass determination
Sphingomyelin mass was assessed with an enzymatic method (25). Cell extracts (50 μg) were incubated, in triplicate, in 100 μl of a reaction buffer containing alkaline phosphatase (10 U/ml), choline oxidase (0.3 U/ml), peroxidase (20 U/ml), alkaline phosphatase (15 U/ml), 4-aminoantipyrine (730 μM), DAOS (730 μM), Tris-HCl (0.05 M), and CaCl2 (0.66 mM) in the absence (negative control) or presence of sphingomyelinase (0.05 U/ml) for 30 min. The colorimetric assay was read at 595 nm in a spectrophotometer. Absorbance of the negative control samples lacking sphingomyelinase were subtracted from samples incubated in the presence of sphingomyelinase. Results are obtained after comparison to a standard curve generated from incubating sphingomyelin (1 to 4 μg, dissolved in methanol).
Sphingosine, sphinganine, and sphingosine-1-phosphate mass determination
Sphingolipid base mass was determined by electrospray ionization tandem mass spectrometry analysis on a Thermo Finnigan TSQ 7000 triple quadrupole mass spectrometer operating in a multiple reaction monitoring positive ionization mode. This analysis was carried out by the Lipidomics analytical core at University of South Carolina, Charleston, SC (26). Sphingoid base mass was normalized to cellular inorganic phosphate (27).
Western analysis
Cells were scraped, pelleted at 1,000 g, and lyzed in 10 mM Tris-HCl, 100 mM NaCl, and 1% SDS, pH 7.6. Samples were boiled for 10 min before electrophoresis, except samples intended for detection of CFTR that were kept for 30 min at room temperature in SDS-containing Laemmli loading buffer. An aliquot of each sample (30–50 μg of protein) was separated by electrophoresis on a 7.5% Tris-glycine gel. The monoclonal antibody against CFTR (monoclonal anti-human CFTR R domain, clone 13-1) was obtained from R and D Systems (Minneapolis, MN); the polyclonal antibody against CFTR was obtained from Cell Signaling Technologies (www.cellsignal.com; # 2269), the LCB1 antibody was obtained from BD Biosciences (San Jose, CA). The ABCA7 expression plasmid (in a pCMV-sport6 vector) and the polyclonal ABCA7 antibody, raised against the last 15 amino acids of mouse ABCA7, were obtained from Dr. Alan Tall (28). Actin antibody was obtained from Sigma-Aldrich. The peroxidase-labeled anti-mouse IgG (NIF 824) was obtained from Amersham (Piscataway, NJ). Detection was performed using Super Signal West Femto maximum sensitivity substrate (Pierce, Rockford, IL). Protein mobilities were compared with prestained broad-range molecular weight standards (Bio-Rad).
Data analysis
Statistical significance was calculated by paired t-tests. Unless otherwise indicated, results are given as mean ± SD. All experiments were repeated on different days at least three times and each time in triplicates.
RESULTS
Decreased CFTR expression or expression of ΔF508CFTR increases sphingolipid synthesis
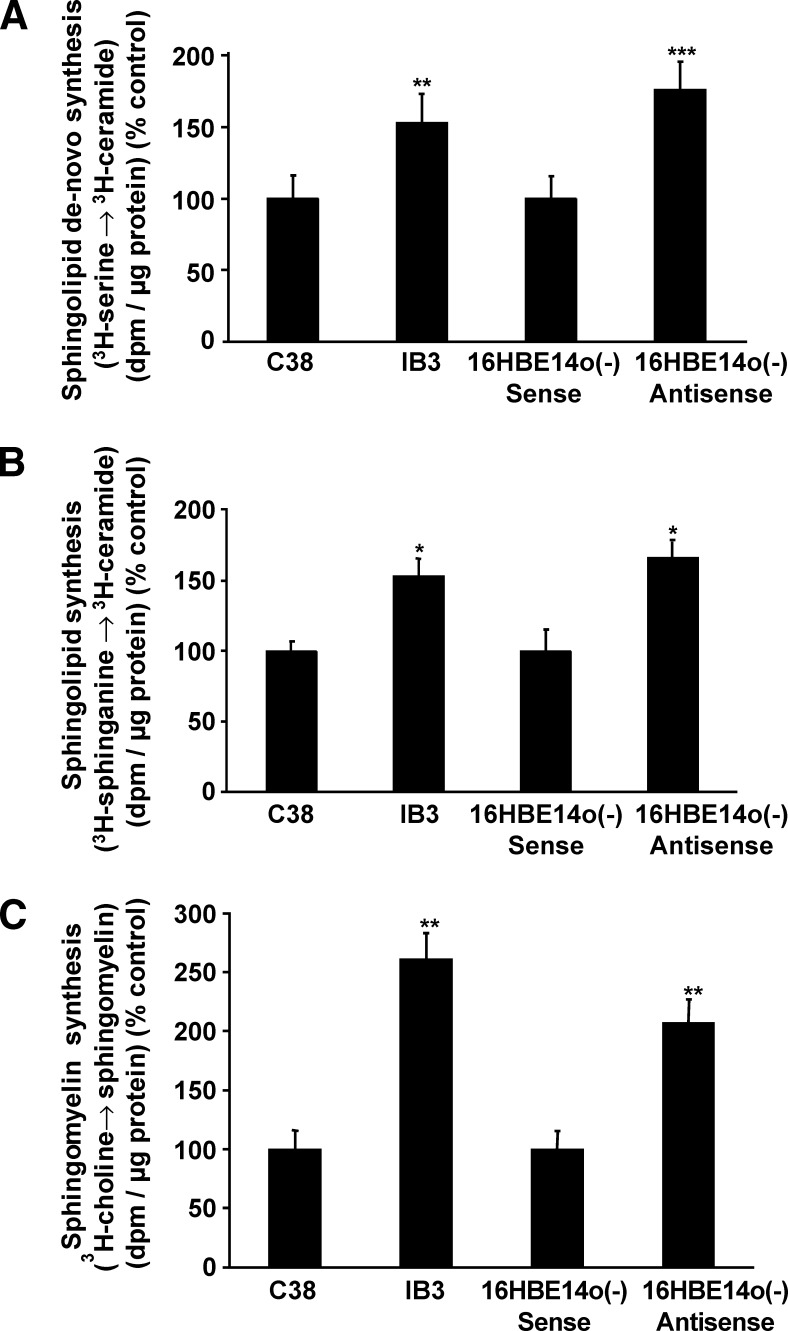
Sphingolipid synthesis was evaluated in two established CF model lines and their respective controls. IB3 cells are bronchoepithelial cells that express ΔF508CFTR, the mutation that is present in ∼80% of patients with CF. The C38 control cell line was generated to express wild-type CFTR by stable infection of IB3 cells with an adeno-associated virus vector (20). In the other bronchoepithelial model cell line, 16HBE14o(−) antisense cells, stable expression of a CFTR antisense construct results in almost complete absence of CFTR expression. The control cell line, 16HBE14o(−) sense, expresses a scrambled control construct. Sphingolipid synthesis was assessed using standard methods that evaluate incorporation of radiolabeled precursors into ceramide. To assess sphingolipid synthesis through recycling pathways, cells were incubated with 3H-sphinganine. To assess SPT-dependent de novo synthesis, cells were incubated with 3H-serine. Sphingolipid synthesis through the de novo pathway was higher (P < 0.02) in IB3 cells compared with C38 cells and higher (P < 0.01) in 16HBE14o(−) antisense cells compared with 16HBE14o(−) sense cells (Fig. 1A). Sphingolipid synthesis through the recycling pathway was higher (P < 0.05) in IB3 compared with C38 cells and higher (P < 0.05) in 16HBE14o(−) antisense cells compared with 16HBE14o(−) sense cells (Fig. 1B). To assess if increased sphingolipid synthesis affects sphingomyelin synthesis, we evaluated the incorporation of 3H-choline into sphingomyelin. Sphingomyelin synthesis was equally increased (P < 0.01) in IB3 and 16HBE14o(−) antisense cells compared with their controls (Fig. 1C). Expression of LCB1, the major subunit of SPT, measured by densitometry and normalized to expression of actin, was increased 1.7-fold (±0.2) in 16HBE14o(−) antisense cells compared with 16HBE14o(−) sense cells (Fig. 2A) and 1.3-fold (±0.1) in IB3 cells compared with C38 cells (Fig. 2B). Expression of CFTR in 16HBE14o(−) antisense is decreased to 10% compared with expression of CFTR in 16HBE14o(−) sense cells. Expression of LCB1/CFTR, both normalized to actin and set to 1 in 16HBE14o(−) sense cells, is increased 16-fold in 16HBE14o(−) antisense cells. In contrast to the large difference in CFTR expression between 16HBE140(−) sense and antisense cells, the difference in expression in IB3 cells compared with C38 control cells is lower. Expression of CFTR in IB3 cells is 90% of CFTR expression in C38 control cells. Expression of LCB1/CFTR, both normalized to actin and set to 1 in C38 cells, is increased to 1.5 in IB3 cells.
Fig. 1.
Expression of ΔF508CFTR and decreased CFTR expression are associated with increased sphingolipid synthesis through recycling and de novo pathways. C38, IB3, 16HBE14o(−) sense, and 16HBE14o(−) antisense cells were incubated for 2 h with 3H-serine to assess sphingolipid synthesis through de novo synthesis or with 3H-sphinganine to assess sphingolipid synthesis through recycling pathways. To measure sphingomyelin synthesis, cells were incubated for 4 h in the presence of 3H-choline. Following lipid extraction and separation by thin-layer chromatography, incorporation of the radioactive tracer into ceramide or sphingomyelin was evaluated by scintillation counting. Sphingolipid synthesis through the de novo pathway is more significantly increased in 16HBE14o(−) antisense cells (P < 0.01) compared with 16HBEo(−) sense control cells than in IB3 cells (P < 0.02) compared with C38 control cells (A). Sphingolipid synthesis though the recycling pathway is equally increased (P < 0.05) in IB3 and 16HBE14o(−) antisense cells compared with their respective controls (B). Sphingomyelin synthesis is equally increased (P < 0.01) in IB3 and 16HBE14o(−) antisense cells compared with their respective controls (C). Asterisks indicate significant difference compared with the respective controls (mean ± SD; * P < 0.05, **P < 0.02, and ***P < 0.01). Data represent the average of at least three experiments carried out in triplicate.
Fig. 2.
Expression of LCB1, the major subunit of the rate-limiting enzyme of de novo sphingolipid synthesis, correlates inversely with expression of CFTR. Expression of CFTR is decreased to ∼10% in 16HBE14o(−) antisense cells compared with expression of CFTR in 16HBE14o(−) sense control cells. LCB1, the major subunit of SPT, the rate-limiting enzyme of sphingolipid de novo synthesis is 1.7-fold (±0.2) higher expressed in 16HBE14o(−) antisense cells than in 16HBE14o(−) sense control cells. Relative expression of LCB1 to CFTR/actin is 0.9 in 16HBE14o(−) sense cells and 16 in 16HBE14o(−) antisense cells (A). IB3 cells express 90% of CFTR expressed by C38 control cells. Expression of LCB1 is 1.3-fold (±0.1) higher in IB3 cells than in C38 controls. Relative expression of LCB1 to CFTR/actin is 0.45 in C38 cells and 0.69 in IB3 cells (B). CFTR was detected with a polyclonal antibody from Cell Signaling.
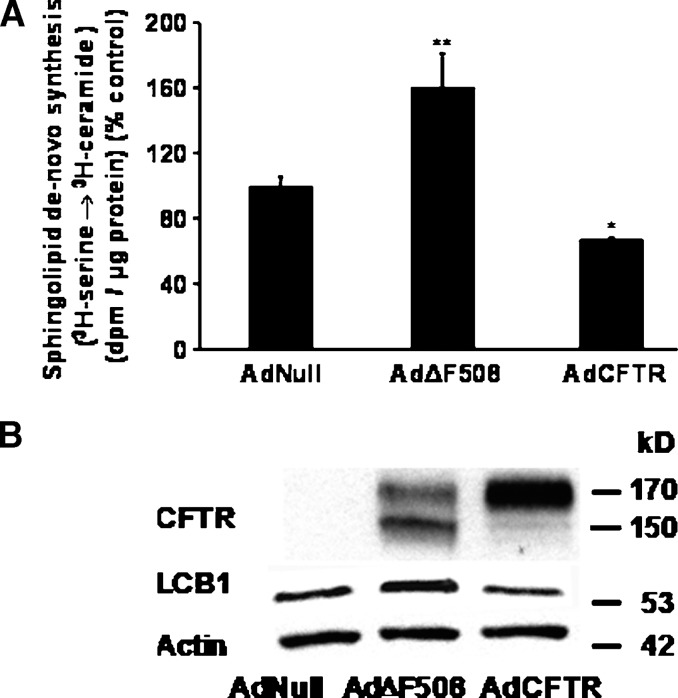
To evaluate if the effects of CFTR on sphingolipid synthesis are cell type dependent, we expressed CFTR and ΔF508CFTR in A549 cells, bronchoalveolar cells that do not express CFTR (21). Compared with controls that were infected with an AdNull control vector, expression of AdΔF508CFTR increased (P < 0.01) sphingolipid de novo synthesis (Fig. 3A) and LCB1 protein mass 1.25-fold (±0.2) (Fig. 3B, lane 2). Expression of AdCFTR decreased sphingolipid de novo synthesis (P < 0.05) (Fig. 3A) and decreased LCB1 protein mass to 0.87 (±0.2) compared with controls (Fig. 3B, lane 3). To evaluate if these effects are specific for CFTR, we overexpressed ABCA7 in A549 cells. A549 cells do not express ABCA7 (Fig. 4, lanes 2, 3). In contrast to cells transduced with AdΔF508CFTR (Fig. 4, lane 3), overexpression of pABCA7 did not increase LCB1 expression (Fig. 4, lane 1) or sphingolipid synthesis (data not shown). Together, the data suggest a specific effect of CFTR on LCB1 expression.
Fig. 3.
Overexpression of ΔF508 CFTR increases sphingolipid synthesis and LCB1 expression. Overexpression of CFTR decreases sphingolipid synthesis. A: A549 cells, a human bronchioalveolar cell line that does not express CFTR, was transduced with 1012 pfu/ml AdNull, AdΔF508, or AdCFTR. Forty-eight hours after infection, sphingolipid de novo was assessed by evaluating incorporation of 3H-serine into ceramide. Compared with controls that were transduced with the AdNull control vector, sphingolipid de novo synthesis is increased (P < 0.01) in A549 cells that express ΔF508CFTR and decreased (P < 0.05) in A549 cells that express CFTR. The data represent the average of three different experiments, carried out in triplicate on different days. B: Expression of CFTR and LCB1 was assessed 48 h after infection. A549 cells do not express CFTR (lane 1). Expression of ΔF508CFTR results in expression of a predominant “B band” pattern of CFTR (∼150 kDa) characteristic for ΔF508CFTR and reflecting the trafficking defect. Expression of ΔF508CFTR increases LCB1/actin expression 1.25-fold (±0.2) (lane 2) compared with control (lane 1). Expression of CFTR results in expression of a predominant “C band” pattern of CFTR (∼170 kDa). Expression of CFTR decreases LCB1/actin expression to 0.87 (±0.2) (lane 3) compared with control (lane 1). CFTR was detected with a monoclonal antibody from R and D systems.
Fig. 4.
Overexpression of ABCA7 does not affect LCB1 expression. A549 cells were transfected with pABCA7 or transduced with AdNull or AdΔF508. Protein expression was measured 48 h later. A549 cells do not express ABCA7 (lanes 2, 3). Overexpression of ABCA7 does not increase LCB1 expression [LCB1/actin 0.82 (±0.2), lane 1] compared with controls transduced with AdNull (LCB1/actin expression set to 1, lane 2). Overexpression of AdΔF508CFTR increases LCB1 expression [LCB1/actin expression 1.6 (±0.3) lane 3] compared with controls. Data are representative of more than three experiments that evaluate the effect of pABCA7 on LCB1 expression.
Decreased CFTR expression or expression of ΔF508CFTR increases sphinganine, sphingosine, and sphingomyelin mass
We determined sphingolipid mass to evaluate the net effect of increased sphingolipid synthesis. Sphingosine, sphinganine, and sphingomyelin mass were higher (P < 0.05) in IB3 compared with C38 cells and higher (P < 0.05) in 16HBE14o(−) antisense cells compared with 16HBE14o(−) sense control cells. By the same token, overexpression of ΔF508CFTR in A549 cells increased (P < 0.05) sphingosine, sphinganine, and sphingomyelin mass, while overexpression of AdCFTR decreased (P < 0.05) sphingosine, sphinganine, and sphingomyelin mass in A549 cells. Although sphingosine-1-phosphate mass was increased in IB3 and 16HBE14o(−) antisense compared with C38 and 16HBE14o(−) sense cells, the differences did not reach significance (Table 1). The data demonstrate increased mass of sphingomyelin and sphingolipids that can function as precursors of lipid signaling molecules in cells that express defective CFTR.
TABLE 1.
Expression of CFTR correlates inversely with sphinganine, sphingosine, and sphingomyelin mass
| SA | SO | S-1-P | SM | |
|---|---|---|---|---|
| C38 | 2.68 (±0.3) | 28.76 (±3.0) | 6.08 (±2.4) | 49 (±7) |
| IB3 | 4.8 (±0.8)* | 38.11 (±6.2)* | 9.37 (±3.8) | 68 (±2)* |
| 16HBE14o sense | 17.49 (±1.6) | 163.49 (±2.8) | 20.63 (±7.9) | 53 (±7) |
| 16HBE14o antisense | 26.28 (±3.4)* | 226.26 (±19.4)* | 25.16 (±6.2) | 87 (±9)* |
| A549 AdCFTR | 1.45 (±0.3)* | 17.50 (±4.3)* | 1.74 (±0.5) | 26 (±9)* |
| A549 AdNull | 3.21 (±0.4) | 49.65 (±6.0) | 3.11 (±0.8) | 32 (±7) |
Expression of defective CFTR (ΔF508CFTR or decreased CFTR) increases (P < 0.05) sphinganine (SA), sphingosine (SO), and sphingomyelin (SM) mass. Overexpression of AdCFTR decreases (P < 0.05) sphinganine, sphingosine, and sphingomyelin mass. Sphingosine-1-phosphate (S-1-P) mass is increased and decreased accordingly, but the differences did not reach significance. Mass of sphinganine, sphingosine, and sphingosine-1-phosphate is expressed in ftmol/nmol inorganic phosphate. Mass of sphingomyelin is expressed in ng/μg protein. Data represent the average of more than three experiments carried out in triplicate (* P < 0.05).
Decreased CFTR expression affects ceramide composition
Compared with controls, mass of C16-dihydroceramide, C22-, and C24-ceramide species was increased in 16HBE14o(−) antisense cells (P < 0.05), and mass of C18-ceramide and C18:1-ceramides was decreased (P < 0.05) (Fig. 5).
Fig. 5.
Decreased CFTR expression affects composition of ceramide mass. Mass of major cellular saturated ceramide species was determined in 16HBE14o(−) cells that express the sense CFTR construct and 16HBE14o(−) cells transfected with the antisense CFTR construct resulting in decreased expression of CFTR. Compared with controls, mass of C16-dihydroceramide, C22-, C24-, and C26-ceramides is higher (P < 0.05) and mass of C18- and C18:1 ceramide species is lower (P < 0.05) in 16HBE14o(−) cells that express the CFTR antisense construct. Data are expressed as a percentage of change compared with 16HBE14o(−) sense controls.
Fenretinide decreases sphingolipid synthesis and LCB1 expression
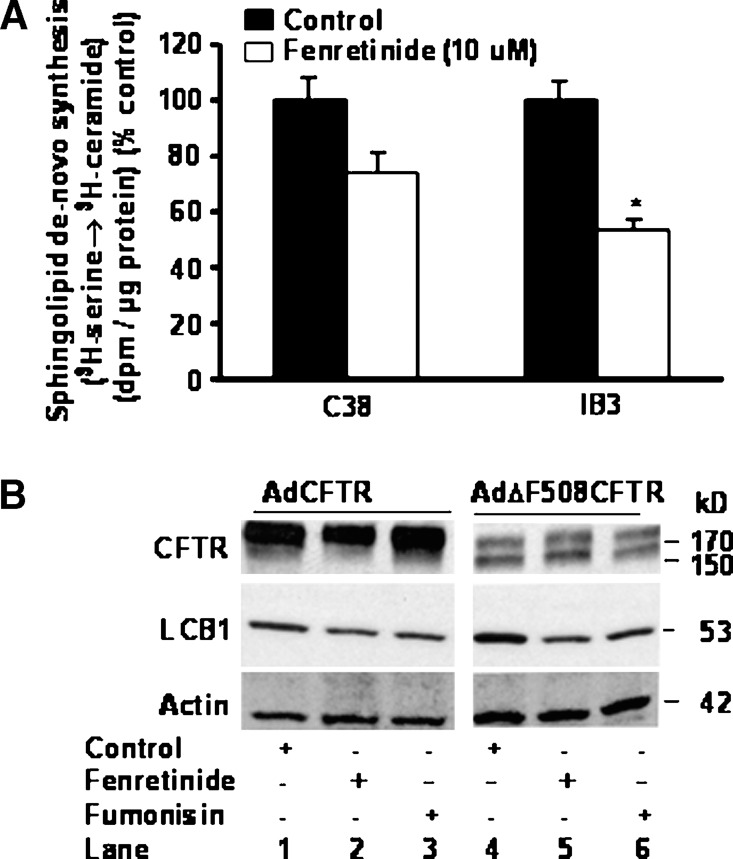
Fenretinide, a synthetic retinoid with multiple actions, including the stimulation of a redox-sensitive ceramide production and an increase of dihydroceramide mass, was shown to inhibit interleukin-8 release in immortalized wild-type and mutant ΔF508CFTR tracheal epithelial cells, decrease mRNA of interleukin-β and S100 calcium binding protein A8 in lung tissue, previously shown to be overexpressed in CFTR KO mice, and prevent development of osteoporosis in a CF mouse model (10, 19, 29, 30). We evaluated if fenretinide affects sphingolipid synthesis and LCB1 expression. Fenretinide decreased (P < 0.05) sphingolipid synthesis in 16HBE14o(−) cells and IB3 cells [data not shown for 16HBE14o(−) cells] (Fig. 6A). To evaluate if the effects of fenretinide are mediated by increasing expression of CFTR, Western analysis was carried out in A549 cells that were transduced with AdNull control vector or AdΔF508CFTR. Because sphingolipids can affect protein trafficking, we used fumonisin, an inhibitor of ceramide synthase that decreases ceramide mass, for comparison. Fenretinide and fumonisin decreased LCB1 expression but did not increase CFTR expression (Fig. 6B). The data suggest that sphingolipid synthesis is amenable to suppression and that the decrease of sphingolipid synthesis and LCB1 occurs through a mechanism that does not affect expression of CFTR.
Fig. 6.
Fenretinide inhibits sphingolipid synthesis and LCB1 expression. A: C38 and IB3 cells were incubated for 6 h in the presence of fenretinide (10 μM), followed by assessment of sphingolipid de novo synthesis, measured by incorporation of 3H-serine into ceramide. After lipid extraction, ceramide was separated by thin-liquid chromatography and counts were measured by scintillation counting. Fenretinide decreases sphingolipid synthesis in IB3 cells. Incubation for 6 h in the presence of fenretinide (10 μM) did not cause apoptosis or cytotoxicity. Asterisks indicate significant difference compared with the respective controls (mean ± SD; * P < 0.05). The data represent average results of three different experiments carried out on different days in triplicate. B: A549 cells were transduced with 1012 pfu/ml AdCFTR or AdΔF508CFTR. Forty-eight hours later, cells were incubated for 6 h with fenretinide (10 μM) or fumonisin B1 (10 μM). Fifty microliters of total protein was separated on a 7.5% Tris-glycine gel, transferred, and probed for LCB1, CFTR, and actin expression. LCB1 expression is higher in cells that express ΔF508CFTR. Fenretinide and fumonisin decrease LCB1 expression.
DISCUSSION
This study identifies a previously unknown regulatory relationship between expression of CFTR and sphingolipid synthesis. The central finding is that expression of defective CFTR, i.e., mutated ΔF508CFTR or decreased CFTR expression, increases sphingolipid synthesis. Increased sphingolipid synthesis results in significantly increased mass of sphingomyelin, sphingosine, and sphinganine, sphingolipids that affect membrane properties and function as parent compounds of signaling lipids (3, 4, 13). The data suggest that the effects of CFTR on sphingolipid metabolism are specific and independent of expression in human lung epithelial model cell lines. Sphingolipid synthesis and mass are increased by overexpression of ΔF508CFTR in cells that do not express CFTR and decreased by overexpression of CFTR, but not of another ABC transporter, ABCA7.
Increased and decreased mass of the sphingolipid ceramide was recently causally implicated in the complex pathophysiology that characterizes the CF phenotype. The effect of CFTR knockout on sphingolipid mass is currently not well defined, as ceramide mass was shown to be decreased in a complete CFTR knockout mouse model and shown to be increased in other CFTR mouse models that differed with regard to background strain, dietary requirements, and residual CFTR expression in the gut (11, 12). Our data show that decreased expression of CFTR in 16HBE14o(−) antisense cells, in contrast to expression of ΔF508CFTR, affected ceramide composition. The mass of four saturated long-chain ceramide species was proportionally increased, and mass of C18:0 and C18:1 saturated fatty acids was decreased, suggesting that failure to express CFTR has distinct effects on ceramide species composition, although exact mechanisms are currently not known. Increased long-chain saturated ceramides could also reflect altered membrane lipid requirements during rapid cell growth (9, 14, 31, 32). Of note is that similar observations with regard to ceramide composition were made in human plasma from CF patients. The observation that six of the seventeen determined ceramide species were significantly lower in human CF plasma that was obtained from a broad range of genotypes therefore support the relevance of the finding made in 16HBE14o(−) antisense cells (11).
Ceramide species are synthesized by ceramide synthases that result in attachment of the fatty acid to the sphingoid long-chain base (33). The six currently known ceramide synthases are specific for groups of different fatty acids; however, the pattern found in the 16HBE14o(−) cells does not match the characteristics of either one, although it cannot be ruled out that ceramide synthase specificity in human bronchoepithelial cells differs from other cell types. A more likely mechanism that affects composition of ceramides implicates sphingolipid synthesis through the de novo pathway, previously shown to affect ceramide species composition (34, 35). In agreement with this literature is that expression of LCB1 and sphingolipid synthesis through the de novo pathway was more significantly increased in 16HBE14o(−) antisense cells, while the increase in LCB1 expression in IB3 cells is minor compared with C38 controls, and de novo sphingolipid synthesis is less significantly increased in IB3 compared with C38 cells. The significance of altered ceramide composition, specifically of increased saturated long-chain ceramides could be their effect on biophysical membrane properties, as the length and saturation of acyl chains determine membrane fluidity and the interaction with other lipids and proteins. Possibly, expression of defective CFTR, previously reported to coincide with characteristics of a destabilized membrane, triggers the synthesis of a class of lipids that promote membrane stabilization (2). If membrane stability were indeed affected by expression of defective CFTR, then an increase in sphingolipid and sphingomyelin synthesis and mass would be a compensatory mechanism directed to increase membrane stability or embedment of CFTR into the sphingolipid and cholesterol-rich membrane regions it has been associated with (3, 4). According to this model, increased sphingolipid synthesis would reflect a state in which significantly decreased CFTR expression, as in 16HBE14o(−) antisense cells, would reflect a state that makes it necessary to maximally increase membrane stability.
In this study, sphinganine, sphingomyelin, and specifically sphingosine mass were significantly higher in the 16HBE14o(−) cells than in the C38 and IB3 cell model. This could be due to the difference in cell models. Mechanisms for this observation are not known, as little is known about the sphingolipid mass composition of human bronchoepithelial cells in general or the combined effect of increased de novo synthesis that characterizes 16HBE14o(−) antisense cells, together with the absence of a trafficking defect mediated by ΔF508CFTR on sphingosine mass. Consistent for each cell type, however, is that the presence of CFTR in control cells, as well as mediated by overexpression of AdCFTR in A549 cells, decreases sphingosine mass almost 3-fold.
Together, our data suggest that CFTR functions within a feedback system that affects the regulation of sphingolipid synthesis. Failure to express wild-type CFTR or overexpression of ΔF508CFTR in cells that do not express CFTR, perhaps by displacement of a functional protein, increases sphingolipid synthesis. Recognition of increased synthesis of sphingolipids that affect membrane lipid composition and can function as signaling molecules is expected to further the understanding of the multiple consequences caused by mutated CFTR.
Acknowledgments
The authors gratefully acknowledge support by the Cystic Fibrosis Foundation (Worgal07I0) and National Institutes of Health 5K08AG025833 (T.S.W.).
Abbreviations
CF, cystic fibrosis
CFTR, cystic fibrosis transmembrane conductance regulator
LCB1, long-chain base subunit 1
SPT, serine-palmitoyl transferase
Published, JLR Papers in Press, January 14, 2009.
References
- 1.Welsh, M., L. Tsui, T. Boat, and A. Beaudet. 1995. The Metabolic Bases of Inherited Disease: Cystic Fibrosis. McGraw-Hill, New York.
- 2.Thelin W. R., Y. Chen, M. Gentzsch, S. M. Kreda, J. L. Sallee, C. O. Scarlett, C. H. Borchers, K. Jacobson, M. J. Stutts, and S. L. Milgram. 2007. Direct interaction with filamins modulates the stability and plasma membrane expression of CFTR. J. Clin. Invest. 117 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kowalski M. P., and G. B. Pier. 2004. Localization of cystic fibrosis transmembrane conductance regulator to lipid rafts of epithelial cells is required for Pseudomonas aeruginosa-induced cellular activation. J. Immunol. 172 418–425. [DOI] [PubMed] [Google Scholar]
- 4.Bates I. R., B. Hebert, Y. Luo, J. Liao, A. I. Bachir, D. L. Kolin, P. W. Wiseman, and J. W. Hanrahan. 2006. Membrane lateral diffusion and capture of CFTR within transient confinement zones. Biophys. J. 91 1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher R. C. 2007. Evidence for airway surface dehydration as the initiating event in CF airway disease. J. Intern. Med. 261 5–16. [DOI] [PubMed] [Google Scholar]
- 6.Zabner J., T. E. Scheetz, H. G. Almabrazi, T. L. Casavant, J. Huang, S. Keshavjee, and P. B. McCray, Jr. 2005. CFTR DeltaF508 mutation has minimal effect on the gene expression profile of differentiated human airway epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 289 L545–L553. [DOI] [PubMed] [Google Scholar]
- 7.Wright J. M., C. A. Merlo, J. B. Reynolds, P. L. Zeitlin, J. G. Garcia, W. B. Guggino, and M. P. Boyle. 2006. Respiratory epithelial gene expression in patients with mild and severe cystic fibrosis lung disease. Am. J. Respir. Cell Mol. Biol. 35 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollard H. B., O. Eidelman, C. Jozwik, W. Huang, M. Srivastava, X. D. Ji, B. McGowan, C. F. Norris, T. Todo, T. Darling, et al. 2006. De novo biosynthetic profiling of high abundance proteins in cystic fibrosis lung epithelial cells. Mol. Cell. Proteomics. 5 1628–1637. [DOI] [PubMed] [Google Scholar]
- 9.Boujaoude L. C., C. Bradshaw-Wilder, C. Mao, J. Cohn, B. Ogretmen, Y. A. Hannun, and L. M. Obeid. 2001. Cystic fibrosis transmembrane regulator regulates uptake of sphingoid base phosphates and lysophosphatidic acid: modulation of cellular activity of sphingosine 1-phosphate. J. Biol. Chem. 276 35258–35264. [DOI] [PubMed] [Google Scholar]
- 10.Vilela R. M., L. C. Lands, B. Meehan, and S. Kubow. 2006. Inhibition of IL-8 release from CFTR-deficient lung epithelial cells following pre-treatment with fenretinide. Int. Immunopharmacol. 6 1651–1664. [DOI] [PubMed] [Google Scholar]
- 11.Guilbault C., J. B. De Sanctis, G. Wojewodka, Z. Saeed, C. Lachance, T. A. Skinner, R. M. Vilela, S. Kubow, L. C. Lands, M. Hajduch, et al. 2008. Fenretinide corrects newly found ceramide deficiency in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 38 47–56. [DOI] [PubMed] [Google Scholar]
- 12.Teichgraber V., M. Ulrich, N. Endlich, J. Riethmuller, B. Wilker, C. C. De Oliveira-Munding, A. M. van Heeckeren, M. L. Barr, G. von Kurthy, K. W. Schmid, et al. 2008. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat. Med. 14 382–391. [DOI] [PubMed] [Google Scholar]
- 13.Hannun Y. A., and L. M. Obeid. 2008. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9 139–150. [DOI] [PubMed] [Google Scholar]
- 14.Carton J. M., D. J. Uhlinger, A. D. Batheja, C. Derian, G. Ho, D. Argenteri, and M. R. D'Andrea. 2003. Enhanced serine palmitoyltransferase expression in proliferating fibroblasts, transformed cell lines, and human tumors. J. Histochem. Cytochem. 51 715–726. [DOI] [PubMed] [Google Scholar]
- 15.Batheja A. D., D. J. Uhlinger, J. M. Carton, G. Ho, and M. R. D'Andrea. 2003. Characterization of serine palmitoyltransferase in normal human tissues. J. Histochem. Cytochem. 51 687–696. [DOI] [PubMed] [Google Scholar]
- 16.Merrill A. H., Jr., E. M. Schmelz, D. L. Dillehay, S. Spiegel, J. A. Shayman, J. J. Schroeder, R. T. Riley, K. A. Voss, and E. Wang. 1997. Sphingolipids–the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol. Appl. Pharmacol. 142 208–225. [DOI] [PubMed] [Google Scholar]
- 17.Hornemann T., S. Richard, M. F. Rutti, Y. Wei, and A. von Eckardstein. 2006. Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. J. Biol. Chem. 281 37275–37281. [DOI] [PubMed] [Google Scholar]
- 18.Hornemann T., Y. Wei, and A. von Eckardstein. 2007. Is the mammalian serine palmitoyltransferase a high-molecular-mass complex? Biochem. J. 405 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saeed Z., C. Guilbault, J. B. De Sanctis, J. Henri, D. Marion, R. St-Arnaud, and D. Radzioch. 2008. Fenretinide prevents the development of osteoporosis in Cftr-KO mice. J. Cyst. Fibros. 7 222–230. [DOI] [PubMed] [Google Scholar]
- 20.Flotte T. R., S. A. Afione, R. Solow, M. L. Drumm, D. Markakis, W. B. Guggino, P. L. Zeitlin, and B. J. Carter. 1993. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J. Biol. Chem. 268 3781–3790. [PubMed] [Google Scholar]
- 21.Renier M., A. Tamanini, E. Nicolis, R. Rolfini, J. L. Imler, A. Pavirani, and G. Cabrini. 1995. Use of a membrane potential-sensitive probe to assess biological expression of the cystic fibrosis transmembrane conductance regulator. Hum. Gene Ther. 6 1275–1283. [DOI] [PubMed] [Google Scholar]
- 22.Gruenert D. C., M. Willems, J. J. Cassiman, and R. A. Frizzell. 2004. Established cell lines used in cystic fibrosis research. J. Cyst. Fibros. 3 (Suppl 2): 191–196. [DOI] [PubMed] [Google Scholar]
- 23.Bligh E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37 911–917. [DOI] [PubMed] [Google Scholar]
- 24.Perry D. K., A. Bielawska, and Y. A. Hannun. 2000. Quantitative determination of ceramide using diglyceride kinase. Methods Enzymol. 312 22–31. [DOI] [PubMed] [Google Scholar]
- 25.Hojjati M. R., and X. C. Jiang. 2006. Rapid, specific, and sensitive measurements of plasma sphingomyelin and phosphatidylcholine. J. Lipid Res. 47 673–676. [DOI] [PubMed] [Google Scholar]
- 26.Bielawski J., Z. M. Szulc, Y. A. Hannun, and A. Bielawska. 2006. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 39 82–91. [DOI] [PubMed] [Google Scholar]
- 27.Van Veldhoven P. P., and R. M. Bell. 1988. Effect of harvesting methods, growth conditions and growth phase on diacylglycerol levels in cultured human adherent cells. Biochim. Biophys. Acta. 959 185–196. [DOI] [PubMed] [Google Scholar]
- 28.Wang N., D. Lan, M. Gerbod-Giannone, P. Linsel-Nitschke, A. W. Jehle, W. Chen, L. O. Martinez, and A. R. Tall. 2003. ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J. Biol. Chem. 278 42906–42912. [DOI] [PubMed] [Google Scholar]
- 29.Zheng W., J. Kollmeyer, H. Symolon, A. Momin, E. Munter, E. Wang, S. Kelly, J. C. Allegood, Y. Liu, Q. Peng, et al. 2006. Ceramides and other bioactive sphingolipid backbones in health and disease: Lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim. Biophys. Acta. 1758 1864–1884. [DOI] [PubMed] [Google Scholar]
- 30.Rehman F., P. Shanmugasundaram, and M. P. Schrey. 2004. Fenretinide stimulates redox-sensitive ceramide production in breast cancer cells: potential role in drug-induced cytotoxicity. Br. J. Cancer. 91 1821–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stutts M. J., S. E. Gabriel, J. C. Olsen, J. T. Gatzy, T. L. O'Connell, E. M. Price, and R. C. Boucher. 1993. Functional consequences of heterologous expression of the cystic fibrosis transmembrane conductance regulator in fibroblasts. J. Biol. Chem. 268 20653–20658. [PubMed] [Google Scholar]
- 32.Schiavi S. C., N. Abdelkader, S. Reber, S. Pennington, R. Narayana, J. M. McPherson, A. E. Smith, H. Hoppe, and S. H. Cheng. 1996. Biosynthetic and growth abnormalities are associated with high-level expression of CFTR in heterologous cells. Am. J. Physiol. 270 C341–C351. [DOI] [PubMed] [Google Scholar]
- 33.Pewzner-Jung Y., S. Ben-Dor, and A. H. Futerman. 2006. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)? Insights into the regulation of ceramide synthesis. J. Biol. Chem. 281 25001–25005. [DOI] [PubMed] [Google Scholar]
- 34.Le Stunff H., P. Giussani, M. Maceyka, S. Lepine, S. Milstien, and S. Spiegel. 2007. Recycling of sphingosine is regulated by the concerted actions of sphingosine-1-phosphate phosphohydrolase 1 and sphingosine kinase 2. J. Biol. Chem. 282 34372–34380. [DOI] [PubMed] [Google Scholar]
- 35.Cowart L. A., Y. Okamoto, X. Lu, and Y. A. Hannun. 2006. Distinct roles for de novo versus hydrolytic pathways of sphingolipid biosynthesis in Saccharomyces cerevisiae. Biochem. J. 393 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]