Abstract
ABCA1 plays a major role in HDL metabolism. Cholesterol secretion by ABCA1 is dependent on the presence of extracellular acceptors, such as lipid-free apolipoprotein A-I (apoA-I). However, the importance of the direct interaction between apoA-I and ABCA1 in HDL formation remains unclear. In contrast, ABCB4 mediates the secretion of phospholipids and cholesterol in the presence of sodium taurocholate (NaTC) but not in the presence of apoA-I. In this study, we analyzed apoA-I binding and NaTC-dependent lipid efflux by ABCA1. ABCA1 mediated the efflux of cholesterol and phospholipids in the presence of NaTC as well as in the presence of apoA-I in an ATP-dependent manner. The Tangier disease mutation W590S, which resides in the extracellular domain and impairs apoA-I-dependent lipid efflux, greatly decreased NaTC-dependent cholesterol and phospholipid efflux. However, the W590S mutation did not impair apoA-I binding and, conversely, retarded the dissociation of apoA-I from ABCA1. These results suggest that the W590S mutation impairs ATP-dependent lipid translocation and that lipid translocation or possibly lipid loading, facilitates apoA-I dissociation from ABCA1. NaTC is a good tool for analyzing ABCA1-mediated lipid efflux and allows dissection of the steps of HDL formation by ABCA1.
Keywords: cholesterol, HDL, Tangier disease
Maintenance of cellular cholesterol homeostasis is important for normal human physiology; its disruption can lead to a variety of pathological conditions, including cardiovascular disease (1). ABCA1, a key protein in cholesterol homeostasis, mediates the secretion of cellular-free cholesterol and phospholipids to an extracellular acceptor, apolipoprotein A-I (apoA-I), to form HDL (2, 3). HDL formation is the only known pathway for the elimination of excess cholesterol from peripheral cells. Defects in ABCA1 cause Tangier disease (4–6), in which patients have a near-absence of circulating HDL, prominent cholesterol-ester accumulation in tissue macrophages, and premature atherosclerotic vascular disease (1, 7).
Cholesterol secretion by ABCA1 is strongly dependent on the presence of extracellular acceptors, such as lipid-free apoA-I. Although a direct interaction between ABCA1 and apoA-I has been shown by several groups via cross-linking experiments (8–12), the importance of this interaction in HDL formation remains unclear. It was reported that only a portion of the apoA-I associated with the cell surface binds directly to ABCA1 (12, 13). It was also proposed that ABCA1 generates a high curvature phospholipid-rich apoA-I binding site on the plasma membrane and that apoA-I binds this site and solubilizes membrane phospholipid and cholesterol (14). Indeed, ABCA1 makes cholesterol available to exogenous cholesterol oxidase and methyl-β-cyclodexitrin (MβCD), even in the absence of apoA-I (15–17). However, it remains unclear whether ABCA1 translocates phosphatidylcholine (PC), together with cholesterol, in the membrane in the absence of apoA-I, although purified ABCA1 reconstituted into PC liposomes shows robust ATPase activity (18). In fact, it has been reported that ABCA1 expression increased the Triton X-100 solubility of cholesterol and sphingomyelin but not that of PC (15).
Human ABCB4 (multidrug resistance 3 P-glycoprotein) is expressed in the canalicular membrane of hepatocytes. ABCB4 is required for PC secretion into bile and for PC translocation across the plasma membrane. We previously showed that ABCB4 mediates the secretion of phospholipids (preferentially PC) and cholesterol in the presence of sodium taurocholate (NaTC) in the medium (19). However, ABCB4 cannot mediate the efflux of phospholipids and cholesterol in the presence of apoA-I (see supplementary Fig. I). The related ABCB1 (multidrug resistance 1) cannot mediate the efflux of phospholipids or cholesterol in the presence of NaTC (19), although it is 75.8% identical and 86.6% similar to ABCB4 in amino acid sequence. Thus, ABCA1 and ABCB4 mediate cholesterol and PC efflux to different extracellular acceptors, apoA-I and NaTC, respectively. However, it has not been determined whether ABCA1 mediates the efflux of cholesterol and PC in the presence of NaTC.
In this study, we demonstrate that ABCA1 mediates the efflux of cholesterol and phospholipids in the presence of NaTC as well as apoA-I, a physiological acceptor. This allowed us to analyze the role of apoA-I in HDL formation in detail.
MATERIALS AND METHODS
Materials
Mouse anti-ABCA1 monoclonal antibody KM3110 was generated against the C-terminal 20 amino acids of ABCA1 (20). Anti-green fluorescent protein (GFP) antibody was purchased from Santa Cruz Biotechnology. NaTC was obtained from Wako Pure Chemicals. TO901317 was purchased from Cayman Chemical. Recombinant apoA-I and Alexa546-conjugated apoA-I were prepared as reported previously (21). Other chemicals were purchased from Sigma-Aldrich, Amersham Biosciences, Wako Pure Chemical Industries, and Nacalai Tesque.
Cell culture
Human embryonic kidney (HEK293) cells and WI-38 fibroblasts were grown in a humidified incubator (5% CO2) at 37°C in DMEM supplemented with 10% heat-inactivated FBS.
Plasmids
The expression vectors for wild-type ABCA1, ABCA1-W590S, and ABCA1-K939M,K1952M (MM), fused to GFP at their C termini, were generated as described previously (3, 18).
Establishment of a stable transformant of ABCA1
HEK293 cells were transfected with each expression vector using LipofectAMINE (Invitrogen) according to the manufacturer's instructions. Cells were selected with G418, and single colonies were isolated.
Western blotting
Cells were washed with PBS and lysed in lysis buffer A (20 mM Tris-Cl, pH 7.5, 1 mM EDTA, 10% glycerol, and 1% Triton X-100) containing protease inhibitors, 100 μg/ml (p-amidinophenyl)methanesulfonyl fluoride, 10 μg/ml leupeptin, and 2 μg/ml aprotinin. Samples were electrophoresed on SDS-polyacrylamide gels, blotted, and probed with the indicated antibodies.
Cellular lipid release assay
Cells were subcultured in poly-l-lysine-coated six-well plates at a density of 8 × 105 cells in DMEM containing 10% FBS. After 24 h incubation, cells were washed twice with DMEM, and the medium was changed to DMEM containing 0.02% BSA and indicated concentrations of NaTC or recombinant apoA-I. The cholesterol and choline phospholipid content in the medium was determined after 24 h incubation using colorimetric enzyme assays as described previously (22).
Biotinylation of cell surface proteins
Cell monolayers were kept on ice for 10 min. Cells were washed with ice-cold PBS+ (PBS containing 0.1 mg/ml CaCl2 and MgCl26H2O) and incubated with 0.5 mg/ml sulfo-N-hydroxysuccinimidobiotin (sulfo-NHS-biotin) solubilized in PBS+ for 30 min on ice in the dark. Cells were washed with PBS+ to remove unbound sulfo-NHS-biotin and lysed in lysis buffer B (20 mM Tris-Cl, pH 7.5, 1% Triton X-100, 0.1% SDS, and 1% sodium deoxycholate) containing protease inhibitors. Immobilized monomeric avidin gel (Pierce) was added to the cell lysate to precipitate biotinylated proteins, which were electrophoresed on a 7% SDS-polyacrylamide gel and immunodetected. Western blots were analyzed using a Fujifilm LAS-3000 imaging system.
Cell viability assay
Cell viability was estimated by measuring the lactate dehydrogenase activity in the media and total cells using a Cyto Tox 96 NonRadioactive Cytotoxicity Assay Kit purchased from Promega.
ApoA-I binding assay
Cells grown on collagen-coated coverslips were incubated with DMEM containing 0.02% BSA and Alexa546-conjugated apoA-I (5 μg/ml) for indicated periods at 27 or 37°C. Cells were then washed, fixed with 4% paraformaldehyde at room temperature for 30 min, and observed with a confocal microscope (LSM 510; Carl Zeiss). The relative amounts of bound apoA-I and the expression levels of ABCA1-GFP were calculated using ImageJ 1.40 software from images of >200 cells. The relative amounts of bound apoA-I were calculated by subtracting the value of bound-apoA-I to HEK293 cells or HEK/ABCA1-MM cells and normalized to the intensity of GFP fluorescence.
ApoA-I dissociation assay
Cells grown on collagen-coated coverslips were incubated with DMEM containing 0.02% BSA and Alexa546-conjugated apoA-I (5 μg/ml) for 15 min at 37°C. The medium was changed to DMEM containing 0.02% BSA and 25 μg/ml unlabeled apoA-I. After incubation for indicated periods at 27°C, cells were fixed and observed with a confocal microscope. The relative amounts of bound apoA-I were calculated as above.
Statistical analysis
Values are presented as means ± SEM. The statistical significance of differences between mean values was analyzed using the nonpaired t-test. Multiple comparisons were performed using the Dunnet test following ANOVA. A value of P < 0.05 was considered statistically significant.
RESULTS
Cholesterol and phospholipid secretion from HEK/ABCA1 cells in the presence of NaTC
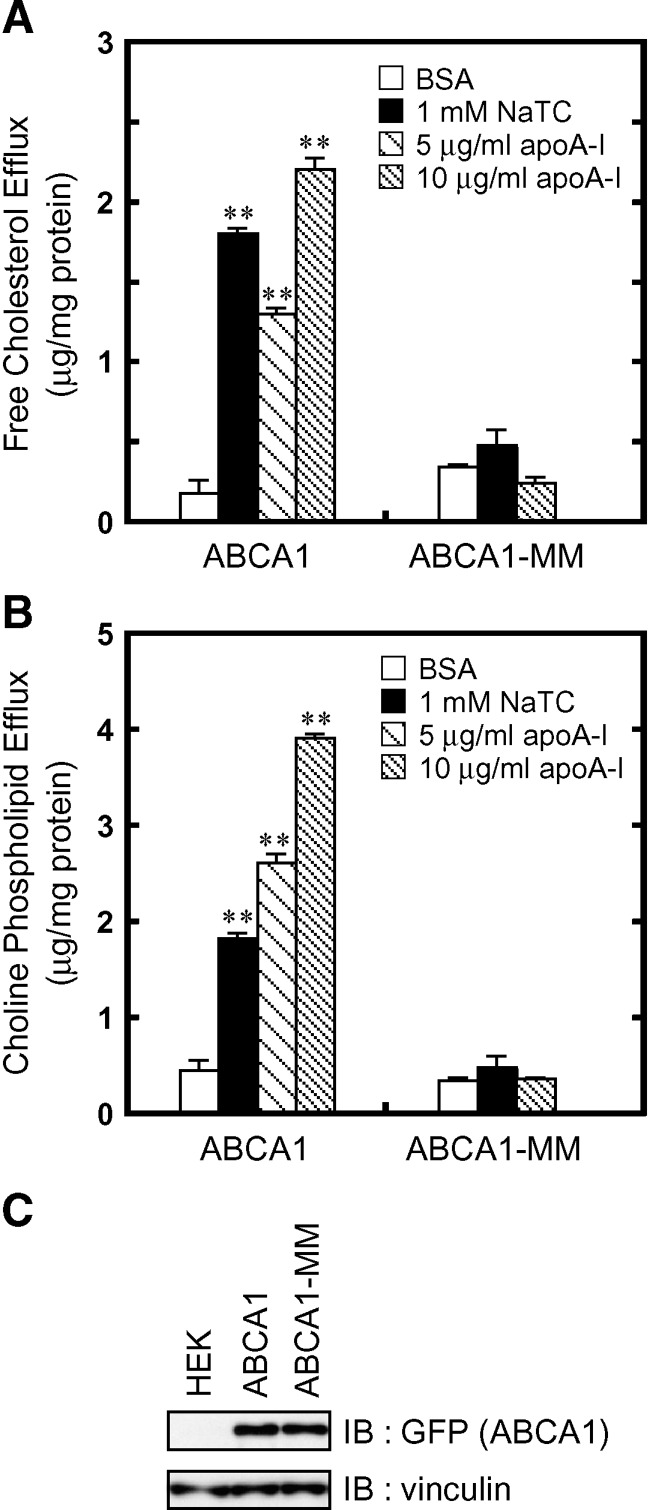
We reported (19) that the efflux of phospholipids from HEK/ABCB4 cells was increased by the addition of NaTC to the medium, and we proposed that NaTC monomers function as acceptors for PC. These results also suggested that NaTC may function as an acceptor for lipids translocated by ABC proteins. Thus, we examined whether NaTC can function as an acceptor for lipids translocated by ABCA1. Indeed, the efflux of cholesterol from HEK/ABCA1-GFP cells was increased by the addition of 1 mM NaTC (Fig. 1A, closed bar), compared with efflux measured in the presence of 0.02% BSA (open bar). In addition, phospholipid efflux from HEK/ABCA1-GFP cells was enhanced in the presence of NaTC (Fig. 1B).
Fig. 1.
NaTC-dependent lipid efflux mediated by ABCA1. The efflux of cellular free cholesterol (A) and choline phospholipids (B) was analyzed. HEK/ABCA1-GFP cells and HEK/ABCA1-MM-GFP cells were incubated for 24 h in DMEM containing 0.02% BSA (open bars), 0.02% BSA and 1 mM NaTC (filled bars), or 0.02% BSA and 5 or 10 μg/ml apoA-I (hatched bars). Experiments were performed in triplicate, and the average values are represented with the SEM. C: Cell lysates (15 μg of protein) were separated by 7% polyacrylamide gel electrophoresis, and ABCA1-GFP was analyzed with anti-GFP antibody. The amount of vinculin was analyzed as a loading control. **P < 0.001, significantly different from values in the presence of BSA.
Next, cholesterol and phospholipid content in the medium of HEK/ABCA1-GFP cells treated with NaTC (1 mM) or apoA-I (5 and 10 μg/ml) were compared. ApoA-I-dependent lipid efflux mediated by ABCA1 increased with increasing concentration of apoA-I from 5 to 10 μg/ml and was nearly saturated at 10 μg/ml (data not shown). In the presence of 1 mM NaTC, cholesterol was secreted from HEK/ABCA1-GFP cells more efficiently than in the presence of 5 μg/ml apoA-I, but less efficiently than in the presence of 10 μg/ml apoA-I. Phospholipid was secreted slightly less efficiently by 1 mM NaTC than by 5 μg/ml apoA-I. These results suggest that 1 mM NaTC is as efficient an acceptor as the physiological lipid acceptor apoA-I and that cholesterol is transferred to NaTC by ABCA1 more efficiently than phospholipids.
NaTC-dependent efflux of phospholipids and cholesterol was not observed in HEK293 cells expressing ABCA1-MM, a mutant in which the Walker A lysines in both nucleotide binding domains are substituted by methionines, although the expression level (Fig. 1C) and surface expression (see supplementary Fig. II) of the mutant were comparable to the wild-type protein. These results suggest that the NaTC-dependent efflux of cholesterol and phospholipids is mediated by ABCA1 in an ATP-dependent manner and that both physiological acceptors, such as apoA-I and apolipoprotein E, and artificial acceptors, such as synthetic amphiphilic helical peptides (23, 24) and NaTC, can serve as receptors for cholesterol and phospholipids translocated by ABCA1.
NaTC concentration dependence of cholesterol and phospholipid secretion by ABCA1
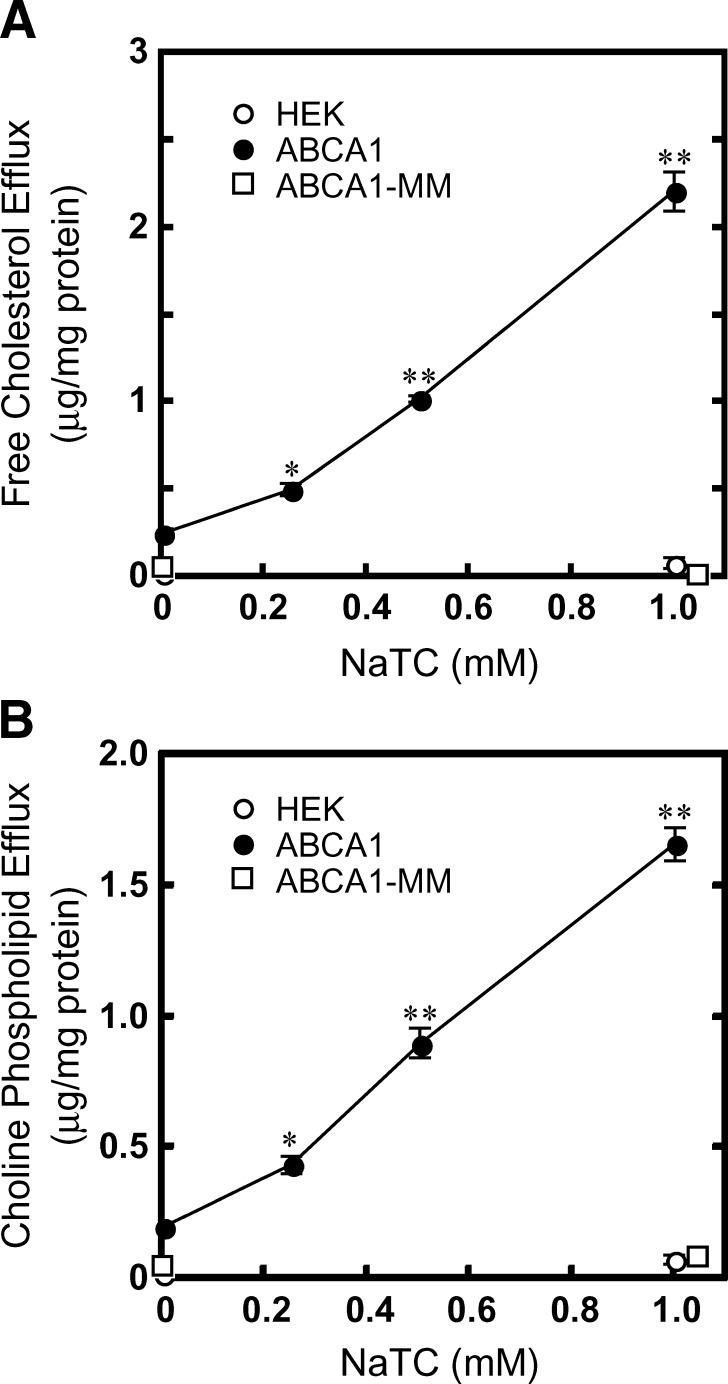
The dependence of lipid secretion from HEK/ABCA1-GFP cells on NaTC concentration was examined. The secretion of cholesterol and phospholipids from HEK/ABCA1-GFP cells increased with increasing concentrations of NaTC and showed concentration dependence from 0.25 to 1 mM NaTC (Fig. 2A, B). The secretion of cholesterol and phospholipid from HEK/ABCA1-GFP cells increased 8.4- and 8.9-fold, respectively, with 1 mM NaTC treatment, while no increase in lipid secretion was observed from HEK293/ABCA1-MM-GFP cells or HEK293 host cells. Time-dependent efflux of cholesterol and phospholipids was observed in the presence of 1 mM NaTC as well as 5 μg/ml apoA-I (see supplementary Fig. III). Incubation with 1 mM NaTC for 24 h did not significantly affect the viability of HEK293 or HEK/ABCA1-GFP cells, as estimated by lactate dehydrogenase release (see supplementary Fig. IV).
Fig. 2.
Concentration dependence of NaTC-mediated lipid efflux. The efflux of cellular-free cholesterol (A) and choline phospholipids (B) was analyzed. HEK cells (open circles), HEK/ABCA1-GFP cells (closed circles), and HEK/ABCA1-MM-GFP cells (open squares) were incubated for 24 h in DMEM containing 0.02% BSA and the indicated concentrations of NaTC. Experiments were performed in triplicate, and the average values are represented with the SEM. *P < 0.05, **P < 0.001, significantly different from the value without NaTC.
We examined whether the cholesterol secretion from HEK/ABCA1-GFP cells was directly mediated by ABCA1. Because the medium of HEK/ABCA1-GFP cells contained about 2 μM PC after 24 h incubation in the presence of 1 mM NaTC, we examined whether cholesterol was extracted from HEK293 host cells in the presence of 1 mM NaTC and 2 μM PC. However, no significant increase in cholesterol secretion was observed compared with the secretion in the absence of NaTC and PC (see supplementary Fig. V).
NaTC-dependent lipid efflux from WI-38 cells mediated by endogenous ABCA1
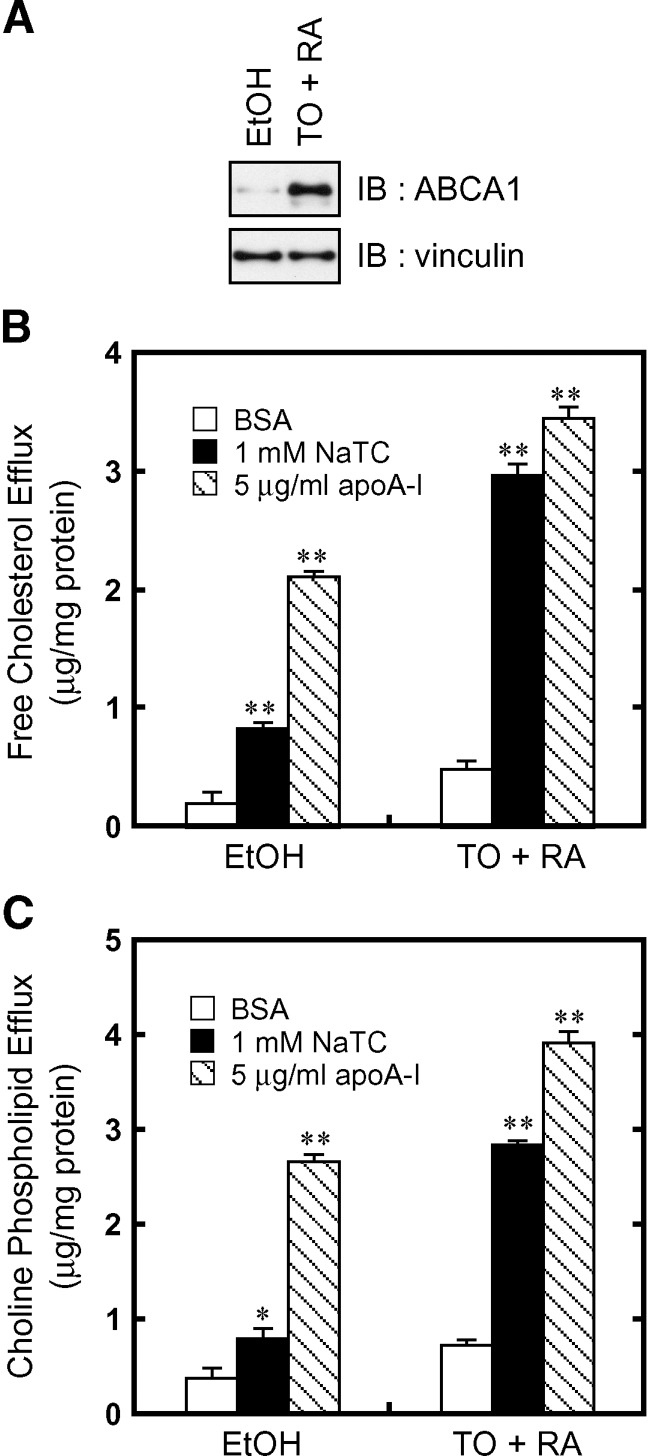
To confirm that NaTC, like apoA-I, can serve as an acceptor for cholesterol and phospholipids translocated by endogenous ABCA1, lipid efflux from WI-38 fibroblast cells was examined. Western blot analysis showed that ABCA1 is weakly expressed in WI-38 cell cultured under normal conditions, and its expression can be greatly induced by the addition of TO901317, a liver X receptor agonist, and 9 cis-retinoic acid (RA), a retinoid X receptor agonist (Fig. 3A).
Fig. 3.
NaTC-dependent lipid efflux by endogenous ABCA1. A: WI-38 cells were treated with TO901317 and RA for 24 h to induce the expression of ABCA1. Cell lysates (2.5 μg of protein) were separated by 7% polyacrylamide gel electrophoresis, and ABCA1 was analyzed with anti-ABCA1 antibody KM3110. The amount of vinculin was analyzed as a loading control. The efflux of cellular free cholesterol (B) and choline phospholipids (C) was analyzed. Cells were incubated for 8 h in DMEM containing 0.02% BSA (open bars), 0.02% BSA and 1 mM NaTC (filled bars), or 0.02% BSA and 5 μg/ml apoA-I (hatched bars). Experiments were performed in triplicate, and the average values are represented with the SEM. *P < 0.05, **P < 0.001, significantly different from the value in the presence of BSA.
Treatment with TO901317 and RA enhanced cholesterol and phospholipid efflux in the presence of NaTC as well as apoA-I (Fig. 3B, C). Cholesterol and phospholipid efflux was much higher in the presence of apoA-I compared with the presence of NaTC without induction of ABCA1 expression. Although it remained unclear why lipid efflux significantly occurred from WI-38 in an apoA-I-dependent manner without induction of ABCA1 expression as reported (25), NaTC-dependent lipid efflux was dependent on the amount of ABCA1. These results suggest that NaTC can serve as an acceptor for cholesterol and phospholipids translocated by endogenous ABCA1.
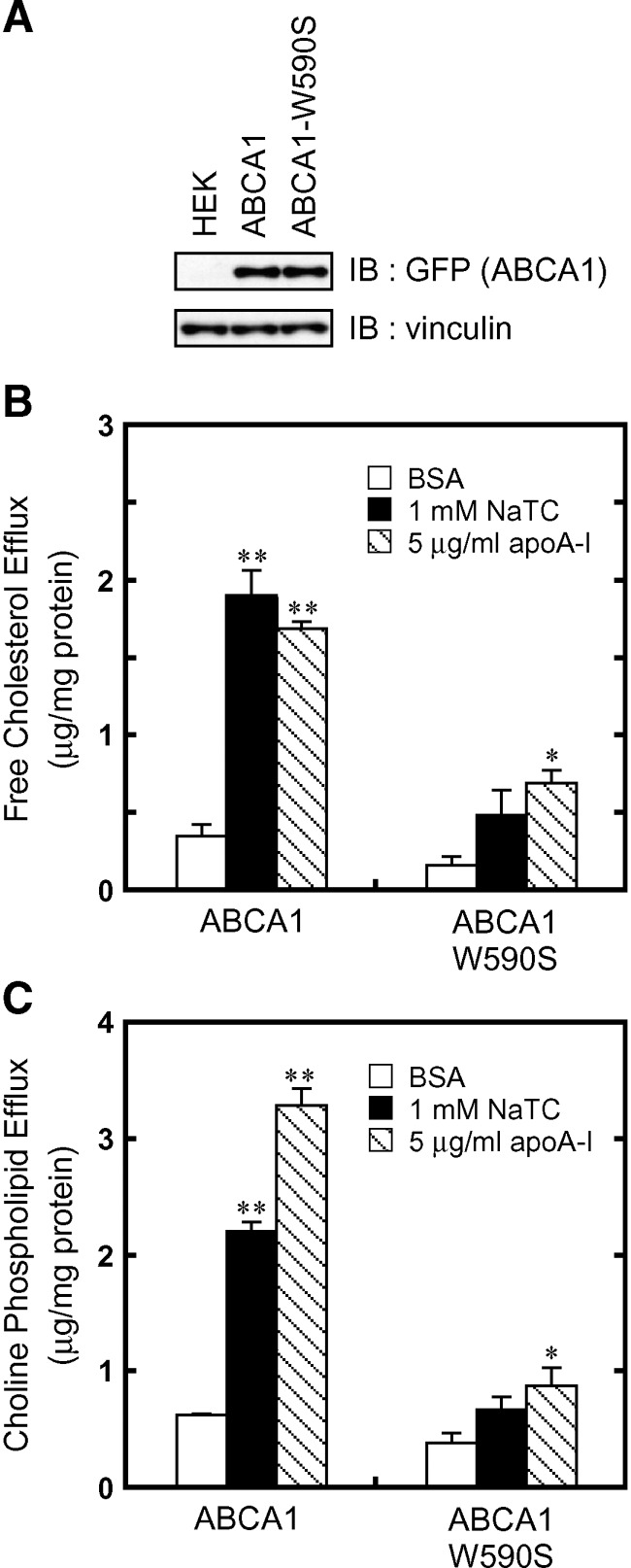
The W590S Tangier mutation impairs NaTC-dependent lipid efflux but not apoA-I binding
It has been reported that ABCA1 carrying the Tangier disease mutation W590S in the first extra cellular domain is correctly targeted to the plasma membrane and interacts with apoA-I but fails to mediate normal apoA-I-dependent lipid efflux (3, 26–28). We compared NaTC-dependent lipid efflux from HEK293 cells stably expressing wild-type or ABCA1-W590S fused to GFP at the C terminus. Western blotting (Fig. 4A) and fluorescence microscopy (see supplementary Fig. II) suggested that the expression and surface localization of the wild type and ABCA1-W590S were similar.
Fig. 4.
The W590S mutation impairs NaTC-dependent lipid efflux by ABCA1. A: Cell lysates (15 μg of protein) were separated by 7% polyacrylamide gel electrophoresis, and ABCA1-GFP was analyzed with anti-GFP antibody. The amount of vinculin was analyzed as a loading control. The efflux of cellular free cholesterol (B) and choline phospholipids (C) was analyzed. HEK/ABCA1-GFP cells and HEK/ABCA1-W590S-GFP cells were incubated for 24 h in DMEM containing 0.02% BSA (open bars), 0.02% BSA and 1 mM NaTC (filled bars), or 0.02% BSA and 5 μg/ml apoA-I (hatched bars). Experiments were performed in triplicate, and the average values are represented with the SEM. *P < 0.05, **P < 0.001, significantly different from the values in the presence of BSA.
Both NaTC-dependent and apoA-I-dependent cholesterol and phospholipid efflux were greatly decreased by the W590S mutation (Fig. 4B, C). As previously reported, apoA-I bound to the cell surface expressing ABCA1-W590S as efficiently as to that expressing wild-type ABCA1 (see supplementary Fig. II). Because apoA-I did not bind to cells expressing ABCA1-MM, apoA-I binding may be an ATP-dependent process. These results suggest that apoA-I binding to the cell surface and lipid translocation, both of which are mediated by ABCA1 in an ATP-dependent manner, are in fact separable and that the W590S mutation impairs only the latter step.
Kinetics of apoA-I binding to ABCA1-expressing cells
To further analyze apoA-I binding mediated by ABCA1, the time-dependence of apoA-I binding and dissociation was analyzed by fluorescence microscopy. Binding of Alexa546-conjugated apoA-I to ABCA1-expressing cells was saturated within 10 min and apoA-I dissociated from cells within 1 min at 37°C (data not shown); these were too fast to analyze. Therefore, we performed apoA-I binding and dissociation experiments at 27°C. ApoA-I-dependent cholesterol secretion from HEK/ABCA1-GFP cells occurred even at 27°C, although the efficiency was ∼80% of that at 37°C (see supplementary Fig. VI). The W590S mutation also impaired cholesterol secretion at 27°C.
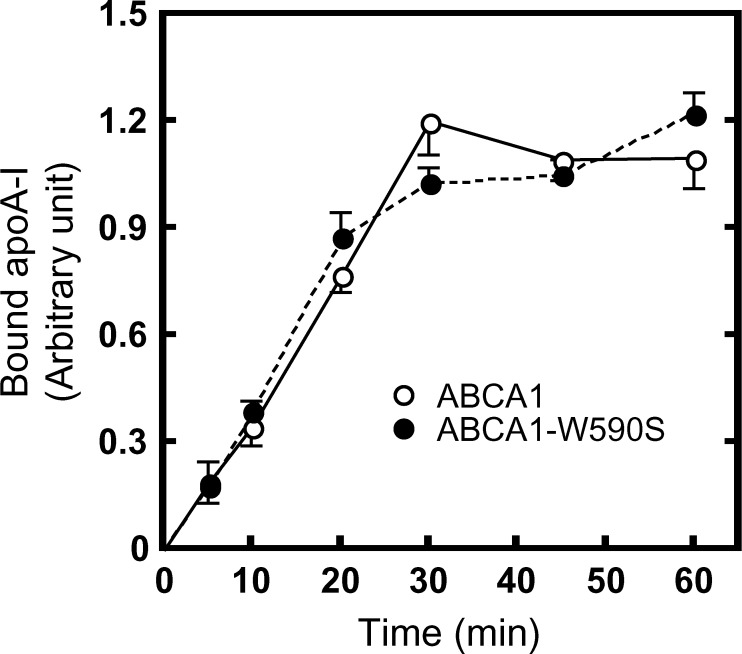
When analyzed at 27°C, the amount of apoA-I bound to cells expressing wild-type ABCA1 increased gradually in a time-dependent manner (Fig. 5; see supplementary Fig. VII). The half-saturation time was estimated to be ∼15 min at 27°C, slower than at 37°C. The kinetics of apoA-I binding to cells expressing ABCA1-W590S was quite similar to that to cells expressing wild-type ABCA1.
Fig. 5.
Time-dependent apoA-I binding to ABCA1-expressing cells. HEK/ABCA1-GFP, HEK/ABCA1-MM-GFP, or HEK/ABCA1-W590S-GFP cells were incubated with 5 μg/ml Alexa546-labeled apoA-I for the indicated times at 27°C. The amount of bound apoA-I was calculated as described in Materials and Methods.
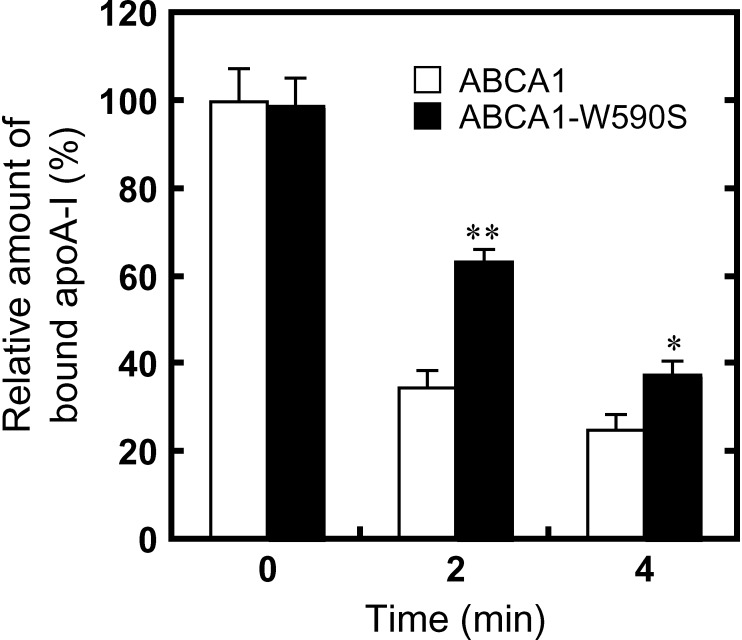
After removing free apoA-I in the medium, >60% of apoA-I dissociated from cells expressing wild-type ABCA1 in 2 min at 27°C (Fig. 6; see supplementary Fig. VIII). In contrast, <40% of apoA-I dissociated from cells expressing ABCA1-W590S in 2 min, and ∼60% of apoA-I dissociated in 4 min. These results suggest that the W590S mutation retards apoA-I dissociation from cells.
Fig. 6.
Time-dependent apoA-I dissociation from cells expressing ABCA1 or ABCA1-W590S. HEK/ABCA1-GFP and HEK/ABCA1-W590S-GFP cells were incubated with 5 μg/ml Alexa546-labeled apoA-I for 15 min at 37°C and further incubated with 25 μg/ml nonlabeled apoA-I for the indicated times at 27°C. The relative amount of bound apoA-I was calculated as described in Materials and Methods with respect to the amount of apoA-I bound to cells expressing wild-type ABCA1 at time 0. *P < 0.05, **P < 0.001, significantly different from the relative amount of apoA-I bound to HEK/ABCA1-GFP cells at each time point.
NaTC increases ABCA1 on the plasma membrane
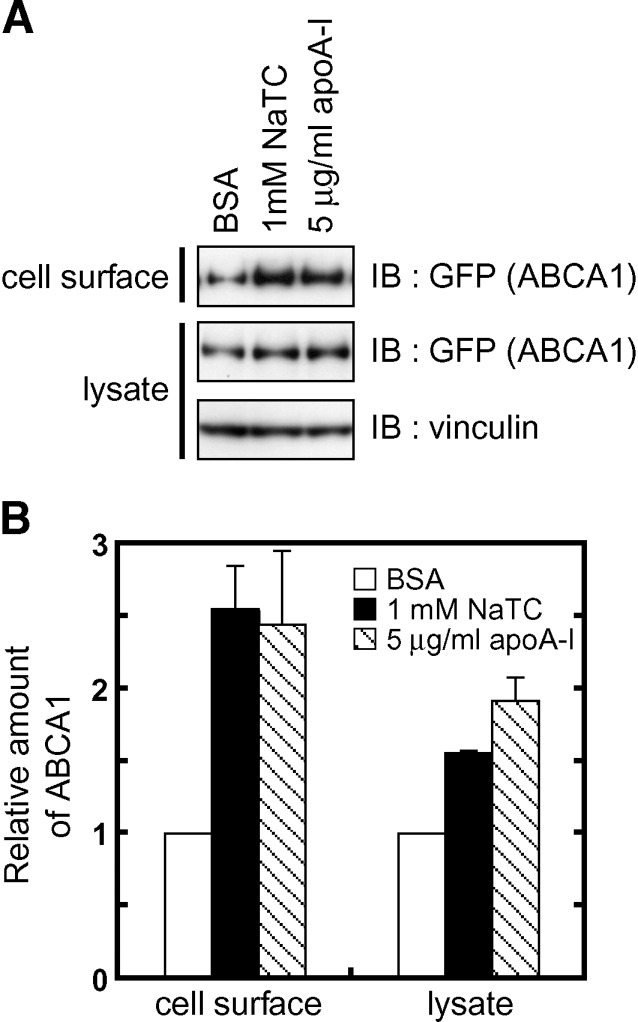
It has been reported that apoA-I suppresses degradation of ABCA1 protein and increases the amount of ABCA1 on the plasma membrane (29, 30). We examined whether NaTC, like apoA-I, increases ABCA1 on the plasma membrane. Biotinylation experiments showed that the amount of ABCA1 on the cell surface increased when cells were cultured for 12 h in the presence of 1 mM NaTC as well as in the presence of 5 μg/ml apoA-I (Fig. 7). These results suggested that degradation of ABCA1 is suppressed when cholesterol and phospholipids are extracted by extracellular acceptors.
Fig. 7.
NaTC increases ABCA1 on the plasma membrane. A: HEK/ABCA1-GFP cells were incubated in the presence of 0.02% BSA, 0.02% BSA and 1 mM NaTC, or 0.02% BSA and 5 μg/ml apoA-I for 12 h. Cells were then treated with sulfo-NHS-biotin, and cell lysates were prepared. Biotinylated cell surface proteins were precipitated with avidin-agarose from 150 μg of cell lysates. Cell lysates (15 μg of protein) and precipitated surface proteins were separated, and ABCA1-GFP and vinculin (loading control) were detected with indicated antibodies. B: Western blots were analyzed using a Fujifilm LAS-3000 imaging system. Experiments were performed in triplicate, and the average values are represented with the SEM.
DISCUSSION
Cholesterol secretion by ABCA1 is dependent on the presence of extracellular acceptors, such as lipid-free apoA-I. However, the mechanisms by which HDL formation is dependent on apoA-I remain unclear. Here, we showed that NaTC can also serve as an acceptor for cholesterol and phospholipids translocated by ABCA1. NaTC (1 mM) was as efficient an acceptor as physiological concentrations of apoA-I. Because it is unlikely that the plasma concentration of NaTC reaches 1 mM, NaTC-dependent efflux of cholesterol and phospholipids mediated by ABCA1 is not physiologically relevant. However, this system allowed us to analyze the role of apoA-I and dissect the steps in HDL formation.
MβCD has been reported to extract more cholesterol from cells expressing ABCA1, and it has been proposed that ABCA1 generates special domains on the plasma membrane, from which MβCD and apoA-I extract lipids (15, 16). However, because MβCD significantly extracts cholesterol from cells that do not express ABCA1 (15) and because MβCD does not extract phospholipids from the membrane (31), it is not the lipid acceptor for ABCA1, which mimics apoA-I, in the real sense of the term. In contrast, NaTC extracts both cholesterol and PC as efficiently as apoA-I from cells expressing ABCA1 and does not extract lipids from HEK293 host cells. Interestingly, significant cholesterol efflux from WI-38 cells was observed without induction of ABCA1 expression in the presence of apoA-I, while NaTC-dependent lipid efflux was strongly dependent on ABCA1 levels (Fig. 3). There may be other proteins (32, 33) in WI-38 cells that mediate cholesterol efflux in the presence of apoA-I. Alternatively, binding of significant amounts of apoA-I on the cell surface may cause spontaneous cholesterol loading on apoA-I. Indeed, greater cholesterol efflux was observed in the presence of apoA-I from cells expressing ABCA1-W590S, which allows apoA-I binding, than in the presence of NaTC, and this effect was strongly suppressed at 27°C (Fig. 4; see supplementary Fig. VI). These results indicate that NaTC is a good tool for analyzing ABCA1-mediated lipid translocation.
NaTC and other bile salts function as physiological acceptors for lipids secreted by ABCB4 (19), and PC secretion by ABCB4 is prerequisite for bile formation (34). ABCB1 and ABCB4 are predicted to contain very short extracellular loops, which are thought not to form specific binding sites for acceptors. Indeed, ABCB4 does not mediate the efflux of phospholipids and cholesterol in the presence of apoA-I (see supplementary Fig. I), and ABCB1 functions as a multidrug transporter in an acceptor-independent manner. It was reported that synthetic amphiphilic helical peptides serve as lipid acceptors as efficiently as apoA-I and that there is no sequence or stereoselective interaction with ABCA1 in the lipid release process (23, 24). NaTC, as well as amphiphilic helical peptides, may therefore extract PC and cholesterol translocated by ABCA1 and ABCB4 without specific interactions with the proteins.
The Tangier mutation W590S, which impairs apoA-I-dependent lipid efflux, greatly decreased NaTC-dependent cholesterol and phospholipid efflux. It has been reported that ABCA1-W590S interacts in a normal manner with ATP (3). The W590S mutation may abolish the coupling of ATP-induced conformational changes of ABCA1 with lipid translocation. The kinetics of apoA-I binding to cells expressing ABCA1-W590S were similar to those of binding to cells expressing wild-type ABCA1, consistent with a previous report showing that the W590S mutation does not impair apoA-I binding (8, 26–28). Thus, the W590S mutation would not affect the conformational changes of ABCA1 required for apoA-I binding but would affect the lipid translocation process.
The W590S mutation retarded the dissociation of apoA-I from ABCA1. This mutation may impair the transition to the conformation required for apoA-I release. Alternatively, lipid translocation by ABCA1 may facilitate dissociation of apoA-I from ABCA1. ApoA-I is reported to undergo a conformational transition in response to lipid (35), and lipidated apoA-I was reported not to interact with ABCA1 (11, 36). We assume that the conformational transition caused by lipid loading by ABCA1 facilitates the dissociation of apoA-I from ABCA1.
It has been reported that apoA-I stabilizes ABCA1 by preventing calpain-mediated degradation of ABCA1 and increases cell surface ABCA1 (29, 30, 37). The synthetic amphiphilic helical peptides that promoted ABCA1-dependent lipid efflux also stabilized ABCA1 (23, 24). In this study, we showed that NaTC stabilizes ABCA1 on the plasma membrane. In addition, ABCA1-W590S was reported not to be stabilized by apoA-I (29). These results suggest that removal of cellular cholesterol and phospholipids by apoA-I and NaTC stabilizes ABCA1, and direct interaction between ABCA1 and apoA-I may not be essential for stabilization of ABCA1.
In this study, we showed that NaTC is a good tool for analyzing ABCA1-mediated lipid efflux, and this system allowed us to dissect the steps of HDL formation by ABCA1. Our results suggest that the W590S mutation impaired NaTC-dependent lipid efflux but not apoA-I binding. Conversely, this mutation retarded the dissociation of apoA-I from ABCA1. These results suggest that the W590S mutation impairs ATP-dependent lipid translocation and that lipid translocation or possibly lipid loading facilitates apoA-I dissociation from ABCA1.
Supplementary Material
Abbreviations
ABC, ATP binding cassette; apoA-I, apolipoprotein A-I
GFP, green fluorescent protein
HEK, human embryonic kidney
MβCD, methyl-β-cyclodexitrin
NaTC, sodium taurocholate
PC, phosphatidylcholine
RA, 9 cis-retinoic acid
sulfo-NHS-biotin, sulfo-N-hydroxysuccinimidobiotin
This work was supported by a Grant-in-aid for Scientific research (S) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation. This work was supported by the World Premier International Research Center Initiative, MEXT, Japan.
Published, JLR Papers in Press, February 8, 2009.
Footnotes
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of eight figures.
References
- 1.Oram J. F., and A. M. Vaughan. 2006. ATP-binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 99 1031–1043. [DOI] [PubMed] [Google Scholar]
- 2.Yokoyama S. 2000. Release of cellular cholesterol: molecular mechanism for cholesterol homeostasis in cells and in the body. Biochim. Biophys. Acta. 1529 231–244. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka A. R., S. Abe-Dohmae, T. Ohnishi, R. Aoki, G. Morinaga, K. Okuhira, Y. Ikeda, F. Kano, M. Matsuo, N. Kioka, et al. 2003. Effects of mutations of ABCA1 in the first extracellular domain on subcellular trafficking and ATP binding/hydrolysis. J. Biol. Chem. 278 8815–8819. [DOI] [PubMed] [Google Scholar]
- 4.Brooks-Wilson A., M. Marcil, S. M. Clee, L. H. Zhang, K. Roomp, M. van Dam, L. Yu, C. Brewer, J. A. Collins, H. O. Molhuizen, et al. 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22 336–345. [DOI] [PubMed] [Google Scholar]
- 5.Bodzioch M., E. Orso, J. Klucken, T. Langmann, A. Bottcher, W. Diederich, W. Drobnik, S. Barlage, C. Buchler, M. Porsch-Ozcurumez, et al. 1999. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22 347–351. [DOI] [PubMed] [Google Scholar]
- 6.Rust S., M. Rosier, H. Funke, J. Real, Z. Amoura, J. C. Piette, J. F. Deleuze, H. B. Brewer, N. Duverger, P. Denefle, et al. 1999. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 22 352–355. [DOI] [PubMed] [Google Scholar]
- 7.Singaraja R. R., L. R. Brunham, H. Visscher, J. J. Kastelein, and M. R. Hayden. 2003. Efflux and atherosclerosis: the clinical and biochemical impact of variations in the ABCA1 gene. Arterioscler. Thromb. Vasc. Biol. 23 1322–1332. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald M. L., A. L. Morris, A. Chroni, A. J. Mendez, V. I. Zannis, and M. W. Freeman. 2004. ABCA1 and amphipathic apolipoproteins form high-affinity molecular complexes required for cholesterol efflux. J. Lipid Res. 45 287–294. [DOI] [PubMed] [Google Scholar]
- 9.Chroni A., T. Liu, M. L. Fitzgerald, M. W. Freeman, and V. I. Zannis. 2004. Cross-linking and lipid efflux properties of apoA-I mutants suggest direct association between apoA-I helices and ABCA1. Biochemistry. 43 2126–2139. [DOI] [PubMed] [Google Scholar]
- 10.Oram J. F., R. M. Lawn, M. R. Garvin, and D. P. Wade. 2000. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 275 34508–34511. [DOI] [PubMed] [Google Scholar]
- 11.Wang N., D. L. Silver, P. Costet, and A. R. Tall. 2000. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 275 33053–33058. [DOI] [PubMed] [Google Scholar]
- 12.Vedhachalam C., A. B. Ghering, W. S. Davidson, S. Lund-Katz, G. H. Rothblat, and M. C. Phillips. 2007. ABCA1-induced cell surface binding sites for ApoA-I. Arterioscler. Thromb. Vasc. Biol. 27 1603–1609. [DOI] [PubMed] [Google Scholar]
- 13.Hassan H. H., M. Denis, D. Y. Lee, I. Iatan, D. Nyholt, I. Ruel, L. Krimbou, and J. Genest. 2007. Identification of an ABCA1-dependent phospholipid-rich plasma membrane apolipoprotein A-I binding site for nascent HDL formation: implications for current models of HDL biogenesis. J. Lipid Res. 48 2428–2442. [DOI] [PubMed] [Google Scholar]
- 14.Vedhachalam C., P. T. Duong, M. Nickel, D. Nguyen, P. Dhanasekaran, H. Saito, G. H. Rothblat, S. Lund-Katz, and M. C. Phillips. 2007. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J. Biol. Chem. 282 25123–25130. [DOI] [PubMed] [Google Scholar]
- 15.Landry Y. D., M. Denis, S. Nandi, S. Bell, A. M. Vaughan, and X. Zha. 2006. ATP-binding cassette transporter A1 expression disrupts raft membrane microdomains through its ATPase-related functions. J. Biol. Chem. 281 36091–36101. [DOI] [PubMed] [Google Scholar]
- 16.Nagao K., K. Takahashi, K. Hanada, N. Kioka, M. Matsuo, and K. Ueda. 2007. Enhanced apoA-I-dependent cholesterol efflux by ABCA1 from sphingomyelin-deficient Chinese hamster ovary cells. J. Biol. Chem. 282 14868–14874. [DOI] [PubMed] [Google Scholar]
- 17.Vaughan A. M., and J. F. Oram. 2003. ABCA1 redistributes membrane cholesterol independent of apolipoprotein interactions. J. Lipid Res. 44 1373–1380. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K., Y. Kimura, N. Kioka, M. Matsuo, and K. Ueda. 2006. Purification and ATPase activity of human ABCA1. J. Biol. Chem. 281 10760–10768. [DOI] [PubMed] [Google Scholar]
- 19.Morita S. Y., A. Kobayashi, Y. Takanezawa, N. Kioka, T. Handa, H. Arai, M. Matsuo, and K. Ueda. 2007. Bile salt-dependent efflux of cellular phospholipids mediated by ATP binding cassette protein B4. Hepatology. 46 188–199. [DOI] [PubMed] [Google Scholar]
- 20.Munehira Y., T. Ohnishi, S. Kawamoto, A. Furuya, K. Shitara, M. Imamura, T. Yokota, S. Takeda, T. Amachi, M. Matsuo, et al. 2004. Alpha1-syntrophin modulates turnover of ABCA1. J. Biol. Chem. 279 15091–15095. [DOI] [PubMed] [Google Scholar]
- 21.Azuma Y., M. Takada, H. W. Shin, N. Kioka, K. Nakayama, and K. Ueda. 2009. Retroendocytosis pathway of ABCA1/apoA-I contributes to HDL formation. Genes Cells. 14 191–204. [DOI] [PubMed] [Google Scholar]
- 22.Abe-Dohmae S., S. Suzuki, Y. Wada, H. Aburatani, D. E. Vance, and S. Yokoyama. 2000. Characterization of apolipoprotein-mediated HDL generation induced by cAMP in a murine macrophage cell line. Biochemistry. 39 11092–11099. [DOI] [PubMed] [Google Scholar]
- 23.Arakawa R., M. Hayashi, A. T. Remaley, B. H. Brewer, Y. Yamauchi, and S. Yokoyama. 2004. Phosphorylation and stabilization of ATP binding cassette transporter A1 by synthetic amphiphilic helical peptides. J. Biol. Chem. 279 6217–6220. [DOI] [PubMed] [Google Scholar]
- 24.Remaley A. T., F. Thomas, J. A. Stonik, S. J. Demosky, S. E. Bark, E. B. Neufeld, A. V. Bocharov, T. G. Vishnyakova, A. P. Patterson, T. L. Eggerman, et al. 2003. Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J. Lipid Res. 44 828–836. [DOI] [PubMed] [Google Scholar]
- 25.Yamauchi Y., S. Abe-Dohmae, and S. Yokoyama. 2002. Differential regulation of apolipoprotein A-I/ATP binding cassette transporter A1-mediated cholesterol and phospholipid release. Biochim. Biophys. Acta. 1585 1–10. [DOI] [PubMed] [Google Scholar]
- 26.Rigot V., Y. Hamon, O. Chambenoit, M. Alibert, N. Duverger, and G. Chimini. 2002. Distinct sites on ABCA1 control distinct steps required for cellular release of phospholipids. J. Lipid Res. 43 2077–2086. [DOI] [PubMed] [Google Scholar]
- 27.Singaraja R. R., H. Visscher, E. R. James, A. Chroni, J. M. Coutinho, L. R. Brunham, M. H. Kang, V. I. Zannis, G. Chimini, and M. R. Hayden. 2006. Specific mutations in ABCA1 have discrete effects on ABCA1 function and lipid phenotypes both in vivo and in vitro. Circ. Res. 99 389–397. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald M. L., A. L. Morris, J. S. Rhee, L. P. Andersson, A. J. Mendez, and M. W. Freeman. 2002. Naturally occurring mutations in the largest extracellular loops of ABCA1 can disrupt its direct interaction with apolipoprotein A-I. J. Biol. Chem. 277 33178–33187. [DOI] [PubMed] [Google Scholar]
- 29.Wang N., W. Chen, P. Linsel-Nitschke, L. O. Martinez, B. Agerholm-Larsen, D. L. Silver, and A. R. Tall. 2003. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. J. Clin. Invest. 111 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arakawa R., and S. Yokoyama. 2002. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J. Biol. Chem. 277 22426–22429. [DOI] [PubMed] [Google Scholar]
- 31.Fukasawa M., M. Nishijima, H. Itabe, T. Takano, and K. Hanada. 2000. Reduction of sphingomyelin level without accumulation of ceramide in Chinese hamster ovary cells affects detergent-resistant membrane domains and enhances cellular cholesterol efflux to methyl-beta -cyclodextrin. J. Biol. Chem. 275 34028–34034. [DOI] [PubMed] [Google Scholar]
- 32.Fidge N. H. 1999. High density lipoprotein receptors, binding proteins, and ligands. J. Lipid Res. 40 187–201. [PubMed] [Google Scholar]
- 33.Martinez L. O., S. Jacquet, J. P. Esteve, C. Rolland, E. Cabezon, E. Champagne, T. Pineau, V. Georgeaud, J. E. Walker, F. Terce, et al. 2003. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 421 75–79. [DOI] [PubMed] [Google Scholar]
- 34.Smit J. J., A. H. Schinkel, R. P. Oude Elferink, A. K. Groen, E. Wagenaar, L. van Deemter, C. A. Mol, R. Ottenhoff, N. M. van der Lugt, M. A. van Roon, et al. 1993. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 75 451–462. [DOI] [PubMed] [Google Scholar]
- 35.Davidson W. S., and T. B. Thompson. 2007. The structure of apolipoprotein A-I in high density lipoproteins. J. Biol. Chem. 282 22249–22253. [DOI] [PubMed] [Google Scholar]
- 36.Mulya A., J. Y. Lee, A. K. Gebre, M. J. Thomas, P. L. Colvin, and J. S. Parks. 2007. Minimal lipidation of pre-beta HDL by ABCA1 results in reduced ability to interact with ABCA1. Arterioscler. Thromb. Vasc. Biol. 27 1828–1836. [DOI] [PubMed] [Google Scholar]
- 37.Lu R., R. Arakawa, C. Ito-Osumi, N. Iwamoto, and S. Yokoyama. 2008. ApoA-I facilitates ABCA1 recycle/accumulation to cell surface by inhibiting its intracellular degradation and increases HDL generation. Arterioscler. Thromb. Vasc. Biol. 28 1820–1824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.