Abstract
The mammalian Δ6-desaturase coded by fatty acid desaturase 2 (FADS2; HSA11q12-q13.1) catalyzes the first and rate-limiting step for the biosynthesis of long-chain polyunsaturated fatty acids. FADS2 is known to act on at least five substrates, and we hypothesized that the FADS2 gene product would have Δ8-desaturase activity. Saccharomyces cerevisiae transformed with a FADS2 construct from baboon neonate liver cDNA gained the function to desaturate 11,14-eicosadienoic acid (20:2n-6) and 11,14,17-eicosatrienoic acid (20:3n-3) to yield 20:3n-6 and 20:4n-3, respectively. Competition experiments indicate that Δ8-desaturation favors activity toward 20:3n-3 over 20:2n-6 by 3-fold. Similar experiments show that Δ6-desaturase activity is favored over Δ8-desaturase activity by 7-fold and 23-fold for n-6 (18:2n-6 vs 20:2n-6) and n-3 (18:3n-3 vs 20:3n-3), respectively. In mammals, 20:3n-6 is the immediate precursor of prostaglandin E1 and thromboxane B1. 20:3n-6 and 20:4n-3 are also immediate precursors of long-chain polyunsaturated fatty acids arachidonic acid and eicosapentaenoic acid, respectively. These findings provide unequivocal molecular evidence for a novel alternative biosynthetic route to long-chain polyunsaturated fatty acids in mammals from substrates previously considered to be dead-end products.
Keywords: polyunsaturated fatty acid biosynthesis, dihomo-γ-linolenic acid, eicosanoid precursor biosynthesis
Long-chain polyunsaturated fatty acids (LCPUFAs) are ubiquitous in mammalian tissue, achieving highest concentrations in the membranes of neural and other excitable tissue (1). LCPUFA of the n-3 and n-6 families, especially eicosapentaenoic acid (EPA; 20:5n-3), docosahexaenoic acid (22:6n-3), and arachidonic acid (20:4n-6), are bioactive components of membrane phospholipids and serve as substrates for signaling molecules (2). The degree of unsaturation of the membranes is determined by the action of enzymes involved in fatty acid biosynthesis and metabolism (3). Most organisms synthesize unsaturated fatty acids, but the pathways are specific to cell types and species.
Fatty acid desaturases are enzymes that catalyze the introduction of cis double bonds at specific positions in a fatty acid chain (4). Desaturases in plants and lower animal species can introduce double bonds near the methyl end. Eukaryotic cells of higher animals, fungi, and dinoflagellates express membrane-bound acyl-CoA front-end desaturases (5, 6) catalyzing double bond introduction into the Δ6, Δ5, Δ8, and Δ4 positions. Mammalian front-end desaturases operate on diet-derived PUFA to synthesize LCPUFA, which can also be derived from the diet but possibly not in sufficient quantities to optimize health (7).
The front-end desaturases are remarkable for their structural similarity and functional diversity. They all contain the N-terminal cytochrome b5 domain (HPGG) as electron donor and three histidine motifs, HXXXH, HXXHH, and QXXHH, conserved from human to microalgae (8). Molecular cloning and isolation of a Δ5-desaturase from Caenorhabditis elegans (9) and Mortierella alpina (10) and a Δ6-desaturase from C. elegans (11), M. alpina (12), rat (13), and mouse (14) have all been reported. The human fatty acid desaturase (FADS) gene cluster at 11q12-q13.1 encodes two desaturases with known function, Δ5-desaturase (FADS1) and Δ6-desaturase (FADS2) (14, 15), as well as a third putative desaturase gene (FADS3) (16), which thus far has no known substrate despite high homology to FADS1 and FADS2.
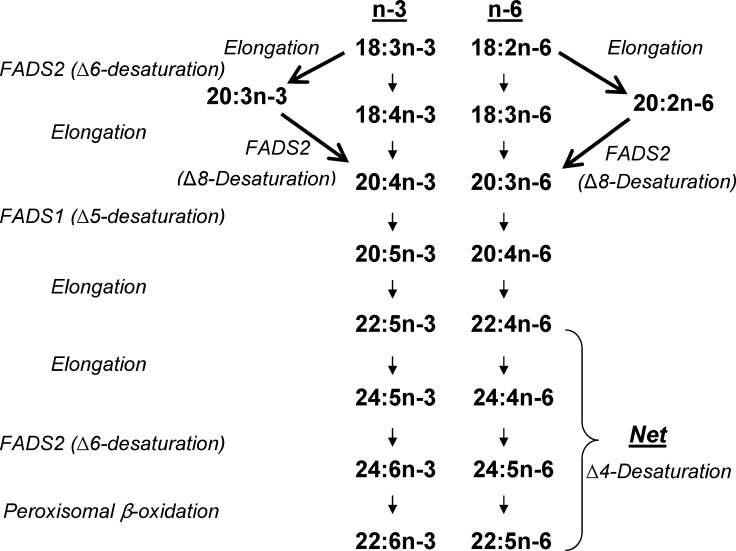
Figure 1 shows the common n-3 and n-6 LCPUFA pathways mediated by Δ6 and Δ5 desaturases. The Δ6-desaturase (FADS2) is known to operate on both 18:3n-3 and 18:2n-6, resulting in the synthesis of 6,9,12,15-18:4 and 6,9,12-18:3 (γ-linolenic acid), respectively. This step is rate limiting and is followed by elongation to 8,11,14,17-20:4 and 8,11,14-20:3 (dihomo-γ-linolenic acid). A rapid Δ5-desaturation (FADS1) on these PUFA produces EPA and arachidonic acid. EPA can be further elongated and desaturated to yield docosahexaenoic acid by the pathway shown, which is accepted as mammalian pathway, or via a Δ4-desaturase as demonstrated in Thraustochytrium (17).
Fig. 1.
Pathways for LCPUFA biosynthesis. The conventional pathway consists of alternating desaturation and elongation leading to LCPUFA. Δ8-Desaturation of 20:2n-6 and 20:3n-3 would yield 20:3n-6 and 20:4n-3, intermediates in the conventional pathway to 20:4n-6 and 20:5n-3, as well as immediate eicosanoid precursors.
The operation of an alternative pathway via C20 fatty acids using a Δ8-desaturase reported in unicellular organisms (18–21) has been verified by molecular cloning and functional characterization studies in Euglena gracilis (22), Acanthamoeba castellanii (23), and Perkinsus marinus (24). There are many reports of Δ8-desaturation activity in mammalian cells (25, 26), in rat and human testes (27, 28), and in mouse liver (29), though it has not been verified by molecular cloning, and the existence of Δ8-desaturation in rat microsomes has been questioned (30). The putative substrate of the Δ8-desaturase, 11,14-eicosadienoic acid (20:2 n-6), is found in human plasma and red cells as well as other tissues, and its concentration has recently been associated with human genetic variation in the FADS gene cluster (31, 32).
The mammalian Δ6-desaturase coded by FADS2 uses at least five substrates, 18:2n-6, 18:3n-3, 24:6n-3, 24:5n-3 (33, 34), and 16:0. Δ6-desaturase in the sebaceous glands catalyzes desaturation of 16:0 to 16:1n-10 (sapienate), the most abundant fatty acid in human sebum, showing that substrate specificity is influenced by the cellular environment in which it is expressed (35). We hypothesized that the primate FADS2 gene product would have Δ8-desaturase activity and cloned baboon FADS2 into Saccharomyces cerevisiae, an organism with no native PUFA biosynthetic capability, to test for gain of Δ8-desaturation activity. Here, we report unambiguous evidence of the existence of Δ8-desaturation in primates, suggesting alternative pathway for LCPUFA biosynthesis.
MATERIALS AND METHODS
RNA isolation and cDNA synthesis
Total RNA from 30 mg neonate baboon liver tissue homogenate was extracted using the RNeasy Mini kit (Qiagen, Valencia, CA). The yield of total RNA was assessed by 260 nm UV absorption. The quality of RNA was analyzed by 260/280 nm ratios of the samples and by agarose gel electrophoresis to verify RNA integrity. One microgram total RNA was reverse transcribed into first-strand cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). The resulting cDNA was used as template for RT-PCR reactions.
Cloning of baboon FADS2 and sequence analysis
To identify baboon FADS2 cDNA sequence, primers were generated using human cDNA sequences for FADS2 (GenBank accession number NM_004265). PCR primers, FADS2 forward (5′-ATGGGGAAGGGAGGGAACCAGGGCGA-3′) and FADS2 reverse (5′-TCATTTGTGAAGGTAGGCGTCCAGCCA-3′) were ordered from Integrated DNA Technologies (Coralville, IA) and were amplified with baboon liver cDNA as template and high-fidelity Taq polymerase (Roche Diagnostics) using Eppendorf gradient thermal cycler. Cycling conditions were as follows: initial denaturation at 95°C for 5 min followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 72°C for 45 s, and extension at 72°C for 1 min, with a final extension at 72°C for 5 min. PCR product was separated by electrophoresis on 2% agarose gel stained with ethidium bromide and band of appropriate size was obtained. The PCR product was gel purified and cloned in pGEM T-Easy vector (Promega) and sequenced using T7 forward and SP6 reverse universal primers at Cornell University Life Sciences Core Laboratories Center using the Applied Biosystems automated 3730 DNA analyzer. We successfully cloned the baboon FADS2 protein coding region (GenBank accession number EU780003). The pGEM T- Easy vector with FADS2 was named pTFADS2.
Transformation into yeast (S. cerevisiae)
The entire coding regions of baboon FADS2 was amplified from pTFADS2 with primers FADS2-KOZAK forward (5′-CCCAAGCTTACCATGGGGAAGGGAGGGAACCAGGGCGA-3′) including the HindIII site and FADS2-KOZAK reverse (5′-CCGCTCGAGTCATTTGTGAAGGTAGGCGTCCAGCCA-3′) including XhoI site. The high-fidelity Taq polymerase (Roche Diagnostics) was used to minimize potential PCR errors. The amplified PCR product containing baboon FADS2 was gel purified, restriction digested, and inserted into HindIII and XhoI sites behind the GAL1 promoter of pYES2 vector (Invitrogen) to yield the plasmid pYFADS2. The constructed plasmid of pYFADS2 was transformed into S. cerevisiase (strain INVSc1 from Invitrogen) using S. c. Easy Comp™ Transformation Kit (Invitrogen), and the transformants were verified by DNA sequencing.
Expression of baboon FADS2
For functional expression characterization, transformed yeast strains with pYES2 (empty vector) as a negative control and pYFADS2 were grown for 24 h in S. cerevisiae minimal media without uracil. As another negative control, wild S. cerevisiae (INVSc1) was cultured in S. cerevisiae minimal medium with uracil. Expression of the transgene was induced when OD600 reached 0.4. At that time, appropriate fatty acids, 1 mM linoleic acid (18:2n-6), α-linolenic acid (18:3n-3), eicosadienoic acid (20:2n-6), and eicosatrienoic acid (20:3n-3), were added in the presence of 1% tergitol-Nonidet P-40 (Sigma-Aldrich) to the cultures and were grown at 30°C with constant shaking. The samples were collected after 48 h for fatty acid analysis. All treatments were performed in duplicate.
Fatty acid analysis
The yeast cells were harvested by centrifugation at 4,000 rpm for 5 min. The cell pellets were washed twice with tergitol-Nonidet P-40 and finally twice with distilled water. Fatty acid methyl esters (FAMEs) were prepared using modified one-step lipid extraction method of Garces and Mancha (36). FAMEs were structurally identified by gas chromatography-covalent adduct chemical ionization tandem mass spectrometry (GC-CACI-MS/MS) (37–39) and quantitatively analyzed by GC-flame ionization detection. An equal weight FAME mixture was used to verify response factors on a daily basis (40). For competition experiments, GC analyses were performed in triplicate.
Materials (Chemicals)
Fatty acids (18:2n-6, 18:3n-3, 20:2n-6, and 20:3n-3) were purchased from Nu-Chek Prep (MN). Uracil dropout SD-U medium and supplement contents including amino acids were obtained from Clontech, TaKaRa Bio. Uracil and tergitol-Nonidet P-40 was from Sigma-Aldrich. pGEM-T Easy Vector II system was purchased from Promega. The pYES2 vector, INVSc1 strain, S. c. Easy Comp™ Transformation Kit, and restriction enzymes (HindIII and XhoI) were obtained from Invitrogen. Total RNA was isolated by using RNeasy Mini kit from Qiagen. The cDNA synthesis kit was purchased from Bio-Rad.
RESULTS
Baboon FADS2
The sequenced PCR product (Baboon FADS2; GenBank EU780003) revealed an open reading frame of 1,335 bp, encoding a protein of 444 amino acids and a stop codon. It shares a 60% homology with baboon FADS1 (EF531577) and 62% homology with the putative baboon FADS3 (EU780002), including HPGG characteristic of a cytochrome b5 domain and three conserved histidine motifs, HXXXH, HXXHH, and QXXHH. Analysis and comparison of amino acid sequence of baboon FADS2 showed 97% identity and 99% similarity with human FADS2 (AAH09011), and 64% identity and 79% similarity with the bifunctional zebrafish desaturase (AAG25710). Baboon FADS2 also shares homology with Δ8-desaturases from unicellular organisms [27% identity and 43% similarity with E. gracilis (AAD45877), 27% identity and 40% similarity with P. marinus (ABF58684), and 23% identity and 38% similarity with A. castellanii (CAO00489)]. Analysis of the baboon FADS2 secondary structure by SOSUI software (41) predicted three transmembrane regions, whereas baboon FADS1 and the putative baboon FADS3 had four transmembrane regions (data not shown).
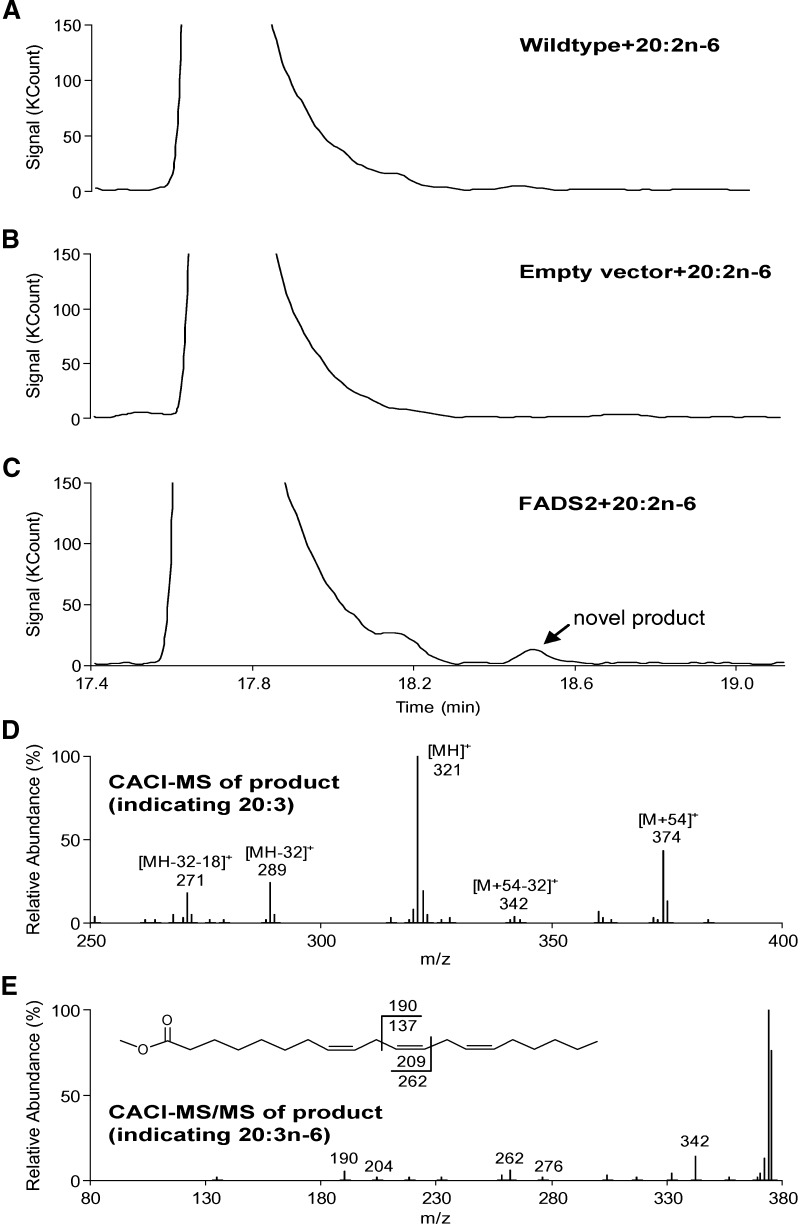
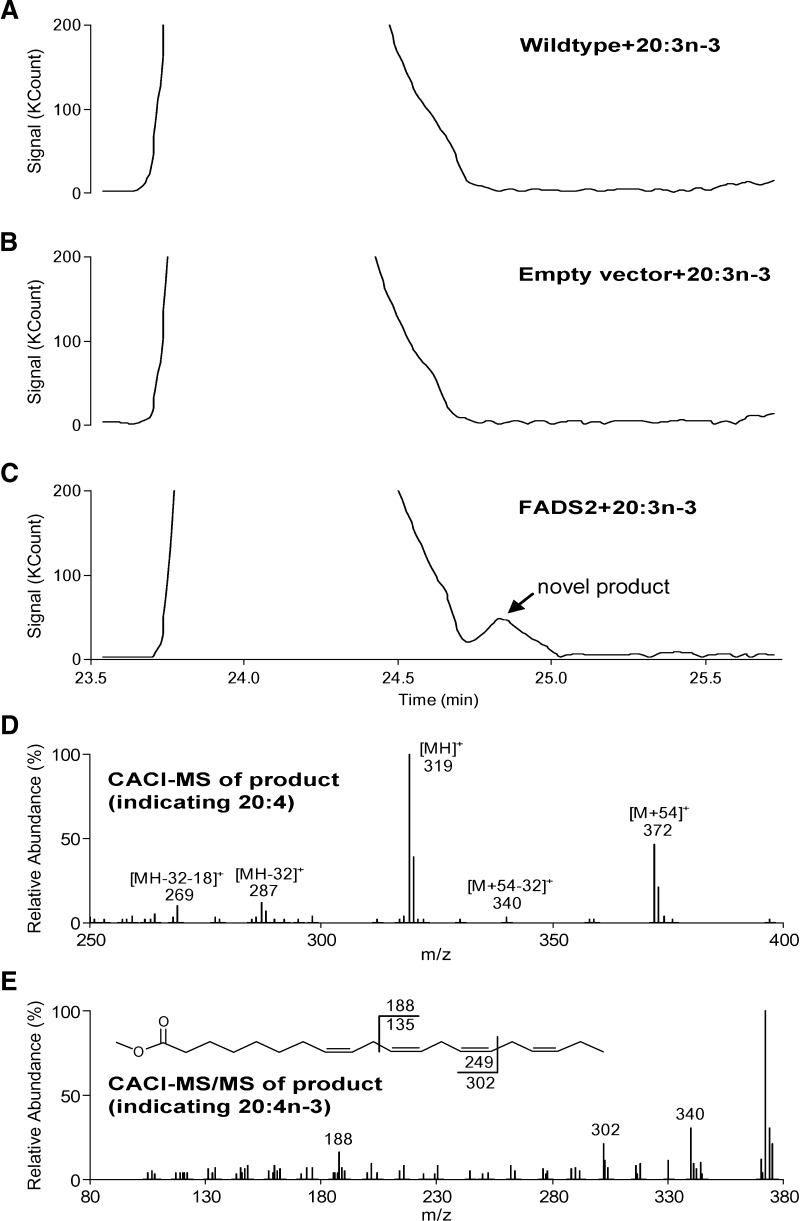
The transformed yeast grown on minimal media were supplemented with various fatty acids, incubated at 30°C and harvested after 48 h. Wild-type (wt) S. cerevisiae and S. cerevisiae containing empty pYES2 vector were used as controls for every replicate. GC-CACI-MS chromatograms of FAME are presented in Figs. 2 and 3 for 20:2n-6 and 20:3n-3 incubations, respectively. A–C of both figures correspond to wt S. cerevisiae, S. cerevisiae containing empty pYES2 vector, and S. cerevisiae containing FADS2, respectively. S. cerevisiae wt and S. cerevisiae with empty pYES2 vector have no activity toward 20:3n-6 and 20:4n-3, as expected. Fig. 2C shows a new product appearing upon incubation with 20:2n-6. Panel D is the MS-1 spectrum showing peaks at m/z 374, 321, 289, and 271, corresponding to the [M+54]+, [MH]+, [MH-32]+, and [MH-32-18]+ ions, respectively, characteristic of a 20:3 FAME. Panel E is the collisional dissociation spectrum of [M+54]+, yielding ions at m/z 262 and 190 corresponding, respectively, to the α and ω diagnostic ions for 20:3n-6 and positively identifying this product. Similarly, Fig. 3D displays MS1 ions characteristic of 20:4 (m/z 372, 319, 287, and 269), and collisional dissociation yields diagnostic ions m/z 302 and 188 in MS/MS, positively identifying 20:4n-3.
Fig. 2.
Data showing FADS2 action on 20:2n-6. A: Reconstructed ion chromatograms of FAME derived from 48 h incubation of 20:2n-6 with wt S. cerevisiae; B: S. cerevisiae transformed with empty vector; and C: S. cerevisiae transformed with FADS2 showing novel product. D: CACI-MS1 spectrum of novel product in C showing diagnostic ions for a 20:3 FAME. E: CACI-MS/MS spectrum of the [M+54]+ showing diagnostic ions characteristic of 20:3. Masses of fragments shown in the structural inset indicate the diagnostic ions characteristic of 20:3n-6 (m/z 190 and 262).
Fig. 3.
Data showing FADS2 action on 20:3n-3. Panels are analogous to those in Fig. 2 and demonstrate synthesis of 20:4n-3.
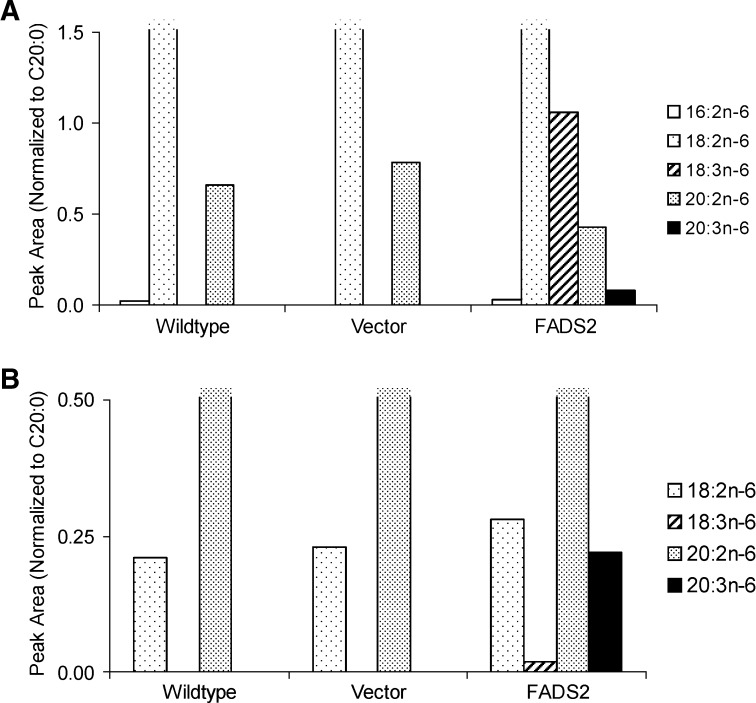
An alternative hypothesis to Δ8-desaturation of 20:2n-6 (20:2n-6 → 20:3n-6) is β-oxidation followed by entry into the normal pathway (20:2n-6 → 18:2n-6 → 18:3n-6 → 20:3n-6). The wt S. cerevisiae contain peroxisomes and mitochondria and thus have native β-oxidation activity, so this pathway is plausible. Figure 4A presents the relative product distribution with 18:2 as a substrate. No Δ6-desaturation is observed for the controls (wt or vector only), while the cells transformed with FADS2 accumulate 18:3n-6, and ∼8% of this product is further elongated to 20:3n-6 (18:2n-6 → 18:3n-6 → 20:3n-6). There is also some elongation of 18:2n-6 to 20:2n-6.
Fig. 4.
Analysis of putative intermediates. A: Peak areas of n-6 PUFA, normalized to 20:0, for wt, vector-only, and FADS2 transformed cells treated with 18:2n-6. A small amount of chain shortening is detected (16:2n-6) in wt and FADS2 cells. The FADS2 cells uniquely show accumulation of the Δ6-desaturated product 18:3n-6, the elongated product 20:2n-6, and a small amount of 20:3n-6, the chain elongated product of 18:3n-6. B: All 20:2n-6 treated cells show the chain-shortened product 18:2n-6, and only the FADS2 cells show a small amount of the elongated product 18:3n-6. The product of Δ8-desaturase activity on 20:2n-6, 20:3n-6 is present only in the FADS2 cells at about 10-fold higher concentration than 18:3n-6.
The putative intermediate of a β-oxidation-mediated alternative pathway, 18:3n-6, was detected in only one of two trials with 20:2n-6 used as a substrate. Fig. 4B presents relative product distribution with 20:2n-6 for that trial. There is indeed some β-oxidation to 18:2n-6 in all treatments. However, Δ6-desaturation of this product to 18:3n-6 is detected in only the FADS2 cells and is ∼7% of the 18:2n-6 product. The Δ8-desaturation product, 20:3n-6, is >10-fold greater in abundance than 18:3n-6, its putative intermediate in the alternative pathway. Considering the data of Fig. 4A, the conversion of 18:3n-6 → 20:3n-6 can only account for a negligible fraction of the 20:3n-6. We conclude that Δ8-desaturation by the FADS2 product mediates direct conversion of 20:2n-6 to 20:3n-6.
Once establishing that FADS2-transformed S. cerevisiae gained the ability to Δ8-desaturate 20-carbon PUFA, the relative activity was tested in competition experiments. Δ8-desaturase activity toward n-3 and n-6 fatty acids was investigated by supplementing media with a 1:1 mixture of 20:2n-6 and 20:3n-3 as substrates. Substrates and products were analyzed quantitatively by GC-flame ionization detection after confirmation of structure by GC-CACI-MS and GC-CACI-MS/MS. Table 1 shows that 20:2n-6 → 20:3n-6 was 0.9 ± 0.16%, and 20:3n-3 → 20:4n-3 was 2.8 ± 0.7% over a 48 h incubation, yielding a relative conversion efficiency of ∼3.1-fold, favoring the n-3 PUFA.
TABLE 1.
Competition between n-6 and n-3 fatty acids
| Substrate Mixture | Reactions | Conversion of Substrate (%) Mean ± SD | Ratio |
|---|---|---|---|
| 20:2n-6+ 20:3n-3 | Δ8-desaturase 20:2n-6 → 20:3n-6 | 0.90 ± 0.16 | 3.1 |
| Δ8-desaturase 20:3n-3 → 20:4n-3 | 2.8 ± 0.7 |
In a second competition experiment, 1:1 mixtures of 18:2n-6+20:2n-6 or 18:3n-3+20:3n-3 were added to media to test the relative Δ6-desaturase and Δ8-desaturase activities. Table 2 shows that conversions 18:2n-6 → 18:3n-6 and 20:2 n-6 → 20:3n-6 were 12.2 ± 0.16% and 1.7 ± 0.54%, respectively, yielding a relative activity of 7.2-fold favoring Δ6-desaturase. The relative conversions 18:3n-3 →18:4n-3 and 20:3n-3 → 20:4n-3 were 24.1 ± 2.4% and 1.03 ± 0.31%, respectively, yielding the substantially greater conversion ratio of 23.
TABLE 2.
Competition between Δ6- and Δ8-desaturae activities
| Substrate Mixture | Reactions | Conversion of Substrate (%) Mean ± SD | Ratio |
|---|---|---|---|
| 18:2n-6+ 20:2n-6 | Δ6-desaturase 18:2n-6 →18:3n-6 | 12.2 ± 0.16 | 7.2 |
| Δ8-desaturase 20:2n-6 → 20:3n-6 | 1.7 ± 0.54 | ||
| 18:3n-3+ 20:3n-3 | Δ6-desaturase 18:3n-3 → 18:4n-3 | 24.1 ± 2.4 | 23 |
| Δ8-desaturase 20:3n-3 → 20:4n-3 | 1.03 ± 0.31 |
DISCUSSION
More than 50 years ago, Thomasson (42) showed that the n-6 PUFA were active in supporting growth of water-deprived young rats raised on a diet with saturates as their only source of fat. The order of relative vitamin F activity was 20:4 > 18:2 = 20:3 > 20:2 (131:100:100:43), with relative activity of <10 for all other fatty acids tested, including n-3s. Within two decades, the pathway from 18:2 to 20:4 by sequential Δ6-desaturation-elongation-Δ5-desaturation (18:2 → 18:3 → 20:3 → 20:4) emerged as the major route of biosynthesis. The alternative, elongation-Δ8-desaturation-Δ5-desaturation (18:2 → 20:2 → 20:3 → 20:4), has been of interest over the years because of the appearance of intermediates in mammalian tissue, as has a third alternative, elongation-Δ5-desaturation-Δ8-desaturation (18:2 → 20:2 → 5,11,14-20:3 → 20:4) because 20:2 is converted to 5,11,14-20:3 (sciadonic acid) by the action of Δ5-desaturase. Experiments involving only activity measurements in mammalian tissue or cells that definitively establish or rule out participation of a Δ8-desaturase are difficult to design because of the low concentration of the intermediates, indicating that molecular techniques capable of isolating a particular biochemical activity are required (30). Indeed, the existence of Δ8-desaturation as an alternative pathway to LCPUFA has been reported periodically, and Δ8-desaturase activity has been found by some and not by others (30). Presently, 20:2n-6 and 20:3n-3 are widely considered dead-end products, in part because their conversion to LCPUFA has not been unequivocally established (43). However, recent studies have associated 20:2n-2 with FADS2 polymorphisms and/or fatty acid compositions in humans (32, 44), including patients with cardiovascular disease (31) and type 2 diabetes mellitus (45). In addition, apolipoprotein D knockout mice, a model for psychiatric disorders, show increased CNS 20:2n-6 and 18:2n-6 compared with wild-type mice (46).
Several fatty acids of chain length 16, 18, and 24 carbons are substrates for the FADS2 gene product. Apart from 18:2n-6 and 18:3n-3, it is also known to Δ6-desaturate 24:5n-3 and 24:4n-6, as required for the coupled microsomal-peroxisomal pathway for 22:6n-3 and 22:5n-6 biosynthesis (33). The FADS2 gene product catalyzes the Δ6-desaturation of 16:0 (palmitic acid) to 16:1n-10 (cis-6-16:1, sapienic acid) when expressed natively in human skin sebocytes (35), and when COS-7 cells were transfected with rat FADS2 they acquired the ability to Δ6-desaturate 16:0 (34). However, there are no previous reports of FADS2 gene product activity toward 20 carbon PUFA.
The alternative synthetic pathways to arachidonic acid from 20:2n-6 are either by sequential action of a Δ8-desaturase and a Δ5-desaturase or vice versa. Initial Δ8-desaturation yields the eicosanoid precursor 20:3n-6, whereas initial Δ5-desaturase activity yields 5,11,14-20:3. There are numerous reports showing that 11,14-20:2 is Δ5-desaturated to 5,11,14-20:3 (30, 47–50). However, no clear evidence for the conversion of 5,11,14-20:3 to 20:4n-6 has been found (30, 47, 49). Sprecher and coworkers have studied the desaturation of 11,14-20:2 with isotope labeling in vitro and in vivo, and consistently find that rat liver does Δ5-desaturate it to 5,11,14-20:3, but they find no evidence of Δ8-desaturation activity on this product (47, 49). Fourteen day feeding of 5,11,14-20:3 led to the accumulation of this PUFA in liver phosphoglycerides where it decreased 20:4n-6, while feeding of 11,14-20:2 did not alter 20:4n-6 levels (30). The production of 5,11,14-20:3 and 5,11,14,17-20:4 in human leukemia K562 cells has been reported in which the Δ5-desaturase is the only active desaturase operating because of the lack of Δ6-desaturase activity in these cells (50). Consistent with this report, we recently found significant amounts of 7,11,14-20:3, 7,11,14,17-20:4, and 9,13,16,19-22:4 in the liver lipids of chow-fed FADS2 null mice, all of which may be synthesized by action of Δ5-desaturase, coded by FADS1, on 18:2n-6 or 18:3n-3, followed by prompt elongation (C. Stroud, P. Lawrence, J. T. Brenna, and M. Nakamura, unpublished observations). These data support the hypothesis that the Δ5-desaturase acts on PUFA only when its preferred substrate is not available, which may well imply that its products found in experimental studies are not relevant in vivo under normal conditions.
Reports of Δ8-desaturase activity in rodent and human testes have appeared (27, 28), and the most recent study shows stable isotope labeling best explained by direct conversion of 11,14-20:2 to 20:4n-6 via Δ8-desaturation, albeit as a minor pathway (30), but there are no existing molecular data to implicate a specific gene responsible for coding for vertebrate Δ8-desaturase activity. Δ8-Desaturation has been shown unequivocally in unicellular organisms where the gene has been cloned and is active when expressed in Arabidopsis thaliana (51). A Δ8-desaturase was first reported in the single cell protist E. gracilis (22) and later, along with a Δ9PUFA-elongase, in Isochrysis galbana (21) as well as the free living amoeba A. castellanii (23). The present report is the first to show that a vertebrate gene product introduces a double bond at the Δ8 position, demonstrating an alternative pathway to LCPUFA biosynthesis.
The competition experiments provide insight as to whether Δ8-desaturase activity of the FADS2 protein product can be important in vivo. The synthesis of 20:4n-3 dominates by 3.1-fold over 20:3n-6, consistent with long-established observations for Δ6-desaturase preference for 18:3n-3 over 18:2n-6 (52–54). These observations are also are consistent with in vitro work showing that the biosynthesis of n-6 PUFA is strongly suppressed by <2% of calories of 18:3n-3, whereas nearly 10 times as much 18:2n-6 is required to equally suppress n-3 PUFA biosynthesis (55), indicating that the affinity of the biosynthetic apparatus favors n-3 PUFA. Our competition experiments (Table 2) also establish that the FADS2 gene product exhibits both Δ6-desaturase and Δ8-desaturase activities when both substrates are available. As expected, the FADS2 gene product showed higher Δ6-desaturase activity by acting on the 18:2n-6 substrate to generate 7-fold more product than for the 20:2n-6. The relative action Δ6/Δ8 activity toward the n-3 was much greater, at 23-fold, indicating that the conventional pathway would be strongly favored when both substrates are available.
In conclusion, baboon FADS2 gene cloned into S. cerevisiae causes gain of Δ8-desaturase activity, in addition to coding for Δ6-desaturase activity. Δ8-Desaturase activity on 20:2n-6 leads directly to 20:3n-6, the immediate precursor of PGE1 and of 20:4n-6. All available evidence indicates that Δ8-desaturation is a minor pathway, but further study may show that it becomes important when there is high demand for eicosanoid synthesis, such as in inflammation or vasodilation, particularly in situations in which specialized tissues require 20:3n-6 as a precursor to prostaglandins E1 and F1α, hydroxyeicosatrienoic acids, or thromboxane B1. This alternative pathway to the eicosanoid precursors may explain data suggesting that 20:2n-6 levels are related to human health.
Abbreviations
EPA, eicosapentaenoic acid
FADS, fatty acid desaturase
FAME, fatty acid methyl ester
GC-CACI-MS/MS, gas chromatography-covalent adduct chemical ionization tandem mass spectrometry
LCPUFA, long-chain polyunsaturated fatty acids
wt, wild type
This work was supported by National Institutes of Health Grant GM071534.
Published, JLR Papers in Press, February 6, 2009.
References
- 1.Brenna J. T., and G. Y. Diau. 2007. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot. Essent. Fatty Acids. 77 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinsella J. E., B. Lokesh, S. Broughton, and J. Whelan. 1990. Dietary polyunsaturated fatty acids and eicosanoids: potential effects on the modulation of inflammatory and immune cells: an overview. Nutrition. 6 24–44 (discussion 59–62). [PubMed] [Google Scholar]
- 3.Voss A., M. Reinhart, S. Sankarappa, and H. Sprecher. 1991. The metabolism of 7,10,13,16,19-docosapentaenoic acid to 4,7,10,13,16,19-docosahexaenoic acid in rat liver is independent of a 4-desaturase. J. Biol. Chem. 266 19995–20000. [PubMed] [Google Scholar]
- 4.Los D. A., and N. Murata. 1998. Structure and expression of fatty acid desaturases. Biochim. Biophys. Acta. 1394 3–15. [DOI] [PubMed] [Google Scholar]
- 5.Tocher D. R., M. J. Leaver, and P. A. Hodgson. 1998. Recent advances in the biochemistry and molecular biology of fatty acyl desaturases. Prog. Lipid Res. 37 73–117. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura M. T., and T. Y. Nara. 2004. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu. Rev. Nutr. 24 345–376. [DOI] [PubMed] [Google Scholar]
- 7.Salem N., Jr., B. Wegher, P. Mena, and R. Uauy. 1996. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc. Natl. Acad. Sci. USA. 93 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperling P., P. Ternes, T. K. Zank, and E. Heinz. 2003. The evolution of desaturases. Prostaglandins Leukot. Essent. Fatty Acids. 68 73–95. [DOI] [PubMed] [Google Scholar]
- 9.Michaelson L. V., J. A. Napier, M. Lewis, G. Griffiths, C. M. Lazarus, and A. K. Stobart. 1998. Functional identification of a fatty acid delta5 desaturase gene from Caenorhabditis elegans. FEBS Lett. 439 215–218. [DOI] [PubMed] [Google Scholar]
- 10.Michaelson L. V., C. M. Lazarus, G. Griffiths, J. A. Napier, and A. K. Stobart. 1998. Isolation of a Delta5-fatty acid desaturase gene from Mortierella alpina. J. Biol. Chem. 273 19055–19059. [DOI] [PubMed] [Google Scholar]
- 11.Napier J. A., S. J. Hey, D. J. Lacey, and P. R. Shewry. 1998. Identification of a Caenorhabditis elegans Delta6-fatty-acid-desaturase by heterologous expression in Saccharomyces cerevisiae. Biochem. J. 330 611–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y. S., S. Chaudhary, J. M. Thurmond, E. G. Bobik, Jr., L. Yuan, G. M. Chan, S. J. Kirchner, P. Mukerji, and D. S. Knutzon. 1999. Cloning of delta12- and delta6-desaturases from Mortierella alpina and recombinant production of gamma-linolenic acid in Saccharomyces cerevisiae. Lipids. 34 649–659. [DOI] [PubMed] [Google Scholar]
- 13.Aki T., Y. Shimada, K. Inagaki, H. Higashimoto, S. Kawamoto, S. Shigeta, K. Ono, and O. Suzuki. 1999. Molecular cloning and functional characterization of rat delta-6 fatty acid desaturase. Biochem. Biophys. Res. Commun. 255 575–579. [DOI] [PubMed] [Google Scholar]
- 14.Cho H. P., M. T. Nakamura, and S. D. Clarke. 1999. Cloning, expression, and nutritional regulation of the mammalian Delta-6 desaturase. J. Biol. Chem. 274 471–477. [DOI] [PubMed] [Google Scholar]
- 15.Cho H. P., M. Nakamura, and S. D. Clarke. 1999. Cloning, expression, and fatty acid regulation of the human delta-5 desaturase. J. Biol. Chem. 274 37335–37339. [DOI] [PubMed] [Google Scholar]
- 16.Marquardt A., H. Stohr, K. White, and B. H. Weber. 2000. cDNA cloning, genomic structure, and chromosomal localization of three members of the human fatty acid desaturase family. Genomics. 66 175–183. [DOI] [PubMed] [Google Scholar]
- 17.Qiu X., H. Hong, and S. L. MacKenzie. 2001. Identification of a Delta 4 fatty acid desaturase from Thraustochytrium sp. involved in the biosynthesis of docosahexanoic acid by heterologous expression in Saccharomyces cerevisiae and Brassica juncea. J. Biol. Chem. 276 31561–31566. [DOI] [PubMed] [Google Scholar]
- 18.Korn E. D. 1964. The polyunsaturated 20-carbon and 22-carbon fatty acids of Euglena. Biochem. Biophys. Res. Commun. 14 1–6. [DOI] [PubMed] [Google Scholar]
- 19.Korn E. D. 1964. Biosynthesis of unsaturated fatty acids in Acanthamoeba Sp. J. Biol. Chem. 239 396–400. [PubMed] [Google Scholar]
- 20.Lees A. M., and E. D. Korn. 1966. Metabolism of unsaturated fatty acids in protozoa. Biochemistry. 5 1475–1481. [DOI] [PubMed] [Google Scholar]
- 21.Qi B., F. Beaudoin, T. Fraser, A. K. Stobart, J. A. Napier, and C. M. Lazarus. 2002. Identification of a cDNA encoding a novel C18-Delta(9) polyunsaturated fatty acid-specific elongating activity from the docosahexaenoic acid (DHA)-producing microalga, Isochrysis galbana. FEBS Lett. 510 159–165. [DOI] [PubMed] [Google Scholar]
- 22.Wallis J. G., and J. Browse. 1999. The Delta8-desaturase of Euglena gracilis: an alternate pathway for synthesis of 20-carbon polyunsaturated fatty acids. Arch. Biochem. Biophys. 365 307–316. [DOI] [PubMed] [Google Scholar]
- 23.Sayanova O., R. Haslam, B. Qi, C. M. Lazarus, and J. A. Napier. 2006. The alternative pathway C20 Delta8-desaturase from the non-photosynthetic organism Acanthamoeba castellanii is an atypical cytochrome b5-fusion desaturase. FEBS Lett. 580 1946–1952. [DOI] [PubMed] [Google Scholar]
- 24.Chu F. L., E. D. Lund, E. Harvey, and R. Adlof. 2004. Arachidonic acid synthetic pathways of the oyster protozoan parasite, Perkinsus marinus: evidence for usage of a delta-8 pathway. Mol. Biochem. Parasitol. 133 45–51. [DOI] [PubMed] [Google Scholar]
- 25.Nakazawa I., J. F. Mead, and R. H. Yonemoto. 1976. In vitro activity of the fatty acyl desaturases of human cancerous and noncancerous tissues. Lipids. 11 79–82. [DOI] [PubMed] [Google Scholar]
- 26.Bardon S., M. T. Le, and J. M. Alessandri. 1996. Metabolic conversion and growth effects of n-6 and n-3 polyunsaturated fatty acids in the T47D breast cancer cell line. Cancer Lett. 99 51–58. [DOI] [PubMed] [Google Scholar]
- 27.Albert D. H., and J. G. Coniglio. 1977. Metabolism of eicosa-11,14-dienoic acid in rat testes. Evidence for delta8-desaturase activity. Biochim. Biophys. Acta. 489 390–396. [DOI] [PubMed] [Google Scholar]
- 28.Albert D. H., R. K. Rhamy, and J. G. Coniglio. 1979. Desaturation of eicosa-11,14-dienoic acid in human testes. Lipids. 14 498–500. [DOI] [PubMed] [Google Scholar]
- 29.Schenck P. A., H. Rakoff, and E. A. Emken. 1996. Delta 8 desaturation in vivo of deuterated eicosatrienoic acid by mouse liver. Lipids. 31 593–600. [DOI] [PubMed] [Google Scholar]
- 30.Chen Q., F. Q. Yin, and H. Sprecher. 2000. The questionable role of a microsomal delta8 acyl-coA-dependent desaturase in the biosynthesis of polyunsaturated fatty acids. Lipids. 35 871–879. [DOI] [PubMed] [Google Scholar]
- 31.Malerba G., L. Schaeffer, L. Xumerle, N. Klopp, E. Trabetti, M. Biscuola, U. Cavallari, R. Galavotti, N. Martinelli, P. Guarini, et al. 2008. SNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular disease. Lipids. 43 289–299. [DOI] [PubMed] [Google Scholar]
- 32.Schaeffer L., H. Gohlke, M. Muller, I. M. Heid, L. J. Palmer, I. Kompauer, H. Demmelmair, T. Illig, B. Koletzko, and J. Heinrich. 2006. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet. 15 1745–1756. [DOI] [PubMed] [Google Scholar]
- 33.D'Andrea S., H. Guillou, S. Jan, D. Catheline, J. N. Thibault, M. Bouriel, V. Rioux, and P. Legrand. 2002. The same rat Delta6-desaturase not only acts on 18- but also on 24-carbon fatty acids in very-long-chain polyunsaturated fatty acid biosynthesis. Biochem. J. 364 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guillou H., V. Rioux, D. Catheline, J. N. Thibault, M. Bouriel, S. Jan, S. D'Andrea, and P. Legrand. 2003. Conversion of hexadecanoic acid to hexadecenoic acid by rat Delta 6-desaturase. J. Lipid Res. 44 450–454. [DOI] [PubMed] [Google Scholar]
- 35.Ge L., J. S. Gordon, C. Hsuan, K. Stenn, and S. M. Prouty. 2003. Identification of the delta-6 desaturase of human sebaceous glands: expression and enzyme activity. J. Invest. Dermatol. 120 707–714. [DOI] [PubMed] [Google Scholar]
- 36.Garces R., and M. Mancha. 1993. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal. Biochem. 211 139–143. [DOI] [PubMed] [Google Scholar]
- 37.Van Pelt C. K., and J. T. Brenna. 1999. Acetonitrile chemical ionization tandem mass spectrometry to locate double bonds in polyunsaturated fatty acid methyl esters. Anal. Chem. 71 1981–1989. [DOI] [PubMed] [Google Scholar]
- 38.Lawrence P., and J. T. Brenna. 2006. Acetonitrile covalent adduct chemical ionization mass spectrometry for double bond localization in non-methylene-interrupted polyene fatty acid methyl esters. Anal. Chem. 78 1312–1317. [DOI] [PubMed] [Google Scholar]
- 39.Michaud A. L., G. Y. Diau, R. Abril, and J. T. Brenna. 2002. Double bond localization in minor homoallylic fatty acid methyl esters using acetonitrile chemical ionization tandem mass spectrometry. Anal. Biochem. 307 348–360. [DOI] [PubMed] [Google Scholar]
- 40.Diau G. Y., A. T. Hsieh, E. A. Sarkadi-Nagy, V. Wijendran, P. W. Nathanielsz, and J. T. Brenna. 2005. The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med. 3 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirokawa T., S. Boon-Chieng, and S. Mitaku. 1998. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics. 14 378–379. [DOI] [PubMed] [Google Scholar]
- 42.Thomasson H. J. 1953. Biological standardization of essential fatty acids; a new method. Int. Z. Vitaminforsch. 25 (Beih): 62–82. [PubMed] [Google Scholar]
- 43.Stubhaug I., D. R. Tocher, J. G. Bell, J. R. Dick, and B. E. Torstensen. 2005. Fatty acid metabolism in Atlantic salmon (Salmo salar L.) hepatocytes and influence of dietary vegetable oil. Biochim. Biophys. Acta. 1734 277–288. [DOI] [PubMed] [Google Scholar]
- 44.Rzehak P., J. Heinrich, N. Klopp, L. Schaeffer, S. Hoff, G. Wolfram, T. Illig, and J. Linseisen. 2009. Evidence for an association between genetic variants of the fatty acid desaturase 1 fatty acid desaturase 2 (FADS1 FADS2) gene cluster and the fatty acid composition of erythrocyte membranes. Br. J. Nutr. 101 20–26. [DOI] [PubMed] [Google Scholar]
- 45.Kusunoki M., K. Tsutsumi, M. Nakayama, T. Kurokawa, T. Nakamura, H. Ogawa, Y. Fukuzawa, M. Morishita, T. Koide, and T. Miyata. 2007. Relationship between serum concentrations of saturated fatty acids and unsaturated fatty acids and the homeostasis model insulin resistance index in Japanese patients with type 2 diabetes mellitus. J. Med. Invest. 54 243–247. [DOI] [PubMed] [Google Scholar]
- 46.Thomas E. A., and J. K. Yao. 2007. Clozapine specifically alters the arachidonic acid pathway in mice lacking apolipoprotein D. Schizophr. Res. 89 147–153. [DOI] [PubMed] [Google Scholar]
- 47.Sprecher H., and C. J. Lee. 1975. The absence of an 8-desaturases in rat liver: a reevaluation of optional pathways for the metabolism of linoleic and linolenic acids. Biochim. Biophys. Acta. 388 113–125. [PubMed] [Google Scholar]
- 48.Takagi T. 1965. The dehydrogenation of all-cis-5,11,14-eicosatrienoic acid to arachidonic acid. Bull. Chem. Soc. Jpn. 38 2055–2057. [Google Scholar]
- 49.Ullman D., and H. Sprecher. 1971. An in vitro and in vivo study of the conversion of eicosa-11,14-dienoic acid to eicosa-5,11,14-trienoic acid and of the conversion of eicosa-11-enoic acid to eicosa-5,11-dienoic acid in the rat. Biochim. Biophys. Acta. 248 186–197. [DOI] [PubMed] [Google Scholar]
- 50.Naval J., M. J. Martinez-Lorenzo, I. Marzo, P. Desportes, and A. Pineiro. 1993. Alternative route for the biosynthesis of polyunsaturated fatty acids in K562 cells. Biochem. J. 291 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi B., T. Fraser, S. Mugford, G. Dobson, O. Sayanova, J. Butler, J. A. Napier, A. K. Stobart, and C. M. Lazarus. 2004. Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat. Biotechnol. 22 739–745. [DOI] [PubMed] [Google Scholar]
- 52.Christiansen K., Y. Marcel, M. V. Gan, H. Mohrhauer, and R. T. Holman. 1968. Chain elongation of alpha- and gamma-linolenic acids and the effect of other fatty acids on their conversion in vitro. J. Biol. Chem. 243 2969–2974. [PubMed] [Google Scholar]
- 53.Brenner R. R., and R. O. Peluffo. 1966. Effect of saturated and unsaturated fatty acids on the desaturation in vitro of palmitic, stearic, oleic, linoleic, and linolenic acids. J. Biol. Chem. 241 5213–5219. [PubMed] [Google Scholar]
- 54.Cook H. W., D. M. Byers, F. B. Palmer, M. W. Spence, H. Rakoff, S. M. Duval, and E. A. Emken. 1991. Alternate pathways in the desaturation and chain elongation of linolenic acid, 18:3(n-3), in cultured glioma cells. J. Lipid Res. 32 1265–1273. [PubMed] [Google Scholar]
- 55.Holman R. T. 1998. The slow discovery of the importance of omega 3 essential fatty acids in human health. J. Nutr. 128 427S–433S. [DOI] [PubMed] [Google Scholar]