Abstract
Sphingolipids are ubiquitous components of eukaryotic cells that regulate various cellular functions. In many cell types, a fraction of sphingolipids contain 2-hydroxy fatty acids, produced by fatty acid 2-hydroxylase (FA2H), as the N-acyl chain of ceramide [hydroxyl fatty acid (hFA)-sphingolipids]. FA2H is highly expressed in myelin-forming cells of the nervous system and in epidermal keratinocytes. While hFA-sphingolipids are thought to enhance the physical stability of specialized membranes produced by these cells, physiological significance of hFA-sphingolipids in many other cell types is unknown. In this study, we report novel roles for FA2H and hFA-sphingolipids in the regulation of the cell cycle. Treatment of D6P2T Schwannoma cells with dibutyryl-cAMP (db-cAMP) induced exit from the cell cycle with concomitant upregulation of FA2H. Partial silencing of FA2H in D6P2T cells resulted in 60–70% reduction of hFA-dihydroceramide and hFA-ceramide, with no effect on nonhydroxy dihydroceramide and ceramide. Under these conditions, db-cAMP no longer induced cell cycle exit, and cells continued to grow and divide. Immunoblot analyses revealed that FA2H silencing prevented db-cAMP-induced upregulation of cyclin-dependent kinase inhibitors p21 and p27. These results provide evidence that FA2H is a negative regulator of the cell cycle and facilitates db-cAMP-induced cell cycle exit in D6P2T cells.
Keywords: sphingolipid, fatty acid α-hydroxylase, hydroxy fatty acids, cell cycle control, cyclin-dependent kinase inhibitor
Sphingolipids are a diverse group of lipids found in all eukaryotic cells. They are major structural components of cell membranes and involved in various cellular functions, such as cell adhesion, signaling, and membrane trafficking (1–3). The structural diversity of sphingolipids arises from >300 distinct carbohydrate head groups as well as modifications on the sphingoid base and the N-acyl chains of ceramide. The sphingoid base in mammalian sphingolipids is predominantly C18 sphingosine, whereas the N-acyl chains are highly diverse with varying chain length (C12–C34), desaturation, and hydroxylation. In mammals, sphingolipids containing 2-hydroxy fatty acids (hFA-sphingolipids) are present at high concentrations in the nervous system, comprising the major myelin lipids galactosylceramide and sulfatide (4). A number of nonneural tissues also contain various hFA-sphingolipids. Examples include hFA-ceramide in the epidermis (5); hFA-sphingomyelin in kidney, intestine, spermatozoa (6–8); and various hFA-glycosphingolipids in liver and intestine (9–12). In addition, aberrant hFA-glycosphingolipids are present in various human tumors, including ovarian tumors (13), neuroblastomas (14), small cell lung carcinomas (15), and colon and liver adenocarcinomas (16, 17). In some studies, a correlation was reported to exist between hFA-sphingolipid levels and the invasive or drug-resistant nature of tumor cells (18, 19). Despite the well-documented prevalence of hFA-sphingolipids, their specific cellular functions are unknown.
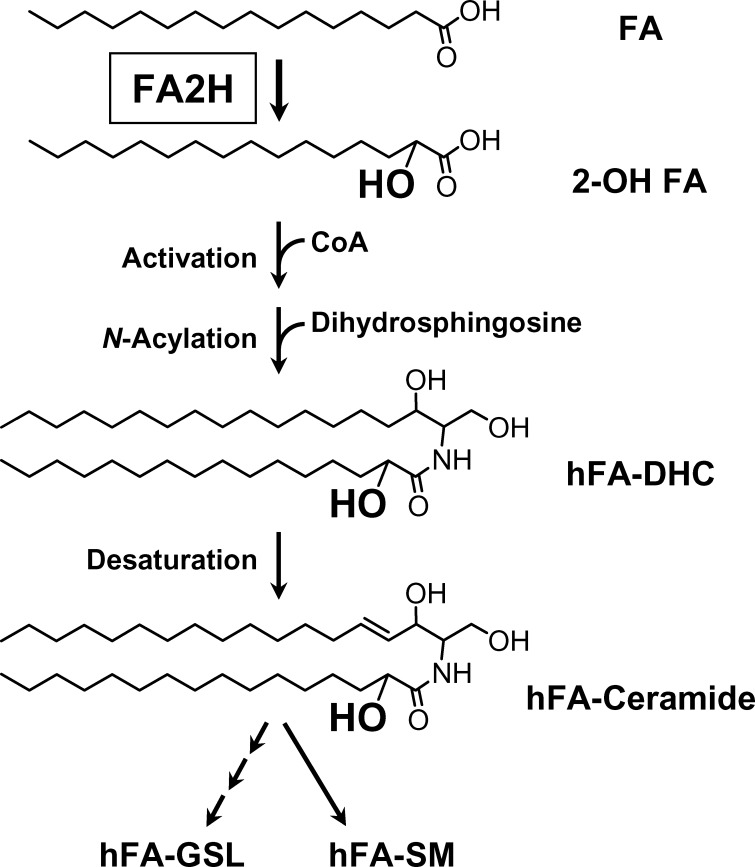
The fatty acid 2-hydroxylase FA2H catalyzes the formation of precursors for hFA-sphingolipids, 2-hydroxy fatty acids (20) (Fig. 1). FA2H is required for the synthesis of hFA-galactolipids in myelin (21–23) and hFA-ceramide and hFA-glucosylceramide in the epidermis (24). During development, FA2H expression is highly upregulated as myelin-forming cells and epidermal keratinocytes begin to produce large quantities of hFA-sphingolipids. Interestingly, silencing FA2H expression in epidermal keratinocytes not only reduced the synthesis of hFA-sphingolipids, but also resulted in profound disturbance of lamellar body formation and intracellular transport (24). In D6P2T Schwannoma cells, migration properties were enhanced by silencing FA2H expression (23). These observations suggest that FA2H and hFA-sphingolipids regulate specific intracellular processes, although underlying molecular mechanisms for these effects are unknown.
Fig. 1.
Proposed biosynthetic pathway for hFA-sphingolipids. The de novo pathway is postulated based on our in vitro and in vivo evidence (20, 22). Salvage pathways (not shown) leading to formation of hFA-sphingolipids have not been studied. In addition to C16 fatty acid, many other fatty acids (C14–C34) can be used. The N-acylation step is catalyzed by any of the six isoforms of ceramide synthase (38). GSL, glycosphingolipid; SM, sphingomyelin.
In this study, we provide evidence that FA2H regulates cAMP-induced cell cycle exit in D6P2T cells. To our knowledge, this is the first report demonstrating the involvement of FA2H in specific intracellular processes.
EXPERIMENTAL PROCEDURES
Materials
Odd chain fatty acids (C15–C21) were purchased from Matreya (Pleasant Gap, PA). Deuterated tetracosanoic acid [3,3,5,5-D4] was purchased from Larodan Fine Chemicals (Malmö, Sweden). Purified human NADPH:cytochrome P450 reductase and NADPH-regenerating system solutions were purchased from BD Biosciences (Bedford, MA). Dibutyryl cAMP (db-cAMP) and anti-β-actin antibodies were purchased from Sigm-Aldrich (St. Louis, MO). Antibodies against p21 and p27 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell cultures
Rat Schwannoma-derived D6P2T cells (25) were purchased from ATCC and grown in DMEM containing 10% FBS. Cells were harvested at ∼60–70% confluency. To silence FA2H expression, ∼2 × 106 cells were transfected with 2.5 μg of control short hairpin RNA (shRNA) or FA2H shRNA expression plasmids (23) using the Nucleofector kit T with program T-20 (Amaxa, Gaithersburg, MD). After 48 h, puromycin (1 μg/ml) was added to the culture medium, and resistant cells were selected for 2 weeks. Stably transfected cells were grown to ∼50% confluence prior to db-cAMP treatment. When indicated, cells were stained with 4 μg/ml calcein AM (Invitrogen) for 30 min at 37°C.
Determination of dihydroceramide and ceramide by LC/MS/MS
Approximately 1 × 106 cells were harvested by trypsin-EDTA treatment and washed with PBS. Internal standards (synthetic ceramides C13 Sph-C16 FA, C17 Sph-C16 FA, and C17 Sph-C24:1 FA) were added to the cells, and lipids were extracted with ethyl acetate/isopropanol/water (60:30:10, v/v). Lipid extracts were brought to dryness and reconstituted in 100 μl of methanol. The reconstituted samples were injected onto a Thermo Finnigan Surveyor liquid chromatography system with a BDS Hypersil C8 column (150 × 3.2 mm, 3 μm particle size) and eluted with a mobile phase consisting of 1 mM methanolic ammonium formate and 2 mM aqueous ammonium formate. Electrospray ionization-MS/MS analysis was performed on a Thermo Finnigan TSQ 7000 triple quadrupole mass spectrometer operating in a multiple reaction monitoring positive ionization mode. Peaks of the target analytes and internal standards were collected and processed using the Xcalibur software. Calibration curves were constructed by plotting peak area ratios of the target analyte to their respective internal standard against concentration using a linear regression model. The molecular species determined are as follows: dihydroceramide (DHC) (C12, C14, C16, C18, C18:1, C20, C20:1, C22, C22:1, C24, C24:1, C26, C26:1), hFA-DHC (HO-C14, HO-C16, HO-C18, HO-C18:1, HO-C20, HO-C22, HO-C24, HO-C24:1, HO-C26, HO-C26:1), ceramide (C18:1, C14, C16, C18, C20, C20:1, C20:4, C22, C22:1, C24, C24:1, C26, C26:1), and hFA-ceramide (HO-C12, HO-C14, HO-C16, HO-C18, HO-C18:1, HO-C20, HO-C22, HO-C24, HO-C24:1, HO-C26, HO-C26:1, HO-C28). DHC and ceramide contents were normalized to the protein contents in each sample.
Quantitative RT-PCR
Total RNA was isolated using the Qiagen RNeasy kit. cDNA was synthesized using the Bio-Rad iScript cDNA synthesis kit. Real-time quantitative PCR was performed on a BioRad MyiQ single-color, real-time PCR detection system as described (23). The abundance of FA2H mRNA was normalized against 18S rRNA content using the ΔΔCt method (26).
FA2H assay
FA2H activity was determined in D6P2T cells using the method described previously (27). Briefly, cell homogenates (50 μg of protein) were added to an assay mixture containing 2.7 mM Tris-HCl, pH 7.6, 1.20 mM NADP+, 3.3 mM glucose 6-phosphate, 3.3 mM MgCl2, 0.2 unit of glucose 6-phosphate dehydrogenase, and 1 μg of human NADPH:cytochrome P-450 reductase, in a total volume of 1.4 ml. The substrate, 1 μg (2.7 nmol) of [3,3,5,5-D4] C24 fatty acid (stock solution: 10 μg/ml in 1.5 mM α-cyclodextrin), was added at time zero and incubated at 37°C for 180 min. At the end of the incubation, 1 pmol of C23 fatty acid was added to each sample as an internal standard, and samples were acidified by an addition of glacial acetic acid (20 μl). Fatty acids were extracted three times with diethyl ether (2 ml), and combined diethyl ether extracts were brought to dryness under N2. Fatty acids were derivatized and quantified by gas chromatography-mass spectrometry as described (27).
Thymidine incorporation assay
Cells were seeded in 24-well plates and labeled with [3H]thymidine (2 μCi/ml) in a serum-free medium for 5 h. Cells were washed three times with PBS and three times with ice-cold 10% TCA. Cells were lysed in 0.2 N NaOH, and [3H]thymidine was quantified by liquid scintillation counting. [3H] counts were normalized to protein contents in unlabeled cells cultured side-by-side.
Immunoblot analysis
Cells expressing control or FA2H shRNA were treated with vehicle only, 0.1, or 1 mM db-cAMP for 24 h. Cell lysates (10 μg protein) were subjected to SDS-PAGE (4–20% gradient gel), transferred to polyvinylidene difluoride membranes, and probed with anti-p21 (1:1,000), anti-p27 (1:1,000), or anti-β-actin (1:200) antibodies. Membranes were incubated with horseradish peroxidase-linked secondary antibodies, followed by chemiluminescent detection (GE Healthcare Life Sciences).
RESULTS
FA2H silencing reduces hFA-DHC and hFA-ceramide
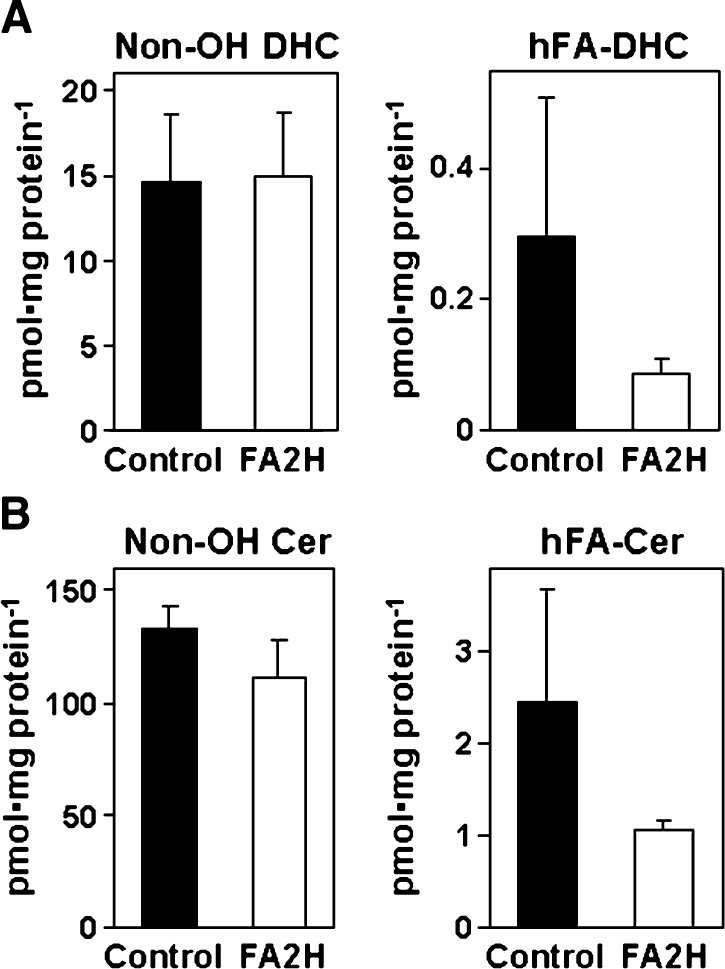
The hydrophobic moiety of all complex sphingolipids is ceramide. In the de novo synthesis of ceramide, dihydrosphingosine is N-acylated to DHC, followed by desaturation to ceramide. Based on our previous observations, we have postulated the de novo synthesis of hFA-sphingolipids as shown in Fig. 1, in which fatty acids are first converted to 2-hydroxy fatty acids prior to the synthesis of hFA-DHC. We previously showed that silencing FA2H by small interfering RNA or shRNA in rat Schwannoma D6P2T cells resulted in ∼60% reduction of total hFA content in the cell (23). Based on the proposed pathway shown in Fig. 1, cellular hFA-DHC and hFA-ceramide content would also be reduced by FA2H silencing. To confirm this point, hFA-DHC/ceramide and nonhydroxy DHC and ceramide were determined in D6P2T cells transfected with control shRNA or FA2H shRNA expression plasmid (Fig. 2). In cells with control shRNA, the total hFA-DHC/hFA-ceramide levels (including hFA-DHC with 10 distinct acyl chains and hFA-ceramide with 12 distinct acyl chains) were ∼2% of the total DHC/ceramide levels (including 13 distinct acyl chains). (See supplementary Table I for the amount of individual molecules.) While nonhydroxy DHC/ceramide levels were not significantly affected by FA2H shRNA, the mean hFA-DHC and hFA-ceramide contents were reduced by 60–70%. Because there was no effect on nonhydroxy DHC/ceramide, any phenotypic changes caused by FA2H silencing could be attributed to reduced hFA-sphingolipids.
Fig. 2.
Effects of FA2H shRNA on DHC and ceramide. D6P2T cells were transfected with control shRNA (black bars) or FA2H shRNA (white bars) plasmid. Transfected cells were selected for puromycin resistance for 2 weeks. Lipids were quantified by LC/MS/MS and normalized against protein contents. The mean and SD of triplicate measurements are shown. (See supplementary Table I for the quantities of individual molecular species.) A: The sum of 13 nonhydroxy DHC and 10 hFA-DHC molecular species. B: The sum of 13 nonhydroxy ceramide and 12 hFA-ceramide molecular species.
db-cAMP-induced upregulation of FA2H in D6P2T cells
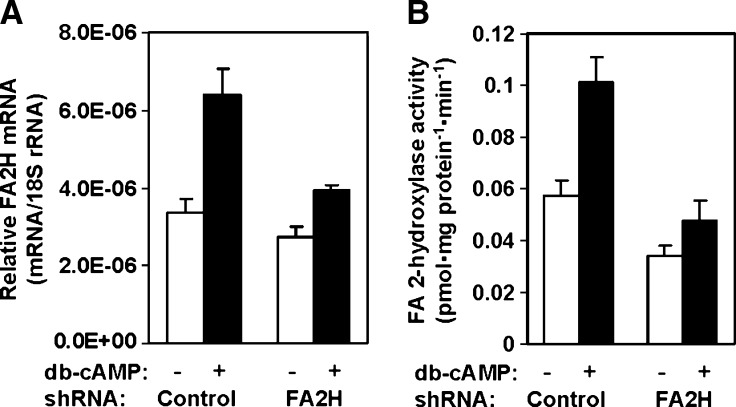
D6P2T cells have been used as a cell culture model for Schwann cell differentiation because they maintain some characteristics of Schwann cells, such as synthesis of hFA-galactolipids and myelin proteins (25, 28). Upon exposure to agents that increase cellular cAMP, D6P2T cells exhibit differentiated phenotypes reminiscent of mature Schwann cells and exit from the cell cycle (29). We have shown previously that in primary cultures of rat Schwann cells, FA2H expression dramatically increased upon exposure to db-cAMP (23). In D6P2T cells, both FA2H mRNA and activity increased 2-fold in response to 1 mM db-cAMP (Fig. 3, control shRNA). With FA2H silencing, basal FA2H mRNA and activity were reduced only modestly (by 20 and 40%, respectively). More importantly, db-cAMP-induced upregulation of FA2H was greatly impaired by FA2H silencing. The FA2H mRNA and activity in FA2H shRNA cells with db-cAMP did not increase above the basal levels in the control cells without db-cAMP treatment. Thus, FA2H silencing with the shRNA expression provided a unique opportunity to investigate specific roles for FA2H and hFA-sphingolipids in db-cAMP-induced phenotypic changes in D6P2T cells.
Fig. 3.
FA2H silencing nullifies db-cAMP-induced upregulation of FA2H. D6P2T cells were transfected with control shRNA or FA2H shRNA plasmid as indicated. Transfected cells were selected for puromycin resistance for 2 weeks and then treated with vehicle only or 1 mM db-cAMP for 48 h. A: FA2H mRNA levels. FA2H mRNA levels were determined by quantitative PCR and normalized against 18s rRNA levels. The mean and SD of triplicate measurements are shown. B: FA2H activity. Crude cell lysates (50 μg protein) were used for FA2H assays. The mean and SD of triplicate measurements are shown.
FA2H silencing prevents db-cAMP-induced cell cycle exit
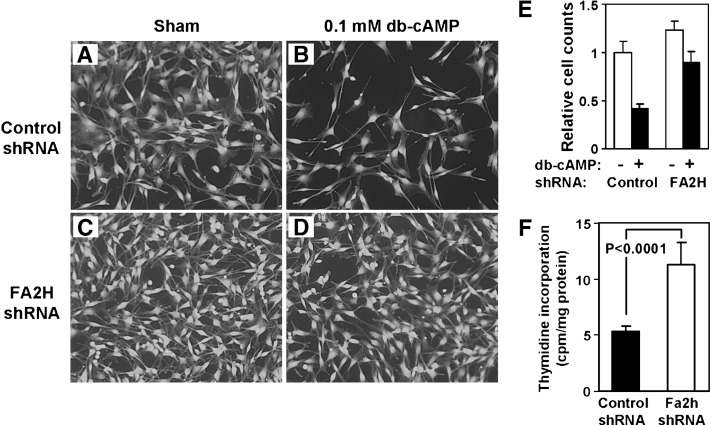
During routine cell cultures, it appeared that cells expressing FA2H shRNA propagated slightly faster than the cells expressing control shRNA. The difference in growth rate became evident when cells were treated with db-cAMP, which is known to induce cell cycle exit in D6P2T cells (30). Control shRNA cells ceased to divide when treated with db-cAMP without any visible signs of cell death or morphological changes (Fig. 4A, B). With FA2H shRNA, cells continued to propagate in the presence of db-cAMP (Fig. 4C, D). Although the difference in basal growth rates (without db-cAMP) was barely recognizable from the cell counts (Fig. 4E), a short-term labeling with [3H]thymidine in a serum-free medium at low confluency showed a 2-fold higher rate of incorporation in cells with FA2H shRNA than control shRNA (Fig. 4F). These results provide clear evidence that FA2H regulates the cell cycle in D6P2T cells.
Fig. 4.
FA2H silencing facilitates cell growth and inhibits db-cAMP-induced growth arrest in D6P2T cells. D6P2T cells were transfected with control shRNA or FA2H shRNA plasmid and selected for puromycin resistance for 2 weeks. A–D: Cells were treated with sham (A, C) or 0.1 mM db-cAMP (B, D) for 24 h before stained with calcein-AM. E: Cell counts in samples A–D. The counts were normalized against the cell number in A. The mean and SD of triplicate counts are shown. F: [3H]thymidine incorporation. Low confluency cells were exposed to [3H]thymidine for 5 h in a serum-free medium. After extensive washing, cells were lysed to quantify radioactivity by liquid scintillation counting. The mean and SD of sextuplicate measurements are shown.
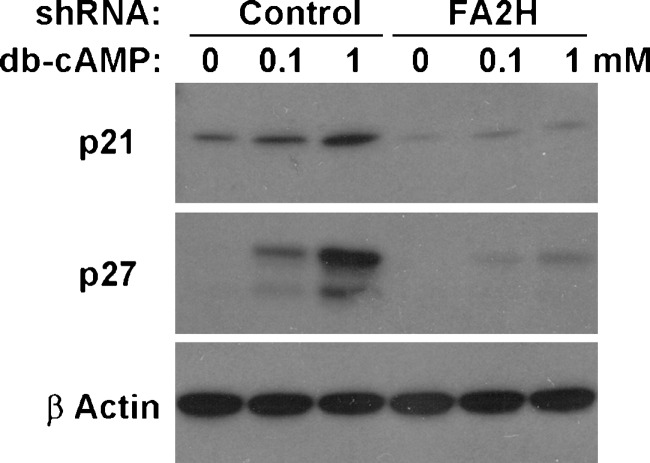
FA2H silencing prevents db-cAMP-induced upregulation of p21 and p27
cAMP-induced cell cycle exit in D6P2T cells is, at least in part, mediated by upregulation of cyclin-dependent kinase (CDK) inhibitor p27 (29). Expression of p27 is also elevated in quiescent (confluent) cultured rat Schwann cells and in rat sciatic nerve at postnatal day 2 (30). Other CDK inhibitors, p21 and p16, are required for withdrawal from the cell cycle during Schwann cell development (31). We therefore examined the levels of p21 and p27 in D6P2T cells in the presence and absence of db-cAMP and examined the effect of FA2H silencing. As shown in Fig. 5, p21 and p27 were markedly upregulated in cells with control shRNA upon db-cAMP treatment. In contrast, the upregulation was greatly diminished in cells with FA2H shRNA, consistent with the continued growth in the presence of db-cAMP. We also examined D6P2T cells overexpressing FA2H to determine if an increase in FA2H expression by itself was sufficient to induce p27. Either the basal level or db-cAMP-induced upregulation of p27 was not affected by FA2H overexpression (data not shown).
Fig. 5.
FA2H silencing prevents upregulation of CDK inhibitors induced by db-cAMP. D6P2T cells were transfected with control shRNA or FA2H shRNA plasmid. Transfected cells were selected for puromycin resistance for 2 weeks. Cells were treated with sham, 0.1 mM, or 1 mM db-cAMP for 48 h and processed for SDS-PAGE (10 μg protein/lane).
DISCUSSION
In this report, we provide evidence that FA2H plays a role in cell cycle control in D6P2T cells. FA2H was upregulated by db-cAMP, which also induced CDK inhibitors p21 and p27, leading to exit from the cell cycle. Silencing FA2H expression significantly reduced hFA-DHC and hFA-ceramide with no effect on nonhydroxy DHC/ceramide levels. Under these conditions, db-cAMP treatment failed to upregulate p21 and p27, and the cells continued to propagate. These results are consistent with a model that hFA-sphingolipids are negative regulators of the cell cycle and that FA2H facilitates the db-cAMP-induced cell cycle exit. For decades, hFA-sphingolipids have been thought to enhance physical stability of specialized membranes, such as myelin and the epidermal cornified envelope, which does not fully account for the prevalence of minute quantities of hFA-sphingolipids in many cell types. Although our previous studies provided some evidence for additional functions beyond the structural role (23, 24), this is the first report of a regulatory function of FA2H in a specific intracellular signaling process. It is notable that a relatively modest reduction of FA2H expression, affecting a small fraction of sphingolipids, could bring about significant phenotypic changes. It exemplifies the sensitivity and complexity of lipid-mediated cell signaling and, at the same time, points to the necessity of defining structural details of bioactive lipids with regard to their acyl chains.
Cellular levels and activities of CDK inhibitors are regulated by mechanisms that involve protein phosphorylation/dephosphorylation at multiple junctures (32, 33). Ceramide is an extensively studied sphingolipid signaling molecule that activates protein kinases and phosphatases (34, 35). There are reports showing the involvement of ceramide in cAMP-mediated signaling pathways (36, 37). It is conceivable that some of the ceramide-activated protein kinases and phosphatases have higher affinity for hFA-ceramide than for nonhydroxy ceramide, rendering the pathway highly responsive to the activity of FA2H. Indeed, our preliminary studies indicate that phosphorylation status of a number of proteins in D6P2T cells is altered by FA2H silencing (unpublished observations). Identification of these proteins, as well as protein kinases/phosphatases responsive to hFA-ceramide, or other hFA-sphingolipids, would provide further insight into cellular function of FA2H.
It should be emphasized that hFA-sphingolipids are more prevalent than commonly recognized. There have been numerous reports describing hFA-sphingolipids in various tissues and cell types in the past 50 years. Northern blot analyses by two groups showed that FA2H is expressed in many organs (20, 21). In a recent study, Mizushima et al. (38) reported that all six isoforms of ceramide synthase (LASS/CerS) can synthesize hFA-ceramide. In our unpublished studies, we have yet to encounter any biological samples (tissues, cells, or serum) with no FA2H or hFA, although their abundance could be diminutively low in some specimens. These observations suggest a possibility that hFA-sphingolipids are ubiquitous bioactive molecules and that FA2H plays important regulatory roles by fine-tuning the level of hFA-sphingolipids.
The low abundance of hFA-sphingolipids in many cell types poses a technical challenge in studying these lipids. Conventional lipid analytical methods sometimes fail to identify hFA in the presence of vastly more abundant nonhydroxy FA. Typically, it requires the use of an electron impact mass spectrometer or an electrospray tandem mass spectrometer to identify and quantify hFA and hFA-sphingolipids. For this reason, it is likely that hFA and hFA-sphingolipids are often overlooked. Some of the bioactivities of sphingolipids may be attributable to a small fraction of hFA-sphingolipids within.
An intriguing question is whether hFA is limited to acyl chains of sphingolipids and not found in glycerolipids in eukaryotes. There is evidence in the literature for hFA-glycerolipids in yeast mutants that cannot synthesize sphingolipids (39). Thus, glycerolipid acyltransferases may be capable of using hFA. This issue warrants further investigation and has the potential to be another exciting area of lipid-mediated cell signaling.
Supplementary Material
Acknowledgments
The authors thank the Medical University of South Carolina Lipidomics Core Facility for the ceramide/DHC measurements. The authors also thank Drs. Chiara Luberto and Steven Rosenzweig for helpful discussions.
Abbreviations
CDK, cyclin-dependent kinase
db-cAMP, dibutyryl adenosine 3′,5′-cyclic monophosphate
DHC, dihydroceramide
FA2H, fatty acid 2-hydroxylase
hFA, hydroxy fatty acid
shRNA, short hairpin RNA
Published, JLR Papers in Press, January 23, 2009.
This work was supported by National Institutes of Health Grants RR-17677 and NS-060807.
Footnotes
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of a table.
References
- 1.Hakomori S. 2003. Structure, organization, and function of glycosphingolipids in membrane. Curr. Opin. Hematol. 10 16–24. [DOI] [PubMed] [Google Scholar]
- 2.Sillence D. J., and F. M. Platt. 2004. Glycosphingolipids in endocytic membrane transport. Semin. Cell Dev. Biol. 15 409–416. [DOI] [PubMed] [Google Scholar]
- 3.Futerman A. H., and Y. A. Hannun. 2004. The complex life of simple sphingolipids. EMBO Rep. 5 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norton, W. T., and W. Cammer. 1984. Isolation and characterization of myelin. In Myelin. P. Morell, editor. Plenum, New York, NY. 147–195.
- 5.Downing D. T. 1992. Lipid and protein structures in the permeability barrier of mammalian epidermis. J. Lipid Res. 33 301–313. [PubMed] [Google Scholar]
- 6.Breimer M. E. 1975. Distribution of molecular species of sphingomyelins in different parts of bovine digestive tract. J. Lipid Res. 16 189–194. [PubMed] [Google Scholar]
- 7.Breimer M. E., K. A. Karlsson, and B. E. Samuelsson. 1975. Presence of phytosphingosine combined with 2-hydroxy fatty acids in sphingomyelins of bovine kidney and intestinal mucosa. Lipids. 10 17–19. [DOI] [PubMed] [Google Scholar]
- 8.Robinson B. S., D. W. Johnson, and A. Poulos. 1992. Novel molecular species of sphingomyelin containing 2-hydroxylated polyenoic very-long-chain fatty acids in mammalian testes and spermatozoa. J. Biol. Chem. 267 1746–1751. [PubMed] [Google Scholar]
- 9.Bouhours J. F., D. Bouhours, and G. C. Hansson. 1993. Developmental changes of glycosphingolipid composition of epithelia of rat digestive tract. Adv. Lipid Res. 26 353–372. [PubMed] [Google Scholar]
- 10.Dahiya R., M. D. Brown, and T. A. Brasitus. 1986. Distribution of glycosphingolipids of monkey small and large intestinal mucosa. Lipids. 21 107–111. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson O., and L. Svennerholm. 1982. Characterization and quantitative determination of gangliosides and neutral glycosphingolipids in human liver. J. Lipid Res. 23 327–334. [PubMed] [Google Scholar]
- 12.Riboni L., D. Acquotti, R. Casellato, R. Ghidoni, G. Montagnolo, A. Benevento, L. Zecca, F. Rubino, and S. Sonnino. 1992. Changes of the human liver GM3 ganglioside molecular species during aging. Eur. J. Biochem. 203 107–113. [DOI] [PubMed] [Google Scholar]
- 13.Kiguchi K., K. Takamatsu, J. Tanaka, S. Nozawa, M. Iwamori, and Y. Nagai. 1992. Glycosphingolipids of various human ovarian tumors: a significantly high expression of I3SO3GalCer and lewis antigen in mucinous cystadenocarcinoma. Cancer Res. 52 416–421. [PubMed] [Google Scholar]
- 14.Ladisch S., C. C. Sweeley, H. Becker, and D. Gage. 1989. Aberrant fatty acyl α-hydroxylation in human neuroblastoma tumor gangliosides. J. Biol. Chem. 264 12097–12105. [PubMed] [Google Scholar]
- 15.Nilsson O., F. T. Brezicka, J. Holmgren, S. Sorenson, L. Svennerholm, F. Yngvason, and L. Lindholm. 1986. Detection of a ganglioside antigen associated with small cell lung carcinomas using monoclonal antibodies directed against fucosyl-GM1. Cancer Res. 46 1403–1407. [PubMed] [Google Scholar]
- 16.Hakomori S., E. Nudelman, S. B. Levery, and R. Kannagi. 1984. Novel fucolipids accumulating in human adenocarcinoma. I. Glycolipids with di- or trifucosylated type 2 chain. J. Biol. Chem. 259 4672–4680. [PubMed] [Google Scholar]
- 17.Yang H. J., and S. I. Hakomori. 1971. A sphingolipid having a novel type of ceramide and lacto-N-fucopentaose III. J. Biol. Chem. 246 1192–1200. [PubMed] [Google Scholar]
- 18.Ugorski M., P. Pahlsson, D. Dus, B. Nilsson, and C. Radzikowski. 1989. Glycosphingolipids of human urothelial cell lines with different grades of transformation. Glycoconj. J. 6 303–318. [DOI] [PubMed] [Google Scholar]
- 19.Kiguchi K., Y. Iwamori, N. Suzuki, Y. Kobayashi, B. Ishizuka, I. Ishiwata, T. Kita, Y. Kikuchi, and M. Iwamori. 2006. Characteristic expression of globotriaosyl ceramide in human ovarian carcinoma-derived cells with anticancer drug resistance. Cancer Sci. 97 1321–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alderson N. L., B. M. Rembiesa, M. D. Walla, A. Bielawska, J. Bielawski, and H. Hama. 2004. The human FA2H gene encodes a fatty acid 2-hydroxylase. J. Biol. Chem. 279 48562–48568. [DOI] [PubMed] [Google Scholar]
- 21.Eckhardt M., A. Yaghootfam, S. N. Fewou, I. Zoller, and V. Gieselmann. 2005. A mammalian fatty acid hydroxylase responsible for the formation of alpha-hydroxylated galactosylceramide in myelin. Biochem. J. 388 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alderson N. L., E. N. Maldonado, M. J. Kern, N. R. Bhat, and H. Hama. 2006. FA2H-dependent fatty acid 2-hydroxylation in postnatal mouse brain. J. Lipid Res. 47 2772–2780. [DOI] [PubMed] [Google Scholar]
- 23.Maldonado E. N., N. L. Alderson, P. V. Monje, P. M. Wood, and H. Hama. 2008. FA2H is responsible for the formation of 2-hydroxy galactolipids in peripheral nervous system myelin. J. Lipid Res. 49 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchida Y., H. Hama, N. L. Alderson, S. Douangpanya, Y. Wang, D. A. Crumrine, P. M. Elias, and W. M. Holleran. 2007. Fatty acid 2-hydroxylase, encoded by FA2H, accounts for differentiation-associated increase in 2-OH ceramides during keratinocyte differentiation. J. Biol. Chem. 282 13211–13219. [DOI] [PubMed] [Google Scholar]
- 25.Bansal R., and S. E. Pfeiffer. 1987. Regulated galactolipid synthesis and cell surface expression in schwann cell line D6P2T. J. Neurochem. 49 1902–1911. [DOI] [PubMed] [Google Scholar]
- 26.Winer J., C. K. Jung, I. Shackel, and P. M. Williams. 1999. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal. Biochem. 270 41–49. [DOI] [PubMed] [Google Scholar]
- 27.Alderson N. L., M. D. Walla, and H. Hama. 2005. A novel method for the measurement of in vitro fatty acid 2-hydroxylase activity by gas chromatography-mass spectrometry. J. Lipid Res. 46 1569–1575. [DOI] [PubMed] [Google Scholar]
- 28.Hai M., N. Muja, G. H. DeVries, R. H. Quarles, and P. I. Patel. 2002. Comparative analysis of Schwann cell lines as model systems for myelin gene transcription studies. J. Neurosci. Res. 69 497–508. [DOI] [PubMed] [Google Scholar]
- 29.Friessen A. J., W. K. Miskimins, and R. Miskimins. 1997. Cyclin-dependent kinase inhibitor p27kip1 is expressed at high levels in cells that express a myelinating phenotype. J. Neurosci. Res. 50 373–382. [DOI] [PubMed] [Google Scholar]
- 30.Tikoo R., G. Zanazzi, D. Shiffman, J. Salzer, and M. V. Chao. 2000. Cell cycle control of schwann cell proliferation: Role of cyclin-dependent kinase-2. J. Neurosci. 20 4627–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atanasoski S., D. Boller, L. De Ventura, H. Koegel, M. Boentert, P. Young, S. Werner, and U. Suter. 2006. Cell cycle inhibitors p21 and p16 are required for the regulation of Schwann cell proliferation. Glia. 53 147–157. [DOI] [PubMed] [Google Scholar]
- 32.Besson A., S. F. Dowdy, and J. M. Roberts. 2008. CDK inhibitors: cell cycle regulators and beyond. Dev. Cell. 14 159–169. [DOI] [PubMed] [Google Scholar]
- 33.Chu I. M., L. Hengst, and J. M. Slingerland. 2008. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat. Rev. Cancer. 8 253–267. [DOI] [PubMed] [Google Scholar]
- 34.Hannun Y. A., and L. M. Obeid. 2008. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9 139–150. [DOI] [PubMed] [Google Scholar]
- 35.Ruvolo P. P. 2003. Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol. Res. 47 383–392. [DOI] [PubMed] [Google Scholar]
- 36.Schneider C., G. M. Rath, N. Delorme, H. El Btaouri, W. Hornebeck, and L. Martiny. 2005. Interleukin-1beta (IL-1beta) induces a crosstalk between camp and ceramide signaling pathways in thyroid epithelial cells. Biochimie. 87 1121–1126. [DOI] [PubMed] [Google Scholar]
- 37.See V., B. Koch, and J. P. Loeffler. 2001. C2-ceramide and reactive oxygen species inhibit pituitary adenylate cyclase activating polypeptide (PACAP)-induced cyclic-AMP-dependent signalling pathway. J. Neurochem. 76 778–788. [DOI] [PubMed] [Google Scholar]
- 38.Mizutani Y., A. Kihara, H. Chiba, H. Tojo, and Y. Igarashi. 2008. 2-hydroxy-ceramide synthesis by ceramide synthase family: enzymatic basis for the preference of FA chain length. J. Lipid Res. 49 2356–2364. [DOI] [PubMed] [Google Scholar]
- 39.Dickson R. C., G. B. Wells, A. Schmidt, and R. L. Lester. 1990. Isolation of mutant Saccharomyces cerevisiae strains that survive without sphingolipids. Mol. Cell. Biol. 10 2176–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.