Abstract
Catabolism of HDL particles is accelerated in type 2 diabetes, leading to a reduction in plasma residence time, which may be detrimental. Rosuvastatin is the most powerful statin to reduce LDL-cholesterol, but its effects on HDL metabolism in type 2 diabetes remain unknown. We performed a randomized double-blind cross-over trial of 6-week treatment period with placebo or rosuvastatin 20 mg in eight patients with type 2 diabetes. An in vivo kinetic study of HDL-apolipoprotein A-I (apoA-I) with 13C leucine was performed at the end of each treatment period. Moreover, a similar kinetic study was carried out in eight nondiabetic normolipidemic controls. Rosuvastatin significantly reduced plasma LDL-cholesterol (−51%), triglycerides (TGs) (−38%), and HDL-TG (−23%). HDL-apoA-I fractional catabolic rate (FCR) was decreased by rosuvastatin (0.25 ± 0.06 vs. 0.32 ± 0.07 pool/day, P = 0.011), leading to an increase in plasma HDL-apoA-I residence time (4.21 ± 1.02 vs. 3.30 ± 0.73 day, P = 0.011). Treatment with rosuvastatin was associated with a concomitant reduction of HDL-apoA-I production rate. The decrease in HDL-apoA-I FCR, induced by rosuvastatin, was correlated with the reduction of plasma TGs and HDL-TG. HDL apoA-I FCR and production rate values in diabetic patients on rosuvastatin were not different from those found in controls. Rosuvastatin is responsible for a 22% reduction of HDL-apoA-I FCR and restores to normal the increased HDL turnover observed in type 2 diabetes. These kinetic modifications may have beneficial effects by increasing HDL plasma residence time.
Keywords: HDL-cholesterol, kinetic, statin
Cardiovascular disease is the major cause of morbidity and mortality in patients with type 2 diabetes, and cardiovascular disease risk is 2- to 4-fold increased over nondiabetic subjects (1–4). Abnormalities of lipid metabolism, observed in type 2 diabetes, are one of the major factors contributing to vascular risk (5, 6). Diabetic dyslipidemia includes increased plasma triglycerides (TGs), decreased HDL-cholesterol levels, and qualitative lipoprotein abnormalities, such as TG enrichment of LDL and HDL particles (7, 8). Low HDL-cholesterol level, in patients with type 2 diabetes, has been shown to be due to increased catabolism of HDL lipoproteins by in vivo kinetic studies using radioisotopes (9) or stable isotopes (10). This increased catabolism of HDL particles leads automatically to significantly decrease their plasma residence time. The cardiovascular protective role of HDL is thought to be mainly due to its role in reverse cholesterol transport (11) and potentially to the antioxidative, anti-inflammatory, antithrombotic, and endothelium-dependent vasorelaxant effects of HDL particles (12). The increased catabolism of HDL lipoproteins is likely to reduce the cardioprotective effects of HDLs in patients with type 2 diabetes.
Rosuvastatin is a statin that has been shown to reduce LDL-cholesterol more than the other statins at an equivalent dose (13). In addition, rosuvastatin significantly decreases plasma TG level with larger effect than pravastatin or simvastatin (13). A modest increase in plasma HDL-cholesterol level, sometimes significant, has been observed with rosuvastatin in patients with hypercholesterolemia (14, 15) or diabetes (16, 17). However, the effects of rosuvastatin on the metabolism of HDL remain unknown in patients with type 2 diabetes. This prompted us to perform an in vivo kinetic study using stable isotopes in patients with type 2 diabetes to assess the effect of 20 mg/day rosuvastatin on the metabolism of apolipoprotein A-I (apoA-I), the major apolipoprotein of HDL. We also aimed to compare the HDL-apoA-I kinetic values obtained in patients with type 2 diabetes on rosuvastatin treatment with those found in normal nondiabetic individuals.
MATERIALS AND METHODS
Subjects
Eight patients with type 2 diabetes (five men and three women) and typical diabetic dyslipidemia defined by TG ≥150 mg/dl (1.71 mmol/l) and HDL-cholesterol <40 mg/dl (1.03 mmol/l) in men and <50 mg/dl (1.29 mmol/l) in women were recruited. These patients were treated with oral glucose lowering agents (metformin alone in four patients and metformin + sulfonylureas or glinides in four patients) for at least 6 months and had stable HbA1c during the last 6 months. Patients with HbA1c >9%, LDL-cholesterol >190 mg/dl (4.90 mmol/l), cardiovascular disease, renal impairment (creatinine clearance <30 ml/min), abnormal liver or muscle enzymes, history of alcohol and/or drug abuse, hyper- or hypothyroidism, insulin treatment, and use of drugs known to affect lipid metabolism (corticoids, retinoids, antiproteases, estrogens, cyclosporin, glitazones, statins other than rosuvastatin, fibrates, cholestyramine, ezetimibe, nicotinic acid, omega 3, or phytosterols) were excluded. No patient but one had microalbuminuria (>20 mg/l). The protocol was approved by the Dijon University Hospital ethics committee, and written informed consent was obtained from each subject before the study.
Eight nondiabetic normolipidemic controls (five men and three women) were also studied. All normal subjects were in good health, with fasting blood glucose <100 mg/dl (5.55 mmol/l) and normal plasma lipid levels. They were not taking any medication. All the women included in the study were not taking oral contraceptives.
Study design
This was a randomized, double blinded, placebo-controlled, and cross-over trial. Eligible patients with type 2 diabetes entered a 4-week placebo maintenance (weight, glucose, lipids) lead-in period followed by randomization to a 6-week treatment period of either 20 mg rosuvastatin or placebo taken orally once daily with cross-over to a further 6-week treatment period. Patients were advised to continue on isocaloric diets and to maintain physical activity constant. Compliance was assessed by tablet count.
Two kinetic studies were performed in each type 2 diabetic patient: the first one at the end of the first treatment period and the second one at the end of the second treatment period. One kinetic study was performed in each control subject.
The day before the kinetic study, each individual was admitted to the diabetology ward in the morning after a 12-h fast for physical examination and blood sampling. The following day, kinetic study was performed in the fed state. Food intake, with a leucine-poor diet (1700 kcal/day, 55% carbohydrates, 39% fats, and 7% proteins), was fractionated in small portions that were provided every 2 h starting 6 h prior to the tracer infusion up to the end of the study to avoid important variations in apolipoprotein plasma concentration, as previously performed by our group (18, 19) and others (20). The endogenous labeling of apoA-I was carried out by administration of l-[1-13C] leucine (99 atom %, w/v; Eurisotop, Saint Aubin, France), dissolved in 0.9% (w/v) NaCl solution. At 08:00 AM, each subject received intravenously a primed infusion of 0.7 mg·kg−1 of tracer, immediately followed by a 16 h constant infusion of 0.7 mg·kg−1·h−1. Blood samples were collected at 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 14, 15, and 16 h after the primed infusion. Serum was separated by centrifugation for 10 min at 4°C and 3,000 g. To avoid the influence of acute exercise on lipid metabolism, all studied subjects were instructed to refrain from strenuous exercise 3 d prior to the kinetic study.
Analytical procedures
Analytical procedures were performed as previously described (18, 21).
Isolation of apolipoproteins
VLDL1 lipoproteins were isolated from plasma by gradient ultracentrifugation using a SW41 rotor in a L90 apparatus (Beckman Instruments, Palo Alto, CA) at a flotation rate (Svedberg flotation 60–400). HDL lipoproteins were isolated from the LDL (Svedberg flotation 0–12) infranant by sequential ultracentrifugation using a 50.4 rotor in a L7 apparatus (Beckman Instruments) at a density range between 1.063 and 1.21 g/ml. VLDL1 and HDL fractions were then dialyzed against a 10 mmol/l ammonium bicarbonate buffer, pH 8.2, containing 0.01% (w/v) EDTA and 0.013% (w/v) sodium azide. VLDL1 and HDL fractions were delipidated 1 h at −20°C using 10 volumes of diethylether-ethanol 3:1. Apolipoproteins from each lipoprotein fraction (VLDL1-apoB100 and HDL-apoA-I) were isolated by preparative discontinuous SDS-PAGE on a 3% (w/v) and 15% (w/v) gel. After staining with Coomassie blue R-250, apolipoprotein bands were excised from polyacrylamide gels and hydrolyzed in 6 M HCl at 110°C for 16 h under nitrogen. Samples were then centrifuged to remove polyacrylamide. Supernatants were lyophilized in a Speed Vac (Savant Instrument, Farmingdale, NY). Lyophilized samples were dissolved in 50% (v/v) acetic acid and applied to an AG-50W-X8 200-400 mesh cation exchange resin (Bio-Rad, Richmond, CA), and amino acids were recovered by elution with 4 N NH4OH.
Determination of leucine enrichment by gas chromatography/combustion/isotope ratio mass spectrometry
Amino acids were converted to N-acetyl O-propyl esters and were analyzed on a Finnigan Mat Delta Plus Advantage isotope ratio mass spectrometer (Finnigan Mat, Bremen, Germany). 13C leucine enrichment was initially expressed in delta ‰ and converted in tracer/tracee ratio prior to modeling (22).
Modeling
Kinetic data were analyzed with the simulation analysis and modeling SAAM II program (23) (SAAM Institute, Seattle, WA). apoA-I and apoB-100 data were analyzed using the following monoexponential function: A(t) = Ap(1−exp[−k(t−d)]), where A(t) is the apolipoprotein enrichment at time t, Ap the enrichment at the plateau of the VLDL1-apoB100 curve, d the delay between the beginning of the experiment and the appearance of tracer in the apolipoprotein, and k the fractional synthetic rate (FSR) of the apolipoprotein (22, 24). It was assumed that the VLDL1-apoB100 tracer/tracee ratio at plateau corresponds to the tracer/tracee ratio of the leucine precursor pool (24). This estimation is made upon the assumption that apoB-100 and the great majority of apoA-I are synthesized by the liver, as previously demonstrated by Ikewaki et al. (24). When VLDL1-apoB enrichment curve did not reach plateau, the plateau variable was introduced into the SAAM model as adjustable variable. In this case, the plateau value was determined by the SAAM program by the fitting of the VLDL1-apoB enrichment curve. In the steady state, the FSR equals the fractional catabolic rate (FCR) (22).
The apoA-I production rate (PR) was calculated as the product of the apoA-I pool size and its FSR divided by body weight. Pool size was estimated by the product of apoA-I plasma concentration and plasma volume, calculated as 4.5% of body weight (21). Because this formula overestimates plasma volume in obese subjects, plasma volume was modified in individuals featuring Body Mass Index >30 by a correction factor as previously reported by many authors (25–27). The parameters were uniquely estimated for every single patient.
Biochemical analysis
Plasma glucose concentrations were measured by an enzymatic method (glucose oxidase) on a Vitros 950 analyzer (Ortho Clinical Diagnostics, Rochester, NY). Glycated hemoglobin A1c (HbA1c) was measured with ion exchange HPLC (Bio-Rad Laboratories). Total LDL and HDL-cholesterol, TG, apoB, and apoA1 concentrations were measured on a Dimension analyzer with dedicated reagents (Dade Behring, Newark, NE). apoB and apoA1 were measured by immunoturbidimetry. The within-run coefficient of variation for that method was <5% at 2 mg/dl. Fructosamine was measured on the Dimension analyzer with ABX Diagnostics reagents (Montpellier, France). Plasma lathosterol measurement was performed by GC-MS analysis using a Hewlett Packard HP6890 gas chromatograph equipped with an HP7683 Injector and a HP5973 mass selective detector. Cholesteryl ester transfer protein (CETP) mass was measured by ELISA as previously reported (28). The ex vivo CETP activity was quantified using the fluorescence Roar kit (Roar Biomedical, New York, NY, USA).
Statistical analysis
Data are reported as mean ± SD. Statistical calculations were performed using the SPSS software package (Chicago, IL). Comparisons of continuous variables on placebo versus on 20 mg rosuvastatin were performed using the nonparametric Wilcoxon matched-pair test. Comparisons of continuous variables between patients with type 2 diabetes and controls were performed by the nonparametric Mann-Whitney U test. Correlation coefficients were calculated by the Spearman test. A two-tailed probability level of 0.05 was accepted as statistically significant.
RESULTS
Glucose and lipid parameters
Baseline characteristics of the patients with type 2 diabetes as their glucose and plasma lipid parameters on placebo and on 20 mg/d rosuvastatin are shown in Table 1. In the same table are also shown characteristics, fasting blood glucose, and lipid parameters in the eight control individuals. Treatment with 20 mg/d rosuvastatin was well tolerated in all patients. Body weight, HbA1c, fasting glycemia, and fructosamine were similar on placebo and on rosuvastatin. Treatment with 20 mg rosuvastatin induced a significant decrease of total cholesterol (−38%), LDL-cholesterol (−51%), TGs (−38%), plasma apoB (−40%), and lathosterol (−85%) (Table 1). Rosuvastatin induced a 5% increase in HDL-cholesterol, which was not statistically significant. Mean plasma apoA-I level was not modified by 20 mg/day rosuvastatin. Treatment with rosuvastatin was accompanied with a reduction of HDL-TGs (Table 1). Rosuvastatin treatment did not modify CETP mass but significantly reduced CETP activity (−21%). Patients with type 2 diabetes on placebo compared with controls had significantly higher levels of plasma TGs, apoB, and HDL-TGs and significantly lower levels of HDL-cholesterol. Patients with type 2 diabetes when treated with 20 mg/day rosuvastatin still had, compared with controls, higher plasma TG and lower HDL-cholesterol levels (Table 1).
TABLE 1.
Baseline characteristics and lipid and glycemic parameters in controls and patients with type 2 diabetes on placebo and on 20 mg/day rosuvastatin
| Patients with T2 DM Placebo | Patients with T2 DM Rosuvastatin | P (PCB vs. Rosuvastatin) | Controls | P (PCB vs. Controls) | P (rosuvastatin vs. Controls) | |
|---|---|---|---|---|---|---|
| Age, years | 55.4 ± 10.2 | 37.0 ± 10.6 | 0.006 | |||
| Sex (M/F) | 5/3 | 5/3 | ||||
| BMI, kg/m2 | 34 ± 4 | 23 ± 1.4 | <0.001 | |||
| Body weight, kg | 97.2 ± 19.9 | 97.2 ± 20.1 | NS | 70.1 ± 10.5 | 0.013 | 0.013 |
| Waist circumference, cm | 113 ± 16 | 113 ± 15 | NS | 83 ± 10 | 0.001 | 0.001 |
| Fasting glycemia, mg/dl (mmol/l) | 156 ± 53 (8.58 ± 2.9) | 163 ± 78 (8.96 ± 4.3) | NS | 91 ± 7 (5.05 ± 0.4) | 0.003 | 0.003 |
| HbA1c, % | 6.9 ± 1.2 | 7.0 ± 1.5 | NS | |||
| Fructosamine, μmol/L | 375 ± 63 | 412 ± 41 | NS | |||
| Total cholesterol, mg/dl (mmol/l) | 214 ± 47 (5.52 ± 1.21) | 133 ± 31 (3.43 ± 0.79) | 0.011 | 192 ± 37 (4.95 ± 0.95) | NS | 0.006 |
| TGs, mg/dl (mmol/l) | 274 ± 124 (3.12 ± 1.41) | 170 ± 70 (1.94 ± 0.80) | 0.011 | 71 ± 15 (0.80 ± 0.17) | <0.001 | 0.005 |
| LDL-cholesterol, mg/dl (mmol/l) | 122 ± 32 (3.14 ± 0.82) | 60 ± 18 (1.55 ± 0.46) | 0.011 | 122 ± 31 (3.14 ± 0.80) | NS | 0.002 |
| HDL-cholesterol, mg/dl (mmol/l) | 37 ± 10 (0.95 ± 0.25) | 39 ± 09 (1.00 ± 0.23) | NS | 58 ± 13 (1.49 ± 0.33) | 0.004 | 0.005 |
| apoB, g/l | 1.12 ± 0.16 | 0.67 ± 0.12 | 0.011 | 0.80 ± 0.19 | 0.007 | NS |
| apoA-I, g/l | 1.29 ± 0.22 | 1.28 ± 0.20 | NS | 1.39 ± 0.19 | NS | NS |
| Lathosterol, mg/l | 4.72 ± 2.47 | 0.74 ± 0.31 | 0.011 | |||
| HDL TG, % of HDL mass | 5.22 ± 1.04 | 4.01 ± 1.54 | 0.049 | 3.33 ± 0.20 | 0.004 | NS |
| CETP mass mg/l | 3.17 ± 1.13 | 2.96 ± 1.25 | NS | |||
| CETP activity pMol/μl·h | 3.10 ± 0.47 | 2.46 ± 0.78 | 0.011 |
Data are shown as mean ± SD. T2 DM, type 2 diabetes mellitus; PCB, placebo; NS, not significant.
ApoA-I kinetic parameters
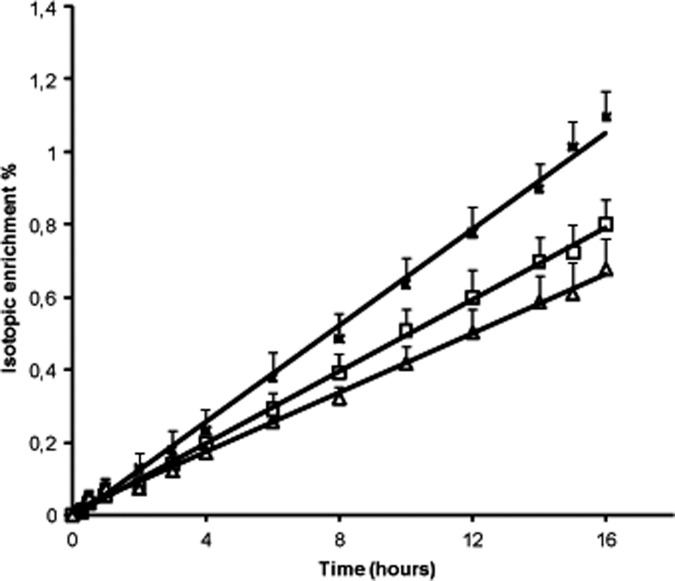
The isotopic enrichment curves for HDL-apoA-I with 13C leucine in the eight patients, when treated with placebo and with 20 mg rosuvastatin, as in controls are shown in Fig. 1. On rosuvastatin, the rate of appearance of tracer within HDL-apoA-I was decreased, indicating a reduction of the corresponding rates of catabolism. The rate of appearance of tracer within HDL-apoA-I was very similar between patients on rosuvastatin and controls.
Fig. 1.
Isotopic enrichment curves of HDL-apoA-I with 13C leucine in the eight patients with type 2 diabetes on placebo (closed squares) and on 20 mg/day rosuvastatin (open squares) as in controls (triangles). Data are expressed as mean ± SEM.
Table 2 gives the kinetic parameters of HDL-apoA-I in patients with type 2 diabetes on placebo and on 20 mg rosuvastatin as in controls. Although plasma apoA-I level remained unchanged with rosuvastatin, both HDL-apoA-I FCR and HDL-apoA-I PR were significantly reduced by rosuvastatin treatment. Compared with placebo, 20 mg rosuvastatin significantly decreased HDL-apoA-I FCR by 22% and HDL-apoA-I PR by 21% (Table 2). As a consequence, plasma HDL-apoA-I plasma residence time was increased by 27% with 20 mg rosuvastatin, corresponding to 22 additional hours.
TABLE 2.
ApoA-I kinetic parameters on placebo and 20 mg/day rosuvastatin in the eight patients with type 2 diabetes and in the eight controls
| Patients with T2 DM Placebo | Patients with T2 DM Rosuvastatin | P (PCB vs. Rosuvastatin) | Controls | P (PCB vs. Controls) | P (Rosuvastatin vs. Controls) | |
|---|---|---|---|---|---|---|
| Plasma apoA-I, g/l | 1.29 ± 0.22 | 1.28 ± 0.20 | NS | 1.39 ± 0.19 | NS | NS |
| apoA-I FCR, pool/day | 0.32 ± 0.07 | 0.25 ± 0.06 | P = 0.011 | 0.20 ± 0.03 | 0.001 | NS |
| apoA-I residence time, day | 3.30 ± 0.73 | 4.21 ± 1.02 | P = 0.011 | 5.04 ± 0.40 | 0.001 | NS |
| apoA-I PR, mg/kg·day | 16.19 ± 4.31 | 12.79 ± 3.98 | P = 0.011 | 12.37 ± 1.74 | 0.035 | NS |
Values are mean ± SD. T2 DM, type 2 diabetes mellitus; PCB, placebo; NS, not significant.
Patients with type 2 diabetes on placebo compared with controls had significantly higher HDL-apoA-I FCR (0.32 ± 0.07 vs. 0.20 ± 0.03 pool/day, P = 0.011) and HDL-apoA-I PR (16.19 ± 4.31 vs. 12.19 ± 1.74 mg/kg/day, P = 0.011) and reduced HDL-apoA-I plasma residence time (3.30 ± 0.73 vs. 5.04 ± 0.40 day, P = 0.011). Interestingly, treatment with 20 mg rosuvastatin, in patients with type 2 diabetes, restored HDL apoA-I FCR, PR, and plasma residence time values, which were not different from controls (HDL-apoA-I FCR: 0.25 ± 0.06 vs. 0.20 ± 0.03 pool/day, P = 0.16; HDL-apoA-I plasma residence time: 4.21 ± 1.02 vs. 5.04 ± 0.40 day, P = 0.16; HDL-apoA-I PR: 12.79 ± 3.98 vs. 12.37 ± 1.74 mg/kg/day, P = 0.75) (Table 2). The response to rosuvastatin treatment was similar in both sexes (all three women and all five men showed a decrease in both HDL-apoA-I FCR and PR with rosuvastatin). However, gender-specific analyses were not performed due to the limited number of participants in each sex category.
The decrease in apoA-I FCR, induced by 20 mg rosuvastatin, was correlated with the reduction of plasma TGs (r = 0.75, P = 0.031) and HDL-TGs (r = 0.84, P = 0.009) as with the increase in plasma HDL-cholesterol level (r = 0.87, P = 0.005). Rosuvastatin-induced changes in apoA-I PR or FCR were not correlated with reduction of plasma LDL-cholesterol.
DISCUSSION
This study provides new information on the effects of rosuvastatin on the metabolism of HDL in patients with type 2 diabetes and diabetic dyslipidemia. We show that rosuvastatin significantly reduces both HDL-apoA-I FCR and HDL-apoA-I PR. The rosuvastatin-induced modification of HDL kinetics leads to a significant 27% increase in HDL-apoA-I plasma residence time. HDL-apoA-I FCR in patients with type 2 diabetes treated with rosuvastatin was not different from controls, indicating that rosuvastatin restores to normal the increased HDL turnover observed in type 2 diabetes.
Previous kinetic studies that have examined the effects of statins on HDL metabolism have not shown consistent effects. In a study performed on eight male patients with mixed hyperlipidemia, 80 mg atorvastatin did not modify apoA-I kinetics (29). Lamon-Fava et al. (30) have not seen significant effects of 20 or 80 mg atorvastatin on apoA-I kinetics in nine patients with combined hyperlipidemia. Mauger et al. (31) reported no modification of HDL-cholesterol concentration in seven men with low baseline HDL-cholesterol levels with 40 mg atorvastatin or 80 mg simvastatin, although they reported an increase in apoA-I production rate with simvastatin compared with atorvastatin. However, it is difficult to draw firm conclusion from this work because no baseline kinetic data before treatment with simvastatin or atorvastatin are available in this study (31). In a kinetic study performed in obese insulin resistant men, Chan et al. (32) did not find any modification of apoA-I kinetics with 40 mg atorvastatin.
This absence of significant effects of statins on HDL-apoA-I kinetics may not be true for rosuvastatin. Indeed, in a recent study, Ooi et al. reported a significant reduction of apoA-I FCR and PR with rosuvastatin in men with the metabolic syndrome but without diabetes (33). In that study, the effect of rosuvastatin on ApoA-I and LpA-I FCRs has been shown to be dose dependent with a maximal effect at 40 mg (33). To date, no study on apoA-I metabolism with rosuvastatin has been performed in patients with type 2 diabetes. Because patients with type 2 diabetes show a significant increase in HDL catabolism (9, 10), it is important to see whether this kinetic abnormality could be modified by rosuvastatin. This study clearly shows that rosuvastatin treatment induces significant modification of HDL-apoA-I kinetics in patients with type 2 diabetes. The main result of our study is the 22% reduction of HDL-apoA-I FCR leading to a significant increase in HDL-apoA-I plasma residence time. We also show, in this study, that the mean HDL-apoA-I FCR obtained after rosuvastatin treatment in our patients with type 2 diabetes is not different from the mean HDL-apoA-I FCR found in controls. Moreover, the mean HDL-apoA-I FCR in our patients with type 2 diabetes on rosuvastatin is very similar to the one observed in the control groups from several kinetic studies (10, 34, 35). Thus, rosuvastatin is able to restore a normal HDL-apoA-I FCR in patients with type 2 diabetes.
HDL-TG enrichment has been shown to be a factor leading to increased HDL FCR in type 2 diabetes (7, 8, 36–38). In type 2 diabetes, the augmented level of plasma TG-rich lipoproteins drives through CETP the transfer of TGs from TG-rich lipoproteins to HDLs leading to the formation of TG-rich HDL particles (39). HDL enriched in TGs become very good substrate for hepatic lipase, whose activity is augmented in type 2 diabetes, leading to increased catabolism of HDL particles (7, 8). In our study, the decrease in apoA-I FCR, induced by rosuvastatin, was correlated with the reduction of plasma TGs and HDL-TGs. The reduction of plasma TG induced by rosuvastatin may be one explanation for the decrease in HDL apoA-I FCR observed with rosuvastatin in patients with type 2 diabetes. Indeed, rosuvastatin is known to significantly decrease plasma TG level with a larger effect than pravastatin or simvastatin (13). In this study, rosuvastatin induced a significant drop in plasma TGs and HDL-TGs. We found that treatment with rosuvastatin was accompanied with a significant drop in CETP activity. This result is in accordance with other studies that have shown significant reduction of CETP activity with rosuvastatin (40, 41) as with atorvastatin (42, 43). This fall in CETP activity may be partly due to the marked reduction of plasma TGs (and TG-rich lipoproteins) induced by rosuvastatin. Thus, the reduced CETP-mediated transfer of TGs from TG-rich lipoproteins to HDLs leads to the formation of HDL particles less rich in TGs and thus more slowly catabolized. This is supported by the correlation we found between decrease in apoA-I FCR and reduction of HDL-TGs following rosuvastatin treatment.
Although rosuvastatin induced a significant decrease in HDL-apoA-I catabolism, plasma apoA-I was not modified. This is due to an associated reduction of HDL-apoA-I PR. This reduction of HDL-apoA-I PR in response to a fall in catabolism may simplistically reflect the operation of balancing feedback mechanisms. How these mechanisms operate is unknown. A similar reduction of both HDL-apoA-I FCR and HDL-apoA-I PR has also been observed with rosuvastatin in men with the metabolic syndrome (32) and with fish oils in men with abdominal obesity (33). Some previous kinetic studies have found a correlation between apoA-I PR and plasma LDL-cholesterol level (44) and a possible link between reduction of plasma LDL-cholesterol with treatments and changes in apoA-I PR has been suggested (45). In this study, changes in apoA-I PR with rosuvastatin were not correlated with reduction of plasma LDL-cholesterol. This absence of association between LDL changes and HDL-apoA-I production rate has been found in several kinetic studies. For instance, decrease in plasma LDL-cholesterol with atorvastatin is not associated with modification of apoA-I PR (29) Furthermore, the increase in plasma LDL-cholesterol level, observed after menopause or with saturated fat diet, is not coupled with any changes in apoA-I PR (46, 47). Our results do not totally exclude that decrease in plasma LDL-cholesterol level induced by rosuvastatin may have played a role in the increase in apoA-I PR, but this effect, if existing, is likely to be minor.
The modification of HDL-apoA-I kinetic induced by rosuvastatin seems different from HDL-apoA-I kinetic changes observed with fibrates. Indeed, an increase in the production of apoA-I with a less important increase in apoA-I catabolism leading to a significant augmentation of apoA-I pool has been reported with fenofibrate (42). The significant increase in apoA-I production reported with fenofibrate is consistent with the Peroxisome Proliferator-Activated Receptor-mediated effect of fenofibrate that increases the transcription of apoA-I in hepatocytes (48). The changes in HDL-apoA-I kinetic induced by rosuvastatin have some similarities with those observed with niacin. The increase in HDL-cholesterol induced by niacin has been shown to be mainly related to a decrease in the FCR of HDL-apoA-I (49). It has been suggested that the decreased clearance of HDL-apoA-I with niacin could be related to a reduction in the expression and activity of CETP. Indeed, niacin has been shown to reduce both CETP mass and activity (50, 51).
The very significant modification of HDL-apoA-I kinetics induced by rosuvastatin in our patients with diabetes with no change in plasma apoA-I and a nonsignificant modest increase in HDL-cholesterol levels have been observed in other kinetic studies (33, 45). In the study performed by Ooi et al. (33) in men with the metabolic syndrome, 10 mg rosuvastatin induced a significant reduction of apoA-I FCR without any significant modification of plasma apoA-I and HDL-cholesterol levels. Fish oils have been shown to significantly decrease both HDL-apoA-I FCR and HDL-apoA-I PR without modifying HDL-cholesterol or plasma apoA-I levels (33). Thus, although rosuvastatin usually induces only a modest increase in plasma HDL-cholesterol level in patients with diabetes (16, 17), it significantly reduces HDL-apoA-I catabolism. This leads to automatically increasing HDL plasma residence time, which may have beneficial effects on the arterial wall. We have to note that the modest effect of rosuvastatin on HDL-cholesterol level does not totally reflect its important impact on HDL metabolism.
Patients with type 2 diabetes show an increased catabolism of HDL particles with, as a consequence, a significant reduction of HDL plasma residence time, which may reduce the anti-atherogenic properties of HDLs. We show, in our study, that rosuvastatin gives 22 additional hours of HDL plasma residence time in patients with type 2 diabetes. We may think that the reduction of HDL-apoA-I FCR induced by rosuvastatin, in patients with type 2 diabetes, restoring a normal HDL-apoA-I plasma residence time could improve reverse cholesterol transport and would give more chance to HDL particles to exert their antioxidative, anti-inflammatory, antithrombotic, and endothelium-dependent vasorelaxant effects. However, even if we think that the increased plasma HDL-apoA-I residence time induced by rosuvastatin may have beneficial effects, it is not possible to assert that these kinetic changes may reduce atherosclerosis. Furthermore, we cannot state that HDL-apoA-I kinetic changes could be part of the benefit of rosuvastatin on the atherosclerotic plaques as observed in ASTEROID (52) and on the cardiovascular events as reported in JUPITER (53).
One limitation of our study is the age difference between the patients with type 2 diabetes and the control subjects. However, we do not think that this difference significantly modifies our results and conclusions. Indeed, HDL apoA-I kinetic does not seem to be modified by age according to data from the literature (47).
We show, in this study, that rosuvastatin induces significant modification of HDL-apoA-I kinetics in patients with type 2 diabetes. Rosuvastatin (20 mg) is responsible for a 22% reduction of HDL-apoA-I FCR leading to a significant increase in HDL-apoA-I plasma residence time. Rosuvastatin restores to normal the increased HDL turnover observed in type 2 diabetes. These kinetic modifications induced by rosuvastatin might have beneficial effects on the arterial wall by increasing HDL plasma residence time. However, further studies are needed to assert that the HDL-apoA-I kinetic changes, induced by rosuvastatin, are beneficial to prevent atherosclerosis.
Acknowledgments
The authors are indebted to Véronique Jost for the preparation of 13C leucine, Cécile Gibassier for dietary assistance, and Elisabeth Niot and Liliane Princep for invaluable technical assistance. The authors thank Dr. Rita Chadarevian for her help during the preparation of the study.
Abbreviations
apoA-I, apolipoprotein A-I
CETP, cholesteryl ester transfer protein
FCR, fractional catabolic rate
FSR, fractional synthetic rate
PR, production rate
TG, triglyceride
Published, JLR Papers in Press, January 22, 2009.
This study was supported by a grant from AstraZeneca.
References
- 1.Haffner S. M., S. Lehto, T. Rönnemaa, K. Pyörälä, and M. Laakso. 1998. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 339 229–234. [DOI] [PubMed] [Google Scholar]
- 2.Pyörälä K., M. Laakso, and M. Uusitupa. 1987. Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab. Rev. 3 463–524. [DOI] [PubMed] [Google Scholar]
- 3.de Vegt F., J. M. Dekker, H. G. Ruhé, C. D. Stehouwer, G. Nijpels, L. M. Bouter, R. J. Heine. 1999. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia. 42 926–931. [DOI] [PubMed] [Google Scholar]
- 4.Kannel W. B., and D. L. McGee. 1979. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 59 8–13. [DOI] [PubMed] [Google Scholar]
- 5.Haffner S. M., S. Lehto, T. Rönnemaa, K. Pyörälä, and M. Laakso. 1998. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 339 229–234. [DOI] [PubMed] [Google Scholar]
- 6.Turner R. C., H. Millns, H. A. Neil, I. M. Stratton, S. E. Manley, D. R. Matthews, R. R. Holman. for the United Kingdom Prospective Diabetes Study Group. 1998. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom prospective diabetes study (UKPDS: 23). BMJ. 316 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taskinen M. R. 2003. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 46 733–749. [DOI] [PubMed] [Google Scholar]
- 8.Vergès B. 2005. New insight into the pathophysiology of lipid abnormalities in type 2 diabetes. Diabetes Metab. 31 429–439. [DOI] [PubMed] [Google Scholar]
- 9.Golay A., L. Zech, M. Z. Shi, Y. A. Chiou, G. M. Reaven, Y. D. Chen. 1987. High density lipoprotein (HDL) metabolism in non insulin dependent diabetes mellitus: measurement of HDL turn-over using tritiated HDL. J. Clin. Endocrinol. Metab. 65 512–518. [DOI] [PubMed] [Google Scholar]
- 10.Duvillard L., F. Pont, E. Florentin, P. Gambert, and B. Vergès. 2000. Inefficiency of insulin therapy to correct apolipoprotein A-I metabolic abnormalities in non insulin-dependent diabetes mellitus. Atherosclerosis. 152 229–237. [DOI] [PubMed] [Google Scholar]
- 11.Sviridov D., and P. Nestel. 2002. Dynamics of reverse cholesterol transport: protection against atherosclerosis. Atherosclerosis. 161 245–254. [DOI] [PubMed] [Google Scholar]
- 12.Link J. J., A. Rohatgi, and J. A. de Lemos. 2007. HDL cholesterol: physiology, pathophysiology, and management. Curr. Probl. Cardiol. 32 268–314. [DOI] [PubMed] [Google Scholar]
- 13.Jones P. H., M. H. Davidson, E. A. Stein, H. E. Bays, J. M. McKenney, E. Miller, V. A. Cain, and J. W. Blasetto; STELLAR Study Group. 2003. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am. J. Cardiol. 92 152–160. [DOI] [PubMed] [Google Scholar]
- 14.Paoletti R., M. Fahmy, G. Mahla, J. Mizan, and H. Southworth. 2001. Rosuvastatin demonstrates greater reduction of low-density lipoprotein cholesterol compared with pravastatin and simvastatin in hypercholesterolaemic patients: a randomized, double-blind study. J. Cardiovasc. Risk. 8 383–390. [DOI] [PubMed] [Google Scholar]
- 15.Olsson A. G., H. Istad, O. Luurila, L. Ose, S. Stender, J. Tuomilehto, O. Wiklund, H. Southworth, J. Pears, and J. W. Wilpshaar. 2002. Rosuvastatin Investigators Group. Effects of rosuvastatin and atorvastatin compared over 52 weeks of treatment in patients with hypercholesterolemia. Am. Heart J. 144 1044–1051. [DOI] [PubMed] [Google Scholar]
- 16.Durrington P. N., J. Tuomilehto, A. Hamann, D. Kallend, and K. Smith. 2004. Rosuvastatin and fenofibrate alone and in combination in type 2 diabetes patients with combined hyperlipidaemia. Diabetes Res. Clin. Pract. 64 137–151. [DOI] [PubMed] [Google Scholar]
- 17.Blasetto J. W., E. A. Stein, W. V. Brown, R. Chitra, and A. Raza. 2003. Efficacy of rosuvastatin compared with other statins at selected starting doses in hypercholesterolemic patients and in special population groups. Am. J. Cardiol. 91 3C–10C. [DOI] [PubMed] [Google Scholar]
- 18.Duvillard L., F. Pont, E. Florentin, P. Gambert, and B. Vergès. 2000. Significant improvement of apoB-containing lipoprotein metabolism by insulin treatment in NIDDM patients. Diabetologia. 43 27–35. [DOI] [PubMed] [Google Scholar]
- 19.Pont F., L. Duvillard, E. Florentin, P. Gambert, and B. Vergès. 2002. Early kinetic abnormalities of apoB-containing lipoproteins in insulin-resistant women with abdominal obesity. Arterioscler. Thromb. Vasc. Biol. 22 1726–1732. [DOI] [PubMed] [Google Scholar]
- 20.Taskinen M. R., C. J. Packard, and J. Sheperd. 1990. Effect of insulin therapy on metabolic fate of apolipoprotein B-containing lipoproteins in NIDDM. Diabetes. 39 1017–1027. [DOI] [PubMed] [Google Scholar]
- 21.Pont F., L. Duvillard, C. Maugeais, A. Athias, L. Persegol, P. Gambert, and B. Vergès. 1997. Isotope ratio mass spectrometry, compared with conventional mass spectrometry in kinetic studies at low and high enrichment levels: application to lipoprotein kinetics. Anal. Biochem. 248 277–287. [DOI] [PubMed] [Google Scholar]
- 22.Pont F., L. Duvillard, B. Vergès, and P. Gambert. 1998. Development of compartmental models in stable isotope experiments: application to lipoprotein metabolism. Arterioscler. Thromb. Vasc. Biol. 18 853–860. [DOI] [PubMed] [Google Scholar]
- 23.Barrett P.H., B. M. Bell, C. Cobelli, H. Golde, A. Schumitzky, P. Vicini, D. M. Foster. 1998. SAAM II: Simulation, Analysis and modeling Software for tracer and pharmacokinetic studies. Metabolism. 47 484–492. [DOI] [PubMed] [Google Scholar]
- 24.Ikewaki K., D. J. Rader, J. R. Schaefer, T. Fairwell, L. A. Zech, and H. B. Brewer, Jr. 1993. Evaluation of apoA-I kinetics in humans using simultaneous endogenous stable isotope and exogenous radiotracer methods. J. Lipid Res. 34 2207–2215. [PubMed] [Google Scholar]
- 25.Cummings M. H., G. F. Watts, C. Pal, M. Umpleby, T. R. Hennessy, R. Naoumova, and P. H. Sönksen. 1995. Increased hepatic secretion of very low density lipoprotein apolipoprotein B100 in obesity: a stable isotope study. Clin. Sci. 88 225–233. [DOI] [PubMed] [Google Scholar]
- 26.Pont F., L. Duvillard, E. Florentin, P. Gambert, and B. Vergès. 2002. High-density lipoprotein apolipoprotein A-I kinetics in obese insulin resistant patients. An in vivo stable isotope study. Int. J. Obes. Relat. Metab. Disord. 26 1151–1158. [DOI] [PubMed] [Google Scholar]
- 27.Dagher F. J., J. H. Lyons, D. C. Finlayson, J. Shamsai, and F. D. Moore. 1965. Blood volume measurement: a critical study. Adv. Surg. 1 69–109. [PubMed] [Google Scholar]
- 28.Guyard-Dangremont V., L. Lagrost, P. Gambert, and C. Lallemant. 1994. Competitive enzyme-linked immunosorbent assay of the human cholesteryl ester transfer protein (CETP). Clin. Chim. Acta. 231 147–160. [DOI] [PubMed] [Google Scholar]
- 29.Bilz S., S. Wagner, M. Schmitz, A. Bedynek, U. Keller, and T. Demant. 2004. Effects of atorvastatin versus fenofibrate on apoB-100 and apoA-I kinetics in mixed hyperlipidemia. J. Lipid Res. 45 174–185. [DOI] [PubMed] [Google Scholar]
- 30.Lamon-Fava S., M. R. Diffenderfer, P. H. Barrett, A. Buchsbaum, N. R. Matthan, A. H. Lichtenstein, G. G. Dolnikowski, K. Horvath, B. F. Asztalos, V. Zago, et al. 2007. Effects of different doses of atorvastatin on human apolipoprotein B-100, B-48, and A-I metabolism. J. Lipid Res. 48 1746–1753. [DOI] [PubMed] [Google Scholar]
- 31.Mauger J. F., P. Couture, M. E. Paradis, and B. Lamarche. 2005. Comparison of the impact of atorvastatin and simvastatin on apoA-I kinetics in men. Atherosclerosis. 178 157–163. [DOI] [PubMed] [Google Scholar]
- 32.Chan D. C., G. F. Watts, M. N. Nguyen, and P. H. Barrett. 2006. Factorial study of the effect of n-3 fatty acid supplementation and atorvastatin on the kinetics of HDL apolipoproteins A-I and A-II in men with abdominal obesity. Am. J. Clin. Nutr. 84 37–43. [DOI] [PubMed] [Google Scholar]
- 33.Ooi E. M., G. F. Watts, P. J. Nestel, D. Sviridov, A. Hoang, and P. H. Barrett. 2008. Dose-Dependent Regulation of High-Density Lipoprotein Metabolism with Rosuvastatin in the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 93 430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vélez-Carrasco W., A. H. Lichtenstein, P. H. Barrett, Z. Sun, G. G. Dolnikowski, F. K. Welty, and E. J. Schaefer. 1999. Human apolipoprotein A-I kinetics within triglyceride-rich lipoproteins and high density lipoproteins. J. Lipid Res. 40 1695–1700. [PubMed] [Google Scholar]
- 35.Pietzsch J., U. Julius, S. Nitzsche, and M. Hanefeld. 1998. In vivo evidence for increased apolipoprotein A-I catabolism in subjects with impaired glucose tolerance. Diabetes. 47 1928–1934. [DOI] [PubMed] [Google Scholar]
- 36.Frénais R., H. Nazih, K. Ouguerram, C. Maugeais, Y. Zaïr, J. M. Bard, B. Charbonnel, T. Magot, and M. Krempf. 2001. In vivo evidence for the role of lipoprotein lipase activity in the regulation of apolipoprotein AI metabolism: a kinetic study in control subjects and patients with type II diabetes mellitus. J. Clin. Endocrinol. Metab. 86 1962–1967. [DOI] [PubMed] [Google Scholar]
- 37.Marsh J. B. 2003. Lipoprotein metabolism in obesity and diabetes: insights from stable isotope kinetic studies in humans. Nutr. Rev. 61 363–375. [DOI] [PubMed] [Google Scholar]
- 38.Vergès B., J. M. Petit, L. Duvillard, G. Dautin, E. Florentin, F. Galland, and P. Gambert. 2006. Adiponectin is an important determinant of apoA-I catabolism. Arterioscler. Thromb. Vasc. Biol. 26 1364–1369. [DOI] [PubMed] [Google Scholar]
- 39.Castle C., S. Kuiper, W. Blake, B. Paigen, K. Marotti, and G. Melchior. 1998. Remodeling of the HDL in NIDDM a fundamental role for cholesteryl ester transfer protein. Am. J. Physiol. 274 E1091–E1098. [DOI] [PubMed] [Google Scholar]
- 40.Caslake M. J., G. Stewart, S. P. Day, E. Daly, F. McTaggart, M. J. Chapman, P. Durrington, P. Laggner, M. Mackness, J. Pears, et al. 2003. Phenotype-dependent and -independent actions of rosuvastatin on atherogenic lipoprotein subfractions in hyperlipidaemia. Atherosclerosis. 171 245–253. [DOI] [PubMed] [Google Scholar]
- 41.Sviridov D., A. Hoang, E. Ooi, G. Watts, P. H. Barrett, and P. Nestel. 2008. Indices of reverse cholesterol transport in subjects with metabolic syndrome after treatment with rosuvastatin. Atherosclerosis. 197 732–739. [DOI] [PubMed] [Google Scholar]
- 42.Watts G. F., P. H. Barrett, J. Ji, A. P. Serone, D. C. Chan, K. D. Croft, F. Loehrer, and A. G. Johnson. 2003. Differential regulation of lipoprotein kinetics by atorvastatin and fenofibrate in subjects with the metabolic syndrome. Diabetes. 52 803–811. [DOI] [PubMed] [Google Scholar]
- 43.Guerin M., T. S. Lassel, W. Le Goff, M. Farnier, and M. J. Chapman. 2000. Action of atorvastatin in combined hyperlipidemia: preferential reduction of cholesteryl ester transfer from HDL to VLDL1 particles. Arterioscler. Thromb. Vasc. Biol. 20 189–197. [DOI] [PubMed] [Google Scholar]
- 44.Welty F. K., A. H. Lichtenstein, P. H. Barrett, G. G. Dolnikowski, and E. J. Schaefer. 2004. Interrelationships between human apolipoprotein A-I and apolipoproteins B-48 and B-100 kinetics using stable isotopes. Arterioscler. Thromb. Vasc. Biol. 24 1703–1707. [DOI] [PubMed] [Google Scholar]
- 45.Schaefer J. R., H. Schweer, K. Ikewaki, H. Stracke, H. J. Seyberth, H. Kaffarnik, B. Maisch, and A. Steinmetz. 1999. Metabolic basis of high density lipoproteins and apolipoprotein A-I increase by HMG-CoA reductase inhibition in healthy subjects and a patient with coronary artery disease. Atherosclerosis. 144 177–184. [DOI] [PubMed] [Google Scholar]
- 46.Matthan N. R., F. K. Welty, P. H. Barrett, C. Harausz, G. G. Dolnikowski, J. S. Parks, R. H. Eckel, E. J. Schaefer, and A. H. Lichtenstein. 2004. Dietary hydrogenated fat increases high-density lipoprotein apoA-I catabolism and decreases low-density lipoprotein apoB-100 catabolism in hypercholesterolemic women. Arterioscler. Thromb. Vasc. Biol. 24 1092–1097. [DOI] [PubMed] [Google Scholar]
- 47.Matthan N. R., S. M. Jalbert, S. Lamon-Fava, G. G. Dolnikowski, F. K. Welty, H. R. Barrett, E. J. Schaefer, and A. H. Lichtenstein. 2005. TRL, IDL, and LDL apolipoprotein B-100 and HDL apolipoprotein A-I kinetics as a function of age and menopausal status. Arterioscler. Thromb. Vasc. Biol. 25 1691–1696. [DOI] [PubMed] [Google Scholar]
- 48.Vu-Dac N., S. Chopin-Delannoy, P. Gervois, E. Bonnelye, G. Martin, J. C. Fruchart, V. Laudet, and B. Staels. 1998. The nuclear receptors peroxisome proliferator-activated receptor alpha and Rev-erbalpha mediate the species-specific regulation of apolipoprotein A-I expression by fibrates. J. Biol. Chem. 273 25713–25720. [DOI] [PubMed] [Google Scholar]
- 49.Shepherd J., C. J. Packard, J. R. Patsch, A. M. Gotto, Jr., and O. D. Taunton. 1979. Effects of nicotinic acid therapy on plasma high density lipoprotein subfraction distribution and composition and on apolipoprotein A metabolism. J. Clin. Invest. 63 858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamanna V. S., and M. L. Kashyap. 2008. Mechanism of action of niacin. Am. J. Cardiol. 101 20B–26B. [DOI] [PubMed] [Google Scholar]
- 51.van der Hoorn J. W., W. de Haan, J. F. Berbée, L. M. Havekes, J. W. Jukema, P. C. Rensen, and H. M. Princen. 2008. Niacin increases HDL by reducing hepatic expression and plasma levels of cholesteryl ester transfer protein in APOE*3Leiden.CETP mice. Arterioscler. Thromb. Vasc. Biol. 28 2016–2022. [DOI] [PubMed] [Google Scholar]
- 52.Nissen S. E., S. J. Nicholls, I. Sipahi, P. Libby, J. S. Raichlen, C. M. Ballantyne, J. Davignon, R. Erbel, J. C. Fruchart, J. C. Tardif, P. Schoenhagen, T. Crowe, V. Cain, K. Wolski, M. Goormastic, E. M. Tuzcu; ASTEROID Investigators. 2006. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 295 1556–1565. [DOI] [PubMed] [Google Scholar]
- 53.Ridker P. M., E. Danielson, F. A. Fonseca, J. Genest, A. M. Gotto, Jr., J. J. Kastelein, W. Koenig, P. Libby, A. J. Lorenzatti, J. G. MacFadyen, et al; JUPITER Study Group. 2008. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359 2195–2207. [DOI] [PubMed] [Google Scholar]