Abstract
Myeloid cell leukemia-1 (Mcl-1) is an anti-apoptotic protein that is regulated by the constitutive androstane receptor (CAR). Activation of CAR can protect the liver against bile acid-induced toxicity and it may have a role in cell death via apoptosis by altering expression of Bcl-2 family proteins such as myeloid cell leukemia-1 (Mcl-1). Our aim was to determine if activation of CAR reduces hepatocellular apoptosis during cholestasis as a mechanism of hepatoprotection. CAR+/+ (WT) and CAR−/− (CAR-null) mice were pre-treated with compounds known to activate CAR prior to induction of intrahepatic cholestasis using the secondary bile acid lithocholic acid (LCA). Pre-treatment with the CAR activators phenobarbital (PB) and TCPOBOP (TC), as well as the non-CAR activator pregnenolone 16α-carbontrile (PCN), protected against LCA-induced liver injury in WT mice, whereas liver injury was more extensive without CAR (CAR-null). Unexpectedly, expression of anti-apoptotic Mcl-1 and Bcl-xL was not increased in hepatoprotected mice. Compared to unprotected groups, apoptosis was decreased in hepatoprotected mice as evidenced by the absence of cleaved caspase 3 (cCasp3). In contrast to the cytoplasmic localization in the injured livers (LCA and oltipraz), Mcl-1 protein was localized in the nucleus of hepatoprotected livers to potentially promote cell survival. This study demonstrates that although apoptosis is reduced in hepatoprotected mice pre-treated with CAR and non-CAR activators; hepatoprotection is not directly a result of CAR-induced Mcl-1 expression.
INTRODUCTION
Cholestasis, or impaired bile flow, is a form of liver disease that has various etiologies and can result in liver failure. Hepatocyte apoptosis is implicated in the pathogenesis of several liver diseases including cholestasis, viral hepatitis and autoimmune diseases (Sola et al., 2006). There are numerous causes of cholestasis, and apoptosis has been proposed as a mechanism of bile acid-induced liver injury (Baskin-Bey et al., 2006; Faubion et al., 1999). Activation of the constitutive androstane receptor (CAR) can protect the liver against bile acid-induced toxicity, however, the precise mechanisms underlying the protection are poorly understood (Saini et al., 2004; Zhang et al., 2004). CAR may have a role in cell death via apoptosis by altering expression of Bcl-2 family proteins such as myeloid cell leukemia-1 (Mcl-1). Considerable research has been conducted to identify how bile acids cause apoptosis, but whether a decrease in apoptosis contributes to hepatoprotection and whether CAR may be involved remains unclear.
Bile acid-induced apoptosis of hepatocytes can occur extrinsically via death receptor activation (Faubion et al., 1999; Sodeman et al., 2000) or intrinsically through oxidative stress and mitochondrial dysfunction (Graf et al., 2002; Rodrigues et al., 1998). Excess hepatic bile acid levels present during cholestasis activate the Fas death receptor to initiate apoptotic signaling resulting in cleavage of caspase 8. Downstream of the mitochondria, effector caspases (caspase-3, -6, -7) are also cleaved and activated into functional proteases resulting in apoptosis (Hengartner, 2000). Effector caspases such as caspase 3 are important in the study of apoptosis as they are considered the actual executioners of apoptosis. The intrinsic and extrinsic death pathways are not mutually exclusive; in fact hepatocytes have been shown to require mitochondrial contribution to amplify the apoptotic signal (Guicciardi and Gores, 2006).
Manipulation of nuclear receptors has recently emerged as a potential mechanism of hepatoprotection during cholestasis. Activation of CAR and the pregnane X receptor (PXR) can protect the liver against bile acid-induced cholestasis via up-regulation of bile acid metabolizing genes such as Cyp3A and Sult2A (Stedman et al., 2005; Kitada et al., 2003; Teng and Piquette-Miller, 2007; Zhang et al., 2004). The nuclear receptor CAR has also been shown to protect against Fas-induced liver injury via up-regulation of the anti-apoptotic protein Mcl-1 (myeloid cell leukemia -1) and down-regulation of pro-apoptotic Bak and Bax (Baskin-Bey et al., 2006). Mcl-1 is a member of the Bcl-2 family of proteins that protects cells from apoptosis by directly inhibiting Ca2+ signaling within the mitochondria to prevent the release of cytochrome c, thus halting the intrinsic signaling pathway (Minagawa et al., 2005). Because bile acids cause apoptosis and CAR has a role in the regulation of anti-apoptotic Mcl-1, there exists a possibility that activation of CAR could confer hepatoprotection by reducing apoptosis during cholestasis.
MATERIALS and METHODS
Materials
Oltipraz (OPZ) was purchased from LKT Laboratories, Inc. (St. Paul, MN). Phenobarbital sodium (PB), lithocholic acid (LCA), 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene [TCPOBOP (TC)] and pregnenolone 16α-carbontrile (PCN) were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies for the following proteins were purchased as follows: Bcl-xL and Bax (AbCam, Inc., Cambridge, MA); Bak, PARP and cCasp3 (Asp 175) (Cell Signaling Technology, Danvers, MA); CAR, lamin B1, α-tubulin and Mcl-1 (Santa Cruz Biotechnology, Santa Cruz, CA).
Animals
Ten-week old male C57BL/6 (Charles River Laboratories, Inc., Wilmington MA) WT mice or CAR-null mice were weight matched into treatment groups (N=4–6 mice/group). Breeding pairs of CAR-null mice in the C57BL/6 background were obtained from Dr. Ivan Rusyn (University of North Carolina, Chapel Hill, NC), which were engineered by Tularik Inc. (South San Francisco, CA) as described previously (Ueda et al., 2002). To induce intrahepatic cholestasis, the secondary bile acid LCA was administered, which has become an increasingly utilized model of cholestasis (Zhang et al., 2004; Kitada et al., 2003). Animals were pre-treated with chemical inducers (PB 80 mg/kg, OPZ 150 mg/kg, PCN 200 mg/kg) or corn oil (CO) for three days via i.p. injection. On the fourth day, LCA treatment was initiated with i.p. injections (125 mg/kg) given twice daily, and the CAR activator treatment, in combination with LCA, continued for another three days. TC (3 mg/kg) pre-treatment was begun on Day 3 and continued during LCA treatment. Approximately 12 hrs following the last treatment, mice were euthanized and the livers removed. Animals were maintained on a 12-hr light/dark cycle at approximately 25°C, with access to food and water ad libitum. The experimental protocol was approved by the University of Kansas Medical Center Institutional Animal Care.
Liver Histology and Enzymes
Mid-sections of the left liver lobe were collected and fixed in 10% neutral buffered formalin. Tissues from two mice per treatment group were embedded in paraffin and 5 micron sections were stained with hematoxylin and eosin. Under treatment-blinded conditions, the tissues were evaluated for liver injury and graded (mild, moderate, severe) by a board-certified veterinary pathologist. Serum alanine aminotransferase (ALT) was measured using an Endocheck Plus Chemistry Analyzer (Hemagen Diagnostics, Inc., Columbia, MD).
Electrophoretic Mobility Shift Assay (EMSA)
The Cyp2B10 nuclear receptor half-site NR-1 (DR4) 5′-TCTGTACTTTCCTGACCTTG-3′ oligonucleotide probe was utilized for the gel shift mobility assay (Honkakoski et al., 1998). Sense and antisense oligonucleotide strands were synthesized with the 5′ end-labeled with IRDye® 700 by Li-Cor Biosciences (Lincoln, NE). The gel shift mobility assay was initiated by incubation of binding buffer (50 mM Hepes pH 7.9, 250 mM KCl, 0.5 mM EDTA, 12.5 mM DTT, 50% glycerol, 0.25% Triton X-100),1 μg poly(dI•dC), nuclear protein, and unlabeled probe for 20 min on ice followed by the addition of labeled probe. An unlabeled double-stranded NR-1 oligonucleotide (Sigma Genosys) was used to confirm competitive protein binding; and the potent, direct-acting CAR ligand TCPOBOP was used as a positive control. The nuclear protein-DNA complex was separated via non-denaturing 5% tris-borate gel electrophoresis, and subsequently scanned for visualization of DNA-protein bands on a Li-Cor Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE).
RNA Isolation and mRNA Expression
Total RNA was isolated from liver using RNA Bee reagent (Tel-Test Inc., Friendswood, TX) according to the manufacturer’s instructions. The concentration of total RNA in each sample was quantified spectrophotometrically at 260 nm. RNA samples were analyzed by agarose gel electrophoresis with ethidium bromide staining, and integrity was confirmed by visualization of intact 18S and 28S rRNA bands under ultraviolet light. The branched DNA (bDNA) assay was used to quantify mRNA expression as previously described (Beilke et al., 2008). Mouse gene sequences of interest were acquired from GenBank and oligonucleotide probe sets were designed using Probe Designer software version 1.0 (Bayer Corp. Emeryville, CA). The development of probe sets for mouse Mcl-1, Bcl-xL, Bak, and Bax is described in Supplementary Table 1.
Western Blot Analysis
Cytosolic and nuclear proteins were isolated as described (Beilke et al., 2008; Merrell et al., 2008). Protein concentrations were determined using a BCA Protein Kit (Pierce Biotechnology, Rockford, IL). Proteins were separated on 10% polyacrylamide gels and transferred onto nitrocellulose membranes (BioRad, Hercules, CA). Blots were blocked with 5% non-fat dry milk in Tris-buffered saline with Tween 20 for 1 hr, and then incubated overnight with primary antibody. Membranes were incubated for 1 hr with a species-appropriate peroxidase-labeled secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) followed by incubation with Amersham ECL Advance® substrate (GE Healthcare, Buckinhamshire, UK). The chemiluminescent image was captured using the ChemiDoc™ XRS system (BioRad, Hercules, CA).
Immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded liver tissue sections were deparaffinized and rehydrated. Following antigen retrieval, endogenous peroxidase activity was blocked by incubation in 1% hydrogen peroxide in methanol for 20 min. Slides were blocked with 1.5% normal goat serum (Vector Laboratories, Burlingame, CA). Negative control slides were prepared without primary antibody. Sections were incubated in primary antibody for 1 hr, washed, and then incubated with secondary antibody (biotinylated goat anti-rabbit IgG, Vector Laboratories, Burlingame, CA). The Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) was used to bind antibodies according to the manufacturer’s instructions. Slides were developed with diaminobenzidine tetracholoride (DAB), counter stained with hematoxylin and mounted. Immunohistochemical staining was observed at 20x with a Nikon Eclipse E400 light microscope equipped with a Sony ExWaveHAD 3CCD color video camera.
Statistical Analysis
For all quantitative data, the mean and standard error of the mean were calculated. Statistical differences were determined using one-way ANOVA followed by Duncan’s multiple range post-hoc test using Statistica software, Version 4.5. (StatSoft, Tulsa, OK). * Indicates p ≤ 0.05 compared to respective CO; † indicates p ≤ 0.05 compared to respective LCA-only.
RESULTS
Markers of Hepatoprotection
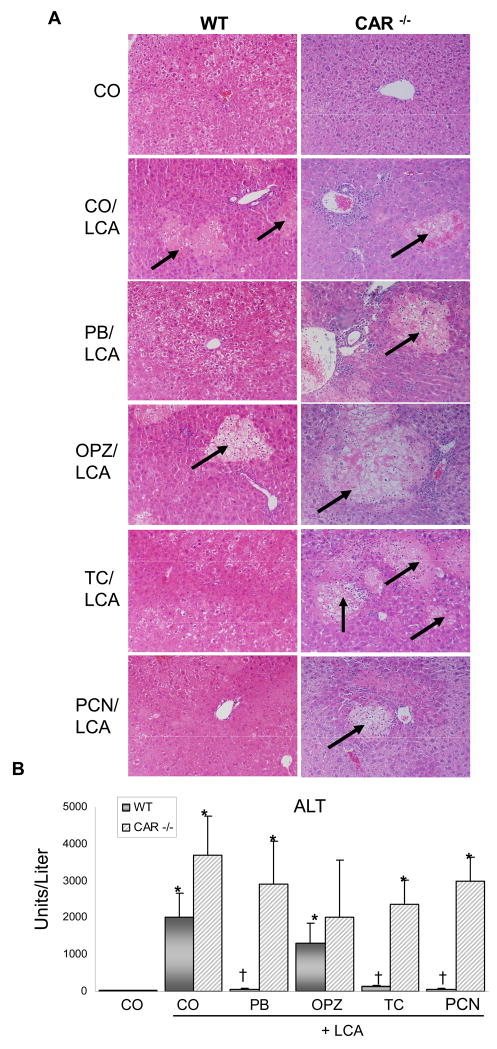
Liver histopathology revealed a similar degree of acute, multifocal hepatic injury with mild to moderate diffuse vacuolization in LCA-treated WT and all CAR-null mice, except CO controls (Fig. 1A). Additionally, in WT mice, OPZ pre-treatment did not alter LCA-induced damage. However, in WT mice with activated CAR (PB, TC) and PXR (PCN) the tissue damage caused by LCA is absent, with liver sections similar in appearance to vehicle controls. PB, TC, and PCN-mediated protection against LCA-induced injury is in line with previous reports (Staudinger et al., 2001; Kitada et al., 2003; Beilke et al., 2008). Most notably, hepatoprotection was absent in CAR-null mice, with all treated groups demonstrating significant liver damage. The effect of PB, OPZ, and TC pre-treatment on liver histology in the absence of LCA co-treatment has been previously demonstrated and is not remarkably different from controls (Annapurna et al., 1989; Davies et al., 1991; Kang et al., 2002; Huang et al., 2005; Baskin-Bey et al., 2007).
Fig 1. CAR activators protect the liver against bile acid-induced toxicity.
(A) A mid-section of the left liver lobe was evaluated and pictures are representative of treatment group pathology. Multifocal hepatic necrosis (arrows) is easily distinguished from surrounding parenchyma. 50× magnification. (B) Serum ALT values with results presented as mean concentration ± S.E.M. * Indicates p ≤ 0.05 compared to respective CO; † indicates p ≤ 0.05 compared to respective LCA-only.
Levels of serum ALT, an indicator of hepatocyte injury, correlated with histological findings (Figure 1B). ALT levels were markedly elevated above CO controls in LCA (73-fold) and OPZ (48-fold) pre-treated WT mice. The increase in ALT caused by LCA treatment was reduced by 94–97% in hepatoprotected WT mice pre-treated with PB, TC and PCN. In CAR-null mice, ALT was increased by LCA (113-fold), and remained elevated above CO controls despite pre-treatments with PB (89-fold), TC (72-fold), and PCN (92-fold). Taken together, data from Figures 1A–B demonstrate a strong correlation between CAR activation and hepatoprotection, pointing to a transcription-mediated mechanism for preventing liver injury.
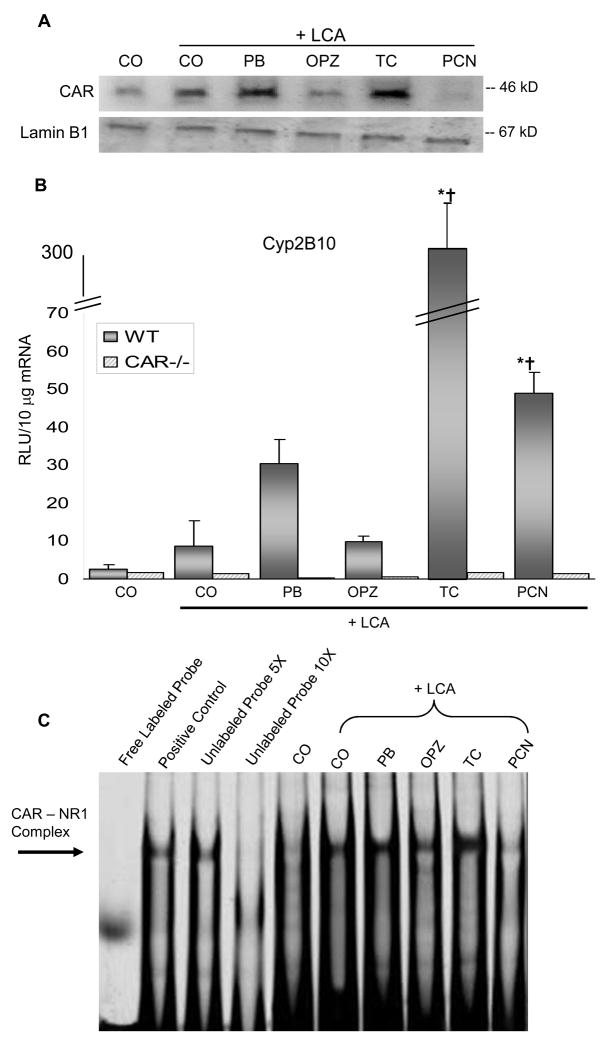
Accumulation and Activation of CAR
The ability of LCA and the various pre-treatments to activate CAR signaling was assessed by measuring nuclear CAR accumulation and binding activity. As expected, PB and TC markedly increased nuclear expression of CAR protein (Fig. 2A). PCN pre-treatment did not alter CAR protein expression, but did enhance PXR protein levels (data not shown). To ensure that nuclear extracts were not contaminated, blots were also screened for the cytosolic enzyme, NADPH quinone oxidoreductase 1 and no protein was detected (data not shown). Induction of the prototypical CAR target gene, Cyp2b10 was also measured and as expected, nuclear expression of CAR correlated with the degree of target gene Cyp2b10 induction (TC > PB=PCN > OPZ=LCA only) (Fig. 2B). Induction of the Cyp2B gene is mediated by the phenobarbital response element (PBRE), where CAR forms a heterodimer with RXR-α and binds to a NR-1 (nuclear receptor-1) site in the PBRE to initiate gene transcription. Binding of CAR to the Cyp2b10 NR-1 site (Fig. 2C) was investigated using the electrophoretic mobility shift assay (EMSA). A competition assay was performed using nuclear protein incubated in the presence of unlabeled NR-1 Cyp2b10 oligonucleotide probe or IR700 dye labeled oligonucleotide probe. The mobility shift assay was conducted with varying concentrations of nuclear protein to ensure the band visualized was in the linear range of detection (data not shown). TC pre-treatment stimulated the greatest binding of CAR to the NR-1 response element, as expected from this potent, direct-acting CAR ligand. As indirect activators of CAR, LCA with or without PB or OPZ also increased NR-1 binding, but to a lesser extent.
Fig. 2. CAR Accumulation and target gene induction.
(A) Nuclear protein was pooled from 3 mice per treatment group and 20 ug of total protein was used for Western blot analysis. (B) mRNA expression of Cyp2b10 as quantified by the bDNA signal amplification assay. * Indicates p ≤ 0.05 compared to respective CO; † indicates p ≤ 0.05 compared to respective LCA-only. (C) EMSA of liver nuclear proteins using a IR700 dye-labeled double-stranded Cyp2b10 NR-1 oligonucleotide probe.
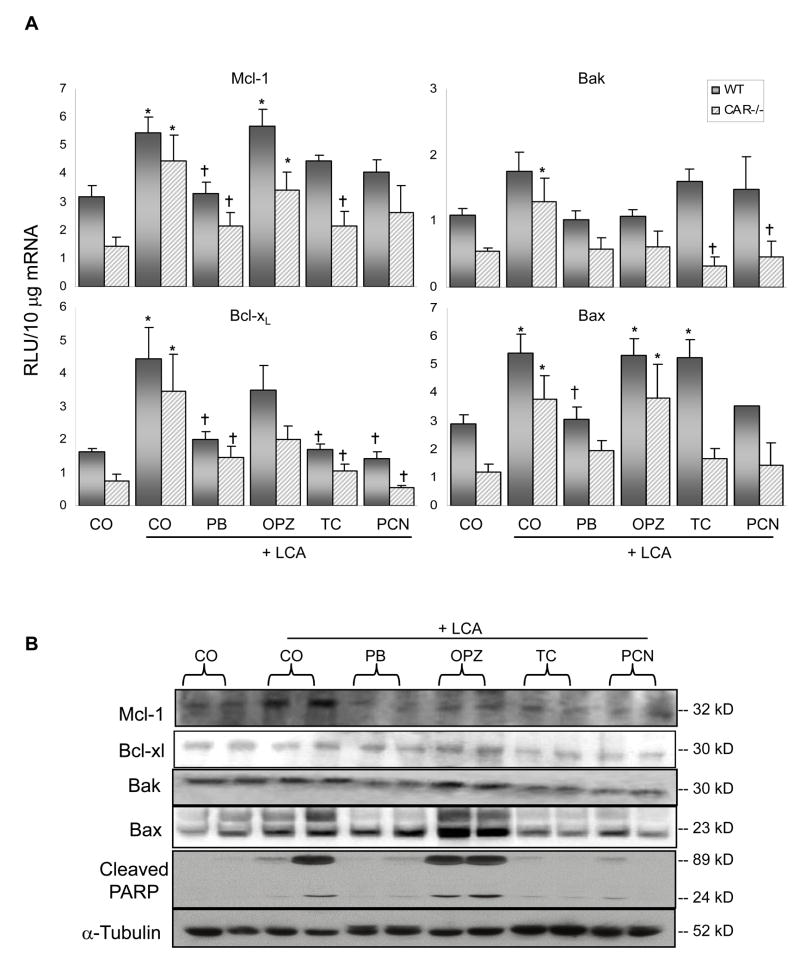
Hepatic Expression of Apoptosis-Related Genes
Increased Mcl-1 expression is generally thought to signify a decrease in apoptotic activity. Interestingly, an increase in Mcl-1 expression did not occur in this study and these findings do not correlate with hepatoprotection (Fig. 3A). On the contrary, Mcl-1 expression was increased in the unprotected WT mice given LCA alone (1.7-fold) or in combination with OPZ (1.8-fold). And rather than the expected increase in expression in the hepatoprotected mice (PB, TC and PCN pre-treated), the levels were unchanged from vehicle controls. Similarly, the expression of another anti-apoptotic gene, Bcl-xL, was increased above control values rather than decreased in unprotected LCA treated (2.7-fold) and OPZ (2.2-fold) pre-treated WT mice, and expression was unchanged from control values in protected PB, TC and PCN pre-treated mice. In CAR-null mice, expression of both Mcl-1 and Bcl-xL was increased in LCA treated mice and reduced closer to control values with inducer pre-treatments. Thus, the changes in anti-apoptotic gene expression were precisely opposite to that expected for an apoptosis-related mechanism of hepatoprotection, where the unprotected groups showed increased anti-apoptotic gene expression and protected groups were unchanged from basal levels.
Fig. 3. Apoptosis-related expression in liver.
(A) mRNA expression of anti-apoptotic Mcl-1 and Bcl-xL, pro-apoptotic expression of Bak and Bax as quantified by the bDNA signal amplification assay. N= 4–6 per group, except for WT PCN, N=3. Data are expressed as relative light units (RLU) ± S.E.M. * Indicates p ≤ 0.05 compared to respective CO; † indicates p ≤ 0.05 compared to respective LCA-only. (B) Cytosolic fractions were isolated and analyzed by Western blotting for protein expression of hepatic Mcl-1, Bcl-xL, Bak and Bax, as well as PARP. Two animals per treatment group are shown.
Treatment of mice with TC has been previously shown to result in decreased expression of pro-apoptotic proteins (Baskin-Bey et al., 2006). Unexpectedly, in the present study pro-apoptotic expression of Bak was largely unchanged in the various groups of WT mice. In CAR-null mice, LCA treatment increased Bak levels 2.4-fold above controls, and this elevation was prevented by the various inducer pre-treatments. Pro-apoptotic expression of Bax in WT mice, which would also be expected to be reduced in hepatoprotected mice, was increased (1.8-fold) following TC pre-treatment. Bax was also increased above control levels by LCA (1.9-fold), and in mice pre-treated with OPZ (1.8-fold). In CAR-null mice, Bax expression was increased 3.2-fold above control values by LCA and similarly following pre-treatment with OPZ (3.2-fold). Again, we did not observe decreases in pro-apoptotic genes in the hepatoprotected mice that would be expected as an indicator of reduced apoptosis.
Hepatic Expression of Apoptosis-Related Proteins
Results of the Western blot analysis are presented in Fig. 3B. Expression of anti-apoptotic Mcl-1 and Bcl-xL was not significantly different between treatment groups.
As an indicator of reduced apoptosis, expression of pro-apoptotic Bak was slightly decreased in hepatoprotected PB, TC and PCN pre-treated mice. And compared to OPZ pre-treated mice, a similar trend was observed with Bax, where expression was decreased in hepatoprotected TC and PCN pre-treated WT mice, and to a lesser extent in PB pre-treated mice. Poly (ADP-ribose) polymerase (PARP) is a nuclear protein involved in DNA repair and apoptosis. PARP cleavage serves as an early marker of apoptosis (Duriez and Shah, 1997; Trucco et al., 1998). Cleaved PARP protein was detected in the cytosol of livers from WT mice treated with LCA with or without OPZ pre-treatment, and these protein levels parallel the cCasp3 staining in livers from these mice. Whereas cleaved PARP protein in hepatoprotected PB, TC and PCN pre-treated mice was faintly noticeable.
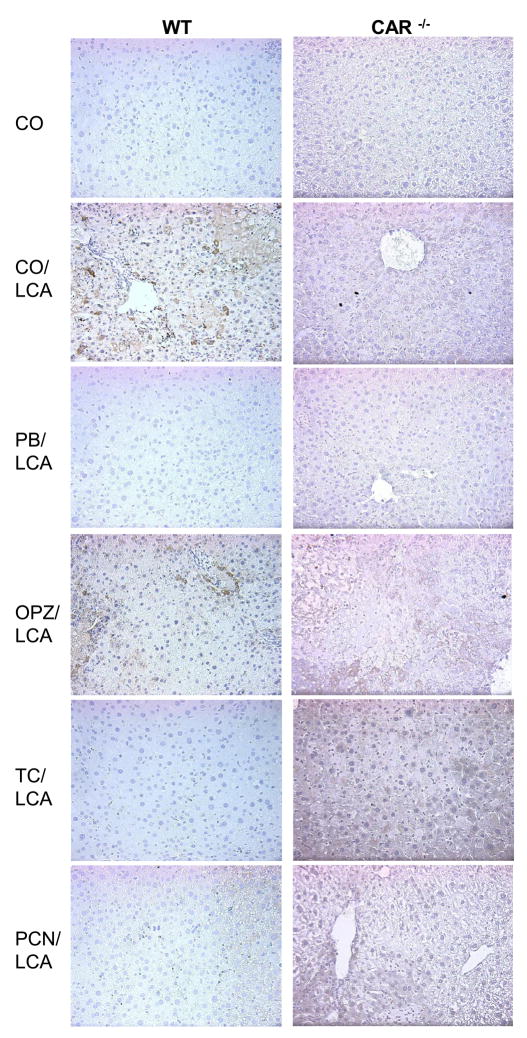
Immunohistochemical Localization of Apoptosis-Related Proteins
In WT mice given LCA with or without OPZ pre-treatment, cCasp3 staining (marker of apoptosis) was localized to the cytoplasm of hepatocytes (Fig. 4). cCasp3 staining was moderate to strong and uniform throughout the liver lobule. No staining of apoptotic cells was observed in the CO control or protected WT mice pre-treated with PB, TC and PCN. No staining was detected in any of the CAR-null groups, suggesting the observed tissue damage in those mice is a result of necrotic and not apoptotic cell death.
Fig. 4. Immunohistochemical localization of cCaspase3.
Caspase-dependent hepatocyte apoptosis was assessed using immunohistochemistry for active caspase 3. A minimum of two animals per treatment group were evaluated for IHC and pictures are representative of treatment group pathology. 20× magnification.
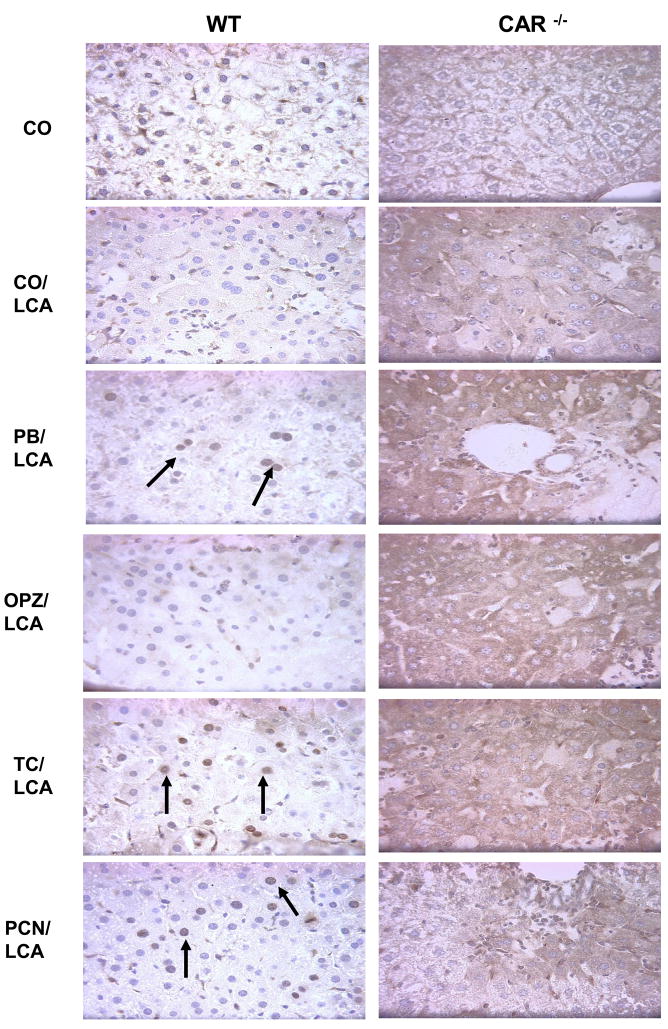
Immunostaining of the anti-apoptotic protein Mcl-1 was localized to the nuclei of CO and hepatoprotected PB, TC and PCN pre-treated WT mice (Fig. 5). Whereas, in unprotected LCA-treated WT and CAR-null groups, the staining was predominantly cytoplasmic. Nuclear Mcl-1 staining in the protected groups was multifocal, and not localized to a specific region of the liver. In liver sections of all the CAR-null treatment groups, Mcl-1 staining was mild and very diffuse. The difference in localization patterns between protected and non-protected mice suggests a possible role for nuclear Mcl-1 in reducing apoptosis.
Fig. 5. Immunohistochemical localization of Mcl-1.
The localization pattern of activated Mcl-1 was altered between the groups with hepatocellular damage (cytoplasmic) and those without damage (nuclear). A minimum of two animals per treatment group were evaluated for IHC and pictures are representative of treatment group pathology. 20× magnification.
DISCUSSION
Previous studies have demonstrated that activation of either CAR or PXR, prior to insult with LCA, protects against the hepatotoxic effects of accumulating bile acids (Staudinger et al., 2001; Kitada et al., 2003; Beilke et al., 2008). Hepatoprotection against LCA toxicity was observed in the present study in WT mice pre-treated with PB, TC and PCN, and a decrease in liver ALT values correlated with histopathology findings. Clearly the importance of CAR during bile acid-induced liver injury has been established in this study, as evidenced by the significant degree of hepatocellular injury observed in the absence of CAR (CAR-null mice). Altered regulation of apoptosis is observed in a number of acute and chronic liver diseases, including cholestasis. Recently, it has been demonstrated that CAR-dependent induction of anti-apoptotic Mcl-1 reduces liver injury (Baskin-Bey et al., 2006).
In the current study, we hypothesized that pre-treatment with CAR activators would increase Mcl-1 expression to decrease apoptosis during LCA-induced cholestasis as a mechanism of hepatoprotection. The classic PXR activator, PCN, was also included to determine whether the observed hepatoprotection was specifically CAR-dependent or whether activation of other nuclear receptors is involved. CAR must translocate to the nucleus to be active, therefore we first determined the intrinsic ability of the various pre-treatments to increase CAR nuclear accumulation. Accumulation of nuclear CAR was highest in PB and TC pre-treated mice. Additionally, CAR binding to the NR-1 site of Cyp2b10 was observed with each of the CAR inducers. Binding was strongest with TC, followed by PB > OPZ = LCA > PCN co-treatments. Interestingly, the strongest CAR activators (PB, TC) offered significant hepatoprotection against LCA in WT mice. Thus, for compounds that activate CAR, it appears that a high degree of CAR accumulation is associated with hepatoprotection, whereas weak accumulation, as with OPZ, is not sufficient to strongly activate CAR target genes and confer protection.
Next, to test our hypothesis that activation of CAR reduces hepatocellular apoptosis via increased Mcl-1 expression as a mechanism of hepatoprotection, we investigated the relative mRNA and protein expression of anti- and pro-apoptotic genes in WT and CAR-null mice. As stated earlier, Mcl-1 was hypothesized to be a key player in conferring CAR-mediated hepatoprotection from LCA-induced injury. Although Mcl-1 is rapidly inducible in response to a variety of stimuli (Zhang et al., 2002), its expression was surprisingly not increased at the mRNA or protein levels in WT mice pre-treated with TC or any of the hepatoprotective treatments. The differences observed in the present study versus Baskin-Bey and colleagues (2006) may be due to differences in the experimental model of liver injury. Where we used bile acid-induced cholestasis, they used the Fas agonist Jo2 as an acute model of liver injury. The liver is especially sensitive to Fas-mediated injury, which may account for the differences in apoptotic protein expression. Collectively, these expression data indicate that treatment with CAR activators during cholestasis does not increase Mcl-1 expression in hepatoprotected mice.
Patterns of anti-apoptotic Bcl-xL expression also did not correlate with hepatoprotection in the present study, indicating up-regulation of Bcl-xL is not necessary for the anti-apoptotic effect. These findings are supported by Baskin-Bey et al. (2006). Although PXR has been implicated as a regulator of Bcl-xL (Zucchini et al., 2005), its expression was not increased by PCN at the mRNA or protein levels. Interestingly, expression levels of pro-apoptotic proteins Bak and Bax did tend to correlate with hepatoprotection, as expression was generally reduced in PB, TC, and PCN pre-treated mice.
Upon establishing a link between strong CAR activation (EMSA) and the degree of hepatoprotection against LCA toxicity (histopathology), studies were aimed at quantifying apoptosis according to caspase 3 activation and PARP cleavage. The initiation and propagation of apoptosis is directed by caspases, and the TUNEL (terminal uridine deoxynucleotidyl transferase dUTP nick end labeling) assay has been a standard model for the evaluation of apoptosis. However, Duan and colleagues (2003) compared the use of activated caspase-3 immunohistochemistry (IHC) with the TUNEL assay and standard H&E histology and found that although the specificity of the three techniques was similar, sensitivity for the detection of apoptosis was greater with activated caspase-3 IHC. Moreover, both apoptosis and necrosis share some initial steps during DNA degradation, such that DNA strand breaks are not unique to apoptosis, resulting in potential false-positive staining with the TUNEL assay (Hayashi, et al., 1998). Therefore, using IHC, we observed a decrease in cCasp3 in each of the hepatoprotected groups (PB, TC and PCN pre-treatments) in comparison to LCA-only and OPZ pre-treated WT mice. Similarly, PARP cleavage, an early marker of apoptosis, was predominantly observed in unprotected LCA-only and OPZ pre-treated WT mice. Researchers have concluded that both apoptosis and necrosis can occur simultaneously (Shimizu et al., 1996; Leist et al., 1997), which was evident in WT mice in the present study. Unprotected LCA and OPZ pre-treated mice demonstrated both necrosis (by histological analysis) and apoptosis (observed using IHC and western blot). The absence of cCasp3 staining in the CAR-null mice suggests that necrosis, and not apoptosis, predominates in the pathology of bile acid-induced toxicity in these mice. Taken together, these findings demonstrate increased apoptosis in unprotected mice (LCA ± OPZ) and decreased apoptosis in hepatoprotected (PB, TC, PCN) pre-treated mice.
The final set of studies yielded the most interesting findings relating to Mcl-1. We investigated Mcl-1 staining to determine if the decreased apoptosis observed with cCasp3 correlated with increased Mcl-1 in hepatoprotected mice. We observed Mcl-1 staining in all groups of WT mice, but rather than the expected increases or decreases in the degree of staining, we observed a difference in the localization pattern between protected and non-protected groups. Mcl-1 was observed in the nucleus of hepatoprotected mice compared to the cytoplasmic localization in unprotected LCA and OPZ pre-treated mice. Interestingly, Mcl-1 is known to interact with other nuclear proteins, such as fortilin, a potent anti-apoptotic protein located in the nucleus (Zhang et al., 2002). Mcl-1 acts as a chaperone and binds and stabilizes fortilin to enable protection of cells from apoptosis by sequestering Ca2+ from downstream apoptotic pathways (Graidist et al., 2007). Since Mcl-1 was localized in the nuclei of hepatoprotected mice, it is possible that decreased apoptosis in these animals is related to its interaction with fortilin to maximize the pro-survival environment. Although we did not observe changes in Mcl-1 gene expression that correlated with hepatoprotection, we did observe notable changes in the localization pattern of Mcl-1.
A role for CAR in the observed hepatoprotection is clearly supported by the increased liver injury in the absence of CAR and the observation that even the PXR activator PCN is not hepatoprotective in the absence of CAR. Additionally, strong activation of CAR is required for the protective effect, as indicated by weak CAR activation by OPZ, which was not hepatoprotective. Apoptosis is reduced in hepatoprotected mice pre-treated with CAR activators; but this is not a direct result of CAR-mediated Mcl-1 up-regulation. Since apoptosis was decreased as indicated by cCasp3 and cleaved PARP but not by induction of anti-apoptotic genes, it is likely that reduced apoptosis is a contributing factor to the overall hepatoprotection in combination with other CAR-mediated effects. Of particular interest, was the change in cellular localization of Mcl-1 in hepatoprotected mice, which suggests that activation of CAR may play a role in directing Mcl-1 to the nucleus. In conclusion, this study demonstrates that apoptosis is reduced by strong activators of CAR and it introduces novel findings in the localization of Mcl-1 and provides direction for future studies on the interaction of Mcl-1 with other nuclear proteins to enhance cell survival as a possible mechanism of protection during cholestasis.
Acknowledgments
FUNDING
This work was supported in part by National Institutes of Health (DK068039 and ES011646 to N.C).
The authors would like to thank Hana Holubec, Parvathi Sinha and Dr. Sally Dickinson for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annapurna V, Mukundan M, Sesikeran B, Bamji M. Effects of ovulen-50, diethylnitrosamine and phenobarbital on liver regeneration in female rats. J Biosci. 1989;14:1–7. [Google Scholar]
- Baskin-Bey E, Huang W, Ishimura N, Isomoto H, Bronk S, Braley K, Craig R, Moore D, Gores G. Constitutive androstane receptor (CAR) ligand, TCPOBOP, attenuates Fas-induced murine liver injury by altering Bcl-2 proteins. Hepatology. 2006;44:252–262. doi: 10.1002/hep.21236. [DOI] [PubMed] [Google Scholar]
- Baskin-Bey E, Anan A, Isomoto H, Bronk S, Gores G. Consitutive androstane receptor agonist, TCPOBOP, attenuates steatohepatitis in the methionine choline-deficient diet-fed mouse. World J Gastroenterol. 2007;13:5635–5641. doi: 10.3748/wjg.v13.i42.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilke L, Besselsen D, Cheng Q, Kulkarni S, Slitt A, Cherrington N. Minimal role of hepatic transporters in the hepatoprotection against LCA-induced intrahepatic cholestasis. Toxicol Sci. 2008;102:196–204. doi: 10.1093/toxsci/kfm287. [DOI] [PubMed] [Google Scholar]
- Davies M, Schamber G, Schnell R. Oltipraz-induced amelioration of acetaminophen hepatotoxicity in hamsters. I Lack of dependence on glutathione. Toxicol Appl Pharmacol. 1991;109:17–28. doi: 10.1016/0041-008x(91)90187-j. [DOI] [PubMed] [Google Scholar]
- Duan W, Garner D, Williams S, Funckes-Shippy C, Spath I, Blomme E. Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J Pathol. 2003;199:221–228. doi: 10.1002/path.1289. [DOI] [PubMed] [Google Scholar]
- Duriez P, Shah G. Cleavage of poly(ADP-ribose) polymerase: a sensitive parameter to study cell death. Biochem Cell Biol. 1997;75:337–349. [PubMed] [Google Scholar]
- Faubion W, Guicciardi M, Miyoshi H, Bronk S, Roberts P, Svingen P, Kaufmann S, Gores G. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137–145. doi: 10.1172/JCI4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf D, Kurz A, Reinehr R, Fischer R, Kircheis G, Haussinger D. Prevention of bile acid-induced apoptosis by betaine in rat liver. Hepatology. 2002;36:829–839. doi: 10.1053/jhep.2002.35536. [DOI] [PubMed] [Google Scholar]
- Graidist P, Yazawa M, Tonquanunt M, Nakatomi A, Lin C, Chang Y, Phongdara A, Fujise K. Fortilin binds Ca2+ and blocks Ca2+-dependent apoptosis in vivo. Biochem J. 2007;408:181–191. doi: 10.1042/BJ20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guicciardi M, Gores G. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2006;54:1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R, Ito Y, Matsumoto K, Fujino Y, Otsuki Y. Quantitative differentiation of both free 3′ –OH and 5′ –OH DNA ends between heat-induced apoptosis and necrosis. J Histochem Cytochem. 1998;46:1051–1059. doi: 10.1177/002215549804600909. [DOI] [PubMed] [Google Scholar]
- Hengartner M. The biochemistry of apoptosis. Nature. 2000;407:770–775. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Moore R, Washburn K, Negishi M. Activation by diverse xenochemicals of the 51-base pair phenobarbital-responsive enhancer module in the CYP2B10 gene. Mol Pharmacol. 1998;53:597–601. doi: 10.1124/mol.53.4.597. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhang J, Washington M, Liu J, Parant J, Lozano G, Moore D. Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol Endocrinol. 2005;19:1646–1653. doi: 10.1210/me.2004-0520. [DOI] [PubMed] [Google Scholar]
- Kang K, Choi S, Ha J, Kim C, Kim S. Inhibition of dimethylnitrosamine-induced liver fibrosis by [5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione] (oltipraz) in rats: suppression of transforming growth factor-b1 and tumor necrosis factor-a expression. Chem Biol Interac. 2002;139:61–77. doi: 10.1016/s0009-2797(01)00286-1. [DOI] [PubMed] [Google Scholar]
- Kitada H, Miyata M, Nakamura T, Tozawa A, Honma W, Shimada M, Nagata K, Sinal C, Guo G, Gonzalez F, Yamazoe Y. Protective role of hydroxysteroid sulfotransferase in lithocholic acid-induced liver toxicity. J Biol Chem. 2003;278:17838–17844. doi: 10.1074/jbc.M210634200. [DOI] [PubMed] [Google Scholar]
- Leist M, Single B, Castoldi A, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in detection between apoptosis and necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell M, Jackson J, Augustine L, Fisher C, Slitt A, Maher J, Huang W, Moore D, Zhang Y, Klaassen C, Cherrington N. The Nrf2 activator oltipraz also activates the constitutive androstane receptor. Drug Metab Dispos. 2008 doi: 10.1124/dmd.108.020867. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa N, Kruglov E, Dranoff J, Robert M, Gores G, Nathanson M. The anti-apoptotic protein Mcl-1 inhibits mitochondrial Ca2+ signals. J Biol Chem. 2005;280:33637–33644. doi: 10.1074/jbc.M503210200. [DOI] [PubMed] [Google Scholar]
- Rodrigues C, Fan G, Ma X, Kren B, Steer C. A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest. 1998;101:2790–2799. doi: 10.1172/JCI1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini S, Sonoda J, Xu L, Toma D, Uppal H, Mu Y, Ren S, Moore D, Xie W. A novel constitutive androstane receptor-mediated and Cyp3A-independent pathway of bile acid detoxification. Mol Pharmacol. 2004;65:292–300. doi: 10.1124/mol.65.2.292. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Eguchi Y, Kamiike W, Waguri S, Uchiyama Y, Matsuda H, Tsujimoto Y. Retardation of chemical hypoxia-induced necrotic cell death by Bcl-2 and ICE inhibitors: possible involvement of common mediators in apoptotic and necrotic signal transductions. Oncogene. 1996;12:2045–2050. [PubMed] [Google Scholar]
- Sodeman T, Bronk S, Roberts P, Miyoshi H, Gores G. Bile salts mediate hepatocyte apoptosis by increasing cell surface trafficking of Fas. Am J Physiol Gastrointest Liver Physiol. 2000;278:G992–G999. doi: 10.1152/ajpgi.2000.278.6.G992. [DOI] [PubMed] [Google Scholar]
- Sola S, Amaral J, Aranha M, Steer C, Rodrigues C. Modulation of hepatocyte apoptosis: cross-talk between bile acids and nuclear steroid receptors. Curr Med Chem. 2006;13:3039–3051. doi: 10.2174/092986706778521823. [DOI] [PubMed] [Google Scholar]
- Staudinger J, Liu Y, Madan A, Habeebu S, Klaassen C. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab Dispos. 2001;29:1467–1472. [PubMed] [Google Scholar]
- Stedman C, Liddle C, Coulter S, Sonoda J, Alvarez J, Moore D, Evans R, Downes M. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci USA. 2005;102:2063–2068. doi: 10.1073/pnas.0409794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng S, Piquette-Miller M. Hepatoprotective role of PXR activation and MRP3 in cholic acid-induced cholestasis. Br J Pharmacol. 2007;151:367–376. doi: 10.1038/sj.bjp.0707235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco C, Oliver F, deMurcia G, Menissier-de Murcia J. DNA repair defect in poly(ADP-ribose) polymerase-deficient cell lines. Nucl Acids Res. 1998;26:2644–2649. doi: 10.1093/nar/26.11.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Hamadeh H, Webb H, Yamamoto Y, Sueyoshi T, Afshari C, Lehmann J, Negishi M. Diverse roles of the nuclear receptor CAR in regulating hepatic genes in response to Phenobarbital. Mol Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- Zhang D, Franklin L, Weidner D, Mnjoyan Z, Fujise K. Physical and functional interactions between myeloid cell leukemia 1 protein (MCL1) and fortilin. J Biol Chem. 2002;277:37430–37438. doi: 10.1074/jbc.M207413200. [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang W, Qatanani M, Evans R, Moore D. The constitutive androstane receptor and pregnane X receptor function coordinately to prevent bile acid-induced hepatotoxicity. J Biol Chem. 2004;279:49517–49522. doi: 10.1074/jbc.M409041200. [DOI] [PubMed] [Google Scholar]
- Zucchini N, de Sousa G, Bailly-Maitre B, Gugenheim J, Bars R, Lemaire G, Rahmani R. Regulation of Bcl-2 and Bcl-xL anti-apoptotic protein expression by nuclear receptor PXR in primary cultures of human and rat hepatocytes. Biochim Biophys Acta. 2005;1745:48–58. doi: 10.1016/j.bbamcr.2005.02.005. [DOI] [PubMed] [Google Scholar]