Abstract
The ability to perform accurate sequential movements is essential to normal motor function. Learning a sequential motor behavior is comprised of two basic components: explicit identification of the order in which the sequence elements should be performed and implicit acquisition of spatial accuracy for each element. Here we investigated the time course of learning of these components for a first sequence (SEQA) and their susceptibility to interference from learning a second sequence (SEQB). We assessed explicit learning with a discrete index, the number of correct anticipatory movements, and implicit learning with a continuous variable, spatial error, which decreased during learning without subject awareness. Spatial accuracy to individual sequence elements reached asymptotic levels only when the whole sequence order was known. Interference with recall of the order of SEQA persisted even when SEQB was learned 24 h after SEQA. However, there was resistance to interference by SEQB with increased initial training with SEQA. For implicit learning of spatial accuracy, SEQB interfered at 5 min but not 24 h after SEQA. As in the case of sequence order, prolonged initial training with SEQA induced resistance to interference by SEQB. We conclude that explicit sequence learning is more susceptible to anterograde interference and implicit sequence learning is more susceptible to retrograde interference. However, both become resistant to interference with saturation training. We propose that an essential feature of motor skill learning is the process by which discrete explicit task elements are combined with continuous implicit features of movement to form flawless sequential actions.
INTRODUCTION
Skilled sequential movements, from dialing a phone number to playing the piano, are central to effective motor behavior. Several laboratory-based sequence-learning paradigms have been developed to investigate how such skilled behaviors are acquired. Two basic components to sequence learning can be defined. The first component is the acquisition of the order of the elements in the sequence; the second is the ability to perform each element fast and accurately and combine them into a single behavior. Paradigms used to study sequence learning tend to emphasize one or another of these components. The popular serial reaction time task (SRTT) has subjects press one of, for example, four buttons, each associated with a different element. Unbeknown to the subject, a repeating sequence of usually 10 to 12 elements is presented; learning of sequence order is inferred from a reduction in response time (Goedert and Willingham 2002; Nissen and Bullemer 1987). A second paradigm has subjects previously learn a short sequence of finger movements and then measures increases in both speed and accuracy (Karni et al. 1998; Walker et al. 2003). Thus this task emphasizes improved performance of the sequence elements rather than acquisition of sequence order. Finally, a set of paradigms requires subjects to explicitly learn a motor sequence. In this type of task, the two components of sequence learning can be quantified separately, the acquisition of the sequence order with a categorical measure, and performance of the sequence elements with continuous measures such as spatial accuracy and movement time (Ghilardi et al. 2000, 2003a,b, 2007, 2008; Hikosaka et al. 1995, 2002).
We hypothesized that further insight into the two component processes of sequence learning could be provided by studying how each consolidates, with consolidation defined as resistance to interference by another sequence. Investigations of sequence consolidation using the SRT task have failed to show evidence for time-dependent resistance to interference by a second sequence (Goedert and Willingham 2002). In contrast, studies of sequential finger tapping have demonstrated consolidation (Walker et al. 2003). A possible explanation for this contradiction is that these studies each investigated one or other of the two components of sequence learning and that these consolidate differently. A similar dissociation for order and performance components has been described for learning rate, generalization across effectors, and off-line gains (Bapi et al. 2000; Ghilardi et al. 2003a; Hikosaka et al. 1995; Savion-Lemieux and Penhune 2005). Our goal here was to show separable consolidation processes during learning of a single sequence task.
Previous work has shown that when target order is not known and subjects are asked to go as soon as possible (reaction time mode), specification of trajectory planning is truncated and therefore incomplete (Ghez et al. 1997; Hening et al. 1988). When subjects develop explicit awareness of sequence order, they make the transition from a reaction time mode into an anticipatory mode of response initiation (Ghilardi et al. 2003a, 2007, 2008). Concurrently, subjects show an implicit increase in spatial accuracy (Ghilardi et al. 2003a, 2007, 2008). Improvement in accuracy as target order is anticipated is likely the result of both increased time for parameter specification and prolongation of movement time.
Here we sought, using a reaching task, to investigate the effect of interference on the time courses for acquisition of explicit sequence order and for improvement in implicit spatial accuracy and thereby determine how these two processes consolidate. We first assessed subjects' ability to learn and recall a single sequence (SEQA). Successful recall after 48 h was measured both in terms of sequence order (explicit) and spatial accuracy (implicit). We then looked for differential sensitivity of these two components to interference by a second sequence (SEQB), learned either 5 min or 24 h after SEQA. Finally, we investigated whether prolonged training with SEQA mitigates interference by SEQB.
METHODS
Subjects
Forty-four subjects volunteered for the study (20 women and 24 men). Ages ranged from 20 to 35 yr (mean ± SD: 28.7 ± 6.5). All subjects signed an institutionally approved consent form and were paid to participate. None of the subjects was aware of the purpose of the experiment. Each subject was randomly assigned to one of eight groups and tested over a 3-day period as reported in Table 1.
TABLE 1.
Experimental protocol for six groups in the study
| Group (n) | Day 1 Train | Day 2 | Day 3 Test |
|---|---|---|---|
| 1 Control (6) | A | A | |
| 2 5-min Interference (6) | A,BBB | A | |
| 3 24-h Interference (6) | A | BBB | A |
| 4 Control (6) | AAA | A | |
| 5 5-min Interference (6) | AAA,BBB | A | |
| 6 24-h Interference (6) | AAA | BBB | A |
A, Sequence A; B, Sequence B.
Motor tasks and experimental protocol
Details about the motor tasks are reported in previous publications (Ghilardi et al. 2003a,b, 2007, 2008). Subjects sat facing a computer monitor at eye level and controlled a screen cursor by moving a handheld indicator across the surface of a horizontal digitizing tablet with their right arm. They made out and back movements from a central starting point to one of eight targets displayed as circles on the computer screen (Fig. 1A). Instructions were to make fast movements without corrections and to reverse inside each target circle. Graying of the target circle indicated successful hits. The target always appeared in synchrony with a tone at 1-s intervals. Distance to the target was 4.8 cm. Testing was done in separate trial blocks of 88 movements (or 11 complete cycles). A computer sampled hand positions at 200 Hz and controlled the experiments.
FIG. 1.
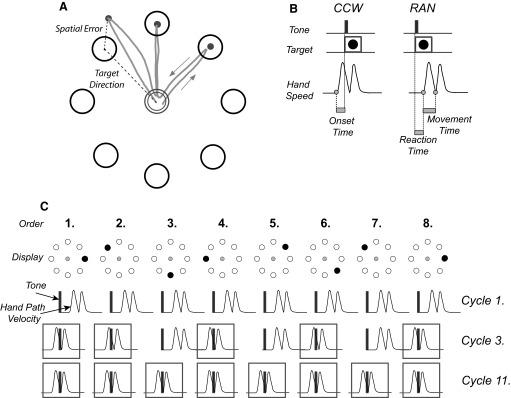
A: target array with representation of spatial error and target direction. B: CCW and RAN tasks (see text for explanation). In CCW, the out and back movements start before tone and target occurrence, resulting in negative onset times. In RAN, movements always start after tone and target presentation. Movement time is measured as the time between the movement onset and the reversal. C: schematic illustration of the development of anticipatory movements during sequence learning (SEQ). Tones and targets are presented at a constant time interval of 1 s (see methods). In the 1st cycle, movements are initiated by responding in reaction time to the target appearance, as in RAN. In the course of learning, movements start before target (boxed hand paths), as in CCW. Five anticipatory movements are already present by the 3rd cycle. In the last (11th) cycle, all target appearances are anticipated.
In all subjects, we first assessed baseline motor performance with two tasks that did not require sequence learning. 1) RAN: a reaction time task in which targets are presented in a pseudorandom, nonrepeating order. Subjects are required to move “as soon as possible,” thereby minimizing reaction time but avoiding target anticipation (Fig. 1B). This task was also used to assess the floor value of reaction time, i.e., the lowest onset time that does not reflect target anticipation. 2) CCW: a timed-response paradigm in which targets appear in a predictable counterclockwise order along with a tone. Subjects are instructed to reach and reverse in the target in synchrony with the tone, thus anticipating its appearance (Fig. 1B). This task represents a situation in which subjects know the target order exactly and so can perfectly anticipate the appearance of each target.
After RAN and CCW, subjects were trained on a sequence (SEQAtrain). We previously used this task to test healthy subjects and patients with basal ganglia disorders (Ghilardi et al. 2003a,b, 2007, 2008). Briefly, during SEQAtrain, the eight targets were presented in a repeating sequence of eight elements (Fig. 1C). Subjects were informed that targets would appear in a particular order and that they should discover this target order. They were also told that when they knew the position of a particular target in the sequence that they should anticipate it. In other words, they were asked to begin in reaction time mode, as in RAN, but to shift to a timed-response mode, as in CCW, as they explicitly acquired the sequence (Fig. 1C).
In the first session, SEQAtrain, subjects were trained with either 11 or 33 cycles. We chose 33 cycles to allow subjects to continue learning even after they had reached asymptote (saturation training). Forty-eight hours later, in a second session, subjects were tested with 11 cycles of the same sequence (SEQAtest). To examine interference, subjects were trained with 33 cycles of a new sequence (SEQB) either 5 min or 24 h after SEQAtrain (subjects were informed that SEQB was different from SEQA). At the end of each SEQ block, subjects were asked to verbally report the sequence order. Responses were scored from 0 to 8 (declarative scores; Ghilardi et al. 2003a,b, 2007, 2008).
Table 1 outlines the experimental protocol for the six groups in the study. All six groups were first tested on RAN and CCW. Two groups (1 and 4) were sequence-learning controls: they trained with either 11 or 33 cycles of SEQA and then were tested with 11 cycles of SEQA 48 h later. To assess interference, two groups were trained with 11 cycles of SEQA and then 33 cycles of SEQB, either five minutes (group 2) or 24h later (group 3). To determine whether extra initial sequence training might make subjects resistance to interference, two groups were trained with 33 cycles of SEQA and then 33 cycles of SEQB, either 5 min (group 5) or 24 h later (group 6). Like the control groups 1 and 4, groups 2, 3, 5, and 6 were tested with 11 cycles of SEQA 48 h after initial training. Based on our results with groups 1–6, we found it necessary to add two more control groups (7 and 8, not shown in Table 1). The rationale for this control experiment is more easily understood in the context of the results. Briefly, we addressed the hypothesis that interference might occur only when there is an imbalance between the training durations for SEQAtrain (11 cycles) and SEQB (33 cycles). Thus two new groups of subjects were trained with 11 cycles of SEQAtrain, but this time followed by only 11 cycles of SEQB, either 5 min (group 7) or 24 h (group 8) later.
Data analysis
Kinematic measures.
As detailed in previous reports (Ghilardi et al. 2000, 2003a,b, 2007, 2008) and shown in Fig. 1, for each movement, onset, peak velocity, peak acceleration, and reversal position were identified and the following measures computed: 1) Spatial error, the distance of the reversal point from the center of the target. 2) Movement extent, the length of the vector from the movement onset to the reversal point. 3) Movement time (MT), the time from movement onset to the endpoint. 4) Onset time, the time from target and tone presentation to movement onset. Depending on the experimental time constraint, this measure corresponds to movement latency or reaction time. Negative values indicate responses that were initiated before the tone.
Explicit knowledge of sequence order.
For both SEQA and SEQB, we computed the number of correct anticipatory movements per cycle (i.e., every eight movements). Correct anticipatory movements were defined as those directed to the correct target and with onset times below the lowest reaction time achieved in RAN for each subject. These movements reflect explicit learning of sequence order (Ghilardi et al. 2003a,b, 2007, 2008) because we have previously found a strong correlation (r2 >0.7) between the number of correct anticipatory movements and the declarative report collected at the end of each block. Thus this correlation provides the basis for our decision to use correct anticipatory movements as a proxy measure for explicit learning. We have previously shown in healthy subjects that acquisition of a new sequence order occurs mostly over cycles 2–5 (Ghilardi et al. 2003a,b, 2007, 2008). Therefore to quantify learning for each sequence, we averaged our learning index over cycles 2–5. The explicit learning index was the number of anticipatory movements, expressed as a percentage of the total number of movements in four cycles (i.e., 32). Both changes in explicit learning of SEQA over sessions 1 and 2 and the difference between learning of SEQA and SEQB in session 1 were assessed as a change in the learning index.
Differences between groups and sessions (training and test) were assessed with mixed ANOVA and post hoc tests (Bonferroni test with correction for multiple comparisons) were considered significant at a value of P < 0.05. All statistical procedures were performed with STATVIEW 5.0 (Abacus Concepts).
RESULTS
Movements in CCW are more accurate than those in RAN
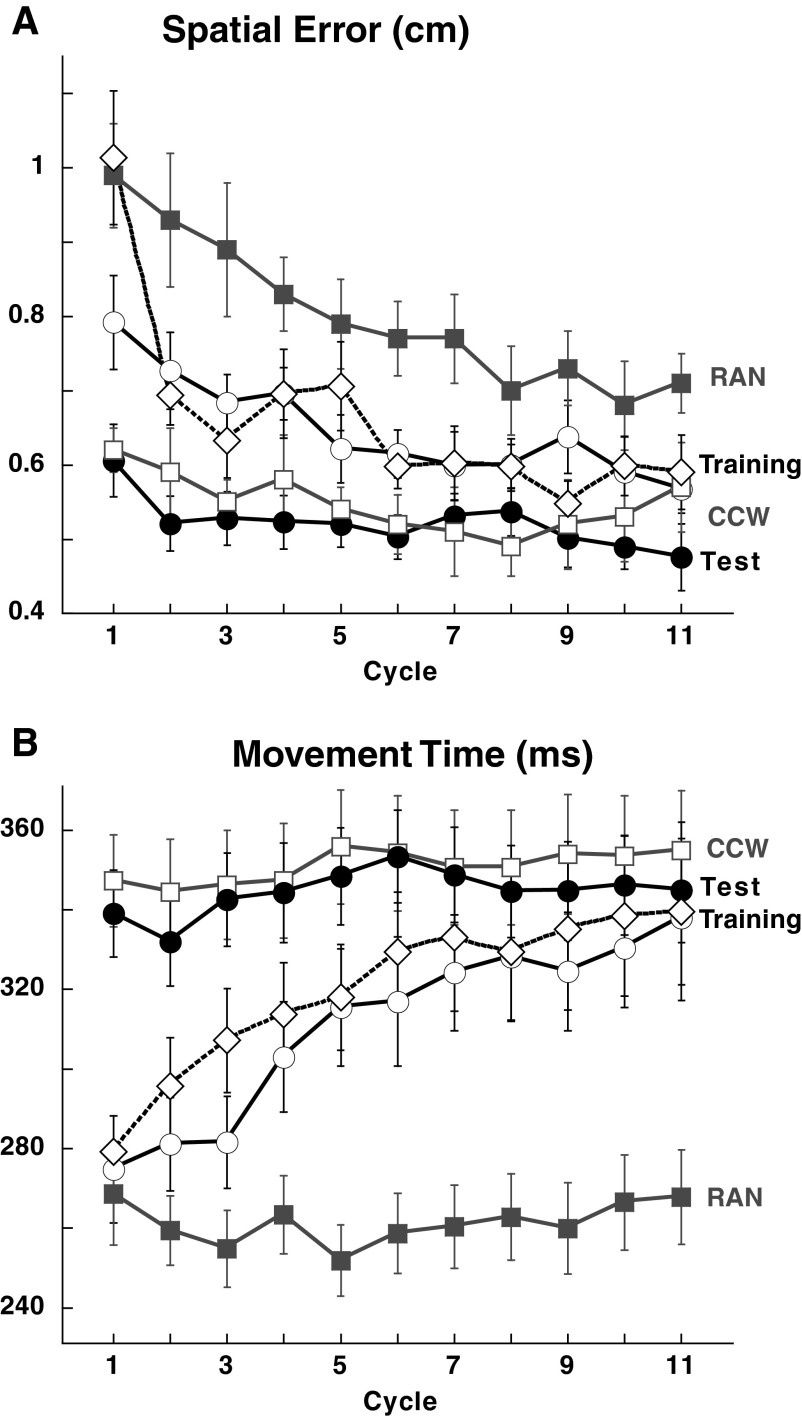
We first analyzed movements in RAN and CCW, the two control tasks in which performance is independent of sequence learning. In RAN, targets were presented randomly and so subjects had to wait for the target to appear, along with the sound of a tone, to specify and start their response. In this task, the instructions to the subjects (“move as soon as possible”) should lead to a truncation of response specification, with a reduction in accuracy compared with when subjects can go “when ready” (Ghez et al. 1997; Hening et al.1988). In CCW, targets were presented sequentially in a counterclockwise order and thus were completely predictable: subjects could specify and start their response before target appearance. As shown in Fig. 2A, spatial accuracy was greater for CCW than for RAN throughout session 1 [ANOVA: effect of task: F(1,142) = 112.0, P < 0.0001]. The spatial accuracy difference between CCW and RAN was paralleled by a longer MT for CCW compared with RAN [ANOVA: effect of task F(1,142) = 57.0, P < 0.0001] (Fig. 2B). The large spatial accuracy and MT differences between CCW and RAN did not change over the course of session 2 [ANOVA: effect of time: spatial accuracy: F(1,142) = 0.23, P = 0.64; MT: F(1,142) = 0.85, P = 0.35] (see Fig. 2A, inset). The marked superiority in spatial accuracy for CCW compared with that of RAN is critical to the approach taken herein with regard to sequence learning. Our core idea was that learning of a sequence reflects the transition of response initiation from a reaction time mode, as in RAN, to an anticipatory mode, as in CCW. This transition affords an increase in spatial accuracy both through fuller specification of movement parameters and prolongation of movement time (Ghilardi et al. 2008). For RAN training in session 1, mean and floor reaction times were similar across all six groups (mean: P = 0.5; floor: P = 0.9). Mean reaction time decreased to a stable asymptote over approximately four cycles (Fig. 3A). In session 2, 48 h later, all six groups maintained this asymptotic reaction time across the entire block [ANOVA: effect of cycle F(1,10) = 4.784, P < 0.0001; cycle × session interaction: F(1,10) = 4.24, P < 0.0001]. Importantly, floor reaction times (see Fig. 3A, inset) did not change across sessions (P = 0.8), suggesting that the reduction in mean reaction time reflects an increase in efficiency of response selection and not a change in strategy. As explained in methods, floor reaction time is used to define anticipatory movements. For CCW, onset time was always anticipatory (Fig. 3B), was similar across all six groups (P = 0.6), did not change across days (P = 0.8), and was of similar magnitude to values reported in previous studies (Ghilardi et al. 2000, 2003a, 2007, 2008).
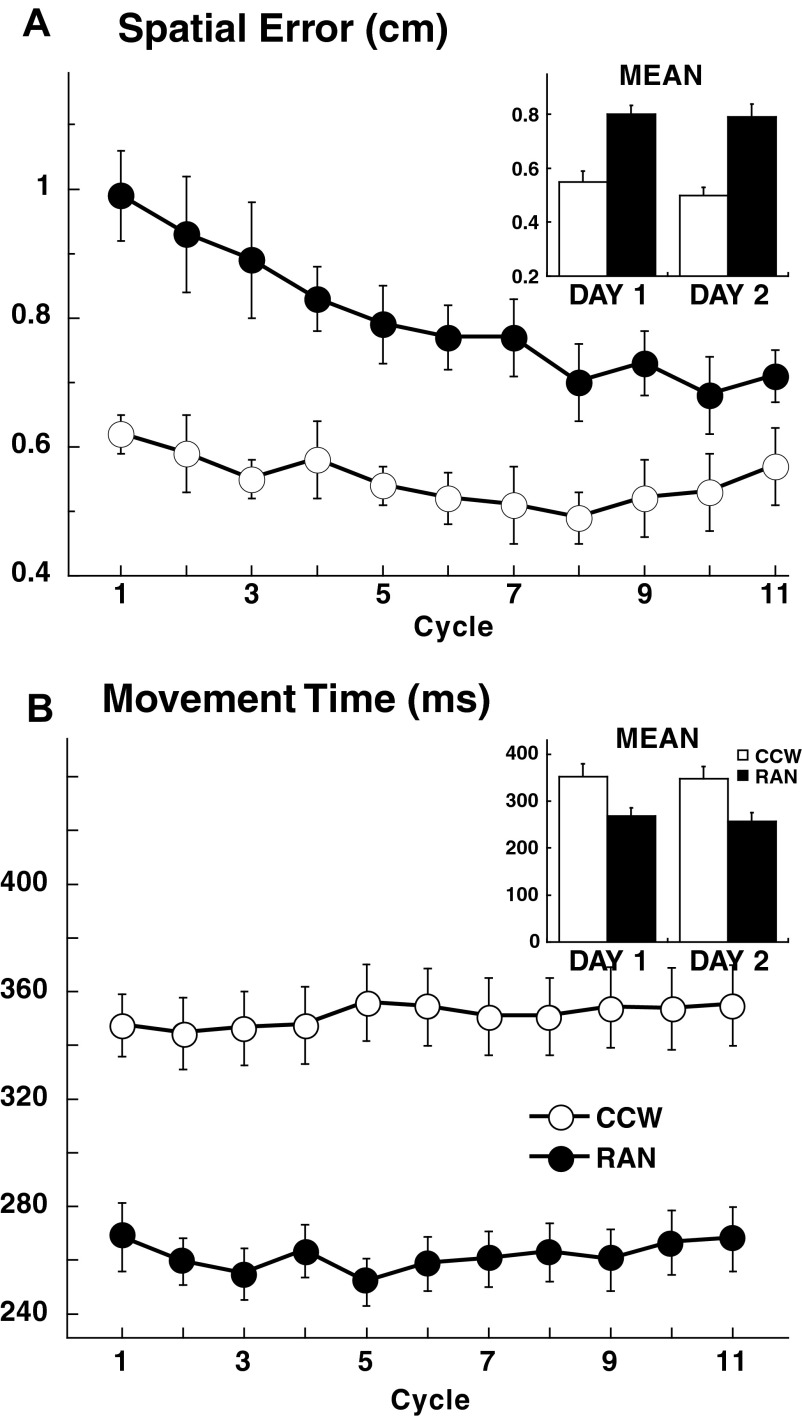
FIG. 2.
Spatial accuracy and movement time (MT) differ in CCW and RAN. A: spatial errors. The time course per cycle (8 movements) plotted for the control groups combined on day 1 and the means for both day 1 and day 2 in the inset, show that spatial error was always higher in RAN than that in CCW. B: MT. The time course and the means (inset) of CCW and RAN MTs for days 1 and 2 show that movement durations were prolonged in CCW compared with RAN, without differences between the 2 days.
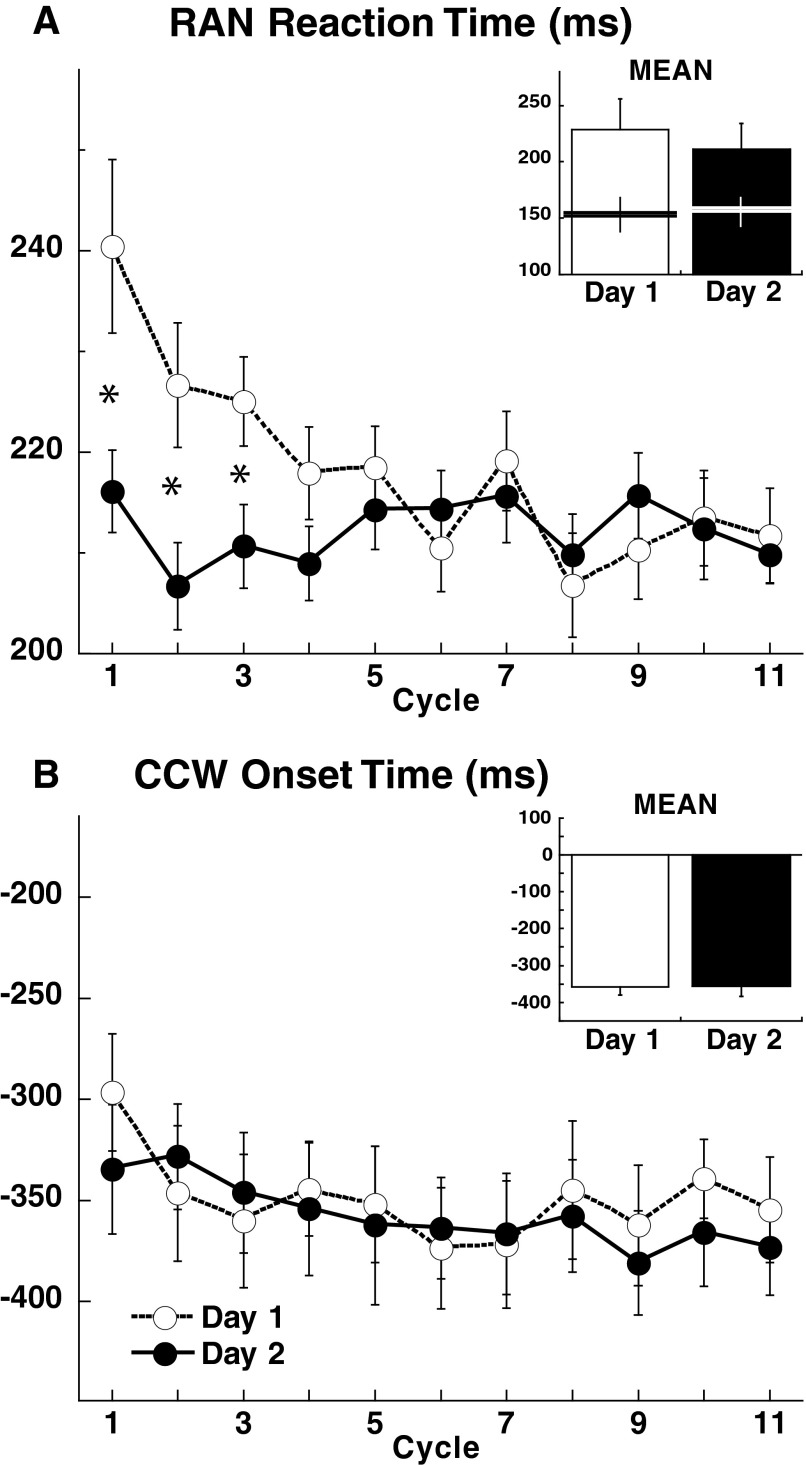
FIG. 3.
A: RAN mean reaction time (±SE) per cycle (8 movements) plotted for all 6 groups combined for day 1 and day 2. On day 1, reaction time decreased over the first 3 cycles to a stable value. Post hoc tests disclosed significant differences between sessions for the first 3 cycles (asterisks, P < 0.001). In the inset, mean reaction time (±SE) of the first 3 cycles for all subjects for both days. The horizontal lines represent average floor reactions times (±SE). Notice that mean reaction times decreased on day 2, whereas floor reaction times did not change. B: CCW mean onset time (±SE) per cycle of all groups combined for day 1 and day 2. In the inset, mean onset time (±SE) on day 1 and day 2. Onset times did not change across either cycles or days.
Acquisition of explicit sequence order was accompanied by an implicit increase in accuracy
Subjects were trained with either 11 (groups 1–3) or 33 cycles (groups 4–6) of SEQAtrain in session 1. As stated in methods, they were instructed to respond to targets in reactive mode, as in RAN, and then to start anticipating a target's appearance, as in CCW, when they had acquired explicit knowledge of its position in the sequence.
Change in onset time.
The transition toward anticipation of target order was evident as a reduction in onset time over the first 11 cycles of SEQA in session 1. Data for the first 11 cycles of SEQ A in session 1 are averaged across the two control groups (1 and 4) and shown in Fig. 4; a similar decrease in onset time was also seen in the other four groups. Indeed the decrease in onset time was not significantly different across all six groups [F(5,300) = 0.65, P = 0.66; group × cycle interaction: F(50,300) = 0.837; P = 0.77]. In the first cycle, mean onset times of SEQAtrain did not significantly differ from RAN reaction times [F(1,30) = 1.4, P = 0.23]. However, by the eleventh cycle, onset times for SEQAtrain were similar to those seen for CCW [F(1,30) = 0.65, P = 0.43], without significant differences between groups [F(5,30) = 0.95, P = 0.47]. There was no further reduction in onset time for the three groups (4–6) trained with 33 cycles. Thus by the end of training, all six groups had the same reduction in onset time for SEQAtrain by the end of session 1.
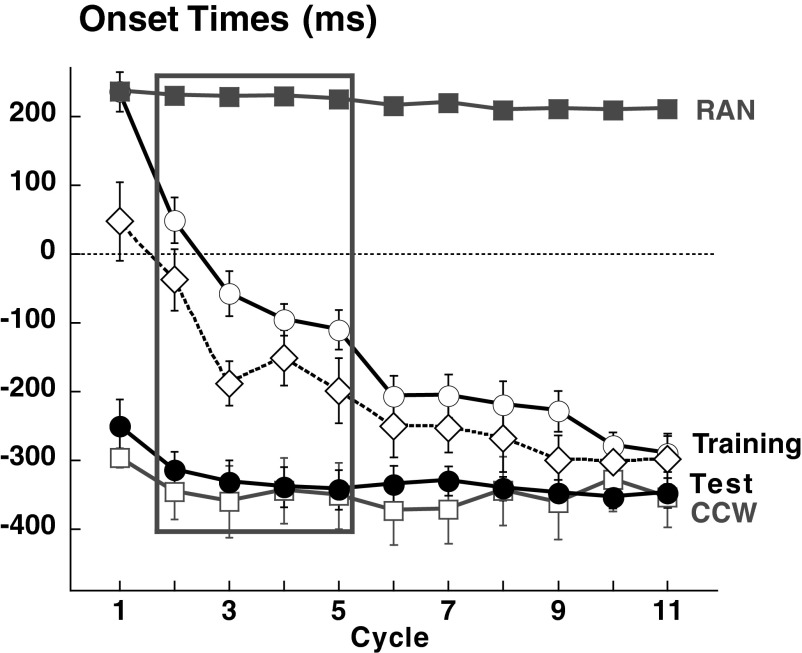
FIG. 4.
Onset times for control groups 1 and 4 combined. Mean onset times (±SE) per cycle plotted for RAN (filled gray squares), CCW (empty gray squares), SEQAtrain (empty circles), and SEQAtest (filled circles). Empty diamonds and dotted lines represent the mean for correct anticipatory movements in SEQAtrain. Data were combined for the 2 control groups because there was no significant difference between them. Learning during SEQAtrain is shown by the progressive reduction in onset time across cycles. There was a significant difference between SEQAtrain and RAN, CCW, and SEQAtest (all P < 0.0001), but not between SEQAtest and CCW (P = 0.98).
Explicit learning of sequence order.
We assessed explicit learning of sequence order with a discrete measure, the number of correct anticipatory movements per cycle, which we have previously shown to be highly correlated with declarative scores (Ghilardi et al. 2003a). Additional evidence that correct anticipatory movements reflect explicit learning is that their onset time (average obtained across all six groups) was already −200 ms (±90 ms) after the second cycle of training. This represents a reduction of about 400 ms within two cycles, which is in stark contrast to the small (∼40- to 120-ms) reductions seen in the SRT tasks and, even then, at the end of an entire training session (see, for instance, Goedert and Willingham 2002; Wilkinson and Shank 2004; Willingham 1989). This magnitude of reduction can plausibly be explained only by explicit recognition of the position of certain targets in the sequence and not by an implicit process whose contribution, even if present, would be small. The two control groups, trained with either 11 cycles (group 1) or 33 cycles (group 4), showed a similar time course of correct anticipatory movements during training and testing with SEQA (P > 0.9) (Fig. 5A). At the end of training, subjects were asked to give verbal report of sequence order and achieved the maximum declarative score of 8, despite not quite reaching perfect anticipatory responses by the end of session 1. At test, 48 h later, there was complete recall of learning from session 1 and further improvement with correct anticipatory responses to all eight targets [ANOVA: effect of session: F(1,220) = 51.1, P < 0.0001; cycles: F(10,220) = 27.2, P < 0.0001; session × cycle: F(10,220) = 18.9, P < 0.0001].
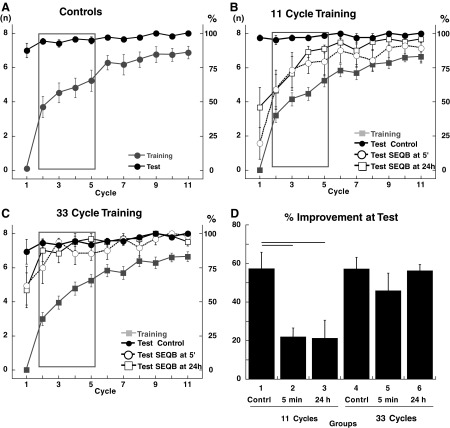
FIG. 5.
Correct anticipatory movements for SEQA. A: control groups 1 (11 cycles of training) and 4 (33 cycles of training). Correct anticipatory movements (mean ± SE) per cycle plotted for SEQAtrain (filled gray circles) and SEQAtest (filled black circles). Data were combined for the 2 control groups because there was no significant difference between them. B: groups 1, 2, and 3 (11 cycles of training). There was no significant difference in SEQAtrain for the 3 groups (P > 0.8) and data were combined (filled gray squares). Correct anticipatory movements for SEQAtest plotted separately for groups 1 (filled black circles), 2 (empty black circles), and 3 (empty black squares). C: groups 4, 5, and 6 (33 cycles of SEQAtrain). There was no significant difference in SEQAtrain for the 3 groups (P > 0.8) and data were combined (filled gray squares). Correct anticipatory movements for SEQAtest plotted separately for groups 1 (filled black circles), 2 (empty black circles), and 3 (empty black squares). D: savings at Test (% improvement in cycles 2–5). Each bar represents the mean ± SE for each group. ANOVA showed a main effect for amount of training [F(1,30) = 10.92, P = 0.002] and a main effect for groups [F(2,30) = 6.4, P = 0.005]. Post hoc tests showed significant (P < 0.003) differences between groups 2 and 3 and groups 1, 4, 5, and 6.
Implicit reduction in spatial error.
In parallel with explicit acquisition of sequence order, subjects also showed a progressive and significant decrease in spatial error over the first 11 cycles of SEQAtrain. This can be seen for the two control groups (1 and 4) in Fig. 6A [ANOVA: effect of cycle: F(10,121) = 2.4, P < 0.014]. In the first cycle, spatial error for SEQAtrain did not significantly differ from RAN reaction times [F(1,30) = 1.4, P = 0.23]. However, by the eleventh cycle, spatial error for SEQAtrain was similar to that seen for CCW. A further decrease of spatial error occurred on day 2 [ANOVA: effect of session: F(1,242) = 53.5, P < 0.0001]. Concomitant with the decrease of spatial error on day 1 was an increase in MT [ANOVA: effect of cycle: F(10,121) = 2.04, P < 0.035] (Fig. 6B). On day 2, MT in SEQAtrain was not different from CCW values (P > 0.5). Thus explicit learning of sequence order led to anticipation of upcoming targets, which allowed subjects to specify responses in advance of target appearance and prolong MT.
FIG. 6.
Implicit learning of SEQA in controls. A: mean spatial errors (±SE) per cycle of control groups 1 and 3 are plotted for RAN (filled gray squares), CCW (empty gray squares), SEQAtrain (filled black circles), and SEQAtest (empty black circles). Empty diamonds and dotted lines represent the mean MT for correct anticipatory movements in SEQAtrain. Data for the 2 control groups were combined because no difference was found between them. Spatial errors decreased over the course of SEQAtrain and further improvement occurred on day 2. B: mean MTs (±SE) per cycle of the 2 control groups combined are plotted for RAN, CCW, SEQAtrain, and SEQAtest and correct anticipatory movements in SEQAtrain. Symbols are the same as in A.
The reduction in spatial error over the first 11 cycles of SEQAtrain suggested a continuous process. This was confirmed by the fact that spatial error showed a similar continuous decrease across cycles when only correct anticipatory movements were included in the analysis (movements that were above the RT floor and/or to the wrong target were excluded) [ANOVA: effect of cycle: F(10,109) = 6.7, P < 0.0001] (Fig. 6A). This result is crucial because the appearance of a continuous decrease in spatial error across cycles could, in fact, have been an artifact of averaging this variable in each cycle, with a single stepwise decrease in error with each additional explicitly anticipated target. In fact, spatial accuracy and MT did not reach asymptotic levels to any given target until the whole target sequence was known (last cycle of session 1 and all of session 2). That is to say, spatial errors to those targets whose position in the sequence had been explicitly identified in early cycles did not reach a minimum until all the target positions were known explicitly. To reiterate, accuracy did not improve in a single step from RAN to CCW levels for each target as its position in the sequence was identified; instead, the entire sequence had to be identified explicitly before spatial performance to any given target could be optimized implicitly to CCW levels. It is of interest to note that spatial error to correctly anticipated targets at the beginning of SEQB (mean ± SE: 0.73 ± 0.07 cm) was higher than that at the end of the first session of SEQA (0.60 ± 0.04 cm, two-tailed t-test: P = 0.009). This is further indication that spatial accuracy can reach its highest level only within the context of a fully known sequence, i.e., the improvement in spatial accuracy cannot be explained by a nonspecific practice effect.
How was spatial accuracy achieved as the sequence order was explicitly acquired? As stated in the introduction, anticipation allows both an increase in response specification time and prolongation of MT. Both of these effects could potentially increase spatial accuracy but disambiguating them was not a primary goal of the current study. This is because, regardless of mechanism, CCW levels of spatial accuracy to any given target were not achieved until the whole sequence was explicitly acquired. This said, certain aspects of the data suggest that MT prolongation was not the sole cause of increase in spatial accuracy. First, as can be seen from Fig. 2, spatial accuracy improved across cycles in RAN without an accompanying increase in MT. Second, correlations of spatial error against MT for individual subjects during initial learning of SEQA showed R values that ranged within 0.17 and 0.4, which suggests that there is considerable variability in spatial accuracy that is not accounted for by MT.
Explicit learning of sequence order did not show a consolidation window
We used an interference paradigm to further determine the differences in the explicit and implicit components of sequence learning. We investigated interference by having subjects learn a second sequence, SEQB, between SEQAtrain and SEQAtest. The interval between SEQAtrain and SEQB was either 5 min or 24 h. The interval between SEQAtrain and SEQtest was always 48 h. We were interested in two types of interference, anterograde and retrograde. We defined these operationally. Interference was considered retrograde if SEQB had more of an effect on SEQAtest when it was closer in time to SEQAtrain, i.e., an interval-dependent gradient of interference between SEQB and SEQAtrain. Interference was considered anterograde when there was more of an effect of SEQB on SEQAtest when it was closer in time to SEQAtest, i.e., an interval-dependent gradient between SEQB and SEQAtest. Interval-independent interference was also considered to be evidence for an anterograde mechanism. We first examined the effect of training with 33 cycles of SEQB after training with only 11 cycles of SEQA [analyses involved groups 1, 2, and 3 (Table 1)]. We then tested the hypothesis that prolonged training with SEQA (33 rather than 11 cycles) would induce resistance to interference from SEQB [analyses involved groups 4, 5, and 6 (Table 1)].
Interference effect of SEQB on explicit recall of SEQAtest.
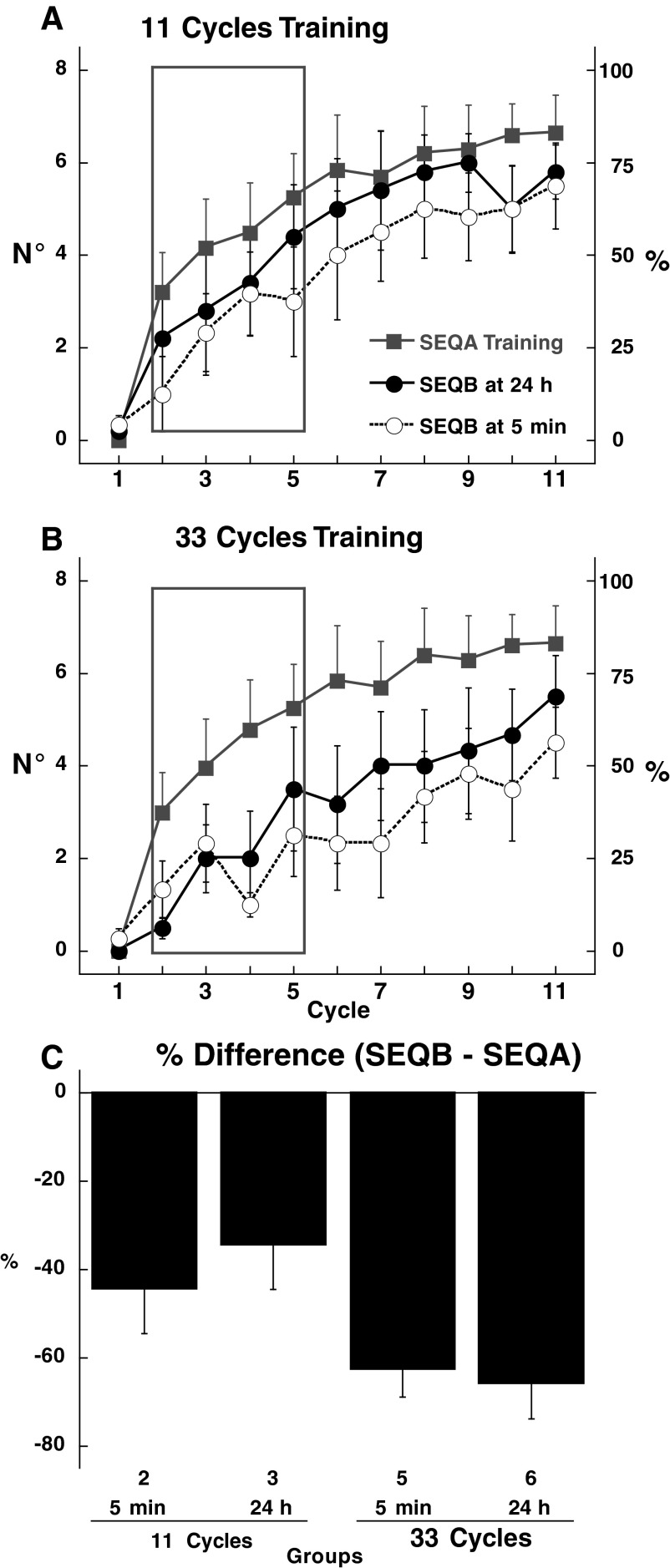
Inspection of the learning curves for subjects trained with 11 cycles of SEQAtrain shows an interference effect of SEQB on SEQAtest (Fig. 5B) [ANOVA: effect of session: F(1,300) = 12.07, P = 0.001; effect of cycle: F(10,300) = 57.4, P < 0.0001; group × cycle: F(20,300) = 2.4, P = 0.001; session × group × cycle: F(20,300) = 2.0, P = 0.007; post hoc comparisons: group 1 vs. groups 2 and 3: P < 0.0004; group 2 vs. group 3: P > 0.8]. Recall of SEQA was still greater than that in session 1, but did not reach the levels seen in the control group: the average improvement in the control group (group 1) was 57.3%, whereas it was only 21.87% for the 5-min interference group (group 2) and 21.25% for the 24-h interference group (group 3, Fig. 5D). Thus the interference effect was of similar magnitude irrespective of whether SEQB was learned 5 min or 24 h after SEQAtrain; a greater interval between SEQAtrain and SEQB did not mitigate the interference effect of SEQB. This result argues against retrograde interference (see discussion). In contrast, when SEQAtrain lasted for 33 cycles, there was similar recall for the control (group 4, 57.14 ± 6.16%), the 5-min interference (group 5, 45.8 ± 9.1%), and the 24-h interference groups (group 6, 56.3 ± 3.3%) (Fig. 5, C and D). These results indicate that the vulnerability of SEQA to interference by SEQB is dependent on the duration of training with SEQA. Sequence order was learned equally well at the end of session 1 with either 11 or 33 cycles; this invariance in the degree of initial learning regardless of the amount of practice has been described previously for explicit sequence learning (Savion-Lemieux and Penhune 2005). Although the full sequence was acquired in 11 cycles, it appears that extended practice (22 more cycles) renders this learning more resistant to interference.
A potential confound, however, is that groups 2 and 3 trained with only 11 cycles of SEQAtrain compared with 33 cycles of SEQB, whereas groups 5 and 6, where interference was not seen, trained with 33 cycles for both SEQAtrain and SEQB. That is to say, interference might occur only when there is an imbalance between the training durations for SEQAtrain and SEQB. To address this issue, two new groups were trained with 11 cycles of SEQAtrain but this time followed by only 11 cycles of SEQB, either 5 min (group 7) or 24 h (group 8) later. Despite this reduction in training with SEQB, interference still occurred: performance improvement 48 h later was reduced in both groups compared with control (group 7: 12.8 ± 3.0%; group 8: 25.1 ± 14.4%) (see Supplemental Fig. S1).1 Therefore persistent interference at both 5 min and 24 h was not attributable to an imbalance between the training SEQB and SEQAtrain training durations.
Interference effect of SEQAtrain on explicit learning of SEQB.
Learning of one sequence may interfere with subsequent learning of a new sequence (anterograde interference). We thus examined the effect of SEQAtrain on explicit learning of SEQB. Anterograde interference could be expected to take the form of elements of SEQB being affected by elements of the previously learned SEQA. Consistent with this, in the four groups that learned SEQB after SEQAtrain (groups 2, 3, 5, and 6), the number of movements directed to the wrong targets increased in SEQB (mean ± SE: 14.68 ± 4.4%) compared with SEQAtrain (8.0 ± 1.9%). This contributed to a decrease in the number of correct anticipatory movements in the first 11 cycles of SEQB (Fig. 7A) [ANOVA: effect of cycle: F(10,220) = 45.1, P < 0.0001; effect of SEQ (SEQB and SEQAtrain): F(1,220) = 2.97, P = 0.09]. The decrement was more evident in the groups who trained with 33 cycles of SEQAtrain (groups 5 and 6) (Fig. 7B) [ANOVA: effect of cycle: F(10,220) = 28.1, P < 0.0001; effect of SEQ (SEQB and SEQAtrain): F(1,220) = 3.57, P = 0.07]. Thus there was a detrimental anterograde effect of SEQAtrain on SEQB in all four interference groups (2, 3, 5, and 6; Table 1), which is summarized in Fig. 7C as the difference in percentage anticipatory movements between SEQB and SEQA [ANOVA: effect of amount of training (11, 33 cycles): F(1,20) = 7.7, P = 0.01; interference interval: F(1,20) = 0.14, P = 0.15]. However, by the end of training, all four groups had learned SEQB as well as SEQAtrain (P > 0.9).
FIG. 7.
Anterograde interference. Correct anticipatory movements for SEQB. A: groups 2 and 3 (11 cycles of training). Correct anticipatory movements for the first block of SEQB are plotted for groups 2 (empty black circles) and 3 (filled black circles). There was no significant difference in SEQAtrain for the 2 groups (P > 0.8) and data were combined (filled gray squares). B: effect of 33 cycles of SEQAtrain (groups 5 and 6). Correct anticipatory movements for the first block of SEQB are plotted for groups 5 (empty black circles) and 6 (filled black circles). There was no significant difference in SEQAtrain for the 2 groups (P > 0.8) and data were combined (filled gray squares). C: percentage performance change between SEQB and SEQAtrain (cycles 2–5) for groups 2, 3, 5, and 6. Each bar represents mean difference (±SE). There was a difference for amount of training but not for interference interval. Post hoc tests did not show any significant differences between the 4 groups.
It is of interest that there was a marked effect of SEQAtrain on SEQB even after 24 h but SEQB had no effect on SEQAtest 24 h later. This marked asymmetry suggests that prior learning of SEQA can prevent anterograde interference by SEQB on SEQAtest and that, even when SEQB does interfere with SEQAtest, interference is mitigated by previous learning (see discussion).
Implicit component of sequence learning showed a consolidation window
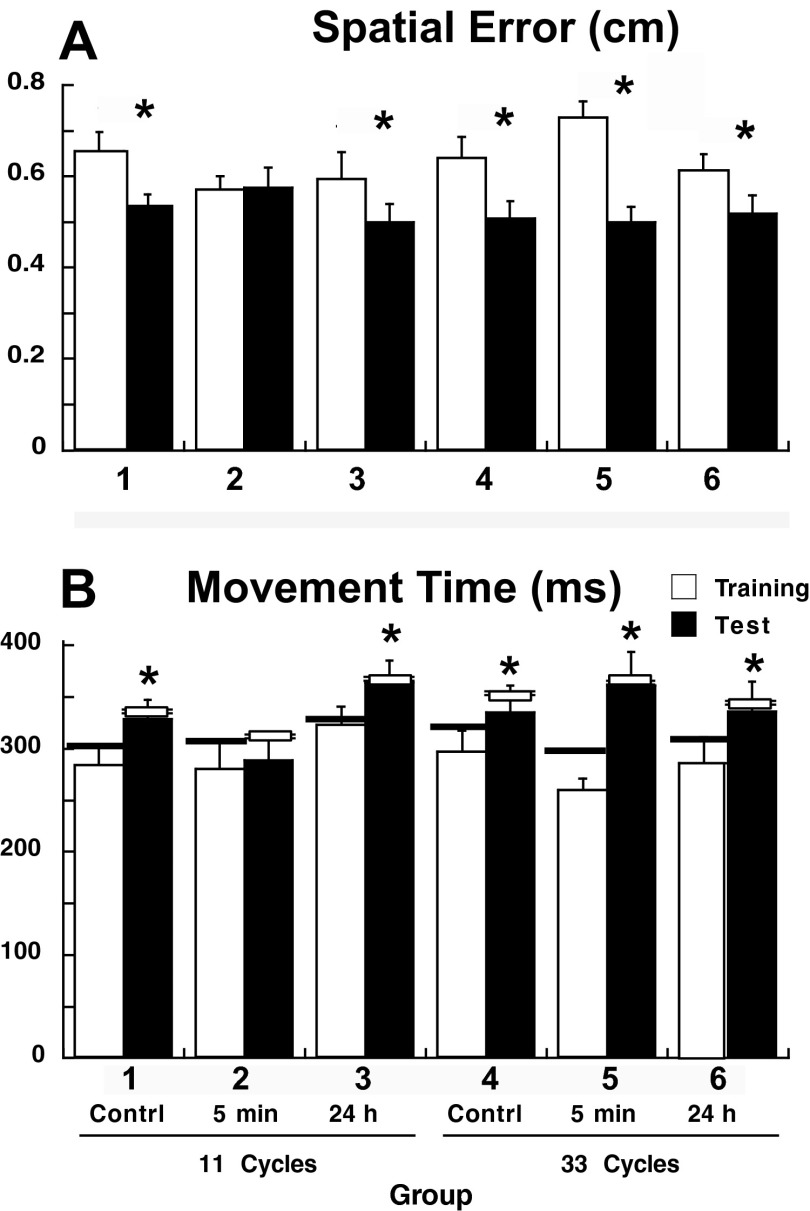
As described previously, we assessed implicit learning as the decrease in spatial error that accompanies the shift from a reactive to an anticipatory mode as the order of SEQA is learned explicitly. We have already reported that, in session 1 (see previous section, Fig. 5A), there was a decrease in spatial error across cycles with similar accuracy levels at the end, in all six groups. In session 2, spatial errors for SEQAtest were significantly lower than those for SEQAtrain but with group differences in the degree of reduction [ANOVA: effect of session: F(1,30) = 49.9, P < 0.0001; group × session: F(5,30) = 3.6, P = 0.01] (Fig. 8A). In the two SEQAtrain controls, groups 1 and 4, the decrease was significant (post hoc test: P < 0.0001).
FIG. 8.
A: mean spatial error (±SE) for the first block of SEQAtrain for all groups (white bars) and SEQAtest (black bars). Bars represent the mean ± SE for cycles 2–5. Similar results were obtained for the mean spatial errors over the entire block. Asterisks indicate significant decreases of spatial error, as revealed by post hoc tests. B: mean MT for correct anticipatory movements in cycles 2–5 (±SE) per group for SEQAtrain (white bars) and SEQAtest (black bars). The horizontal bars represent the MT mean for the entire block. Post hoc tests revealed significant prolongation of MT in all groups (asterisks, P < 0.05 corrected) with the exception of group 2.
Interference effect of SEQB on implicit recall of SEQAtest.
In the four groups that trained with SEQB, spatial error decreased in session 2 to a degree comparable to that of the controls (P < 0.01), except for group 2, who trained with SEQB 5 min after SEQAtrain [the five (P > 0.8) who did not improve beyond their session 1 level of performance] (Fig. 8A). Thus with only 11 cycles of training, implicit learning of SEQA was vulnerable to interference at 5 min but not at 24 h. However 22 extra cycles of SEQAtrain led to resistance to interference even after 5 min. These findings are in distinct contrast to learning of explicit sequence order, in which 11 cycles of training remained vulnerable to interference even at 24 h. These results were confirmed when we compared spatial errors for correct anticipatory movements over cycles 2 to 5 in all six groups. Similar results were obtained for MT (see Figs. 6B and 8B). In session 2, MT for SEQAtest was significantly higher than that for SEQAtrain in all except the 5-min interference group (group 2) (Fig. 8B) [ANOVA: effect of session: F(1,30) = 41.2, P < 0.0001; session × group: F(5,30) = 3.4, P = 0.01; post hoc tests for training vs. test: all groups except group 2: P < 0.05 corrected, group 2: P = 0.6]. The observation that interference was also seen with respect to MT supports the notion that improvements in spatial accuracy are partially mediated by adjustments in MT.
Interference effect of SEQAtrain on implicit learning of SEQB.
Finally, we examined the anterograde effects of SEQAtrain on the rate of reduction in spatial error for SEQB. Spatial error in SEQB, which was learned either 5 min (groups 2 and 5) or 24 h (groups 3 and 6) after SEQAtrain, also decreased across cycles [ANOVA: effect of cycle: F(10,385) = 10.18, P < 0.0001] but at a slower rate than SEQAtrain in the controls [ANOVA: cycle × sequence type: F(10,1) = 2.62, P < 0.005]. This anterograde interference effect on spatial error was not significantly different between the four groups who trained with SEQB (P > 0.4). These results demonstrate that in the case of explicit sequence order, an anterograde effect of SEQAtrain on SEQB was detectable in all four groups, despite the fact that in three of these groups there was no comparable effect of SEQB on SEQAtest.
DISCUSSION
The experiments presented here sought to investigate two components of sequence learning—order and spatial accuracy—with respect to their time course and their differential susceptibility to interference from learning of another sequence. In our task, the acquisition of sequence order occurred explicitly and increase in accuracy occurred implicitly. We assessed the interference effect of SEQB on sequence order with a discrete measure, the number of correct anticipatory movements, and on motor performance with a continuous spatial accuracy measure. These two measures appear to successfully isolate the two processes: correct anticipatory responses to any given target were represented by changes in onset time far larger than would be expected from implicit learning. Likewise, spatial accuracy continued to improve after the position of the target was already known explicitly. We found that explicit recall of SEQA was interfered with to the same degree whether SEQB was learned after 5 min or 24 h. In contrast, spatial accuracy became resistant to interference by SEQB at 24 h. However, both the explicit and implicit components of sequence learning showed a dissociation between interference with new learning versus interference with recall. Finally, both order and spatial accuracy became resistant to interference by SEQB, even at 5 min, when subjects underwent prolonged initial training with SEQA. These results indicate that the two components of sequence learning are interfered with by distinct time-dependent mechanisms but undergo a similar training-related stabilization process.
Interference with learning of explicit sequence order
We chose a discrete measure, the number of correct anticipatory movements, to capture the explicit acquisition of sequence order. Correct anticipatory movements constitute a reliable measure of explicit learning both because their number is highly correlated with declarative scores (Ghilardi et al. 2003a) and their onset times are close to CCW values. The main finding was that there was an equal interference effect of training with SEQB at 5 min and 24 h when subjects received only 11 cycles of initial training with SEQA. The absence of an interference window (i.e., a graded effect of interval with more interference after 5 min compared with after 24 h) is not consistent with a retrograde interference mechanism but is consistent with an anterograde mechanism. It may be argued that the absence of an interval-dependent gradient of resistance does not exclude the possibility that it might have been seen with longer intervals between SEQAtrain and SEQB. However, a gradient was seen for the implicit measure. Thus we found a dissociation between interference mechanisms for explicit and implicit learning using intervals of 5 min and 24 h. It should be emphasized that retrograde interference can be inferred only by the presence of effects inherent to its definition: the effect of a task B on recall of task A diminishes as the interval between initial training with task A and task B increases. It is of course possible to posit a retrograde mechanism despite the absence of such a gradient but this cannot be proven unless a new operational definition is provided.
Our finding of no interference window is in partial agreement with a recent SRT study, where anterograde effects of SEQA on SEQB were present even after 24 h (Goedert and Willingham 2002). The results of the SRT study differ from ours in that, after interference, compared with day 1, they showed a worsening of performance, whereas we saw only a lesser degree of improvement. It is possible that, in our task, more complete learning occurred on day 1 and thus greater retention occurred at test compared with the SRTT study. We reported a similar interval-independent interference effect with visuomotor rotation learning (Krakauer et al. 2005). However, in that case, after interference with a counter-rotation task, performance at test was back at naïve levels, whereas for explicit sequence learning, performance was always better at test than on day 1. These results suggest a retained memory of SEQA. When we compared the effect of SEQB on recall of SEQA with the effect of SEQA on learning of SEQB, we found that learning of SEQB was worse than naïve learning of SEQA even when the interval between them was 24 h. This asymmetry in interference suggests that the degree of interference caused by SEQB on the recall of SEQA is the sum of savings for SEQA in the control group minus some degree of anterograde interference by SEQB on the recall of SEQA. This phenomenon of a competition for retrieval between two memories is known as “cue overload” and is well described for paired-associates word learning (Wixted 2004).
Interference with spatial accuracy
We chose a continuous measure—spatial accuracy—to capture an implicit aspect of sequence learning. We have previously shown that subjects are more accurate when they can anticipate target order than when they are in reaction time mode (Ghilardi et al. 2000, 2003a, 2007, 2008). Our hypothesis was that this is a form of implicit learning: once target appearance can be anticipated, subjects can better specify movement parameters and prolong movement time, both of which will optimize spatial accuracy. It should be emphasized that, although our task explicitly required the subject to hit the correct target, there was no requirement for or display indicating spatial accuracy. Thus it can safely be assumed that both adjustments in spatial accuracy and movement time were achieved implicitly, i.e., without subject awareness. The change in movement time over the course of session 1 was about 40 ms, meaning even smaller increments per cycle. Subjects do not explicitly detect nor can they intentionally produce changes in movement time of this small a magnitude. Our main finding was that interference with implicit learning by SEQB occurred only when SEQAtrain lasted 11 cycles and with an interval between SEQAtrain and SEQB of 5 min. Savings in spatial accuracy comparable to that of controls occurred when the interval between the two sequences was extended to 24 h. This result is in agreement with studies of sequential finger tapping, which showed with an implicit measure that learning a second sequence 5 min after the first caused retrograde interference (Walker et al. 2003). We also found a retrograde interference effect for rotation tasks (Krakauer et al. 2005), where learning occurs implicitly (Mazzoni and Krakauer 2006). Our results suggest a true consolidation process for learning of spatial accuracy that occurs through a graded effect of time. The dichotomy between consolidation processes for sequence order and spatial accuracy is consistent with previous findings that show a difference in the time course for order and motor performance measures during initial sequence learning (Bapi et al. 2000; Hikosaka et al. 1995).
Hikosaka and colleagues (2002) suggested that sequence learning is a serial process in which the order is first fully acquired and then improvement in execution of the learned sequence occurs. Our results are in agreement with this idea because, although spatial accuracy did start to improve to individual target elements before the sequence was fully known, it did not reach CCW levels until the whole sequence was known explicitly. An interesting addition to this idea is made by our finding of a dissociation, in the 24-h interference group, between interference with recall of sequence order but not with spatial accuracy. This result suggests that consolidation of spatial performance can be maintained for individual elements of a particular sequence even when there is interference with explicit recall of other elements in that sequence. This result is intriguing because it indicates the existence of two independent sequence-specific learning processes, one explicit and one implicit, that can be thought of as layered over each other at the end of training. A similar point has been made with respect to recent SRT experiments (Willingham et al. 2002).
Resistance to interference with saturation training
Prolonged SEQ training prevented interference even at 5 min for both components of sequence learning. These results are reminiscent of our recent results for rotation learning, in which prolonged training with a counterclockwise rotation prevented interference by a clockwise rotation 5 min later (Krakauer et al. 2005). The mechanism of interference by which SEQB interferes with recall of the explicit order of SEQA is likely to be anterograde because there was no effect of time interval. An anterograde mechanism of interference by SEQB implies an effect on retrieval rather than on consolidation, an effect that is mitigated by prolonged training with SEQA. The behavioral difference between training with 11 versus 33 cycles of SEQA is that in the latter case, subjects essentially experienced 22 extra cycles of near-asymptotic or saturated levels of performance. This suggests that the explicit order component of sequence learning either becomes resistant to anterograde interference during the saturation phase of training or saturated training is required for resistance to occur in the subsequent interval between SEQA and SEQB. The fact that resistance to interference appeared to be equal after 5 min and 24 h supports the former interpretation. A similar effect of saturation of early learning and consolidation has been seen for other explicit tasks of working-memory enumeration (Hauptmann and Karni 2002; Hauptmann et al. 2005). Thus for explicit sequence order, consolidation per se probably occurs rapidly and it is only memory retrieval that is vulnerable to anterograde interference. Only saturation training and not time interval provides protection from anterograde interference. In contrast, recall of implicit spatial learning seems to be vulnerable only to retrograde effects and both time interval and saturation effects lead to resistance to this form of interference.
Overall, the results suggest that saturation training can induce resistance to two distinct interference mechanisms, one operating on explicit sequence order and the other on implicit spatial learning. Support for an effect of saturation training on recall and consolidation of implicit motor learning has been shown for prism and rotation adaptation (Krakauer et al. 2005; Yin and Kitazawa 2001). Likewise, an effect of saturation training on consolidation of explicit learning has been described (Hauptmann and Karni 2002; Hauptmann et al. 2005).
With regard to neuroanatomical correlates, our results are consistent with studies that suggest a separation for circuits involved in implicit and explicit sequence learning (Willingham et al. 2002), with the idea that memory consolidation may, at least initially, occur in the same areas involved in the acquisition. There is evidence that for accurate performance of sequential finger movements, which we would argue is analogous to improvements in spatial accuracy in our task, consolidation is mediated by changes in primary motor cortex (Karni et al. 1998; Muellbacher et al. 2002). In contrast, explicit learning of sequence order is associated with activation in prefrontal cortex and anterior cingulate cortex (Destrebecqz et al. 2005; Ghilardi et al. 2000). We are not aware of any imaging studies that have directly addressed consolidation for explicit sequence learning.
Conclusions
Our results show that the learning of explicit sequence order and that of implicit spatial accuracy are distinct in their susceptibility to interference from learning of another sequence. Explicit learning of a second sequence interferes in an anterograde manner with the retrieval of the first sequence. Learning of an implicit spatial accuracy is interfered with in a retrograde manner by a second sequence. This difference helps to reconcile the apparent contradiction between results that have shown no evidence for retrograde interference and consolidation in the SRT task (Goedert and Willingham 2002), but evidence for both with sequential finger tapping (Walker et al. 2003). Specifically, our results would suggest that the SRTT assesses for acquisition of sequence order, whereas finger sequencing tasks test for spatiotemporal accuracy of combined sequence elements. Interestingly, both types of sequence learning are made resistant to their respective interference mechanisms by saturation training.
Our proposed dichotomy between explicit acquisition of sequence order and implicit acquisition of spatial accuracy raises an apparent contradiction with respect to the SRTT, which is widely assumed to reflect implicit, not explicit, acquisition of sequence order (Robertson 2007), although there are dissenting views (Moisello et al. 2008; Shanks and Johnstone 1998). Assuming that target order can indeed be acquired implicitly, our consolidation dichotomy for sequence order and spatial accuracy would still hold. It would be interesting to determine in future experiments whether spatial accuracy can improve when target order is acquired implicitly using an SRTT paradigm and, if so, whether they show different susceptibilities to interference. The answer to this question is not currently known because spatial accuracy measures are not easily acquired with the keyboard tasks typically used in the SRTT literature.
On the basis of this study we propose that motor skill learning, such as serving in tennis, be considered the process by which explicit knowledge of the order required for an action sequence is combined with the implicit ability to accurately execute the elements of the sequence. Such skill appears to be distinct from forms of motor adaptation in which errors are reduced implicitly, without awareness.
GRANTS
This work was supported by a McDonnell Foundation grant and a National Parkinson Foundation grant to M. F. Ghilardi, and National Institute of Neurological Disorders and Stroke Grants R01-NS-054864 to M. F. Ghilardi, R01-NS-055185 to G. Tononi, and K02-NS-048099 to J. W. Krakauer.
Supplementary Material
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Bapi 2000.Bapi RS, Doya K, Harner AM. Evidence for effector independent and dependent representations and their differential time course of acquisition during motor sequence learning. Exp Brain Res 132: 149–162, 2000. [DOI] [PubMed] [Google Scholar]
- Destrebecqz 2005.Destrebecqz A, Peigneux P, Laureys S, Degueldre C, Del Fiore G, Aerts J, Luxen A, Van Der Linden M, Cleeremans A, Maquet P. The neural correlates of implicit and explicit sequence learning: interacting networks revealed by the process dissociation procedure. Learn Mem 12: 480–490, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez 1997.Ghez C, Favilla M, Ghilardi MF, Gordon J, Bermejo R, Pullman S. Discrete and continuous planning of hand movements and isometric force trajectories. Exp Brain Res 115: 217–233, 1997. [DOI] [PubMed] [Google Scholar]
- Ghilardi 2003b.Ghilardi MF, Carbon M, Silvestri G, Dhawan V, Tagliati M, Bressman S, Ghez C, Eidelberg D. Impaired sequence learning in carriers of the DYT1 dystonia mutation. Ann Neurol 54: 102–109, 2003b. [DOI] [PubMed] [Google Scholar]
- Ghilardi 2003a.Ghilardi MF, Eidelberg D, Silvestri G, Ghez C. The differential effect of PD and normal aging on early explicit sequence learning. Neurology 60: 1313–1319, 2003a. [DOI] [PubMed] [Google Scholar]
- Ghilardi 2007.Ghilardi MF, Feigin AS, Battaglia F, Silvestri G, Mattis P, Eidelberg D, Di Rocco A. L-Dopa infusion does not improve explicit sequence learning in Parkinson's disease. Parkinsonism Relat Disord 13: 146–151, 2007. [DOI] [PubMed] [Google Scholar]
- Ghilardi 2000.Ghilardi MF, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, Antonini A, Eidelberg D. Patterns of regional brain activation associated with different forms of motor learning. Brain Res 871: 127–145, 2000. [DOI] [PubMed] [Google Scholar]
- Ghilardi 2008.Ghilardi MF, Silvestri G, Feigin A, Mattis P, Zgaljardic D, Moisello C, Crupi D, Marinelli L, Dirocco A, Eidelberg D. Implicit and explicit aspects of visuomotor sequence learning in pre-symptomatic carriers of Huntington's disease. Parkinsonism Relat Disord 14: 457–464, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert 2002.Goedert KM, Willingham DB. Patterns of interference in sequence learning and prism adaptation inconsistent with the consolidation hypothesis. Learn Mem 9: 279–292, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann 2002.Hauptmann B, Karni A. From primed to learn: the saturation of repetition priming and the induction of long-term memory. Brain Res Cogn Brain Res 13: 313–322, 2002. [DOI] [PubMed] [Google Scholar]
- Hauptmann 2005.Hauptmann B, Reinhart E, Brandt SA, Karni A. The predictive value of the leveling off of within session performance for procedural memory consolidation. Brain Res Cogn Brain Res 24: 181–189, 2005. [DOI] [PubMed] [Google Scholar]
- Hening 1988.Hening W, Favilla M, Ghez C. Trajectory control in targeted force impulses. V. Gradual specification of response amplitude. Exp Brain Res 71: 116–128, 1988. [DOI] [PubMed] [Google Scholar]
- Hikosaka 1995.Hikosaka O, Rand MK, Miyachi S, Miyashita K. Learning of sequential movements in the monkey: process of learning and retention of memory. J Neurophysiol 74: 1652–1661, 1995. [DOI] [PubMed] [Google Scholar]
- Hikosaka 2002.Hikosaka O, Rand MK, Nakamura K, Miyachi S, Kitaguchi K, Sakai K, Lu X, Shimo Y. Long-term retention of motor skill in macaque monkeys and humans. Exp Brain Res 147: 494–504, 2002. [DOI] [PubMed] [Google Scholar]
- Karni 1998.Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA 95: 861–868, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer 2005.Krakauer JW, Ghez C, Ghilardi MF. Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci 25: 473–478, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan 1988.Logan GD Toward an instance theory of automatization. Psychol Rev 95: 492–497, 1988. [Google Scholar]
- Mazzoni 2006.Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26: 3642–3645, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisello.Moisello C, Crupi D, Tunik E, Quartarone A, Bove M, Tononi G, Ghilardi MF. The serial reaction time task revisited: a study on motor sequence learning with an arm-reaching task. Exp Brain Res In press. [DOI] [PMC free article] [PubMed]
- Muellbacher 2002.Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature 415: 640–644, 2002. [DOI] [PubMed] [Google Scholar]
- Nissen 1987.Nissen M, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cogn Psychol 19: 1–32, 1987. [Google Scholar]
- Robertson 2007.Robertson EM The serial reaction time task: implicit motor skill learning? J Neurosci 27: 10073–10075, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savion-Lemieux 2005.Savion-Lemieux T, Penhune VB. The effects of practice and delay on motor skill learning and retention. Exp Brain Res 161: 423–431, 2005. [DOI] [PubMed] [Google Scholar]
- Shanks 1998.Shanks DR, Johnstone T. Implicit knowledge in sequential learning tasks. In: Handbook of Implicit Learning, edited by Stadler MR, Frensch PA. Thousand Oaks, CA: Sage Publications, 1998, p. 533–572.
- Todorov 2004.Todorov E Optimality principles in sensorimotor control. Nat Neurosci 7: 907–915, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker 2003.Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature 425: 616–620, 2003. [DOI] [PubMed] [Google Scholar]
- Wilkinson 2004.Wilkinson L, Shanks DR. Intentional control and implicit sequence learning. J Exp Psychol Learn Mem Cogn 30: 354–369, 2004. [DOI] [PubMed] [Google Scholar]
- Willingham 1989.Willingham DB, Nissen MJ, Bullemer P. On the development of procedural knowledge. J Exp Psychol Learn Mem Cogn 15: 1047–1060, 1989. [DOI] [PubMed] [Google Scholar]
- Willingham 2002.Willingham DB, Salidis J, Gabrieli JD. Direct comparison of neural systems mediating conscious and unconscious skill learning. J Neurophysiol 88: 1451–1460, 2002. [DOI] [PubMed] [Google Scholar]
- Wixted 2004.Wixted JT The psychology and neuroscience of forgetting. Annu Rev Psychol 55: 235–269, 2004. [DOI] [PubMed] [Google Scholar]
- Yin 2001.Yin PB, Kitazawa S. Long-lasting aftereffects of prism adaptation in the monkey. Exp Brain Res 141: 250–253, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.