Abstract
Fragile X syndrome (FXS) is the most common form of inherited mental retardation. The syndrome results from the absence of the fragile X mental retardation protein (FMRP), which is encoded by the fragile X mental retardation 1 (FMR1) gene. FMR1 and its two paralogs, fragile X–related genes 1 and 2 (FXR1 and -2), form the Fmr1 gene family. Here, we examined long-lasting synaptic plasticity in Fmr1 knockout, Fxr2 knockout, and Fmr1/Fxr2 double knockout mice. We found that metabotropic glutamate receptor–dependent long-term depression (mGluR-LTD) in the hippocampus was affected in Fmr1 knockout, Fxr2 knockout, and Fmr1/Fxr2 double knockout mice at young ages (4–6 wk old). In addition, Fmr1/Fxr2 double knockout mice showed significant deficiencies relative to either Fmr1 or Fxr2 knockout mice in baseline synaptic transmission and short-term presynaptic plasticity, suggesting FMRP and FXR2P may contribute in a cooperative manner to pathways regulating presynaptic plasticity. However, compared with wild-type littermates, late-phase long-term potentiation (L-LTP) was unaltered in all knockout mice at 4–6 mo of age. Interestingly, although Fmr1/Fxr2 double knockout mice exhibited a more robust enhancement in mGluR-LTD compared with that in Fmr1 knockout mice, Fxr2 knockout mice exhibited reduced mGluR-LTD. Furthermore, unlike Fmr1 knockout mice, mGluR-LTD in Fxr2 knockout mice required new protein synthesis, whereas mGluR-LTD in Fmr1/Fxr2 double knockout mice was partially dependent on protein synthesis. These results indicated that both FMRP and FXR2P function in synaptic plasticity and that they likely operate in related but independent pathways.
INTRODUCTION
Fragile X syndrome (FXS) is the most common form of inherited mental retardation (Warren and Nelson 1994). Typical fragile X patients display physical and behavioral abnormalities, such as a long and narrow face, large ears, macroorchidism, cognitive impairment, hyperactivity, and autistic behaviors (Hagerman 1996). The most common mutation causing the syndrome is an expansion mutation of a CGG triplet repeat in the 5′ untranslated region of the FMR1 gene, which results in the transcriptional silencing of the gene and the subsequent loss of fragile X mental retardation protein (FMRP) (Verkerk et al. 1991). FMRP is an RNA-binding protein that associates with translating polyribosomes (Corbin et al. 1997; Eberhart et al. 1996; Feng et al. 1997a). Fmr1 mRNA and FMRP are expressed ubiquitously and more abundantly in the brain and testes (Devys et al. 1993), which are tissues consistent with the symptoms of FXS. Although FMRP can shuttle between nucleus and cytoplasm, it is predominantly localized in the cytoplasm (Feng et al. 1997b) and is found in neuronal dendrites near translation complexes close to spines (Weiler et al. 1997).
Drosophila and mouse models have been generated to study the mechanisms of FXS. Unlike its mammalian counterpart, the Drosophila genome has a single Fmr1 ortholog termed Drosophila FMR1 (dfmr1) or Drosophila Fmr1-related gene (dfxr) (Wan et al. 2000). Both fly and mouse models of FXS display an excess of immature and tortuous dendritic spines, suggesting slowed maturation and pruning (Dockendorff et al. 2002; Morales et al. 2002; Zhang et al. 2001). Fmr1 mRNA and FMRP were detected in synaptoneurosomes and protein levels were significantly increased following stimulation with (RS)-3,5-dihydroxyphenylglycine (DHPG), a selective group 1 metabotropic glutamate receptor (mGluR) agonist (Weiler et al. 1997), suggesting the involvement of FMRP in synaptic plasticity. In Fmr1 knockout mice, hippocampal mGluR-dependent long-term depression (mGluR-LTD), a form of long-lasting, protein synthesis-dependent synaptic plasticity, was found to be enhanced compared with wild-type mice (Huber et al. 2002). Surprisingly, in contrast to wild-type mice, mGluR-LTD in Fmr1 knockout mice does not require new protein synthesis (Nosyreva and Huber 2006). This difference in protein synthesis dependence between wild-type and Fmr1 knockout mice suggests that there are distinct mechanisms that regulate mGluR-LTD that depend on the existence of FMRP.
FMRP has two autosomal paralogs, FXR1P and FXR2P, that have been well conserved in mammalian evolution (Kirkpatrick et al. 2001; Siomi et al. 1995; Zhang et al. 1995). They share high amino acid similarity with FMRP and have similar functional domains, including two KH domains and an RGG box. FXR1P and FXR2P are mainly localized in the cytoplasm and are able to form homo- and heterotypic interactions with FMRP (Zhang et al. 1995). All three proteins are expressed in neurons and can be found in dendrites. Fxr1 and Fxr2 knockout mouse models have been described previously (Bontekoe et al. 2002; Mientjes et al. 2004). Some of the neurobehavioral phenotypes in Fxr2 knockout mice are similar to those of Fmr1 knockout mice and exaggerated behavioral abnormalities are observed in Fmr1/Fxr2 double knockout mice (Spencer et al. 2006). Thus FXR2P has been postulated to compensate for the loss of FMRP in the fragile X condition. However, FXR2P levels are not significantly altered in the absence of FMRP (Bakker et al. 2000; Tamanini et al. 1997). Furthermore, FXR1P and FXR2P are expressed widely during embryonic development, but display different expression patterns from FMRP in some tissues. For example, FXR1P is highly expressed in muscle and heart, whereas FMRP shows almost no expression in these tissues (Coy et al. 1995). In contrast to FMRP, FXR2P has a nucleolar localization signal (NoS) and shuttles between the cytoplasm and the nucleolus (Tamanini et al. 2000). Some FMRP-binding proteins, such as cytoplasmic FMR1-interacting protein 2 (CYFIP2), also associate with both FXR1P and FXR2P, whereas others (cytoplasmic FMR1 interacting protein 1 [CYFIP1]) are specific to FMRP (Schenck et al. 2001). These findings suggest that FXR1P and FXR2P, although structurally similar, may have different functions.
The function of FXR2P has been less well characterized, and Fxr2 knockout mice have been found to exhibit learning and memory impairments (Bontekoe et al. 2002). Thus it is of interest to determine whether FXR2P itself is required for normal synaptic plasticity. Here we report that mGluR-LTD in Fxr2 knockout mice differs from that in both wild-type and Fmr1 knockout mice and that Fmr1/Fxr2 double knockout mice exhibited more severe electrophysiological alterations than either single knockout model, which suggests that FMRP and FXR2P may function, both together and separately, to regulate synaptic plasticity.
METHODS
Animals
Fmr1 knockout and Fxr2 knockout mice were generated as described (Bakker et al. 1994; Bontekoe et al. 2002). For the current study, wild-type, Fmr1 knockout, Fxr2 knockout, and Fmr1/Fxr2 double knockout mice were obtained by mating female Fmr1+/−/Fxr2+/− with male Fxr2+/− C57Bl/6J mice. Male Fmr1 knockout and double knockout mice and Fxr2 knockout and wild-type mice from both sexes were used for the experiments. Genotyping was performed by polymerase chain reaction (PCR) analysis. For detection of the Fmr1 wild-type allele, PCR was performed on DNA from tails with primers Fmr1_S1 (5′-GTG GTT AGC TAA AGT GAG GAT GAT-3′) and Fmr1_S2 (5′-CAG GTT TGT TGG GAT TAA CAG ATC-3′). The Fmr1 knockout allele was detected by PCR with the Fmr1_S1 primer and primer Fmr1_N2 (5′-GTG GGC TCT ATG GCT TCT GAGG-3′). For detection of the Fxr2 wild-type allele, PCR was performed with primers FXR-F (5′-GTG ACA GTT TCC TGC TTT ACA GTCC-3′) and Fxr2-R (5′-TCT GCC TGT TTC CTG AGT GTTG-3′). The Fxr2 knockout allele was detected by PCR with the FXR-F primer and primer NEO-R (5′-CGC CTT CTA TCG CCT TCT TGAC-3′). All mice were housed in a room in the Transgenic Mouse Facility of the Baylor College of Medicine with a 12-h light/dark cycle. The mice had unrestricted access to food and water. All procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Hippocampal slice preparations
Hippocampal slices from Fmr1 knockout, Fxr2 knockout, Fmr1/Fxr2 double knockout mice and their wild-type controls were prepared as previously described (Hou et al. 2006). In short, slices were placed in saline solution (110 mM sucrose, 60 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 28 mM NaHCO3, 5 mM d-glucose, 0.5 mM CaCl2, and 7 mM MgCl2, gassed with 95% O2-5% CO2, pH 7.4) for 30 min at room temperature and were then perfused for 1–2 h with oxygenated artificial CSF (ACSF: 125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 2 mM CaCl2, 1 mM MgCl2, 25 mM d-glucose saturated 95% O2-5% CO2, pH 7.4) in an interface tissue slice chamber at 30–32°C.
In vitro electrophysiology
Basal synaptic transmission (input–output relationship), paired-pulse facilitation (PPF), metabotropic glutamate receptor–dependent long-term depression (mGluR-LTD) and late-phase long-term potentiation (L-LTP) were examined in these studies. Field excitatory postsynaptic potentials (fEPSPs) were elicited by stimulation of the Schaffer collateral/commissural afferents and recorded in the CA1 stratum radiatum. Within each fEPSP waveform, the fiber volley was used to determine the input value (amplitude of the fiber volley). The range of input values and their respective output values (measured as the fEPSP slope) were plotted as a means to characterize basal synaptic transmission in wild-type and knockout mice. Stable baseline synaptic transmission was collected using a stimulus intensity of 40–50% of the maximum fEPSP. fEPSP responses were recorded every 20 s and presented as the average of six individual traces by Patch Clampex analysis software. PPF, a form of presynaptic facilitation induced by two stimuli presented in rapid succession, was measured by examining the ratio of the fEPSP slope of stimulus 2 to stimulus 1. mGluR-LTD was induced with 50 μM DHPG (RS form) for 5 min. L-LTP was induced by four 1-s trains of high-frequency stimulation (HFS) delivered at 100 Hz with a 5-min intertrain interval. In mGluR-LTD and L-LTP experiments, stable baseline transmission was recorded for ≥20 min before either drug application or HFS.
Data analysis
The slope of the fEPSP was expressed as a percentage of the baseline average. Normalized data were averaged and expressed as the means ± SE. Significant differences between groups were determined using either one-way or two-way ANOVA with P ≤ 0.05 as significance criterion.
RESULTS
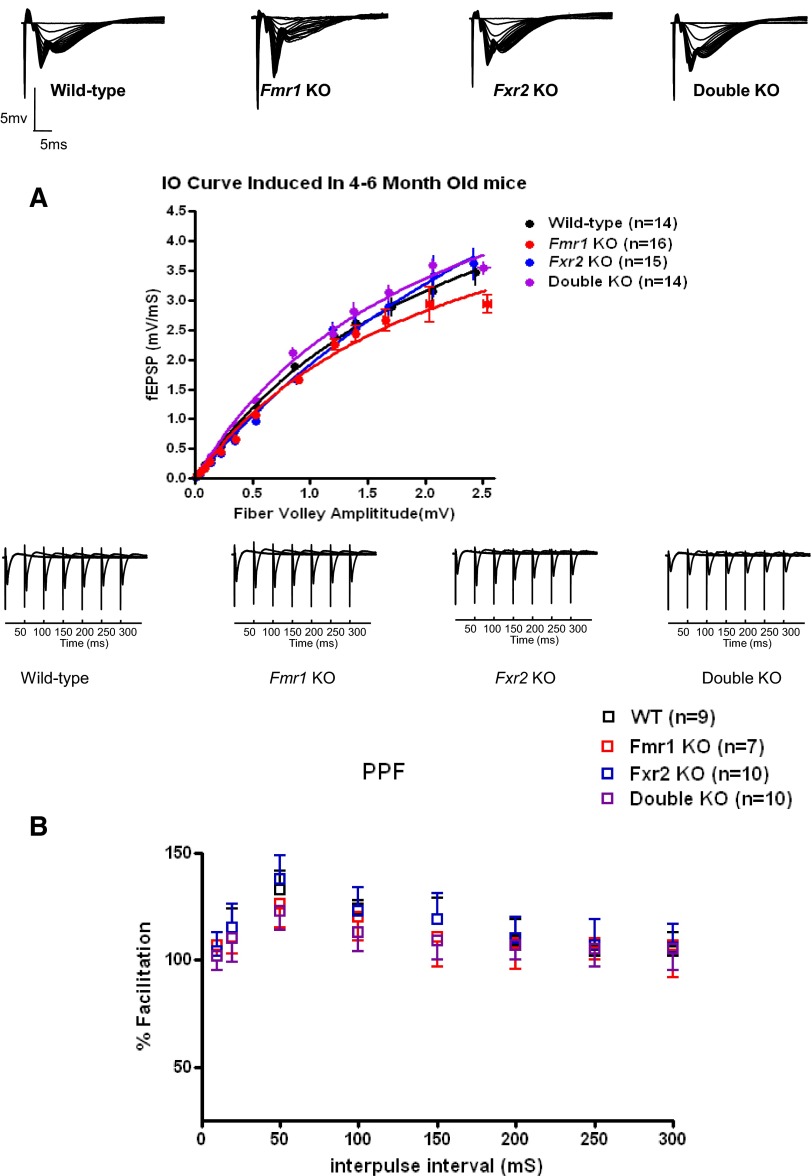
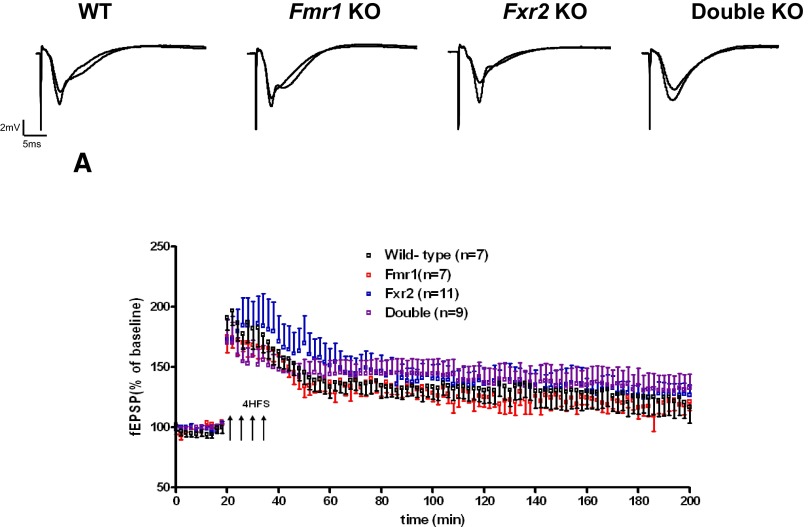
Basal synaptic transmission, PPF, and L-LTP are normal in 4- to 6-mo-old Fmr1, Fxr2, and Fmr1/Fxr2 double knockout mice
To determine whether basal synaptic transmission, paired-pulse faciliation (PPF), and late-phase long-term potentiation (L-LTP) were altered in Fmr1 knockout, Fxr2 knockout, and Fmr1/Fxr2 double knockout mice, we examined 4- to 6-mo-old Fmr1 knockout, Fxr2 knockout, Fmr1/Fxr2 double knockout mice, and their nonmutant wild-type littermate controls at Schaffer collateral synapses in area CA1 of hippocampal slices. Previous studies indicated that basal synaptic transmission, PPF, and L-LTP were normal in Fmr1 knockout mice (Godfraind et al. 1996; Paradee et al. 1999) (Fig. 1). Similar electrophysiological experiments using Fxr2 and Fmr1/Fxr2 double knockout mice showed basal transmission and PPF indistinguishable from those of their wild-type littermates (Fig. 1). These findings suggest that there are no gross differences in synaptic organization or baseline synaptic transmission in the knockout mice at this age. In addition, we found no significant differences in L-LTP, a long-lasting protein synthesis-dependent form of synaptic potentiation, between Fxr2 knockout or Fmr1/Fxr2 double knockout and their wild-type littermates (Fig. 2). These findings suggest that the translation regulatory machinery required for L-LTP is intact in the absence of either FMRP or FXR2P alone, or both together, in mice 4–6 mo of age.
FIG. 1.
Basal synaptic transmission and short-term plasticity are normal in 4- to 6-mo-old Fmr1 knockout, Fxr2 knockout, and Fmr1/Fxr2 double knockout mice. Input–output relationship (A) of wild-type, Fmr1 knockout, Fxr2 knockout, and Fmr1/Fxr2 double knockout mice and nonlinear regression analysis show no difference in basal synaptic transmission in these 4 genotypes (P > 0.05 by 2-way ANOVA, followed by Bonferroni's post hoc tests). Error bars indicate SE for 13 determinations (B); paired-pulse facilitation (PPF) is unaltered in either single or double knockout mice (P > 0.05 by one-way ANOVA). The percentage of facilitation is shown at interpulse intervals ranging from 10 to 300 ms. Samples of field excitatory postsynaptic potentials (fEPSPs) from hippocampal slices of wild-type, Fmr1 knockout, Fxr2 knockout, and double knockout are shown above.
FIG. 2.
Facilitated late-phase long-term potentiation (L-LTP) is normal in 4- to 6-mo-old Fmr1 knockout, Fxr2 knockout, and Fmr1/Fxr2 double knockout mice. Four 100-Hz trains evoked L-LTP in wild-type and knockout slices that decayed toward baseline after 3 h. Samples of fEPSPs from hippocampal slices of wild-type, Fmr1 knockout, Fxr2 knockout, and double knockout are shown above.
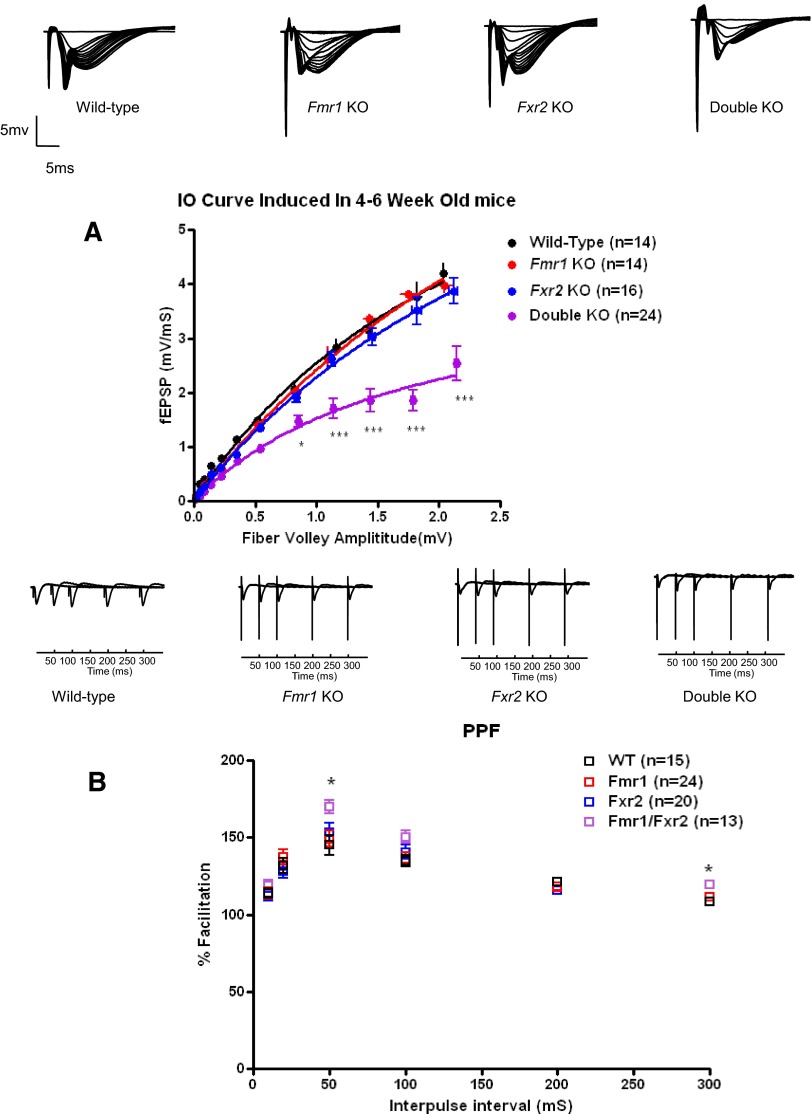
Basal synaptic transmission and PPF are impaired in 4- to 6-wk-old Fmr1/Fxr2 double knockout mice but are unaltered in the same age of Fxr2 knockout mice
Previous studies showed that Fmr1 knockout mice have more immature dendritic spines than those of wild-type mice. However, differences in spine morphology between wild-type and mutant mice decreased with brain development (Nimchinsky et al. 2001), which suggests that spine maturation is delayed in the absence of FMRP. Although we did not detect any synaptic deficiencies in 4- to 6-mo-old Fmr1 knockout, Fxr2 knockout, or Fmr1/Fxr2 double knockout mice, we proceeded to examine whether synaptic plasticity was intact in younger (4–6 wk old) knockout mice. Similarly, we examined basal synaptic transmission by then measuring the input–output (IO) relationship in wild-type and Fmr1 or Fxr2 single and Fmr1/Fxr2 double mutants. We found that there was an abnormal IO relationship in Fmr1/Fxr2 double knockout mice (P < 0.05 and P < 0.001, by two-way ANOVA), whereas both single knockout mouse models showed normal IO curves (Fig. 3 A). This alteration in baseline transmission suggests that presynaptic terminals were not responding normally to single stimuli in the absence of both FMRP and FXR2P in 4- to 6-wk-old mice.
FIG. 3.
Basal synaptic transmission and short-term plasticity are abnormal in 4- to 6-wk-old Fmr1/Fxr2 double knockout mice, but are normal in either Fmr1 knockout or Fxr2 knockout mice. Input–output relationship (A) of wild-type, Fmr1 knockout, Fxr2 knockout, and Fmr1/Fxr2 double knockout mice and nonlinear regression analysis show a change in basal synaptic transmission of Fmr1/Fxr2 double knockout mice (*P < 0.05, ***P < 0.001 by a two-way ANOVA, followed by Bonferroni's post hoc tests), but no difference was observed in Fmr1 or Fxr2 single knockouts. Error bars indicate SE for 12 determinations. B: PPF is enhanced in Fmr1/Fxr2 double knockout mice, but is unaltered in either Fmr1 or Fxr2 knockout mice. The percentage of facilitation is shown at interpulse intervals ranging from 10 to 300 ms (*P < 0.05, by one-way ANOVA). Samples of fEPSPs from hippocampal slices of wild-type, Fmr1 knockout, Fxr2 knockout, and double knockout are shown above.
To examine potential presynaptic abnormalities in the absence of either FMRP or FXR2P, or the absence of FMRP and FXR2P together, we examined PPF in hippocampal slices from Fmr1, Fxr2, Fmr1/Fxr2 double knockout mice, and their wild-type littermates. PPF is typically induced by two stimuli delivered to the same slice in rapid succession and is thought to result from an increase in transmitter release probability triggered by calcium influx into the presynaptic terminal during the response to the first stimulus. We found that slices from Fmr1/Fxr2 double knockout mice had increased PPF compared with that of their wild-type littermates at interpulse intervals of 50 and 300 ms, whereas there were no detectable differences between the single knockout mice and wild-type mice (Fig. 3B, for wild-type slices, mean ratios were 145 ± 7%, n = 15; 108 ± 1.4%, n = 15 at interpulse intervals 50 and 300 ms, respectively; for double knockout slices, mean ratios were 170 ± 4%, n = 13, P < 0.05; 120 ± 2%, n = 13, P < 0.05, at interpulse intervals 50 and 300 ms, respectively). The enhancement of PPF in the double knockout mutant slices, and in particular an increase at longer interpulse intervals, could be explained either by an increase in the facilitation itself or by a decrease in synaptic inhibition.
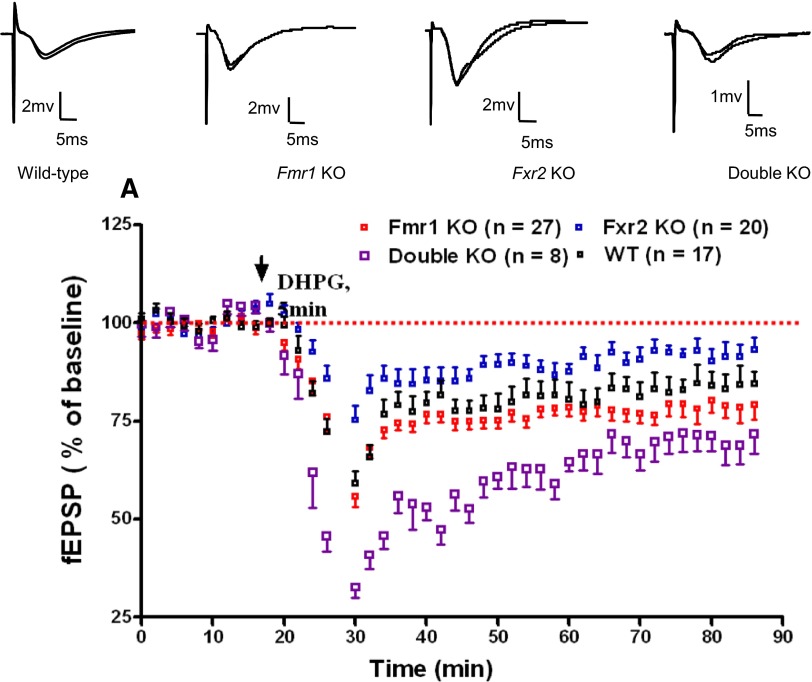
mGluR-LTD induced by DHPG is reduced in Fxr2 knockout mice, but is enhanced in Fmr1 and Fmr1/Fxr2 double knockout mice
Several lines of evidence have shown that mGluR-LTD induced by DHPG, a selective group I mGluR agonist, is enhanced in the absence of FMRP (Huber et al. 2002). In agreement with previous studies, we observed an enhanced mGluR-LTD in Fmr1 knockout mice (wild-type: 83 ± 1%, n = 17; Fmr1 knockout: 78 ± 1%, n = 27, P < 0.01) (Fig. 4). We examined this form of LTD in Fxr2 knockout and Fmr1/Fxr2 double knockout mice by applying DHPG to hippocampal slices. Interestingly, in contrast to Fmr1 knockout slices, Fxr2 knockout slices showed a much reduced initial depression of synaptic transmission (maximal acute depression wild-type: 59 ± 3% of pre-DHPG baseline, Fmr1 knockout: 56 ± 3% of baseline, P < 0.05 by one-way ANOVA, Fxr2 knockout: 75 ± 4% of baseline, P < 0.001), whereas Fmr1/Fxr2 double knockout displayed a more enhanced acute depression (maximal acute depression double knockout: 33 ± 3%, P < 0.001) (Fig. 4). The differences in the maximal acute depression between Fmr1/Fxr2 single and double knockout, and wild-type mice suggests a presynaptic defect in the absence of either FMRP/FXR2P alone or FMRP and FXR2P together. After washout of DHPG, the fEPSP slope increased gradually in all four genotypes. In wild-type slices, the fEPSP slope was reduced to 83 ± 1% of the baseline average value during the last 20 min of the experiment (n = 17), whereas the fEPSP slope in Fmr1 knockout, Fxr2 knockout, and double knockout slices had recovered to 78 ± 1% (n = 27, P < 0.01 by one-way ANOVA), 91 ± 1% (n = 20, P < 0.01), and 70 ± 2% (n = 8, P < 0.01), respectively. The opposite alterations of mGluR-LTD in Fmr1 and Fxr2 knockout mice suggest that FMRP and FXR2P are both involved, but likely have distinct roles, in mGluR-LTD.
FIG. 4.
Metabotropic glutamate receptor–dependent long-term depression (mGluR-LTD) is enhanced in Fmr1 knockout mice and is enhanced to a greater degree in Fmr1/Fxr2 double knockout mice; however, mGluR-LTD is reduced in Fxr2 knockout mice. mGluR-LTD was induced by 50 μM (RS)-3,5-dihydroxyphenylglycine (DHPG) for 5 min. The percentage of facilitation is shown over the entire time course of the experiments. Samples of fEPSPs from hippocampal slices of wild-type, Fmr1 knockout, Fxr2 knockout, and double knockout are shown above.
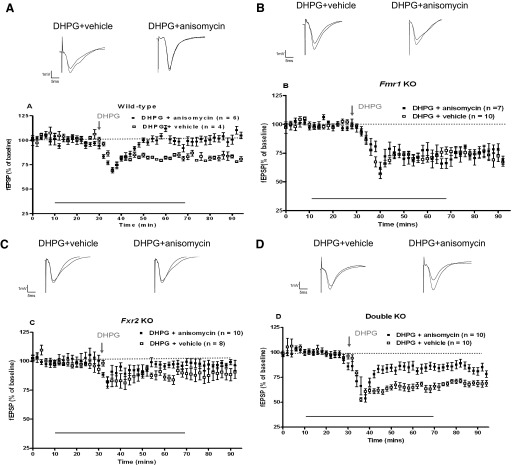
Reduced mGluR-LTD is dependent on protein synthesis in Fxr2 knockout mice, but is partially dependent on protein synthesis in Fmr1/Fxr2 double knockout mice
Recent studies indicate that in contrast to wild-type mice, mGluR-LTD in Fmr1 knockout mice is independent of new protein synthesis (Hou et al. 2006). To determine whether mGluR-LTD in Fxr2 knockout and Fmr1/Fxr2 double knockout mice is protein synthesis dependent, we preincubated the wild-type and knockout hippocampal slices with protein synthesis inhibitor anisomycin (20 μM) before applying DHPG. As observed previously, preincubation of anisomycin with slices from wild-type mice inhibited mGluR-LTD, whereas anisomycin had no effect on mGluR-LTD in the slices from Fmr1 knockout mice (wild-type: DHPG + anisomycin: 99 ± 3% of baseline average value the last 20 min of the experiment, n = 6; DHPG + vehicle: 83 ± 2% of baseline, n = 4, P < 0.0001 by t-test, two-tailed; Fmr1 knockout: DHPG + anisomycin: 75 ± 3% of baseline, n = 7; DHPG + vehicle: 74 ± 2% of baseline, n = 10, P > 0.5) (Fig. 5, A and B). In contrast to Fmr1 knockout mice, anisomycin blocked mGluR-LTD in Fxr2 knockout mice (DHPG + anisomycin: 97 ± 1% of baseline, n = 10; DHPG + vehicle: 89 ± 1% of baseline, n = 8, P < 0.0001) (Fig. 5C). Interestingly, anisomycin partially inhibited mGluR-LTD in Fmr1/Fxr2 double knockout mice (DHPG + anisomycin: 85 ± 2% of baseline, n = 10; DHPG + vehicle: 69 ± 2% of baseline, n = 10, P < 0.0001) (Fig. 5D). The difference in the protein synthesis dependence of mGluR-LTD in Fmr1 knockout, Fxr2 knockout, and double knockout mice suggests that FMRP and FXR2P are not involved in the same molecular pathway to regulate mGluR-LTD, consistent with the findings presented in Fig. 4.
FIG. 5.
Protein synthesis inhibitor does not block the enhanced mGluR-LTD in Fmr1 knockout mice, but it blocks the reduced mGluR-LTD in Fxr2 knockout mice and it partially blocks the considerably more enhanced mGluR-LTD in Fmr1/Fxr2 double knockout mice. A: mGluR-LTD was induced by incubation of hippocampal slices from wild-type (A), Fmr1 knockout (B), Fxr2 knockout (C), and double knockout (D) with 50 μM DHPG for 5 min in the presence of either vehicle or anisomycin (20 μM). Either the vehicle or the anisomycin was present in the perfusing solution 20 min before, during, and 30 min after application of DHPG, as indicated with the line above the x-axis. The percentage of facilitation is shown over the entire time course of the experiments. Samples of fEPSPs from hippocampal slices of wild-type, Fmr1 knockout, Fxr2 knockout, and double knockout mice are shown above each figure.
DISCUSSION
The absence of FMRP causes FXS. FMRP, FXR1P, and FXR2P form a family of RNA-binding proteins that interact with themselves and with each other (Bontekoe et al. 2002; Zhang et al. 1995). These three proteins have been shown to partially overlap with respect to their tissue expression and it has been postulated that FXR1P or FXR2P could, at least partially, compensate for the loss of FMRP in FXS. However, our results indicate that although FMRP and FXR2P are both involved in hippocampal synaptic plasticity, they likely function in distinct molecular pathways underlying the plasticity.
It has been shown that the disruption of either the Fmr1 or the Fxr2 gene in mice produces learning and memory impairments (Bontekoe et al. 2002). To better characterize FXR2P and its potential functional interaction with FMRP, we generated Fmr1/Fxr2 single and double knockout mice and examined both short-term and long-term synaptic plasticity in the mutant mice and their wild-type littermates at two ages (one group was 4–6 mo old; the other group was 4–6 wk old). Our results indicate that in the absence of both FMRP and FXR2P, hippocampal slices from 4- to 6-wk-old mice displayed significant impairments in baseline synaptic transmission (IO curves), short-term synaptic plasticity (PPF), and long-term synaptic plasticity (mGluR-LTD), relative to the loss of either single protein. The less robust phenotypes in Fmr1 and Fxr2 single mutant mice compared with double knockout mice suggest that FMRP and FXR2P compensate for the loss of one another.
Basal transmission and PPF defects are detected only in 4- to 6-wk-old Fmr1/Fxr2 double knockout mice
Herein, we have shown that, unlike the Fmr1 and Fxr2 single knockout mice, the Fmr1/Fxr2 double knockout mice exhibited impaired baseline synaptic transmission and short-term presynaptic plasticity as measured by the IO relationship and PPF, respectively. Alterations in the IO relationship and PPF usually indicate a presynaptic site of action (McKernan and Shinnick-Gallagher 1997). Thus the impairment of both the IO relationship and PPF suggests presynaptic deficiencies are present in Fmr1/Fxr2 double knockout mice. The lack of a presynaptic impairment in the single mutant mice could be explained by very mild alterations that are undetectable using the techniques we used in these studies. A recent study using female heterozygous Fmr1 knockout mice suggests that FMRP has a significant role in presynaptic neurons that is required for the development of normal synapses, underscoring the potential value of characterizing presynaptic functions for the Fxr gene family (Hanson and Madison 2007).
Presynaptic defects in the Fmr1/Fxr2 double knockout mice are transient phenotypes
Interestingly, we did not detect alterations in baseline synaptic transmission (IO), short-term synaptic plasticity (PPF), or long-term synaptic plasticity (L-LTP) in 4- to 6-wk-old single and double mutant mice. Our finding that impaired synaptic plasticity occurs in younger rather than older knockout mice suggests that the role of FXR proteins in synaptic plasticity is critical during earlier stages of neuronal development. It will be of interest to investigate whether removal of FXR proteins at different developmental time points causes similar phenotypes and whether the reintroduction of FXR proteins into FXR-deficient animals at different developmental stages will rescue these phenotypes. We have developed conditional mutations in the Fmr1 and Fxr2 genes that will assist in answering these questions (Koekkoek et al. 2005; Mientjes et al. 2006).
FMRP and FXR2P are both involved in hippocampal mGluR-LTD, but they could play distinct roles in response to mGluR activation
The “metabotropic glutamate receptor (mGluR) theory” has been proposed as a model for FMRP function at synapses (Bear et al. 2004; Chang et al. 2008; Desai et al. 2006; Dolen et al. 2007; McBride et al. 2005; Todd et al. 2003; Weiler et al. 1997; Yan et al. 2005). FMRP is localized to translating polyribosomes at the base of dendritic spines and, in response to mGluR activation, FMRP levels are increased (Comery et al. 1997; Feng et al. 1997b; Weiler et al. 1997), which suggests a role for FMRP in regulating local protein synthesis and thus in controlling synaptic plasticity. There are two main types of long-term synaptic plasticity: long-term potentiation (LTP) and long-term depression (LTD). It has been found that LTP and N-methyl-d-aspartate receptor-dependent LTD (which is reversible and independent of protein synthesis in the early phase) are normal in the hippocampus of Fmr1 knockout mice. However, another type of hippocampal LTD, which is triggered by the activation of group I metabotropic glutamate receptors and requires new protein synthesis, is enhanced in the hippocampus of Fmr1 knockout mice (Bear et al. 2004; Huber et al. 2002). Based on the assumption that FMRP functions as a translational repressor, it has been proposed that FMRP functions as a brake to inhibit the synthesis of more “LTD” proteins in response to the activation of mGluRs. However, in the absence of FMRP, the brake is removed and the synthesis of more “LTD” proteins results in enhanced mGluR-LTD. One prediction of the “mGluR theory” was that excessive mGluR5 signaling could contribute to the altered neuronal development in fragile X syndrome (Bear et al. 2004). Consistent with this prediction, a recent study demonstrated that a 50% reduction of mGluR5 expression can rescue several phenotypes in Fmr1 knockout mice, including dendritic spine abnormalities in cortical pyramidal neurons and elevated basal protein synthesis levels in the hippocampus (Dolen et al. 2007). These results further validate the “mGluR theory” and suggest that mGluRs are therapeutic targets for the treatment of Fragile X syndrome.
In the present study, our results indicate that in the absence of both FMRP and FXR2P, 4- to 6-wk-old mice showed a more severe impairment in mGluR-LTD, relative to the loss of either single protein. The milder phenotypes observed in Fmr1 and Fxr2 single mutant mice compared with the double knockout mice suggest that FMRP and FXR2P can compensate for each other in group I mGluR-dependent signaling in dendrites. However, our findings that mGluR-LTD in Fmr1 knockout mice is enhanced whereas mGluR-LTD is reduced in Fxr2 knockout mice indicates that, although FMRP and FXR2P contribute to the same ultimate phenotype, they may function in distinct pathways.
Consistent with the idea that FMRP and FXR2P have different functions in mGluR-LTD, hippocampal slices from Fmr1 and Fxr2 single and Fmr1/Fxr2 double knockout mice exhibited different protein synthesis dependence in mGluR-LTD. Unlike mGluR-LTD in Fmr1 knockout hippocampal slices, in Fxr2 knockout slices mGluR-LTD requires protein synthesis, suggesting that mGluR-LTD is regulated by distinct “LTD” mechanisms in the various Fxr genotypes. The different altered mGluR-LTDs and their distinct dependence on protein synthesis in Fmr1 or Fxr2 knockout mice suggest that both FMRP and FXR2P are involved in the normal “LTD” protein synthetic machinery, but likely function in distinct molecular pathways. In contrast to either Fmr1 or Fxr2 knockout mice, mGluR-LTD in Fmr1/Fxr2 double knockout mice could be partially blocked by a protein synthesis inhibitor, indicating that a different group of “LTD” proteins is likely regulated in the absence of both FMRP and FXR2P. Both FMRP and FXR2P are RNA-binding proteins and each associates with polyribosomes. They appear to be involved in the processing, transport, and/or translational control of RNAs that regulate neuronal function. In normal conditions, FMRP and FXR2P can interact with each other to form homo- or heteromultimers, and in the absence of FMRP, FXR2P/FXR1P can independently associate with ribosomes (Tamanini et al. 1999; Zhang et al. 1995). Thus our findings with mGluR-LTD can be explained by a simple model wherein the FMRP/FXR2P-deficient condition, different FXR-polyribosome complexes form and thus target a different group of RNAs/proteins that are required for the expression of this form of synaptic plasticity. This differential in the LTD RNAs/proteins could result in more α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor endocytosis in Fmr1/Fxr2 double knockout mice compared with Fmr1 knockout mice (Nosyreva and Huber 2006), but less AMPA receptor endocytosis in Fxr2 single knockout mice compared with Fmr1 knockout mice. Recent studies have demonstrated that activity-regulated cytoskeleton-associated protein (Arc/Arg 3.1), which associates with FMRP, can stimulate mGluR-dependent AMPA receptor endocytosis in the mouse hippocampus (Park et al. 2008; Waung et al. 2008). Therefore it would be interesting to determine whether the expression of Arc differs between Fmr1 knockout mice and Fmr1/Fxr2 double knockout mice and whether such a difference could explain the distinct mGluR-LTD phenoytpes observed in these mice.
FMRP and FXR2P play both redundant and nonredundant roles in hippocampal synaptic plasticity
Our results suggest that FMRP and FXR2P play both overlapping (short-term synaptic plasticity) and nonoverlapping (mGluR-dependent long-term synaptic plasticity) roles in neuronal functions. These data are consistent with some behavioral studies carried out in Fmr1/Fxr2 double knockout mice. For example, double knockout mice display a very robust phenotype in circadian activity exhibiting behavioral arrythmicity even in light/dark conditions, whereas Fmr1 or Fxr2 single knockout mice show only an accelerated circadian period (Zhang et al. 2008). These findings suggest that FMRP and FXR2P play overlapping roles in regulating the circadian clock pathway. Another example is found in examination of hotplate sensitivity in Fmr1/Fxr2 single and double knockout mice, which indicated that FMRP and FXR2P could not compensate for one another (Spencer et al. 2006). In addition to FMRP and FXR2P, FXR1P, another member of the Fmr1gene family, may play a compensatory role in Fmr1/Fxr2 double knockout mice. Due to the early postnatal death of Fxr1 knockout mice, a conditional Fxr1 knockout model was produced (Mientjes et al. 2004). The generation of Fmr1/Fxr1/Fxr2 triple knockout mice will provide more information on potential functional interactions among these three proteins and may offer a more direct comparison with the Drosophila loss of function model, where a single FMRP-like protein may perform many of the functions distributed among the three mammalian genes. Taken together, our results open new perspectives for the roles of members of the Fmr1 gene family in neuronal function.
GRANTS
This work was supported in part by National Institutes of Health Grants HD-38038 to D. L. Nelson and NS-047384 to E. Klann, the Baylor College of Medicine Mental Retardation and Developmental Disabilities Research Center Grant HD-24064, and FRAXA Research Foundation grants to D. L. Nelson and E. Klann.
REFERENCES
- Bakker et al. 2000.Bakker CE, de Diego Otero Y, Bontekoe C, Raghoe P, Luteijn T, Hoogeveen AT, Oostra BA, Willemsen R. Immunocytochemical and biochemical characterization of FMRP, FXR1P, and FXR2P in the mouse. Exp Cell Res 258: 162–170, 2000. [DOI] [PubMed] [Google Scholar]
- Bakker et al. 1994.Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, Bygrave A, Hoogeveen AT, Oostra BA. The Dutch–Belgian Fragile X Consortium. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell 78: 23–33, 1994. [PubMed] [Google Scholar]
- Bear et al. 2004.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci 27: 370–377, 2004. [DOI] [PubMed] [Google Scholar]
- Bontekoe et al. 2002.Bontekoe CJ, McIlwain KL, Nieuwenhuizen IM, Yuva-Paylor LA, Nellis A, Willemsen R, Fang Z, Kirkpatrick L, Bakker CE, McAninch R, Cheng NC, Merriweather M, Hoogeveen AT, Nelson D, Paylor R, Oostra BA. Knockout mouse model for Fxr2: a model for mental retardation. Hum Mol Genet 11: 487–498, 2002. [DOI] [PubMed] [Google Scholar]
- Chang et al. 2008.Chang S, Bray SM, Li Z, Zarnescu DC, He C, Jin P, Warren ST. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat Chem Biol 4: 256–263, 2008. [DOI] [PubMed] [Google Scholar]
- Comery et al. 1997.Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci USA 94: 5401–5404, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin et al. 1997.Corbin F, Bouillon M, Fortin A, Morin S, Rousseau F, Khandjian EW. The fragile X mental retardation protein is associated with poly(A)+ mRNA in actively translating polyribosomes. Hum Mol Genet 6: 1465–1472, 1997. [DOI] [PubMed] [Google Scholar]
- Coy et al. 1995.Coy JF, Sedlacek Z, Bachner D, Hameister H, Joos S, Lichter P, Delius H, Poustka A. Highly conserved 3′ UTR and expression pattern of FXR1 points to a divergent gene regulation of FXR1 and FMR1. Hum Mol Genet 4: 2209–2218, 1995. [DOI] [PubMed] [Google Scholar]
- Desai et al. 2006.Desai NS, Casimiro TM, Gruber SM, Vanderklish PW. Early postnatal plasticity in neocortex of Fmr1 knockout mice. J Neurophysiol 96: 1734–1745, 2006. [DOI] [PubMed] [Google Scholar]
- Devys et al. 1993.Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet 4: 335–340, 1993. [DOI] [PubMed] [Google Scholar]
- Dockendorff et al. 2002.Dockendorff TC, Su HS, McBride SM, Yang Z, Choi CH, Siwicki KK, Sehgal A, Jongens TA. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron 34: 973–984, 2002. [DOI] [PubMed] [Google Scholar]
- Dolen et al. 2007.Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron 56: 955–962, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart et al. 1996.Eberhart DE, Malter HE, Feng Y, Warren ST. The fragile X mental retardation protein is a ribonucleoprotein containing both nuclear localization and nuclear export signals. Hum Mol Genet 5: 1083–1091, 1996. [DOI] [PubMed] [Google Scholar]
- Feng et al. 1997a.Feng Y, Absher D, Eberhart DE, Brown V, Malter HE, Warren ST. FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol Cell 1: 109–118, 1997a. [DOI] [PubMed] [Google Scholar]
- Feng et al. 1997b.Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci 17: 1539–1547, 1997b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind et al. 1996.Godfraind JM, Reyniers E, De Boulle K, D'Hooge R, De Deyn PP, Bakker CE, Oostra BA, Kooy RF, Willems PJ. Long-term potentiation in the hippocampus of fragile X knockout mice. Am J Med Genet 64: 246–251, 1996. [DOI] [PubMed] [Google Scholar]
- Hagerman 1996.Hagerman RJ Physical and behavioral phenotype. In: Fragile X Syndrome: Diagnosis, Treatment, and Research (2nd ed.), edited by Hagerman RJ, Cronister A. Baltimore, MD: Johns Hopkins Univ. Press, 1996, p. 3–87.
- Hanson and Madison 2007.Hanson JE, Madison DV. Presynaptic FMR1 genotype influences the degree of synaptic connectivity in a mosaic mouse model of fragile X syndrome. J Neurosci 27: 4014–4018, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou et al. 2006.Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron 51: 441–454, 2006. [DOI] [PubMed] [Google Scholar]
- Huber et al. 2002.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA 99: 7746–7750, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick et al. 2001.Kirkpatrick LL, McIlwain KA, Nelson DL. Comparative genomic sequence analysis of the FXR gene family: FMR1, FXR1, and FXR2. Genomics 78: 169–177, 2001. [DOI] [PubMed] [Google Scholar]
- Koekkoek et al. 2005.Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, Willemsen R, Ikeda T, Kakizawa S, Onodera K, Nelson DL, Mientjes E, Joosten M, De Schutter E, Oostra BA, Ito M, De Zeeuw CI. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in fragile X syndrome. Neuron 47: 339–352, 2005. [DOI] [PubMed] [Google Scholar]
- McBride et al. 2005.McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT, McDonald TV, Jongens TA. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 45: 753–764, 2005. [DOI] [PubMed] [Google Scholar]
- McKernan and Shinnick-Gallagher 1997.McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature 390: 607–611, 1997. [DOI] [PubMed] [Google Scholar]
- Mientjes et al. 2006.Mientjes EJ, Nieuwenhuizen I, Kirkpatrick L, Zu T, Hoogeveen-Westerveld M, Severijnen L, Rife M, Willemsen R, Nelson DL, Oostra BA. The generation of a conditional Fmr1 knock out mouse model to study Fmrp function in vivo. Neurobiol Dis 21: 549–555, 2006. [DOI] [PubMed] [Google Scholar]
- Mientjes et al. 2004.Mientjes EJ, Willemsen R, Kirkpatrick LL, Nieuwenhuizen IM, Hoogeveen-Westerveld M, Verweij M, Reis S, Bardoni B, Hoogeveen AT, Oostra BA, Nelson DL. Fxr1 knockout mice show a striated muscle phenotype: implications for Fxr1p function in vivo. Hum Mol Genet 13: 1291–1302, 2004. [DOI] [PubMed] [Google Scholar]
- Morales et al. 2002.Morales J, Hiesinger PR, Schroeder AJ, Kume K, Verstreken P, Jackson FR, Nelson DL, Hassan BA. Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron 34: 961–972, 2002. [DOI] [PubMed] [Google Scholar]
- Nimchinsky et al. 2001.Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in Fmr1 knock-out mice. J Neurosci 21: 5139–5146, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva and Huber 2006.Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol 95: 3291–3295, 2006. [DOI] [PubMed] [Google Scholar]
- Paradee et al. 1999.Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience 94: 185–192, 1999. [DOI] [PubMed] [Google Scholar]
- Park et al. 2008.Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, Huganir RL, Linden DJ, Worley PF. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron 59: 70–83, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck et al. 2001.Schenck A, Bardoni B, Moro A, Bagni C, Mandel JL. A highly conserved protein family interacting with the fragile X mental retardation protein (FMRP) and displaying selective interactions with FMRP-related proteins FXR1P and FXR2P. Proc Natl Acad Sci USA 98: 8844–8849, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi et al. 1995.Siomi MC, Siomi H, Sauer WH, Srinivasan S, Nussbaum RL, Dreyfuss G. FXR1, an autosomal homolog of the fragile X mental retardation gene. EMBO J 14: 2401–2408, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer et al. 2006.Spencer CM, Serysheva E, Yuva-Paylor LA, Oostra BA, Nelson DL, Paylor R. Exaggerated behavioral phenotypes in Fmr1/Fxr2 double knockout mice reveal a functional genetic interaction between fragile X-related proteins. Hum Mol Genet 15: 1984–1994, 2006. [DOI] [PubMed] [Google Scholar]
- Tamanini et al. 1999.Tamanini F, Bontekoe C, Bakker CE, van Unen L, Anar B, Willemsen R, Yoshida M, Galjaard H, Oostra BA, Hoogeveen AT. Different targets for the fragile X-related proteins revealed by their distinct nuclear localizations. Hum Mol Genet 8: 863–869, 1999. [DOI] [PubMed] [Google Scholar]
- Tamanini et al. 2000.Tamanini F, Kirkpatrick LL, Schonkeren J, van Unen L, Bontekoe C, Bakker C, Nelson DL, Galjaard H, Oostra BA, Hoogeveen AT. The fragile X-related proteins FXR1P and FXR2P contain a functional nucleolar-targeting signal equivalent to the HIV-1 regulatory proteins. Hum Mol Genet 9: 1487–1493, 2000. [DOI] [PubMed] [Google Scholar]
- Tamanini et al. 1997.Tamanini F, Willemsen R, van Unen L, Bontekoe C, Galjaard H, Oostra BA, Hoogeveen AT. Differential expression of FMR1, FXR1 and FXR2 proteins in human brain and testis. Hum Mol Genet 6: 1315–1322, 1997. [DOI] [PubMed] [Google Scholar]
- Todd et al. 2003.Todd PK, Mack KJ, Malter JS. The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc Natl Acad Sci USA 100: 14374–14378, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk et al. 1991.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65: 905–914, 1991. [DOI] [PubMed] [Google Scholar]
- Wan et al. 2000.Wan L, Dockendorff TC, Jongens TA, Dreyfuss G. Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol Cell Biol 20: 8536–8547, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren and Nelson 1994.Warren ST, Nelson DL. Advances in molecular analysis of fragile X syndrome. JAMA 271: 536–542, 1994. [PubMed] [Google Scholar]
- Waung et al. 2008.Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron 59: 84–97, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler et al. 1997.Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci USA 94: 5395–5400, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan et al. 2005.Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 49: 1053–1066, 2005. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 2008.Zhang J, Fang Z, Jud C, Vansteensel MJ, Kaasik K, Lee CC, Albrecht U, Tamanini F, Meijer JH, Oostra BA, Nelson DL. Fragile X-related proteins regulate mammalian circadian behavioral rhythms. Am J Hum Genet 83: 43–52, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. 1995.Zhang Y, O'Connor JP, Siomi MC, Srinivasan S, Dutra A, Nussbaum RL, Dreyfuss G. The fragile X mental retardation syndrome protein interacts with novel homologs FXR1 and FXR2. EMBO J 14: 5358–5366, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. 2001.Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell 107: 591–603, 2001. [DOI] [PubMed] [Google Scholar]