Abstract
Hemodynamic changes in the brain are often used as surrogates for epileptic neuronal activity in both the laboratory and the clinic (e.g., intrinsic signal, functional magnetic resonance imaging and single-photon emission computed tomography) in spite of the fact that perfusion-based signals have been shown to overestimate the population of spiking neurons. In addition, mechanisms of neurovascular coupling that apply during normal cortical processing may not be relevant in pathological circumstances such as epilepsy. For these reasons, we investigated the spatiotemporal dynamics of epileptic neurovascular coupling using voltage-sensitive dyes (VSDs) to generate spatial maps of excitatory membrane activity and intrinsic optical spectroscopy (IOS) to measure deoxy-hemoglobin and total hemoglobin, i.e., cerebral blood volume (CBV), in vivo during interictal spikes in rat neocortex to examine their spatiotemporal correlations. We hypothesized that the IOS signal would correlate spatially with subthreshold excitatory activity, which involves a larger area of cortex than suprathreshold neuronal spiking. However, we found that both perfusion and oximetric signals spatially overshot the extent of the excitatory VSD signal by ∼2x. Nevertheless, a high correlation could be found at specific time points in the evolution and dissolution of the hemodynamic signals. The increase in deoxy-hemoglobin reached the highest correlation with the excitatory VSD signal earlier than CBV signals although CBV signals correlated equally well at certain time points. The amplitude of the hemodynamic signals had a linear correlation with the amplitude of the VSD signals except for small nonlinearities in the very center of the focus and in the periphery of the surround, indicating a tight spatial coupling. Our data suggest that hemodynamic signals can accurately define the spatial extent of excitatory interictal epileptiform subthreshold membrane activity at specific time points in their evolution.
INTRODUCTION
In recent years, the field of brain mapping has witnessed the growth of a variety of techniques that measure hemodynamic changes in the brain as a surrogate for neuronal activity. This is particularly true in the clinical domain, such as the treatment of epilepsy, where therapeutic decisions are often influenced by the results of single-photon emission computed tomography (SPECT) and functional magnetic resonance imaging (fMRI) scans (Bagshaw et al. 2006; Van Paesschen 2004). Hemodynamic signals, generally based on perfusion and/or oximetry, are attractive because they often can be measured noninvasively. However, these signals also have a relatively slow time course, on the order of seconds, and the techniques used to measure them generally also suffer from low temporal and spatial resolution. Because few studies have simultaneously examined neuronal activity and associated hemodynamic signals during epileptic activity in the same preparation with high spatial and temporal resolution as well as wide spatial sampling, our understanding of the correlation between the spatial extent of perfusion, oximetry and neuronal activity is limited (Hirase et al. 2004). Several recent studies have attempted to correlate hemodynamic signals with extracellular field potential and multiunit recordings during normal cortical processing (Bosking et al. 1997; Lauritzen 2001; Logothetis et al. 2001; Sheth et al. 2004). However, it is unclear if the neurovascular coupling mechanisms that function in the normal brain apply during epilepsy. In addition, normal functional brain architecture is relatively static compared with epileptic events that evolve spatially over time.
A useful technique for measuring membrane voltage that overcomes the sampling limitations of microelectrodes is voltage-sensitive dyes (VSDs). The dye molecules bind to the membranes of neurons and change their fluorescence in a linear response to changes in membrane potential (Cohen et al. 1974; Grinvald and Hildesheim 2004). VSDs have a temporal resolution on the order of microseconds and can sample large areas of cortex simultaneously using optical methods. VSD imaging can provide a high-resolution map of membrane potential changes in vivo during an epileptiform event (Ma et al. 2004). This map can be used as a “gold standard” of the spatial extent of excitatory activity with which hemodynamic signals can be compared.
Intrinsic optical spectroscopy (IOS) is a separate technique that can provide direct quantitative measurements of the spatial extent of changes in deoxygenated hemoglobin (Hbr) and total hemoglobin (Hbt), the latter correlating quite closely with cerebral blood volume (CBV) (Malonek et al. 1997; Sheth et al. 2004). Although hemodynamic signals evolve more slowly, changes in perfusion and oximetry can be measured by IOS within 100–200 ms of a brain event with broad spatial sampling and a resolution of 100–200 μm (Polimeni et al. 2005).
It has been previously demonstrated that both the IOS signal and the focal alterations in perfusion and hemoglobin oxygenation from which it arises involve a larger area of cortex than the population of neurons firing action potentials (Bosking et al. 1997; Das and Gilbert 1995; Grinvald et al. 1994; Toth et al. 1996). Hence maps based on these signals should presumably overestimate the size of the epileptic focus, particularly the cells firing bursts of action potentials. We hypothesized that these hemodynamic-based optical signals might have a higher correlation with excitatory subthreshold membrane potential change, which arise from the extensive dendritic arbor around the epileptic neurons. If the spatial correlation between the VSD signal and the IOS signal was high, it would imply that the metabolic demand associated with reestablishing the resting dendritic membrane potential might be the primary driving factor in the spatial specificity of epileptic neurovascular coupling mechanisms, which could provide us with a glimpse of the electrical events underlying the images created by our clinical imaging tools. On the other hand, even if hemodynamic signals overestimated the size of this subthreshold excitatory activity, we supposed that at specific time points in the evolution of the hemodynamic signals, they could be used as spatial surrogates for epileptic excitatory membrane activity. In addition, by recording two-dimensional maps of both neuronal and hemodynamic activity, it should be possible to derive a response function between the two events at the resolution of a single pixel (∼33 μm) to determine the resolution of their spatial coupling.
To address these questions, we measured the spatial extant of the excitatory membrane potential changes with VSDs and correlated them over time with hemodynamic changes measured with IOS at 570 and 610 nm using a modified Beer-Lambert Law to calculate Hbr and Hbt. We specifically looked at excitatory neuronal activity because the clinically significant aspect of the epileptic focus is the excitatory region, which is contained by inhibitory activity in the surrounding normal brain (Prince and Wilder 1967).
METHODS
Animal preparation
All experimental procedures were approved by the Weill Cornell Medical College Animal Care and Use Committee following National Institutes of Health guidelines. Adult male Sprague-Dawley rats (250–380 g) were used in our study. Two anesthesia paradigms were used. In the first group, eight rats were anesthetized with intraperitoneal injection of ketamine (90 mg/kg) and xylazine (4.0 mg/kg) and then sustained with urethan (1.25 g/kg), decadron (0.17 mg/kg), mannitol (0.83 g/kg), and atropine (0.067 mg/kg). Additional doses of urethan (0.3 g/kg) were administered to by intraperitoneal injection as to maintain the anesthetic level when needed. To determine the effect of anesthesia on our results, another seven rats were anesthetized with 1.5, 2.0, or 2.5% isoflurane mixed with 70% N2 and 30% O2 in an random sequence. Imaging commenced 20 min after any change in anesthetic level.
Body temperature was maintained at 37°C with a regulated heating blanket (Harvard Apparatus, Holliston, MA). The heart rate, pO2, and ETCO2 were carefully monitored with a small-animal capnograph (Surgivet, Waukesha, WI) and were maintained stable throughout the experiment (heart rate: 250–300 pulse/min, pO2: >90%, ETCO2: ∼25–28 mmHg). The head was fixed in a sterotaxic frame. A ∼5 × 8-mm cranial window was opened over the left hemisphere, between lambda and bregma, centered over somatosensory cortex. A temporal well was built around the craniotomy window with Vaseline oil. In the first group, the VSD, RH-795 solution (Molecular Probes, 0.6 mg/ml in 0.9% NaCl saline), was applied to the exposed dura for ∼45 min (Ma et al. 2004). After staining, the dura was washed with saline for ∼15 min to remove unbound dye, and the temporal well was removed. A small slit was made in the dura for electrophysiology recording. In the second isoflurane group, the dura was removed, and the cortex was stained with new blue dye, RH-1692 solution (1 mg/ml), for ∼90 min. After staining, the cortex was washed with saline for ∼10 min to remove unbound dye.
Electrophysiology
A glass electrode (4–6 MΩ) filled with a solution of bicuculline methiodide (BMI) (5 mM in 165 mM NaCl, pH 3.0; Sigma-Aldrich, St. Louis, MO) was positioned into the II–III layers. Interictal foci were induced by iontophoresis of BMI using a micro-iontophoresis dual current generator (WPI, Sarasota, FL). A second glass electrode (2–4 MΩ) filled with 0.9% saline was positioned <500 μm from the bicuculline electrode to record local field potential (LFP). During imaging, the exposed cortex was covered with 1% agar and a cover slip.
Optical recording
A tandem lens setup, with two 50-mm lenses, was used for imaging. A removable filter cube was placed between the two lenses. The camera was focused ∼400 μm below the cortex surface to sample an area of ∼33 mm2. The camera (Imager 3001, Optical Imaging) had a spatial resolution of 252 × 120 pixels, and each pixel sampled an area of 33 × 33 μm. For VSD recording, the illumination from a 100-W halogen lamp was filtered with a band-pass filter (530 ± 10 nm for RH-795 and 630 ± 10 nm for RH-1692), reflected onto the cortex by a dichroic mirror (580 nm for RH-795 and 650 nm for RH-1692), and the fluorescent image was collected after long-pass filter (610 nm for RH-795 and 665 nm for RH-1692). The temporal resolution was 340 Hz. For intrinsic signal recording, the dichroic mirror and long-pass filter were removed. Illumination was provided by two fiber-optic light guides filtered at either 570 ± 10 nm or 610 ± 10 nm. The temporal resolution was 34 Hz. For each animal, imaging was performed during four sequential 5-min blocks: block1 = VSD; block2 = 570 nm; block3 = 610 nm; block4 = VSD. The two VSD data sets were first analyzed separately. If there was a significant difference in the amplitude and spatial extent of the VSD blocks at their peak amplitude, the data were excluded. Otherwise, both VSD blocks were averaged together for subsequent data analysis.
A modified Beer Lambert law was used to calculate Hbr and Hbt changes from the 570- and 610-nm data using Eq. 1 (Dunn et al. 2005; Kohl et al. 2000).
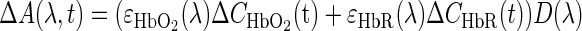 |
where A(λ,t) = log[Ro/R(t)] is the attenuation at each wavelength, Ro and R(t) are the measured reflectance intensities at baseline and time t respectfully, ΔCHbO2 and ΔCHbr are the changes in the concentrations of HbO2 and Hbr, respectively, and ɛHbO2 and ɛHbr are the molar extinction coefficients. Equation 1 was solved for ΔCHbO2 and ΔCHbr using a least-squares approach. The differential path length factor, D(λ), accounts for the fact that each wavelength travels a slightly different path length through the tissue due to the wavelength dependence of scattering and absorption in the tissue, and was estimated using the approach of Kohl et al. (Kohl et al. 2000) through Monte Carlo simulations of light propagation in tissue. The wavelength dependency of the molar extinction coefficients and the path length factors were drawn from work by Dunn et al. (2005).
Data analysis
The VSD and hemodynamic signals were analyzed in a similar fashion using frame division triggered by the onset of the interictal spike (IIS). A 300-frame window (50 frames before and 250 frames after) was averaged over all IISs in each block (75 ∼171 IISs depending on the spike frequency). This resulted in an average spike triggered movie of reflection or fluorescence change over time. To calculate the fractional change of the signal, all the frames were divided by an average of the three frames prior to each IIS, which functioned as a baseline (Schwartz and Bonhoeffer 2001; Suh et al. 2005). A 20-Hz low-pass filter was performed on each averaged, baseline-divided data. Spatial low-pass filtering was achieved by convolution with a Gaussian kernel (σ = 3 pixels, ∼100 μm).
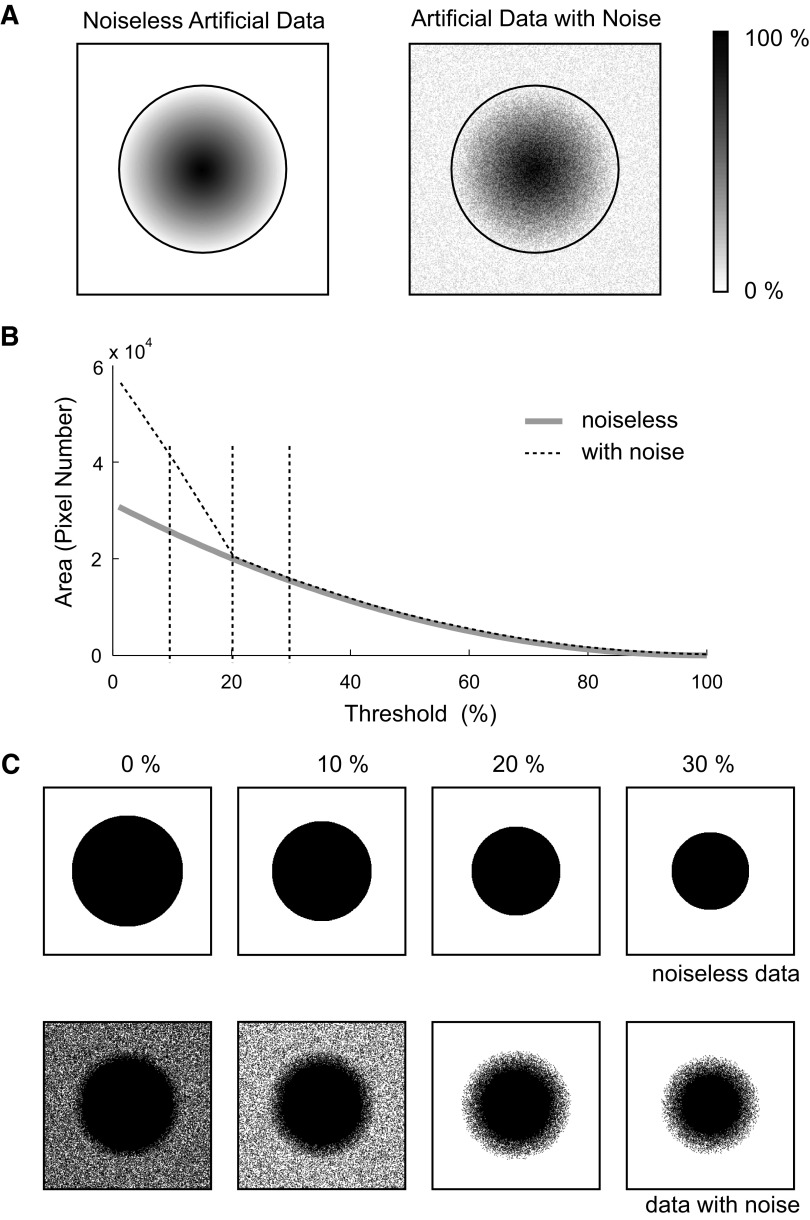
To determine the spatial extent of the optical signals, we thresholded the data using a percentage of the maximum pixel value, as previously described (Berwick et al. 2008; Chen-Bee et al. 1996). The threshold was set at 25% of the maximal amplitude, and all pixels above this threshold were defined as activated pixels. Although the determination of the threshold is somewhat arbitrary, we chose 25% based on the signal-to-noise ratio of our actual data. For example, if artificial conical data are generated with a white noise level set at ±20% of the maximal amplitude of the signal (Fig. 1A), the area of the signal can then be calculated at different thresholds (Fig. 1B). When the threshold is set above the level of the noise, the thresholded images closely approximate the area of artificial data (Fig. 1C). At higher thresholds the areas are also equivalent but underestimated. As can be appreciated in Fig. 1B, when the threshold is set above the noise level (20%), there is “turning point” in the curve. The area of spread was then calculated by multiplying the number of thresholded pixels by the area of each pixel (∼33 × 33 μm). The geometrical center of the thresholded area was defined as epicenter. To calculate this center, the x and y coordinates of all the thresholded pixels were averaged.
FIG. 1.
Detecting the optimal threshold for artificial data. A: artificial conical data with a 100-pixel radius is generated with Matlab (left). The amplitude of the conical center is set at 100%. The amplitude of other pixels is linearly reduced as the distance from the center increases. When the distance is >100 pixels, the pixel amplitude is set to 0% (baseline). A white-noise frame (±20% of the maximal amplitude) is overlapped to the artificial data (right). The activated area was marked with a black circle. B: the area of the noiseless data and the data with noise are plotted at different thresholds. Notice the “turning point” in the curve of the data with noise when the threshold is set at the maximal amplitude of the noise. C: the area of the noiseless data and data with noise at different thresholds.
Regions of interest (ROIs) were selected for data presentation in a variety of ways to characterize different aspects of our data. To illustrate the temporal correlation between the LFP and VSD signals, we used a 5 × 5-pixel ROI centered at the LFP electrode. This ROI provides averaged optical data from the same area where the LFP was recorded. To demonstrate the spatiotemporal evolution of the VSD and IOS signals in this same animal, we chose three random pixels of interest (POIs). The first (POI1) was at the BMI electrodes to demonstrate signal change in the center of the IIS focus. POI2 and POI3 were 885 and 1,797 μm (chosen randomly) away from POI1, respectively. Random selection of the pixel locations eliminates assumptions and bias regarding the size and shape of the focus. Finally, we used concentric rings of interest (RiOI) to demonstrate the spatiotemporal evolution of the signals in multiple directions simultaneously. Ring diameters were arbitrarily set at 0.4, 0.8, 1.2, 1.6, and 2.0 mm to encompass the spatial extent of the data. Separate IIS foci from different animals were grouped together superimposing the region where the tip of the BMI electrode entered the cortex.
VSD/IOS cross-correlation
Because the VSD signals evolve much more rapidly that the IOS signals, to compare the temporal evolution of the spatial extents, we thresholded the VSD map at its maximum to which we compared the evolution of the hemodynamic signals using a two-dimensional cross-correlation over time. Because the VSD signal reached its peak amplitude and dissipated prior to the evolution of the hemodynamic signals, it made sense to compare the evolution of the hemodynamic signals to the maximum spatial extent of the VSD signal based on the hypothesis that this rapid membrane potential change triggered subsequent alterations in perfusion and hemoglobin oxygenation.
To explore the spatial specificity of epileptic neurovascular coupling mechanisms, we first superimposed each IIS over each other by aligning the site of the tip of the BMI electrode. We averaged the values of the amplitude of the VSD signal in each pixel at the moment in time where the amplitude was maximal to create a composite average BMI focus. The respective Hbr and Hbt maps were similarly aligned, and composite average maps were made of the peak amplitude of the hemodynamic responses. We then plotted the relative values of VSD amplitude versus IOS amplitude at this moment in time on a pixel-by-pixel basis for all animals pooled together and performed a best-fit regression of the resulting plot to determine the relative hemodynamic response to membrane potential change for each pixel. Because the BMI electrodes were placed in different areas in the cortex, suitable data could only be obtained in a 114 × 84 pixel (3.76 × 2.77 mm) rectangle around the BMI electrode.
RESULTS
Periodic (∼0.3 Hz) biphasic IISs were induced with BMI iontophoresis. The mean amplitude and width of the IISs was −1.47 ± 0.09 mV and 63.2 ± 2.3 ms (mean ± SD, n = 8 animals), respectively. These measurements were stable during the entire recording period (<1 h) and were not significantly different between blocks for any animal. Likewise the amplitude and spatial extent of the VSD signal were not significantly different between Block1 and Block4 for any experiment (t-test, P < 0.001).
Spatiotemporal evolution of the voltage and hemodynamic signals
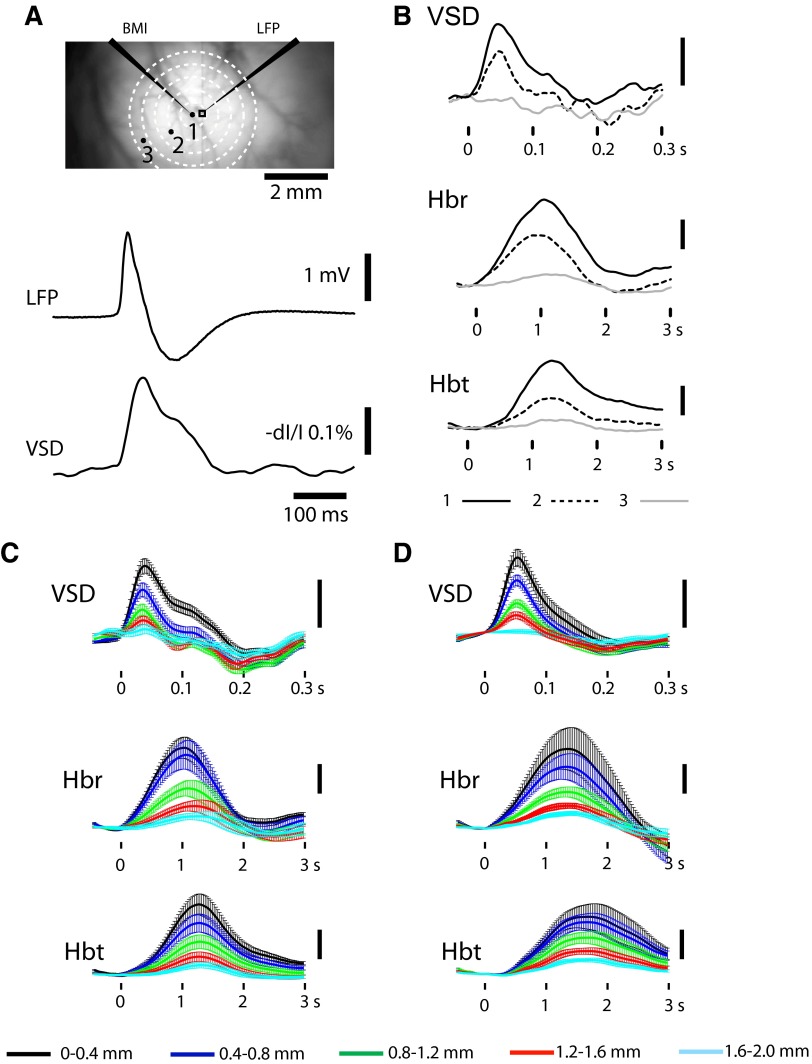
The VSD signal recorded from a ROI adjacent to the BMI infusion had a waveform and time course closely correlating with those of the LFP recorded from the same location (Fig. 2A). The VSD amplitude was highest at POI1 and diminished as a function of the distance from the focus (Fig. 2B). At the farthest POI3, inhibitory membrane potential change was recorded. On average, the excitatory VSD signal in the focus peaked at 61.3 ± 5.2 ms (n = 8 animals).
FIG. 2.
Experimental setup and imaging data in representative animals. A: illustration of relative locations of bicuculline methiodide (BMI) and local field potential (LFP) electrodes as well as region of interest (ROI), pixels of interest (POIs), and rings of interest (RiOIs) in rat cortex. The tips of the LFP and BMI iontophoresis electrodes are ∼300 μm apart. The black rectangle around the LFP electrode indicates a ROI for comparison with the LFP (below). The time course of the LFP signal and the voltage-sensitive dye (VSD) signal recorded from the ROI are similar. Three points (l, 2, 3) indicate the location of 3 POIs. POI1 is in the center of the focus. POI2 and POI3 are 885 and 1,797 μm away from POI1 (see methods). The time courses of the optical signals recorded from these 3 pixels are presented with a black solid line, a black dashed line, and a gray solid line, respectively in B. RiOIs are used to demonstrate lateral spread in multiple directions simultaneously in C and D. B: VSD, deoxygenated hemoglobin (Hbr) and total hemoglobin (Hbt) signals recorded from the POIs marked in A. The Hbr and Hbt tracings are calculated with a modified Beer Lambert equation with pathlength correction (see methods). C: average ± SD amplitude of optical signal change in each RiOI for single animal shown in A. D: example from all animals (n = 8) averaged together Vertical bars in B–D are: 1% of dI/I for VSD and 1% of fractional changes for hemodynamic signals.
The time course of the hemodynamic signals was slower than the VSD signals (Fig. 2B). On average the Hbr signal peaked at 1.5 ± 0.3 s and the Hbt signal peaked at 1.8 ± 0.4 s. The increase in Hbr was consistent with the previously described “dip” in oxygenation during epileptiform events (Suh et al. 2005). Because the IIS frequency was high, there was insufficient time for the late overshoot in hemoglobin oxygenation (Hbr decrease) to occur (Suh et al. 2005). At more distant POIs, a small increase in Hbr was still recorded. Hbr increases were clearly recorded at POI3 where no excitatory VSD signal was detected. The Hbt increase showed similar distribution as Hbr, peaking at the focus and diminishing as a function of distance. Like the Hbr signal, a positive Hbt signal was still present at POI3. The difference between the VSD and hemodynamic signals at POI3 indicates that hemodynamic response extends horizontally further than the VSD signal.
Because single points (POIs) provide limited spatial sampling, we used concentric RiOIs to demonstrate the spatiotemporal evolution of the optical signals in multiple directions simultaneously (see methods). Representative data from a single animal and all animals averaged together are shown in Fig. 2, C and D, respectively. In all animals, the peak amplitude of the optical data were significantly different between rings [repeated-measure 1-way ANOVA F(4,39) = 13.463, P < 0.0001 (VSD); F(4,39) = 10.884, P < 0.0001 (Hbr); F(4,39) = 14.478, P < 0.0001 (Hbt)]. Bonferroni's post hoc test found significant difference between the center ring and other rings (P < 0.05). These data demonstrate that the amplitude of the voltage and hemodynamic signals decrease circumferentially with increasing distance from the focus.
Spatial extent of the voltage and hemodynamic signals
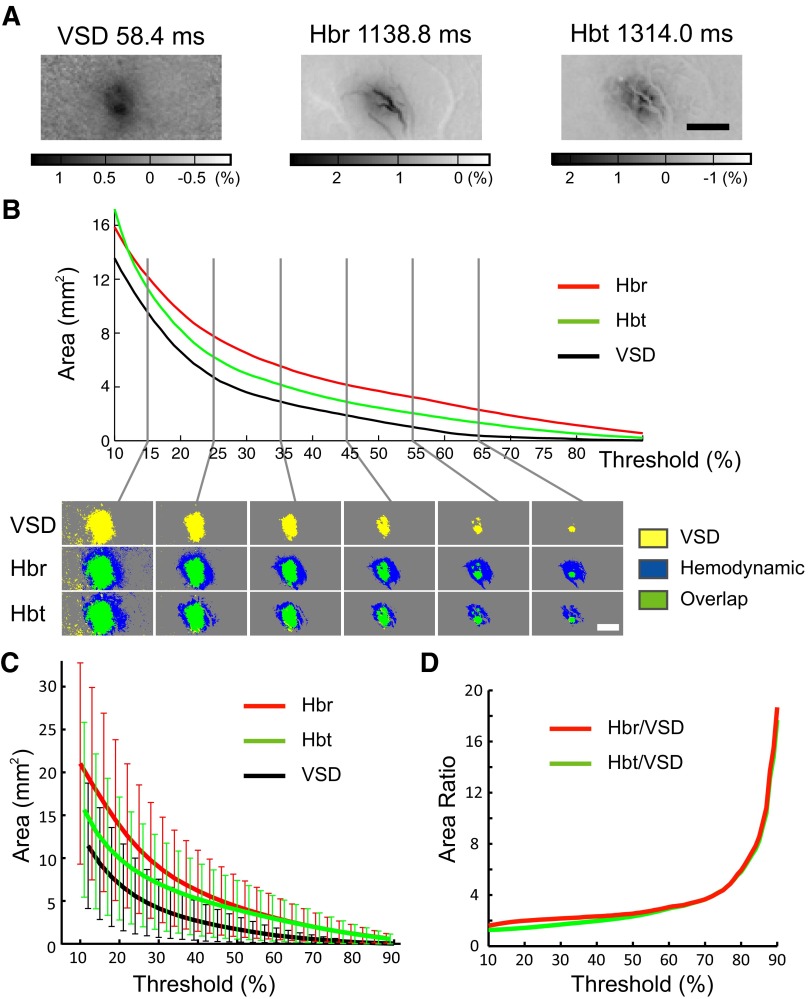
Because hemodynamic signals are often used as surrogates for excitatory neuronal activity in clinical epilepsy, we wished to examine the spatial correlation between the two phenomena. As described in methods, we determined the appropriate threshold for our data by calculating the amplitude of the noise. For all data (VSD, Hbr, and Hbt), the noise during the baseline recording varied from 14 to 22% of the maximal amplitude of the signal recorded in the central ROI after the IIS. Hence we set the threshold at 25% of the maximal amplitude. The threshold was confirmed by graphing the area of the signal at a range of thresholds. A “turning point” similar to what was found in the artificial data occurred below 25% (Fig. 3). Thresholding at 25% of the maximal pixel value, the average area of the VSD, Hbr, and Hbt signal was 5.3 ± 42, 11.5 ± 7.8, and 8.3 ± 6.2 mm2, respectively. At their maximal amplitudes, the Hbr and Hbt signals were 2.0 and 1.6 times the area of the VSD signal (t-test, P < 0.05). Even when the threshold was varied, in the range from 25 to 50%, the relative size of the hemodynamic signals compared with the VSD signals only changed slightly from 2.0 to 2.5 times for Hbr and from 1.6 to 2.3 times for Hbt (Fig. 3D). At higher thresholds, the hemodynamic signals became equivalent and the VSD signal became relatively smaller, because of the lower amplitude of its signal (see discussion).
FIG. 3.
Spatial evolution of the voltage and hemodynamic signals. A: representative example in a signal animal of the raw data at their maximal amplitudes. Scale bar: 2 mm. B: plot of the area of the VSD, Hbr, and Hbt signals at different thresholds and the actual thresholded images (below). Scale bar: 2 mm. C: area (mean ± SD) of VSD, Hbr, and Hbt signals at a range of thresholds (n = 8 animals). D: the relative area of the hemodynamic signals at their maximal amplitude compared with the VSD signals over a range of thresholds. Note the overlap between the hemodynamic areas and the relative decrease in the VSD area at high thresholds.
Temporal correlation between voltage and hemodynamic signals
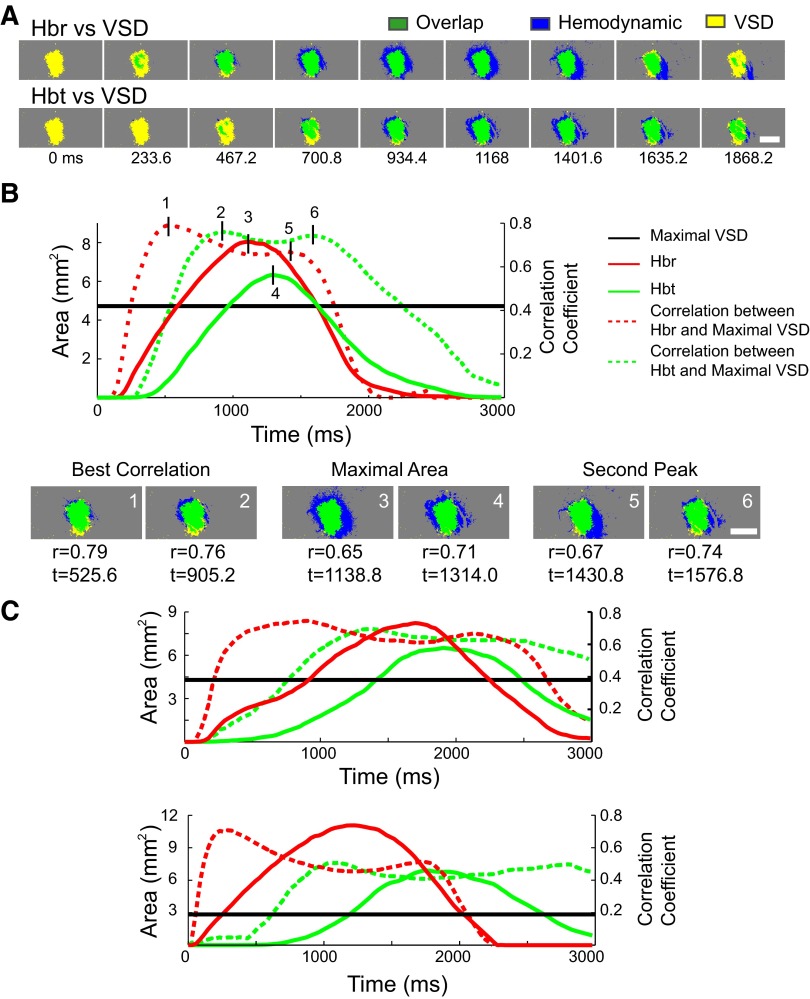
Because the hemodynamic signals evolved slowly over time, we wished to determine the specific time points at which the hemodynamic signals spatially correlated best with the maximal spread of the increase in membrane potential that had triggered the hemodynamic signal. To answer this question, we performed a two-dimensional cross-correlation over time between the thresholded area of the maximal excitatory membrane voltage change and the thresholded area of the Hbr and Hbt signals as they evolved over time. This analysis presumes that the maximal spatial extent of the excitatory membrane change, which occurs at ∼50 ms, will represent the area of greatest metabolic demand, producing the influx in Hbt and the increase Hbr, which occur in a delayed fashion over the next few seconds. An example of this correlation in a few representative animals is shown in Fig. 4. Although different animals show slightly different variations in their correlation curves, the best spatial correlation between the hemodynamic and VSD signals always occurred before the hemodynamic signals reached their maxima (Fig. 4). A second peak in the cross-correlation occurred at a later time point as the hemodynamic signals dissipated. On average, the thresholded Hbr area correlated best with the maximal VSD area (r = 0.67 ± 0.14) 1.1 ± 0.4 s after the onset of IIS. The area of the Hbt signal correlated best with the VSD signal (r = 0.66 ± 0.16) later at 1.5 ± 0.4 s after the IIS, which is significantly slower than Hbr signal (t-test, P < 0.01). The second peaks for Hbr occurred at 1.8 ± 0.5 s (r = 0.59 ± 0.15) and for Hbt at 2.3 ± 0.6 s (r = 0.55 ± 0.16). The second peak for Hbr is also significantly earlier than Hbt (t-test, P < 0.01).
FIG. 4.
Spatiotemporal cross-correlation among VSD, Hbr, and Hbt signals. A: the overlap between the thresholded VSD signal at its maximum area and the thresholded area of the Hbr and Hbt signals at selected arbitrary time points in their evolution. Scale bar: 2 mm. B, top: temporal evolution of the absolute areas and cross-correlations between Hbr and Hbt signals and the maximal area of the VSD signal over time from a single representative animal. The black horizontal line demonstrates the area of the maximal spread of the VSD signal. Six short vertical lines indicate the timepoints when Hbr (1) and Hbt (2) signals correlate best with VSD signal as the hemodynamic signals increase over time, the correlation when Hbr (3) and Hbt (4) signals reach the maximal area, and the best correlation when the Hbr (5) and Hbt (6) signals decrease in size as they dissipate. Bottom: the overlapping images at these 6 static time points. Scale bar: 2 mm. C: the temporal evolution and the correlation curves from 2 other animals show the overall similarity in the patterns of correlation as well as the variability in the precise timing. Although Hbr always reached its highest correlation before Hbt, the degree of correlation by Hbr and Hbt was not significantly different when all animals were combined.
These results indicate that hemodynamic signals may be reasonable surrogates for excitatory neuronal activity at specific time points in their evolution and dissolution or alternatively can be measured at their maximum to estimate the area of excitatory neuronal activity.
Correlation between the epicenter of the voltage and hemodynamic signals
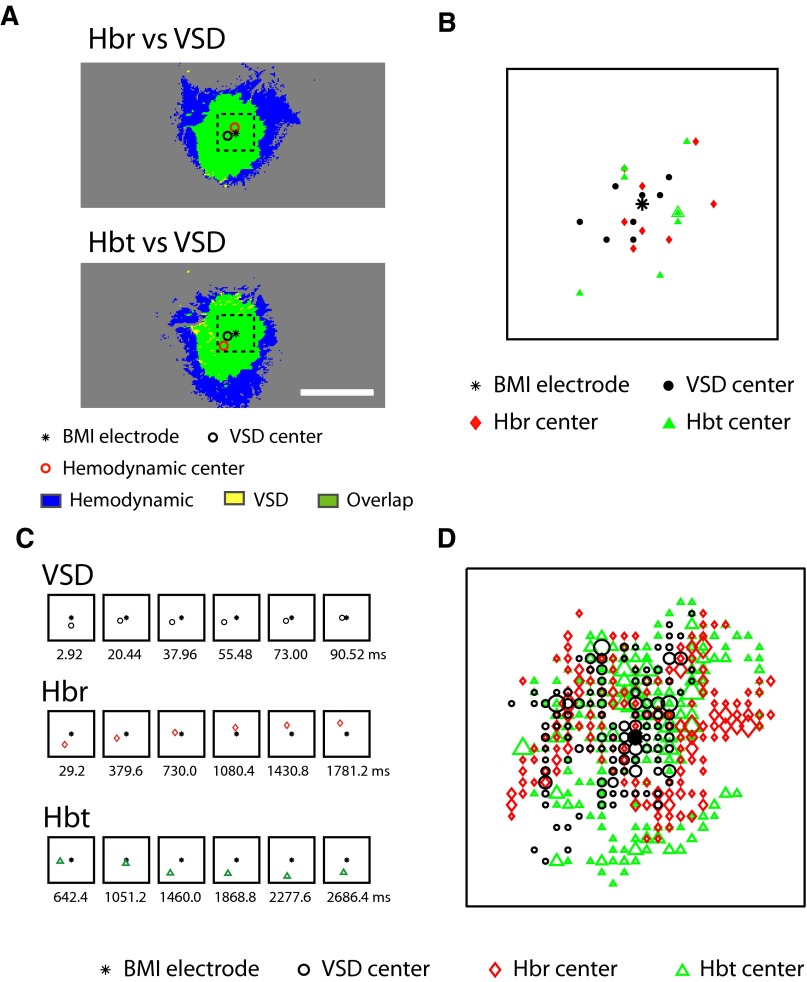
Another measure of the spatial overlap between the voltage and hemodynamic signals is the location of the epicenter of the signal, which can also evolve over time. The epicenter was defined as the geometric center of the thresholded area. We used the location of the BMI electrode as the “gold standard” and calculated the epicenter of the thresholded VSD, Hbr, and Hbt signals at their maximal amplitude and over time (Fig. 5). At their maximum amplitude, the average distances (n = 8 animals) between the BMI electrode and the VSD, Hbr, and Hbt epicenters were 134 ± 69, 174 ± 86, and 216 ± 109 μm, respectively (Fig. 5, A and B). Although the epicenter of the VSD signal was on average closest to the BMI electrode, this was not statistically significant [1-way ANOVA, F(2,23) = 1.839; P = 0.164; Bonferroni's post hoc test found no significant difference, P > 0.05]. The average distance between the VSD epicenter and the Hbr epicenter was 201 ± 81 μm, which was also not significantly different from the distance between the VSD epicenter and Hbr epicenter, which was 218 ± 82 μm (t-test, P = 0.29).
FIG. 5.
The overlap of the epicenter of the voltage and hemodynamic signals A: the location of the BMI electrode as well as the epicenter of the VSD and hemodynamic signals at their maximal spatial spread in a representative animal. The distances between BMI electrode and VSD, Hbr, and Hbt centers in this example were 242, 149, and 407 μm, respectively. All the epicenters stay within a 1 × 1-mm dashed box, centered at the BMI electrode. Subfigures B–D are all magnifications of this same 1 × 1-mm area. Scale bar: 2 mm. B: the relative locations of BMI electrode and epicenters of the VSD and hemodynamic signals at their maximal spatial spread in 8 animals. The locations of BMI electrode from all animals are overlapped and the epicenters are shifted accordingly. C: the temporal evolution of VSD, Hbr, and Hbt epicenters in the same animal as A compared with the location of the BMI electrode. Images at arbitrary time points marked with milliseconds were displayed. D: the relative locations of BMI electrode and all epicenters during the evolution of the VSD and hemodynamic signals in eight animals. All the detected epicenters during the temporal evolution of VSD, Hbr, and Hbt signals from 8 animals were plotted in the same way as B with the locations of the BMI electrode superimposed and the epicenters shifted accordingly. A total of 348 VSD epicenters, 394 Hbr epicenters, and 611 Hbt epicenters were displayed depending on the relative durations of the signals and the acquisition frame rates. The size of the markers indicates the number of overlapping epicenters. Range of size corresponds to VSD: 1–12, Hbr: 1–12, Hbt: 1–19, respectively.
We then investigated the evolution in the epicenters over time (Fig. 5C). For each recorded time point during the evolution of VSD and hemodynamic signals, an epicenter could be recorded. Although the VSD and hemodynamic epicenters shifted as the signal developed, the location of the epicenters was always close to the BMI electrode, and there did not appear to be any reproducible pattern. However, a plot of the locations of all epicenters of the VSD signal as it evolved for all eight animals was more closely packed around the BMI electrode than the evolving Hbr and Hbt epicenters (Fig. 5D). The average distance between the VSD epicenters and BMI electrode was 164 ± 92 μm compared with 217 ± 84 and 233 ± 95 μm for Hbr and Hbt, respectively. In spite of this significant difference [1-way ANOVA, F(2,1352) = 29.85, P < 0.001; Boniferroni's post hoc found significant difference between groups (P < 0.05)], the greatest distance between the epicenters of the VSD and hemodynamic signals and the BMI electrode was only 427 μm, indicating that the locations of the signals epicenters were quite similar throughout their evolution.
Spatial coupling between the amplitude of voltage and hemodynamic signals
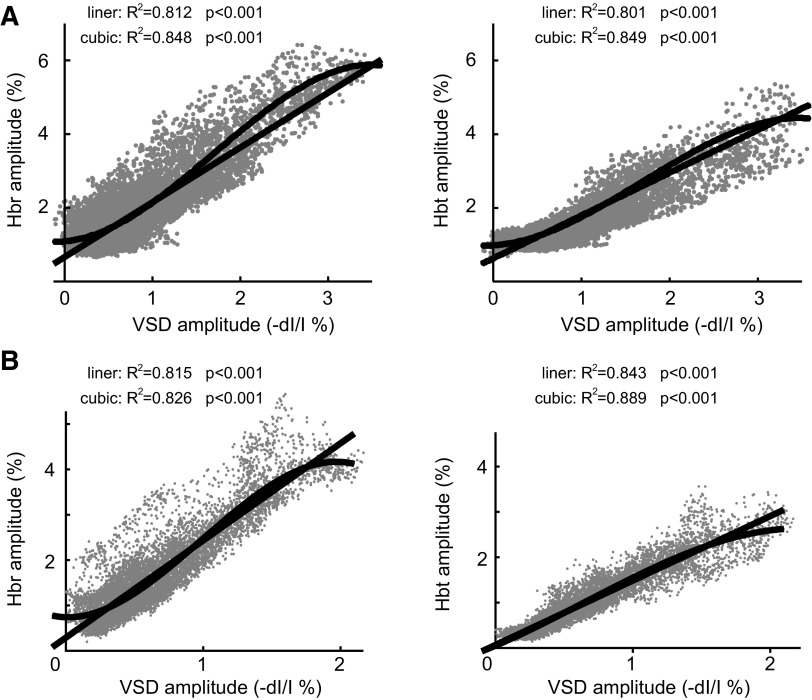
Because membrane potential change drives the increase in metabolic demand that triggers neurovascular coupling mechanisms, we wished to examine the spatial specificity of these mechanisms in epilepsy. To determine the response function between membrane voltage and hemodynamic response, we plotted the amplitude of the hemodynamic changes and the amplitude of the VSD signal from each pixel at their maximum amplitude (Fig. 6). The plot in a single animal shows that the amplitude of the hemodynamic signals positively correlates with VSD changes (Fig. 6A). The slight sigmoidal shape of the curve is demonstrated in the improved correlation between a cubic compared with a linear regression. Similar results are obtained when we average data from all animals (see methods). The R2 for the linear regression between both Hbr and Hbt compared with VSD changes was highly significant (Fig. 6B). The slope of the Hbt-VSD relationship (mean slope = 1.40) was less than that of the Hbr-VSD relationship (mean slope = 1.83), indicating that progressive increases in excitatory membrane potential change elicited a fractionally greater increase in Hbr than Hbt. When the distribution was fitted with cubic equation, the R2 for the regression between both Hbr and Hbt compared with VSD changes improved over the linear fit (Fig. 6B). In addition, the regression curves all cross the y axis above zero because the spatial spread of the hemodynamic signals surpasses the spread of the VSD signal. The good fit of the linear regression indicates that epileptic neurovascular coupling is linear in most of the IIS focus. However, the slight sigmoidal shape of the curve and the cubic regression statistics show that at the extremes of the curves, where the VSD change is highest (focus) and lowest (surround) coupling becomes spatially nonlinear and begins to plateau.
FIG. 6.
Spatial coupling between the amplitudes of the hemodynamic and VSD signals. A: plot of the amplitude of the maximal hemodynamic vs. maximal VSD signal for each pixel in 1 animal. The linear and cubic regression lines are both represented on the graphs. B: the plot of the linear and cubic regression lines for all 8 animals combined showing the relationship between the amplitude of each pixel of the hemodynamic and VSD signals, Hbr (left) and Hbt (right). The cubic regressions fit more closely.
Influence of choice of VSD and level of anesthesia on neurovascular coupling
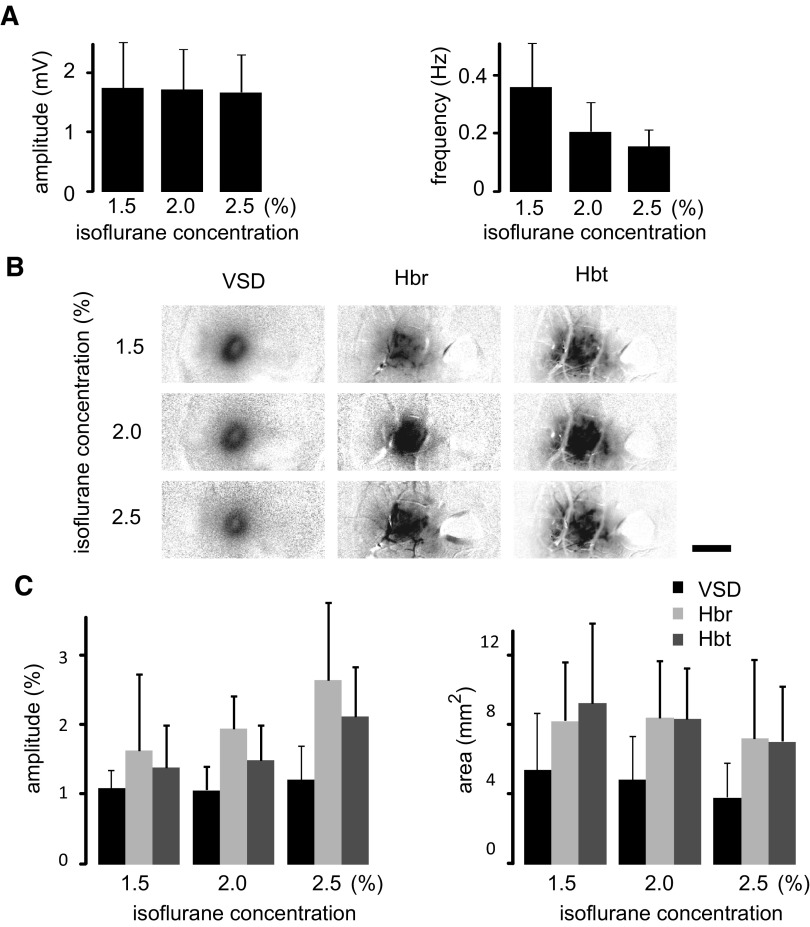
In a second group of experiments, IIS were recorded from animals (n = 7) using the newer blue dye, RH-1692, under 1.5 and 2% isoflurane anesthesia. In a subset of these animals (n = 4), recordings were also made at 2.5% isoflurane anesthesia. The frequency of IIS decreased with increasing levels of anesthesia [Fig. 7 A; repeated-measures 1-way ANOVA, F(2,17) = 4.718, P = 0.026; Boniferroni's post hoc test found significant difference between groups, P < 0.05]. However, the amplitude of the LFP signal was not significantly different at different levels of anesthesia. [Fig. 7A; 1-way ANOVA, F(2,11) = 0.12, P = 0.988]. Similar to the LFP recording, the maximal amplitude of the VSD signal was not significantly different at different levels of anesthesia [F(2,17) = 0.233, P = 0.795]. Although the amplitude of the hemodynamic signal slightly increased with the increase in anesthetic level, this was not significant [F(2,17) = 2.142, P = 0.152 (Hbr) and F(2,17) = 1.671, P = 0.221 (Hbt); Boniferroni's post hoc test found no significant difference, P > 0.05 for both Hbr and Hbt]. The activated area thresholded at 25% of the maximal were also not significantly different at different levels of anesthesia [Fig. 7; F(2,17) = 0.268, P = 0.768 (VSD); F(2,17) = 0.57, P = 0.945 (Hbr); F(2,17) = 0.229, P = 0.798 (Hbt); Boniferroni's post hoc test found no significant difference, P > 0.05 for all the signals]. The averaged area were not different from the result obtained with the dye RH-795 [t-test, P = 0.831 (VSD); P = 0.787 (Hbr); P = 0.624 (Hbt)].
FIG. 7.
The electrophysiologic, VSD, and hemodynamic responses using the blue dye RH-1692 and varying the level of anesthesia. A: average amplitude and frequency of IISs recorded with LFP. B: representative example in a single animal of the VSD and hemodynamic responses at their maximal amplitudes under 3 levels of anesthesia. Scale bar: 2 mm. C: the maximal amplitude and area of VSD and hemodynamic responses under different levels of anesthesia. Note that the relationship between the VSD and hemodynamic responses remain relatively constant.
DISCUSSION
We used VSD and IOS to obtain spatial data on membrane voltage, Hbr, and Hbt during IISs and report four significant findings relevant to an IIS. 1) Hemodynamic signals consistently overestimate the area of subthreshold excitatory neuronal activity and thus may not be reliably used to precisely map the horizontal extent of the dendritic arbor associated with the IIS focus. 2) Hemodynamic signals are a useful estimate of subthreshold excitatory activity particularly at specific time points in their evolution (early) and dissolution (late). Maps derived from Hbr signals reach this peak earlier than maps derived from Hbt although the accuracy of both maps are comparable. Regardless of the time, the centers of the hemodynamic and VSD signal were close to each other and hence hemodynamic signals are useful in determining the center of the epileptic focus. 3) Neurovascular coupling is remarkably linear, spatially specific and homogeneous throughout the majority of the IIS focus, except for small nonlinearities in the very center of the focus and at the edge of the hemodynamic signal where no excitatory voltage change is measured. 4) Acute IIS excitatory membrane potential changes elicit a fractionally greater increase in Hbr than Hbt. These findings are extremely significant because there is little high-resolution data on the spatial aspects of neurovascular coupling during epilepsy in spite of the current use of hemodynamic surrogates of neuronal activity in clinical practice and are not influence by the choice of dye or the level of anesthesia.
Etiology of the VSD and IOS signals during the IIS
VSDs measure membrane potential in the dendrites and nonmyelinated axons (Grinvald and Hildesheim 2004). Because the overall membrane area of the dendrites is far greater than the area of the axons and because the images in vivo blur the two compartments together, the vast majority of the signal arises from synaptic activity in the dendrites. Petersen et al. (2003) showed that the initial increase in VSD signal correlates with the population of neurons firing action potentials with rapid spread laterally to adjacent cortical areas overlapping with their dendritic arbor where subthreshold excitatory postsynaptic potentials (EPSP) are recorded.
Iontophoresis of BMI, like penicillin, elicits paroxysmal bursts of action potentials in a focal population of interconnected neurons that appears as an IIS in the LFP (Goldensohn and Salazar 1986; Prince and Wilder 1967). Intracellular recordings demonstrate depolarizing shifts (PDS) in a majority of adjacent neurons, consisting of a burst of action potentials superimposed on a giant EPSP surrounded by inhibitory (I)PSPs in the more distant adjacent cortex (Goldensohn and Salazar 1986; Prince and Wilder 1967). Hence the excitatory VSD signal demonstrates the spatial extent of the synaptic activity underlying the PDS as well as the horizontal spread of the adjacent subthreshold EPSPs associated with the IIS. Because the inhibitory activity occurs in a delayed fashion, the area of excitatory activity at its peak amplitude represents the horizontal extent of the epileptic focus and the dendritic arbor of the epileptic neurons.
IOS, by contrast, measures changes in perfusion and oximetry elicited by alterations in neuronal activity. Hence it is not surprising that the epicenter of the VSD signal had a higher correlation with the location of the BMI electrode compared with the hemodynamic signals. Previous investigations of the “point spread” of the intrinsic signal, on which IOS is based, in vivo have shown that although the signal is roughly centered on the cells firing action potentials, there is considerable additional lateral spread. As much as 50% of the signal is thought to represent subthreshold activity in an area 5–10 times larger than the area of spiking cells and possibly the extent of long-range horizontal connections (Bosking et al. 1997; Das and Gilbert 1995; Grinvald et al. 1994; Toth et al. 1996). The corresponding “point spread” of the intrinsic signal during epileptic events is unknown. However, a random sampling of cells from maps derived from intrinsic signal imaging at wavelengths sensitive to light scatter demonstrated epileptiform bursting (Schwartz and Bonhoeffer 2001). At the wavelengths measured in the present study, however, the IOS maps reflect the increase in cerebral metabolism of oxygen (CMRO2) that elicits a focal increase in cerebral blood flow (CBF) to provide oxygenated hemoglobin to hypermetabolic neurons, with a corresponding increase in Hbt. Because supply does not equal demand, the concentration of Hbr increases (Suh et al. 2005). When neurons are active, the major determinant of CMRO2 is the re-establishment of ionic concentration gradients via the Na+-K+ ATPase related to synaptic activity (Takahashi et al. 1995). The large concentration of mitochondria in the dendrites are a source for the increases in metabolic demand in this compartment and simultaneous LFP and fMRI studies demonstrate a high correlation between hemodynamic signals and synaptic activity (Logothetis et al. 2001; Mathiesen et al. 2000).
For these reasons, we hypothesized that the spatial overlap between the hemodynamic signals and the membrane potential changes would be quite high. Two prior studies have compared VSD with IOS imaging in the same preparation in rat somatosensory cortex and found a relatively close spatial overlap but did not quantify the spatiotemporal aspects of the relationship in any detail (Devor et al. 2007; Takashima et al. 2001). In our current study, in contrast, we found that during an IIS, perfusion and oxygenation changes were spatially approximately two times as large as the extent of the subthreshold excitatory membrane activity. These data may indicate that at their peak maximal spread, hemodynamic signals are not good surrogates for excitatory membrane activity and that the forces driving the hemodynamic response are more complex than just the re-establishment of ionic gradients. Alternatively, the extent of the hemodynamic spread may be specific to epileptic IISs, which are a unique condition of an intermittent cortical stimulus that continuously repeats. As previously demonstrated in normal somatosensory cortex, the frequency and duration of cortical stimulation can profoundly affect the hemodynamic response (Berwick et al. 2008; Chen-Bee et al. 2007). Because the hemodynamic response is slow, in our IIS experiments, an interspike baseline is never attained compared with the faster VSD responses that return to baseline between each IIS. Additionally, the VSD signal and its measurement may be intrinsically more difficult to resolve at its lateral extent for technical reasons, and our data may underestimate its true size. However, we used the same camera to measure both signals albeit at a different frame-rate and even utilized different dyes. Likewise we thresholded based on a percentage of the peak amplitude, which is an accepted standard in the literature (Berwick et al. 2008; Chen-Bee et al. 1996). The limitation of this method is that larger amplitude events will have a higher threshold value which results in a smaller areal extent (Chen-Bee et al. 1996, 2007). However, because the IOS signal had a larger percent change compared with the VSD signal, the method would have biased our data to increase the size of the VSD areal extent, so it is more likely that we overestimated rather than underestimated its size. Finally, level of anesthesia has been documented to influence the hemodynamic response (Ances 2004), so we used two separate anesthetics and varied their concentration to ascertain if this played a role in our data, which it did not.
Spatial evolution of hemodynamic signals in IISs
What are some of the possible explanations for the spatial blurring of the IOS compared with excitatory VSD signals? Mechanistically, there are several possible biological explanations for the overshoot of the hemodynamic signals beyond the excitatory membrane potential changes. In general, hemodynamic events depend on complex signaling mechanisms among neurons, astrocytes, pericytes, and vascular cells that form a functional unit (Iadecola 2004; Peppiatt et al. 2006; Takano et al. 2006). For example, the orientation and distribution of local vasculature may influence the spatial distribution of increases in CBF (Disbrow et al. 2000). Likewise, the passage of Hbr into draining veins will decrease spatial specificity of the oximetry signals over time (Fukuda et al. 2006). Alternatively, vasodilators such as lactate, carbon dioxide, nitrous oxide or potassium may diffuse to areas at a distance from the activated cortex, causing spatial blurring of perfusion responses (Erinjeri and Woolsey 2002; Iadecola et al. 1997; Kuschinsky and Wahl 1978; Lauritzen 2001). Similarly, neurotransmitters such as acetylcholine, GABA, catecholamines, neuropeptides, and adenosine are vasoactive and can diffuse through the extracellular space (Iadecola 2004). Another mechanism might involve astrocyte-mediated vasodilatation spread via calcium waves (Takano et al. 2006). Finally, neuronal inhibition in the surrounding cortex may require energy and elicit increases in CBV and deoxy-hemoglobin.
Regardless of the mechanism, the lateral extent of the hemodynamic events and excitatory membrane activity are not spatially specific at the time point when the maximal amplitude of each signal is reached although the epicenters of the signals overlap spatially. Nevertheless the horizontal distribution of the amplitude of the VSD signal was linearly correlated with the hemodynamic signals except for in the very center of the focus and at the margin of the surround. These minor nonlinearities are not as surprising as the remarkable linearity in the VSD/IOS amplitude response function identified through the majority of the focus. Although linear and nonlinear hemodynamic response functions are reported in animal models of sensory stimulation, with a plateau at high levels of stimulation, these studies measure neuronal activity with a single LFP electrode with no spatial data (Jones et al. 2004; Martin et al. 2006; Sheth et al. 2003, 2004). Our study is the first to use VSDs to obtain spatial data on the hemodynamic response function. The resolution of our data was the size of a pixel, roughly 33 × 33 μm2. Therefore we can conclude that neurovascular coupling during IISs functions on a level smaller than this size. Such tight spatial coupling can be explained by the mechanisms described in the preceding text such as pericytes or astrocyte foot processes that can regulate flow at the level of the capillary (Peppiatt et al. 2006; Takano et al. 2006).
The nonlinearities in the margin represent areas of cortex where the hemodynamic signal is present in the absence of a measurable change in membrane voltage. This phenomenon may have both a technical and physiological explanation. The technical etiology may reside in the different amplitude of the two biological signals, the different responses in the dye versus the light reflection or the different signal-to-noise ratios. The biological explanation may rest in the nonspecific nature of the hemodynamic signal, driven by dilation of upstream arterioles and incidental flow to nonactivated vascular territories. This latter reasoning does not explain the increase in Hbr in areas without VSD signal, although metabolic demands of the ring of inhibition may come into play. Nonlinearity in the focus, on the other hand, may be caused by the saturation of hemodynamic responses (i.e., maximal vasodilatation).
Using perfusion and oximetry as surrogates for interictal epileptiform activity
Our data clearly show that hemodynamic events associated with the IIS are dynamic processes that evolve over space and time. The utility of these signals as surrogates for membrane potential appears to depend on their ability to capture moments in time during their evolution when there is a high degree of spatial overlap. Perhaps more importantly, at its maximum, the hemodynamic signal overestimates the excitatory interictal synaptic activity by approximately two times. We show that both Hbr and Hbt are equally useful as surrogates for excitatory interictal synaptic activity although the initial dip (Hbr increase) provides the fastest response with the higher amplitude and response function.
These findings in epileptiform events have distinct similarities and differences compared with the spatiotemporal evolution of neurovascular coupling mechanisms found during normal cortical processing. Although early studies reported that oximetry signals, particularly the initial dip, were a more spatially localized reflection of underlying neuronal activity (Frostig et al. 1990; Malonek et al. 1997), subsequent studies have demonstrated that the perfusion-based signals (Hbt, CBV, or CBF) can be as focal, or even more focal than the early increase in Hbr, if measured early in their development at the level of the capillary rather than the arteriole (Culver et al. 2005; Sheth et al. 2004; Vanzetta et al. 2005). Likewise, a late focal CBV signal has been demonstrated to correlate well with neuronal activity during long-duration sensory activation (Berwick et al. 2008). However, IISs are short-lived and the later CBV map we define occurs much earlier than the late focal CBV signal described in rat barrel cortex (Berwick et al. 2008).
In contrast to normal cortical events, however, the amplitude and spatial spread of the initial dip (Hbr) during IISs appeared proportionally larger than the Hbt signal. We attribute this finding to the sudden dramatic increase in metabolic demand elicited by interictal spikes in which a large population of adjacent cells all are firing simultaneously (Matsumoto and Marsan 1964). Because this metabolic surge is not met by an adequate increase in cerebral perfusion (Bruehl et al. 1998), the increase in Hbr is larger and more extensive than that which is found during normal cortical function (Malonek et al. 1997). Focal acute pharmacologically induced animal models of IISs are likely more hypermetabolic than either chronic models or human epilepsy, in which hypometabolism is generally measured (Engel et al. 1982; Hagemann et al. 1998).
In summary, our results indicated that hemodynamic-based techniques for imaging interictal events will be spatially blurred unless the signals can be captured at early time points in their evolution or later moments when the signal is decreasing. In addition, deoxygenated hemoglobin provides a faster, higher-amplitude signal than cerebral blood volume. For this reason, we recommend caution in interpreting perfusion and oximetry based scans in patients with epilepsy unless a faster temporal resolution can be achieved and the technique can differentiate hemoglobin oxygenation from CBV. Nevertheless, the potential for using hemodynamic surrogates to map brief excitatory epileptiform and nonepileptiform events in the clinic is excellent, assuming a faster temporal resolution can be achieved.
GRANTS
This project was supported by National Institute of Neurolgical Disorders and Stroke Grant RO1 NS-49482 and by the Epilepsy Foundation through the generous support of the American Epilepsy Society.
Acknowledgments
We thank Drs. Amiram Grinvald and Jian-Young Wu for comments on the manuscript and S. Shariff and A. Geneslaw for technical assistance. We also thank Drs. Chris Schaffer and Peifang Tian for assistance with data analysis.
Present address of M. Suh: Dept. of Biological Science, Sungkyunkwan University, Suwon, South Korea.
REFERENCES
- Ances 2004.Ances BM Coupling of changes in cerebral blood flow with neural activity: what must initially dip must come back up. J Cereb Blood Flow Metab 24: 1–6, 2004. [DOI] [PubMed] [Google Scholar]
- Bagshaw et al. 2006.Bagshaw AP, Kobayashi E, Dubeau F, Pike GB, Gotman J. Correspondence between EEG-fMRI and EEG dipole localisation of interictal discharges in focal epilepsy. Neuroimage 30: 417–425, 2006. [DOI] [PubMed] [Google Scholar]
- Berwick et al. 2008.Berwick J, Johnston D, Jones M, Martindale J, Martin C, Kennerley AJ, Redgrave P, Mayhew JE. Fine detail of neurovascular coupling revealed by spatiotemporal analysis of the hemodynamic response to single whisker stimulation in rat barrel cortex. J Neurophysiol 99: 787–798, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosking et al. 1997.Bosking WH, Zhang Y, Schofield B, Fitzpatrick D. Orientation selectivity and the arrangement of horizontal connections in tree shrew striate cortex. J Neurosci 17: 2112–2127, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl et al. 1998.Bruehl C, Hagemann G, Witte OW. Uncoupling of blood flow and metabolism in focal epilepsy. Epilepsia 39: 1235–1242, 1998. [DOI] [PubMed] [Google Scholar]
- Chen-Bee et al. 2007.Chen-Bee CH, Agoncillo T, Xiong Y, Frostig RD. The triphasic intrinsic signal: implications for functional imaging. J Neurosci 27: 4572–4586, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Bee et al. 1996.Chen-Bee CH, Kwon MC, Masino SA, Frostig RD. Areal extent quantification of functional representations using intrinsic signal optical imaging. J Neurosci Methods 68: 27–37, 1996. [PubMed] [Google Scholar]
- Cohen et al. 1974.Cohen LB, Salzberg BM, Davila HV, Ross WN, Landowne D, Waggoner AS, Wang CH. Changes in axon fluorescence during activity: molecular probes of membrane potential. J Membr Biol 19: 1–36, 1974. [DOI] [PubMed] [Google Scholar]
- Culver et al. 2005.Culver JP, Siegel AM, Franceschini MA, Mandeville JB, Boas DA. Evidence that cerebral blood volume can provide brain activation maps with better spatial resolution than deoxygenated hemoglobin. Neuroimage 27: 947–959, 2005. [DOI] [PubMed] [Google Scholar]
- Das and Gilbert 1995.Das A, Gilbert CD. Long-range horizontal connections and their role in cortical reorganization revealed by optical recording of cat primary visual cortex. Nature 375: 780–784, 1995. [DOI] [PubMed] [Google Scholar]
- Devor et al. 2007.Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci 27: 4452–4459, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disbrow et al. 2000.Disbrow EA, Slutsky DA, Roberts TP, Krubitzer LA. Functional MRI at 1.5 tesla: a comparison of the blood oxygenation level-dependent signal and electrophysiology. Proc Natl Acad Sci USA 97: 9718–9723, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn et al. 2005.Dunn AK, Devor A, Dale AM, Boas DA. Spatial extent of oxygen metabolism and hemodynamic changes during functional activation of the rat somatosensory cortex. Neuroimage 27: 279–290, 2005. [DOI] [PubMed] [Google Scholar]
- Engel et al. 1982.Engel J Jr, Brown WJ, Kuhl DE, Phelps ME, Mazziotta JC, Crandall PH. Pathological findings underlying focal temporal lobe hypometabolism in partial epilepsy. Ann Neurol 12: 518–528, 1982. [DOI] [PubMed] [Google Scholar]
- Erinjeri and Woolsey 2002.Erinjeri JP, Woolsey TA. Spatial integration of vascular changes with neural activity in mouse cortex. J Cereb Blood Flow Metab 22: 353–360, 2002. [DOI] [PubMed] [Google Scholar]
- Frostig et al. 1990.Frostig RD, Lieke EE, Ts'o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci USA 87: 6082–6086, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda et al. 2006.Fukuda M, Wang P, Moon CH, Tanifuji M, Kim SG. Spatial specificity of the enhanced dip inherently induced by prolonged oxygen consumption in cat visual cortex: implication for columnar resolution functional MRI. Neuroimage 30: 70–87, 2006. [DOI] [PubMed] [Google Scholar]
- Goldensohn and Salazar 1986.Goldensohn ES, Salazar AM. Temporal and spatial distribution of intracellular potentials during generation and spread of epileptogenic discharges. Adv Neurol 44: 559–582, 1986. [PubMed] [Google Scholar]
- Grinvald and Hildesheim 2004.Grinvald A, Hildesheim R. VSDI: a new era in functional imaging of cortical dynamics. Nat Rev Neurosci 5: 874–885, 2004. [DOI] [PubMed] [Google Scholar]
- Grinvald et al. 1994.Grinvald A, Lieke EE, Frostig RD, Hildesheim R. Cortical point-spread function and long-range lateral interactions revealed by real-time optical imaging of macaque monkey primary visual cortex. J Neurosci 14: 2545–2568, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann et al. 1998.Hagemann G, Bruehl C, Lutzenburg M, Witte OW. Brain hypometabolism in a model of chronic focal epilepsy in rat neocortex. Epilepsia 39: 339–346, 1998. [DOI] [PubMed] [Google Scholar]
- Hirase et al. 2004.Hirase H, Creso J, Buzsaki G. Capillary level imaging of local cerebral blood flow in bicuculline-induced epileptic foci. Neuroscience 128: 209–216, 2004. [DOI] [PubMed] [Google Scholar]
- Iadecola 2004.Iadecola C Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci 5: 347–360, 2004. [DOI] [PubMed] [Google Scholar]
- Iadecola et al. 1997.Iadecola C, Yang G, Ebner TJ, Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol 78: 651–659, 1997. [DOI] [PubMed] [Google Scholar]
- Jones et al. 2004.Jones M, Hewson-Stoate N, Martindale J, Redgrave P, Mayhew J. Nonlinear coupling of neural activity and CBF in rodent barrel cortex. Neuroimage 22: 956–965, 2004. [DOI] [PubMed] [Google Scholar]
- Kohl et al. 2000.Kohl M, Lindauer U, Royl G, Kuhl M, Gold L, Villringer A, Dirnagl U. Physical model for the spectroscopic analysis of cortical intrinsic optical signals. Phys Med Biol 45: 3749–3764, 2000. [DOI] [PubMed] [Google Scholar]
- Kuschinsky and Wahl 1978.Kuschinsky W, Wahl M. Local chemical and neurogenic regulation of cerebral vascular resistance. Physiol Rev 58: 656–689, 1978. [DOI] [PubMed] [Google Scholar]
- Lauritzen 2001.Lauritzen M Relationship of spikes, synaptic activity, and local changes of cerebral blood flow. J Cereb Blood Flow Metab 21: 1367–1383, 2001. [DOI] [PubMed] [Google Scholar]
- Logothetis et al. 2001.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157, 2001. [DOI] [PubMed] [Google Scholar]
- Ma et al. 2004.Ma HT, Wu CH, Wu JY. Initiation of spontaneous epileptiform events in the rat neocortex in vivo. J Neurophysiol 91: 934–945, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malonek et al. 1997.Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, Grinvald A. Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proc Natl Acad Sci USA 94: 14826–14831, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin et al. 2006.Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage 32: 33–48, 2006. [DOI] [PubMed] [Google Scholar]
- Mathiesen et al. 2000.Mathiesen C, Caesar K, Lauritzen M. Temporal coupling between neuronal activity and blood flow in rat cerebellar cortex as indicated by field potential analysis. J Physiol 523: 235–246, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto and Marsan 1964.Matsumoto H, Marsan CA. Cortical cellular phenomena in experimental epilepsy: interictal manifestations. Exp Neurol 80: 286–304, 1964. [DOI] [PubMed] [Google Scholar]
- Peppiatt et al. 2006.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature 443: 700–704, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen et al. 2003.Petersen CC, Grinvald A, Sakmann B. Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions. J Neurosci 23: 1298–1309, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimeni et al. 2005.Polimeni JR, Granquist-Fraser D, Wood RJ, Schwartz EL. Physical limits to spatial resolution of optical recording: clarifying the spatial structure of cortical hypercolumns. Proc Natl Acad Sci USA 102: 4158–4163, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince and Wilder 1967.Prince DA, Wilder BJ. Control mechanisms in cortical epileptogenic foci. “Surround” inhibition. Arch Neurol 16: 194–202, 1967. [DOI] [PubMed] [Google Scholar]
- Schwartz and Bonhoeffer 2001.Schwartz TH, Bonhoeffer T. In vivo optical mapping of epileptic foci and surround inhibition in ferret cerebral cortex. Nat Med 7: 1063–1067, 2001. [DOI] [PubMed] [Google Scholar]
- Sheth et al. 2003.Sheth S, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Evaluation of coupling between optical intrinsic signals and neuronal activity in rat somatosensory cortex. Neuroimage 19: 884–894, 2003. [DOI] [PubMed] [Google Scholar]
- Sheth et al. 2004.Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Linear and nonlinear relationships between neuronal activity, oxygen metabolism, and hemodynamic responses. Neuron 42: 347–355, 2004. [DOI] [PubMed] [Google Scholar]
- Suh et al. 2005.Suh M, Bahar S, Mehta AD, Schwartz TH. Temporal dependence in uncoupling of blood volume and oxygenation during interictal epileptiform events in rat neocortex. J Neurosci 25: 68–77, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi et al. 1995.Takahashi S, Driscoll BF, Law MJ, Sokoloff L. Role of sodium and potassium ions in regulation of glucose metabolism in cultured astroglia. Proc Natl Acad Sci USA 92: 4616–4620, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano et al. 2006.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 9: 260–267, 2006. [DOI] [PubMed] [Google Scholar]
- Takashima et al. 2001.Takashima I, Kajiwara R, Iijima T. Voltage-sensitive dye versus intrinsic signal optical imaging: comparison of optically determined functional maps from rat barrel cortex. Neuroreport 12: 2889–2894, 2001. [DOI] [PubMed] [Google Scholar]
- Toth et al. 1996.Toth LJ, Rao SC, Kim DS, Somers D, Sur M. Subthreshold facilitation and suppression in primary visual cortex revealed by intrinsic signal imaging. Proc Natl Acad Sci USA 93: 9869–9874, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Paesschen 2004.Van Paesschen W Ictal SPECT. Epilepsia 45, Suppl 4: 35–40, 2004. [DOI] [PubMed] [Google Scholar]
- Vanzetta et al. 2005.Vanzetta I, Hildesheim R, Grinvald A. Compartment-resolved imaging of activity-dependent dynamics of cortical blood volume and oximetry. J Neurosci 25: 2233–2244, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]