Abstract
Optical imaging of cortical signals enables the mapping of functional organization across large patches of cortex with good spatial resolution. But techniques for the quantitative analysis and interpretation of these images are limited. Frequently the functional architecture of the cortex is inferred from the visible topography of cortical reflectance images averaged or differenced across stimulus conditions and scaled or color-coded for presentation. Such qualitative assessments have sometimes led to divergent conclusions particularly about the organization of spatial and temporal frequency preferences in the primary visual cortex. We applied quantitative methods derived from signal detection theory to objectively interpret optical images. The differential response to any two arbitrary stimuli was represented at each pixel as the probability of discriminating between the two stimuli given the reflectance values at that pixel. These probability maps reduced false alarms and provided better signal-to-noise ratio in fewer trials than difference maps. We applied these methods to optical images of primate primary visual area (V1) obtained in response to sinusoidal gratings of different orientations and spatiotemporal frequencies. Clustering by orientation preference was stronger than that for spatial frequency, whereas clustering by temporal frequency preference was the weakest, largely in agreement with a previous electrophysiological study that quantified the degree of clustering of neurons for various response properties using uniform, quantitative criterion. We suggest that probability maps can extend the applicability of optical imaging to investigations of finer aspects of cortical functional organization through better signal-to-noise ratio and uniform, quantitative criteria for interpretation.
INTRODUCTION
Since the 1980s, optical imaging has revealed the functional organization of sensory cortical areas in rich detail (rev. Bonhoeffer and Grinvald 1996; Zepeda et al. 2004). Building on past successes, recent studies have used optical imaging to investigate finer and more complex aspects of cortical feature maps. This has led to an increased recognition that the low signal-to-noise ratio (SNR) of optical images can be a major limiting factor (Everson et al. 1998; Issa et al. 2000; Khaytin et al. 2008; Sirovich and Uglesich 2004). We applied methods based on signal-detection theory to analyze and interpret the weak signals captured by optical imaging. These methods offer an objective scale for representing processed optical-imaging data in a physiologically and behaviorally meaningful manner. These methods can also facilitate the objective interpretation of optical images by prescribing the statistical significance of the inferences drawn and have the potential to mitigate problems associated with low SNR. The main aim of this study was to empirically determine the general scope of applicability of these methods to optical images and their limitations thereof. We also tested the resolving power of these methods by applying them to the problem of mapping spatiotemporal frequency preferences across the surface of the primary visual cortex (Basole et al. 2003; Bonhoeffer et al. 1995; Hubener et al. 1997; Issa et al. 2000; Khaytin et al. 2008; Shoham et al. 1997; Sirovich and Uglesich 2004; Xu et al. 2007).
Optical imaging of intrinsic signals and voltage-sensitive dyes in vivo has made it possible to test models of cortical organization that could not be easily tested earlier (reviewed by Bonhoeffer and Grinvald 1996). For instance, computational modeling of the results of Hubel and Wiesel (1963) suggested a “pinwheel” pattern of arrangement for orientation maps (Braitenberg and Braitenberg 1979). Optical imaging has visibly demonstrated the existence of orientation pinwheels in cortical areas with orderly orientation maps (Bartfeld and Grinvald 1992; Blasdel 1992a,b; Bonhoeffer and Grinvald 1991; Bosking et al. 1997; Crair et al. 1997; Hubener et al. 1997; Rao et al. 1997; Xu et al. 2004, 2005, 2007). Optical imaging has also shown that orientation pinwheels and high orientation gradient sites tend to lie near the centers of ocular dominance columns in cats and primates (Blasdel and Salama 1986; Bartfeld and Grinvald 1992; Crair et al. 1997; Hubener et al. 1997; Obermayer and Blasdel 1993; Xu et al. 2004, 2005; Yu et al. 2005) largely in agreement with computational models proposed for the formation of cortical feature maps (Farley et al. 2007; Obermayer et al. 1990; Swindale 1991, 2004; Yu et al. 2005).
In contrast to these clear successes, optical imaging has led to divergent conclusions about other aspects of the functional organization of the visual cortex. For example, different imaging studies have suggested different patterns of organization for spatiotemporal frequency preferences across the primary visual cortex of cats, prosimians, and monkeys (Bonhoeffer et al. 1995; Everson et al. 1998; Hubener et al. 1997; Issa et al. 2000; Khaytin et al. 2008; Shoham et al. 1997; Sirovich and Uglesich 2004; Xu et al. 2007). Early optical-imaging studies concluded that only two distinct spatiotemporal frequency domains exist, one corresponding to high spatial frequency (SF) and low temporal frequency (TF) and the other corresponding to low SF and high TF (Bonhoeffer et al. 1995; Hubener et al. 1997; Shoham et al. 1997). This result was interpreted as a manifestation of distinct inputs from the ascending X and Y pathways. A similar view has been supported by Sirovich and Uglesich (2004), who quantified the SF response at each pixel using singular value decomposition after reducing contamination from nonspecific components of the hemodynamic response. But at least two other studies have found evidence for multiple domains representing multiple SFs. Everson et al. (1998) used principal-components analysis on the images and observed pinwheel structures among multiple SF domains. Issa et al. (2000) systematically explored different ways of estimating and representing the SF preference of each pixel in the image, and in doing so, they also found evidence for a continuum of SF preference domains. But no SF pinwheels were obtained in this study. This result, obtained in cats, was later extended to primates as well (Xu et al. 2007). Finally, although Shoham et al. (1997) found evidence in cats for domains with high and low temporal frequency preference at low and high spatial frequencies, respectively, Khaytin et al. (2008) found no temporal frequency preference domains in primates.
These discrepant conclusions about the nature of spatial frequency maps are not specific to optical-imaging studies. They mirror a similar discrepancy among previous results obtained using single-unit electrophysiology (De Valois et al. 1979; Maffei and Fiorentini 1973, 1977; Tolhurst and Thompson 1982) and activity-dependent 2DG radiographic labeling (Tootell et al. 1981). These earlier studies found an orderly organization of SF preference, but they disagreed about the exact nature of this organization, variously suggesting laminar grouping by SF (e.g., Maffei and Fiorentini 1977), clustering (e.g., Tolhurst and Thompson 1982), and a columnar organization (e.g., Tootell et al. 1981). But more recently, De Angelis et al. (1999) used a reverse-correlation technique to estimate spatiotemporal maps of receptive fields for pairs of simultaneously recorded neurons. From these maps, they extracted quantitative measures of a “clustering index” concurrently for orientation, SF, and TF preferences. It was found that the tendency for neurons to cluster together by orientation preference was the strongest, TF preference clustering was the weakest, and clustering by SF preference was at an intermediate strength. These results suggest that the segregation of a cortical feature map into domains could be a matter of degree with orientation maps manifesting strong domains for different orientations and spatiotemporal frequency maps containing weakly expressed domains. If this were true, then one cause of the discrepancy between the results obtained by the different optical imaging studies of spatiotemporal frequency maps could be the different methods of analysis and representation used on the images and consequently, the different levels of sensitivity with which the domains were detected and classified.
The most common method used for analyzing and interpreting optical images is to average the cortical reflectance images across one stimulus condition and, when possible or necessary, divide or difference the result by the average image for another condition. The presence or absence of activation domains is then inferred from the topography of the resulting image, often by direct visual inspection and judgment of the contrast between a potential activation domain and its surround. This method has three potential problems. First, the contrast or color intensity of the resultant image, which is taken to be a measure of the strength of activation of the domains, is not a veridical representation of the original reflectance values. Due to preprocessing, dividing/differencing, and postprocessing of the images, the visible differences in the final image may not be linearly or proportionally related to the measured reflectance values. Second, the differential map, which represents the difference between average images for two stimulus conditions, fails to capture the variance of the reflectance values and hence the statistical significance of the difference. Third, as the contrast or color difference between a potential activation domain and its surround decreases, there will be considerable variability in experimenters' subjective judgments about the presence of a domain.
Is it possible to represent the results of an optical imaging experiment so that the visible intensity is an objective quantity that also takes into account the variance of the reflectance values? We addressed this problem using signal detecting theory by simply extending the receiver operating characteristic (ROC) analysis to the image as a whole and producing probability maps. We compared SNR, false alarm rate, estimation bias, efficiency, and consistency for probability maps and differential activity maps. These comparisons showed the overall statistical reliability to be better for probability maps. We found that probability maps required fewer trials to reach a given SNR than differential activity maps. We determined that 20–25 outlier-filtered images per stimulus condition suffice to compute a reliable differential probability map regardless of stimulus conditions. Finally, we applied this method to the problem of mapping spatiotemporal frequency preferences and found that orientation domains are more strongly expressed than SF domains and that TF domains were the weakest of the three. These results are similar to those obtained by De Angelis et al. (1999) using single-unit electrophysiology.
METHODS
Animal preparation and surgery
Twelve bush babies (Otolemur garnetti) of both sexes were used in these experiments according to approved protocols from the IACUC at Vanderbilt University. Our surgical and optical imaging procedures were previously described (Khaytin et al. 2007; Xu et al. 2004, 2005) and are only briefly summarized here. All experiments were conducted under Propofol/nitrous oxide anesthesia. Neuromuscular blockade (NMB) was induced with an injection of 0.7–1.0 mg/kg of vencuronium bromide. Animals were respired with a ventilator that delivered a 3:1 mixture of NO2 and O2 at a sufficient rate to maintain expired CO2 at 4%. During surgery, anesthesia was maintained using NO2 as described and Propofol delivered at ∼10 mg·kg−1·h−1, which was reduced during the experiments to 4–7 mg·kg−1·h−1. NMB was maintained throughout the experiments with vencuronium bromide (0.6 mg·kg−1·h−1) in a 5.0% dextrose lactated Ringer solution. An opening ∼10 mm in diameter was made over V1 centered at 8 mm from the posterior pole and 9 mm from the midline. Dura was removed and replaced with Tecoflex as described by Sakas et al. (1990). The opening was covered with 1% agarose in saline and sealed with a glass cover-slip. Pupils were dilated with 2% cyclopentolate (Cyclogyl) and/or 1% atropine drops, and contact lenses with sufficient power and 3-mm pupils were used to make the retina conjugate with the viewing distance to the monitor of 28.5 cm. Retinal landmarks including the optic disks and area centralii were plotted using back reflection from the tapetum at the beginning of each experiment.
Optical imaging
Our imaging equipment and procedures have been previously described (Bosking et al. 1997; Khaytin et al. 2008; Xu et al. 2004, 2005). Cortical reflectance changes following stimulus presentation were imaged using an Optical Imager 2001 system (Optical Imaging, Mountainside, NJ). Visual stimuli were presented on a 21-in monitor (Sony FD Trinitron, F400, Sony, Tokyo, Japan) at 120 Hz (noninterlaced mode, background luminance = 30 cd/m2) using the VSG 2/5 stimulus generator (Cambridge Research Systems, Rochester, UK) synchronized with the imaging system. A macroscope consisting of two front-to-front tandem Nikon lenses (50 mm/50 mm or 50 mm/135 mm) magnified the cortex for the CCD camera. The image was focused at ∼500–600 μm below the cortical surface and the diaphragm was closed by one or two f-stops. The light source was filtered at 540 nm to acquire a reference image of the surface vasculature and 611 nm to collect functional data. Raw image for each trial was normalized by the image acquired during a blank screen condition at the start of the trial (Crair et al. 1997).
Visual stimuli
The stimuli used in these experiments have been previously described in detail (Khaytin et al. 2008; Xu et al. 2005, 2007). Full-screen sine wave and square wave gratings at different orientations, SFs, and TFs were used. Unless stated otherwise in the following text, orientation maps were obtained for 0, 45, 90, and 135°, SF maps were obtained for 0.2, 0.5, 0.8, 1.2, and 1.6 cycle/deg, and TF maps were obtained for 1, 2, 5, and 10 Hz. These values were selected based on our previous electrophysiological estimates of the range of spatial and temporal frequency preferences of V1 neurons in bush babies (Bonds et al. 1987; DeBruyn et al. 1993). In each experiment, the different orientations and either spatial or temporal frequencies were randomly presented. The SF maps were obtained using sine wave gratings only and at the fixed TF of 2 Hz. TF maps were obtained using square wave gratings of 0.5 cycle/deg fundamental spatial frequency and 20% duty cycle. To determine the effect of higher harmonic spatial frequencies on the imaged TF maps, in one additional animal, we used sine wave grating stimuli at the same temporal frequencies, at 0.5 cycle/deg spatial frequency, and 50% contrast. In each trial, stimuli were presented in the following sequence: 5 s of uniform gray screen of mean luminance 30 cd/m2, followed by 8 s of a drifting grating, followed by 12 or 17 s of the uniform gray screen. Each stimulus was pseudo randomly repeated 10–30 times.
ROC analysis
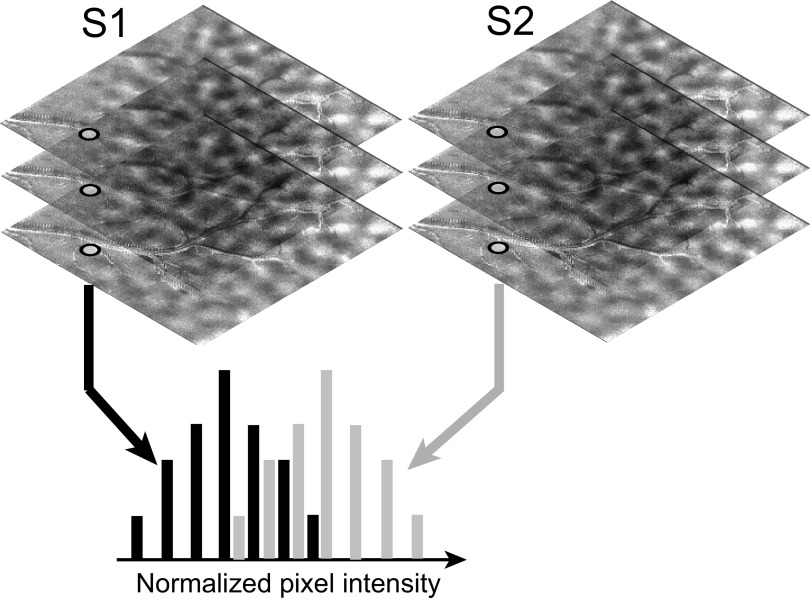
Consider two sets of optical images obtained for two stimulus conditions, S1 and S2 (Fig. 1). Each image in a set is a measurement of the cortical reflectance obtained in one trial for the corresponding stimulus. Assume that a pixel belongs to an activation domain for stimulus S1 but lies outside the activation domains for S2. Following the presentation of a stimulus, cortical reflectance decreases in regions where there is an increase in neural activity (Grinvald et al. 1988). Therefore, ideally, the reflectance of this pixel will be lower for stimulus S1 than for S2. Suppose an ideal observer is given one reflectance value from a trial in which stimulus S1 was presented and another from a trial in which S2 was presented. Suppose also that the ideal observer is naive as to which reflectance value comes from which trial. In the absence of noise, the ideal observer will be 100% correct if the observer consistently predicts that the lower of the two reflectance values comes from the trial in which S1 was presented. If the observer uses the opposite rule, then the observer will be 100% incorrect. Thus, if the probability of correct answers from the ideal observer is either 1.0 or 0.0, then we can conclude that the two stimuli can be perfectly discriminated based only on the reflectance values at this pixel. In other words, there is a full 1 bit of information in the reflectance value about the stimulus. If the probability of correct answers from the observer is near 0.5 for either rule, then we can conclude that there is no information (0 bit) in these reflectance values about the stimulus.
FIG. 1.
Schematic illustrating the computation of a probability map. Images of cortical response to 2 stimulus conditions, S1 and S2, are sorted into 2 groups. For each pixel, reflectance values from all the trials for a stimulus condition are grouped into 1 histogram. The area under the receiver operating characteristic (ROC) curve for this pair of histograms is taken as a measure of the ideal observer's ability to determine the stimulus condition based only on the cortical reflectance at this pixel.
This discrimination probability, i.e., the performance of the ideal observer, was evaluated as shown in Fig. 1. The reflectance values for stimulus S1 were grouped together into a histogram that provided (when normalized) an estimate of the conditional probability density of the reflectance given that stimulus S1 was presented, p(r/S1). Similarly, reflectance values for S2 were grouped into another histogram the normalized value of which provided an estimate of the conditional probability density for the reflectance values given S2, p(r/S2). If an ideal observer used the rule that the lower of the two reflectance values is due to S1, then the probability of correct answers for the ideal observer should equal the probability of the pixel intensity being greater for S2, given by
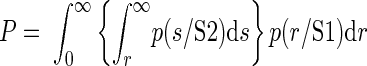 |
This quantity was nonparametrically computed as the area under the ROC curve for the pair of normalized histograms p(r/S1) and p(r/S2). By performing these computations for all the pixels and representing these probability values as an image, we created a differential probability map.
Note that if either the observer used the opposite rule or the pixel belonged to an activation domain for S2 but not to the activation domains of S1, then the probability of correct answers for the ideal observer should be 1 − P. Therefore, the probability maps created in these cases will be contrast-reversed versions of the ones computed above, and they will not provide any additional information about the activation domains for S1 and S2. Thus, it suffices to compute just one probability map for a given pair of stimuli.
Preprocessing data for ROC analysis
To remove gross fluctuations in reflectance due to variations in the physiological state of the animal and movement artifacts associated with respiration, our system automatically normalizes each trial image by dividing it with the response to a blank screen presented at the beginning of the trial. In addition, for orientation maps alone we sometimes z-scored each image with its own mean and variance (see Smoothing, clipping, and SNR). Because each image contains a full range of responses to an orientation, this normalization sometimes improved results for both difference and probability maps. For spatial and temporal frequency maps, because the response level can vary across different frequencies, it is not appropriate to z-score the images individually.
RESULTS
Differential probability maps for orientation
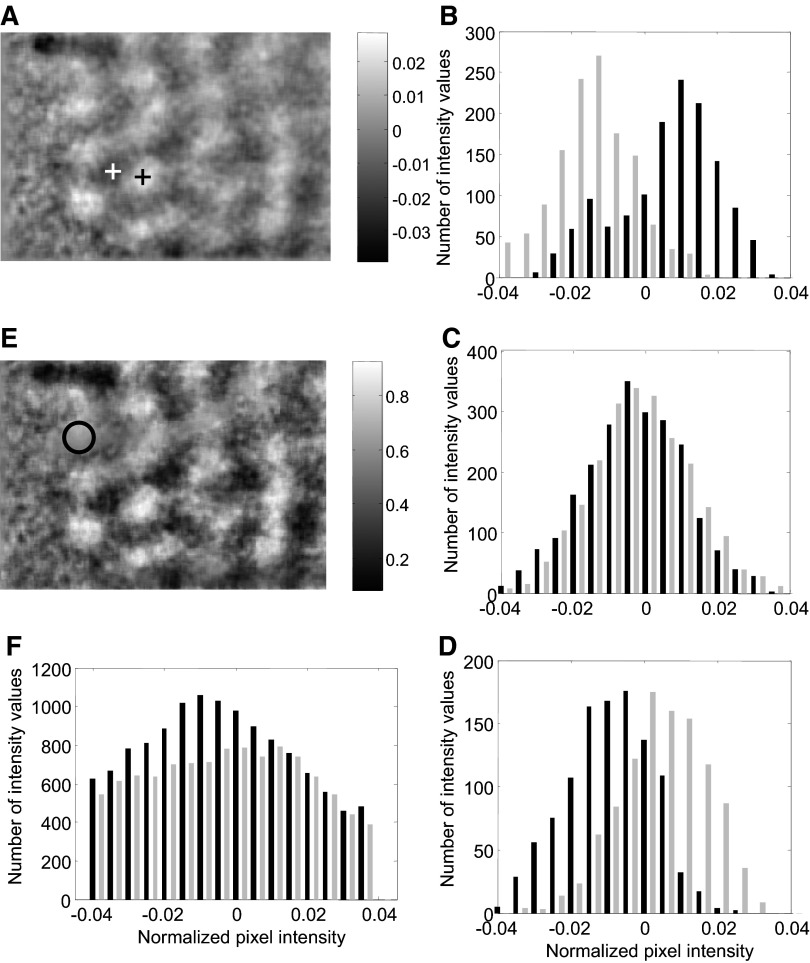
Following the presentation of a stimulus, cortical reflectance subtly decreases in regions where there is an increase in neural activity (Grinvald et al. 1988). But the overall reflectance from the cortical surface also varies from trial to trial due to a variety of extraneous factors (Kalatsky and Stryker 2003; Vnek et al. 1999). It is therefore common to compute “difference maps” that capture the differences in reflectance between regions of high neural activity in one image and low neural activity in another (Blasdel and Salama 1986; Frostig et al. 1990; Grinvald et al. 1986). To investigate the reliability of such maps and to compare them with differential probability maps, we computed a difference map by subtracting the mean of the images for 0° orientation from the mean image for 90° orientation (Fig. 2A). Dark regions show selectivity for 90° and bright regions for 0° orientation.
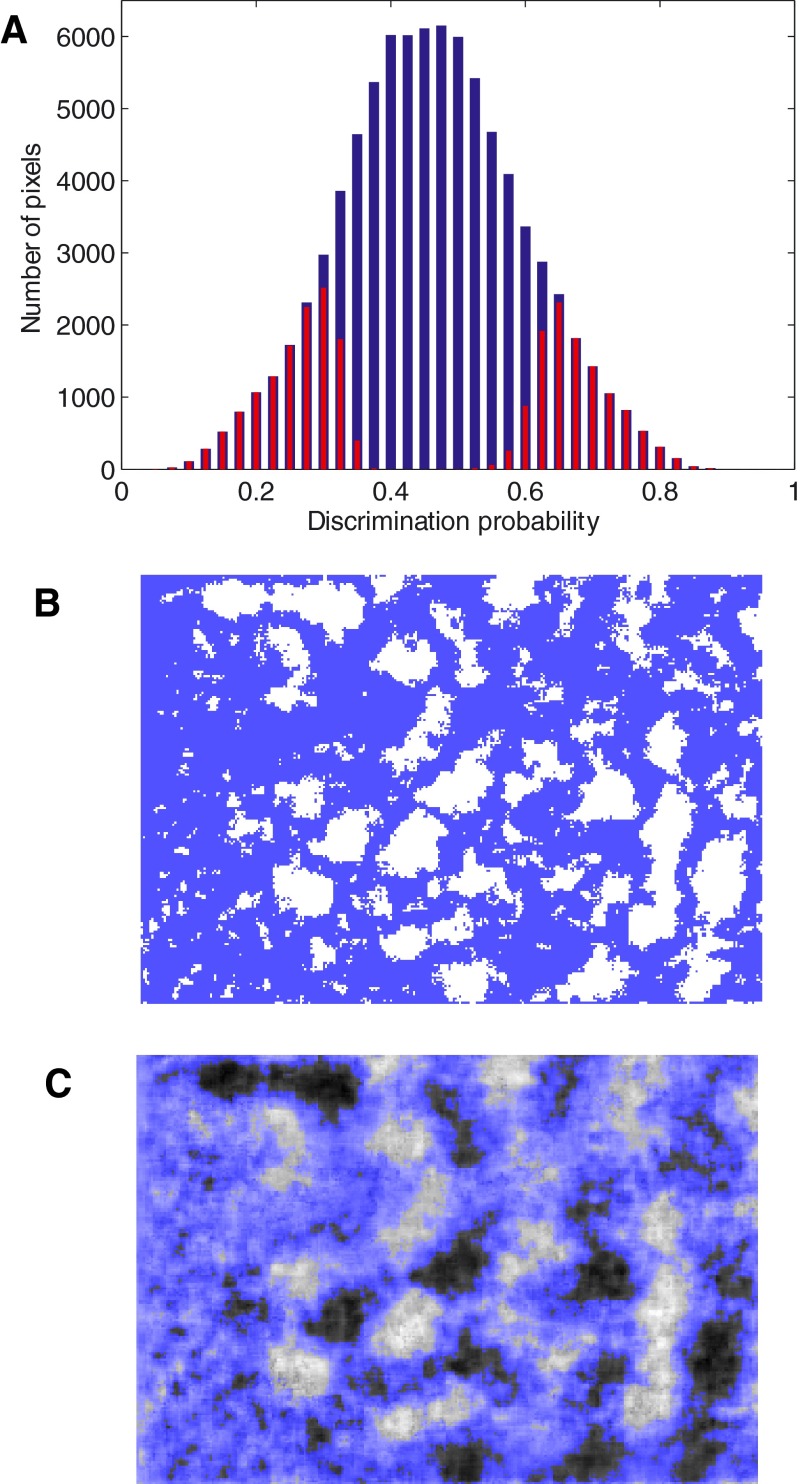
FIG. 2.
Analysis of a sample differential probability map. A: a conventional difference map is shown for reference. Dark regions show selectivity for 90° orientation and bright regions for 0°. B: reflectance distributions are shown for pixels marked with black cross in A. The distribution for 0° orientation (gray histogram) is shifted below that for 90° orientation (black) as would expected for pixels lying inside an activation domain for 0° orientation. C: for pixels lying between the 2 activation domains, reflectance distributions for 0° (gray) and 90° (black) orientations almost overlap. D: for pixels at the center of an activation domain for 90° (marked by the white cross in A), reflectance distribution for 90° (black) is shifted below that for 0° (gray). E: probability map computed from the same reflectance images. F: reflectance distribution for pixels shown circled in E (see text for details).
We compared the distribution of reflectance values for the two orientations at three representative regions on the map. For pixels near the center of a bright region (activation domain for 0° orientation), the distribution of reflectance values for the 0° orientation lay to the left of the distribution for 90° (Fig. 2B). Near the center of a dark region, the opposite trend was seen (Fig. 2D). In between these two points, the two distributions almost completely overlapped (Fig. 2C). These comparisons showed that reflectance distributions at each pixel can be used to predict the orientation preference of the pixel. We computed the probability with which orientation preference can be correctly predicted from reflectance. The correct orientation prediction probability for each pixel was estimated as the area under the ROC curve for the pair of reflectance histograms belonging to that pixel (see methods). For pixels that lay between two activation domains, because the two histograms overlapped, the correct prediction probability was close to 0.5, i.e., it was at chance level (Fig. 2C). For pixels near the center of a bright region (activation domain for 0° orientation), the probability of the reflectance value for 0° orientation being smaller than that for 90° was >0.5 (e.g., 0.8 for Fig. 2B). Near the center of dark region, this probability was <0.5 (e.g., 0.2 for Fig. 2D). The probability of the reflectance for 0° being lower than the reflectance for 90° is shown for all the pixels in the differential probability map (Fig. 2E).
A number of differences are apparent between the difference map (Fig. 2A) and the differential probability map (Fig. 2E). We discuss these differences, their potential causes, and their implications in detail in the following sections.
False alarm reduction
One major difference between the difference map and the differential probability map can be seen within the region shown circled on the differential probability map (Fig. 2E). This region exhibits a more prominent domain in the difference map than in the differential probability map. An examination of the distributions of reflectance values within this region revealed the cause of this discrepancy (Fig. 2F). The reflectance values for both orientations were broadly distributed. The mean reflectance value for 90° was −0.0121, whereas that for 0° was −0.0016 and both variances were large. As mentioned in the introduction, the difference map represents only the difference between the means and ignores the variance. Therefore the difference map shows an average positive intensity of ∼0.0105 in this region. But the area under the ROC curve for this pair of distributions, which takes the variances into account, was ∼0.5, near chance. Consequently, the domain is not emphasized in the differential probability map as much as it is in the difference map.
The maximum difference in the cortical reflectance values between any two pixels that can be obtained in a single trial is typically very small, on the order of 0.1% of the mean reflectance across the image (Blasdel and Salama 1986; Bonhoeffer and Grinvald 1991; Grinvald et al. 1986). Hence even after averaging across several trials, difference maps can be noisy and visual judgments about the presence or absence of a domain can be quite variable between observers and experimental conditions. The preceding example shows that differential probability maps can reduce such false alarms because the variance information is automatically incorporated into them. In the following section, we quantify the false alarm rate and blood vessel artifacts.
Smoothing, clipping, and SNR
A comparison of the difference map with the differential probability map for the above example also shows a slightly higher SNR for the latter, i.e., the contrast between adjacent activation domains in the differential probability map is slightly greater than the same for the difference map. We investigated if this difference in SNR was real or if it was simply due to a difference in the scale of representation. We used different scaling methods to improve the contrast of the difference map and compared the results with the differential probability map.
As noted in methods, the raw image obtained in each trial was normalized by the image acquired during a blank screen condition at the start of the trial. However, if this normalization had been inadequate, then it could have resulted in a lower SNR for the difference map. Therefore, in addition to this original normalization, we z-scored each image with its own mean and variance. We then computed a difference map with these normalized images (Fig. 3B). A comparison of this new difference map with the original difference map (reproduced in Fig. 3A) showed that this additional normalization did not improve the contrast significantly.
FIG. 3.
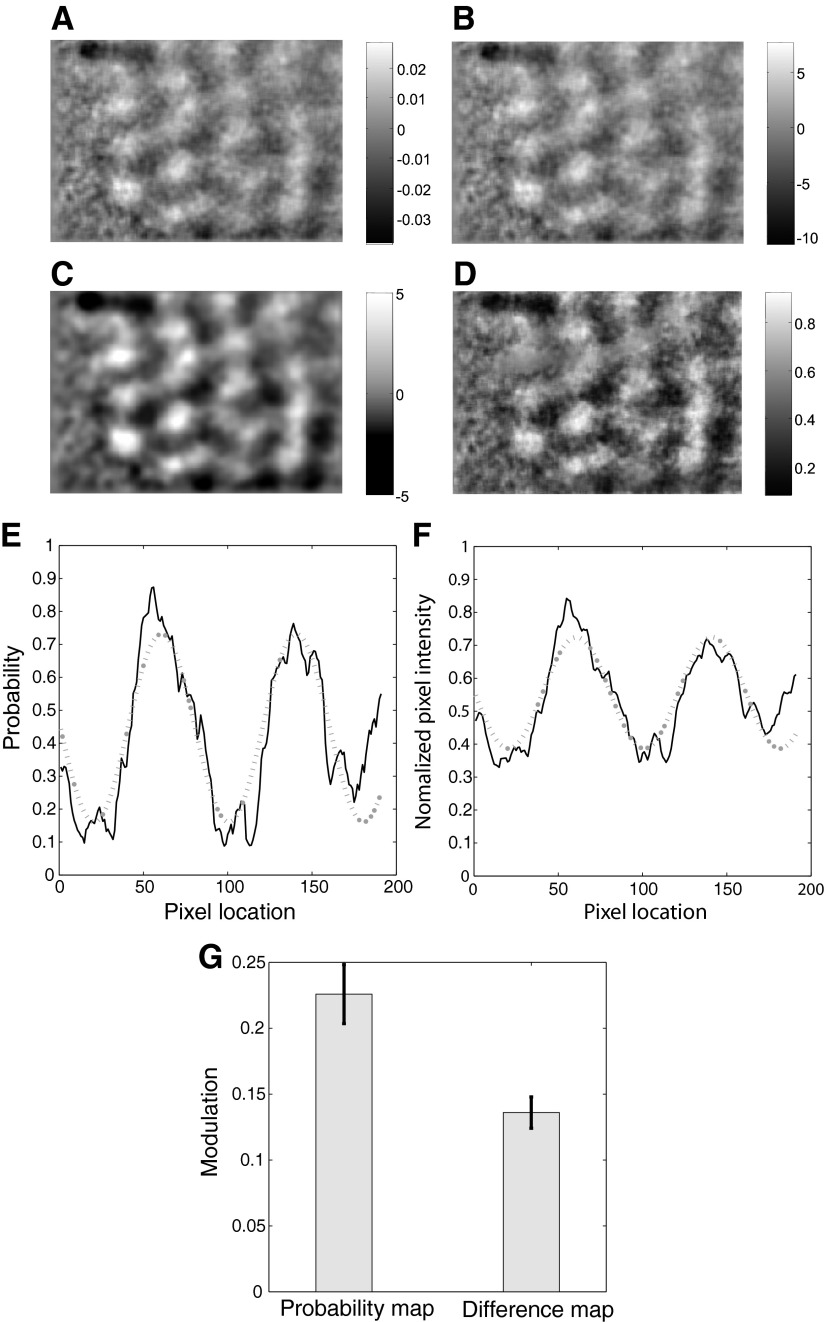
Signal-to-noise ratio (SNR) for differential activity and probability maps Differential activity maps computed from base-normalized images (A), base-normalized and z-scored images (B), base-normalized, z-scored images and further smoothed and clipped (C). D: differential probability map. E: discrimination probabilities are shown for a horizontal section through two activation domains (—) along with the best-fitting sinusoidal curve (···). F: normalized difference between the mean activation values are shown for the same set of pixels (—) along with the best-fitting sinusoidal curve (···). G: mean modulation strengths for the differential probability map and the difference map are shown.
Next we smoothed the new difference map using a two-dimensional (2D) Gaussian filter of SD ≈ 44 μm and clipped the resulting image at both ends of the scale. This smoothing and clipping are frequently used on difference maps to improve contrast (Khaytin et al. 2008). The resulting image (Fig. 3C) has significantly higher contrast and looks smoother than the original (A). The contrast of the smoothed-clipped difference map is now better than that of the differential probability map. However, the “domain” in the top left corner, which was identified as statistically insignificant in the previous section, appears very prominent in Fig. 3C. Therefore, this traditional method for filtering noise and amplifying the contrast of the difference map (also see discussion) does not overcome the basic problems associated with difference map analysis: because the variances of the reflectance values are ignored, when signal gets amplified, so does the noise.
These analyses suggested that the greater contrast seen in the differential probability map is not merely due to a difference in the scale of representation because changing the scale on the difference map produced qualitatively different results. We estimated the frequency of false positives by comparing activation regions with intensity values outside 2σ in the difference map with the corresponding ROC values. Those regions with mean ROC values between 0.45 and 0.55 were treated as false positives. Over eight animals, difference maps of orientations had an average false positive rate of 11%. Of these false “domains,” 80% were on or near major blood vessels.
Quantitative comparison of SNR
To estimate the improvement in SNR offered by the ROC method, we quantified SNR for both probability and difference maps using the same method and compared them. Local changes in pixel intensity values in both maps were well fitted by sine curves (Fig. 3, E and F). Discrimination probability values are shown plotted for a horizontal slice of the image passing over a few domains along with the best-fitting sine curve (Fig. 3E). We performed nonlinear least-squares curve fitting using the interior reflective Newton method (Coleman and Li 1996) to optimize the scale factor (amplitude of the sine), shift factor (DC or mean value), frequency, and phase of the sine function. Similar result for the difference map is shown for the same pixels in Fig. 3F. Note that the “noise” we are concerned about here is not the speckle noise but the overall changes in reflectance that reduce the visible contrast in the difference and probability maps. Therefore, we took the mean modulation strength over the whole image, i.e., average of the amplitudes of the best-fitting sine waves over all horizontal (or vertical) slices, as a measure of the SNR of the image. In our illustrative example, the amplitude of the best-fitting sine for the probability values was 0.29 while that for the normalized differential activities was 0.17, thus indicating a SNR improvement of 71% for the ROC method for this slice of the image. Across all horizontal slices, the mean modulation value for the probability map was 0.23 while that for the difference map was 0.14, indicating that the ROC method gave a 66% improvement in SNR for this particular case. Across eight animals, the ROC method yielded an improvement in SNR ranging from 36 to 73% for orientations maps in both low and high resolution.
Reliability of the differential probability map
The preceding analyses showed that differential probability maps are, in some ways, more reliable than difference maps. But the reliability of the probability maps also needed to be investigated. Several factors, including the limited number of data points (∼20–30 points per distribution in our experiments, see methods) that were available for estimating the discrimination probability, could have contributed to errors in the estimated discrimination probabilities. Our first concern was that the random sample of limited size drawn from the true distributions (unknown) could have given rise to skewed probability estimates that were either above or below chance while the true distributions were such that the discrimination probability was near chance. This could have led to false positives for activation regions. We deal with this issue in this section. In the following sections, we address other concerns about the statistical reliability of the probability estimates.
We compared discrimination probabilities in dark and bright regions (shown circled in Fig. 4A) with the distribution of probability estimates for all the pixels in the differential probability map (Fig. 4B). The centers of activation regions had discrimination probabilities that were distinct from chance value (P < 0.001). But for the overall image, the distribution of the probability estimates tended toward a mean value of ∼0.5, i.e., near chance level. Our goal was to determine which of the estimated probability values in the map could have arisen by chance. Previous electrophysiological studies have successfully used a simple permutation test (Efron and Tibshirani 1998) to determine the significance of deviation of each estimate of the area under the ROC curve from chance level (Britten et al. 1996; Cook and Maunsell 2002; Purushothaman and Bradley 2005; Uka and DeAngelis 2004). We used two modified versions of this test to delimit reliably activated regions of the cortex.
FIG. 4.
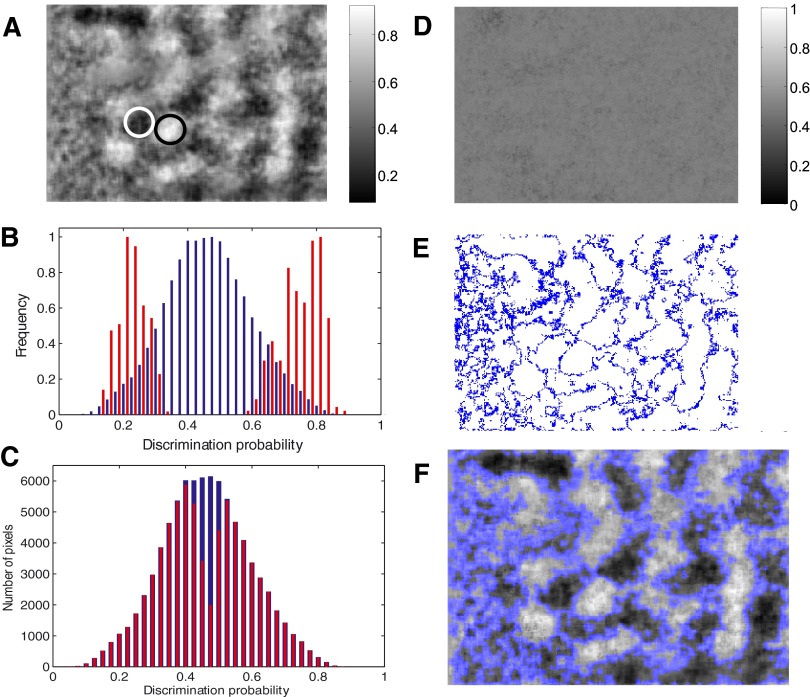
A permutation test for identifying statistically significant activation regions. A: sample domains for 90° (white circle) and 0° (black circle) are shown on the probability map. B: distributions of the probability values are shown for the 2 circled regions (red histograms) and for the whole image (blue histogram). C: probability estimates that were shown to be significantly different from chance by the permutation test are shown (red) against the distribution of probability estimates for the whole image (blue). D: a sample image of probability estimates for the permuted distributions shows all pixels having chance-level discrimination probability. E: pixels that did not pass the permutation test are shown in blue. F: superposition of image E on image A.
In the first test, the area under the ROC curve for the two reflectance distributions was first estimated for each pixel. Then the two distributions were permuted so that the reflectance values from both distributions were randomly but equally split into two new distributions. The area under the ROC curve for the pair of permuted distributions was then estimated. This computation was repeated 100 times, and a distribution of discrimination probabilities was obtained in the absence of any consistent relationship between the reflectance value and the stimuli presented. Each original estimate of the discrimination probability was tested to see if it was significantly different from the mean of the estimates obtained for the permuted distributions (Student's t-test, P < 0.01). Figure 4C shows the original probability estimates superimposed on those that passed this permutation test. A majority of these mean values were close to 0.5 (Fig. 4D). Figure 4E shows pixels with significant discrimination probability values in white and those that did not pass this permutation test in blue. When superimposed on the original differential probability map (Fig. 4F), this image shows statistically significant activation regions.
In the second test, we required the discrimination probability for each pixel to lie outside the central 95% of the distribution of the permuted estimates (Britten et al. 1996). Figure 5A shows that significantly fewer discrimination probability estimates were selected in this case compared with the previous test (Fig. 4C). Consequently, this test also selected fewer pixels as belonging to significant activation regions (Fig. 5, B and C).
FIG. 5.
A stringent permutation test. A: probability estimates that passed the permutation test (red) are shown superimposed on the distribution of estimates for the whole image (blue). B: pixels that did not pass the permutation test are shown in blue. C: superposition of B on Fig. 4A.
We compared the statistically significant activation regions detected by the permutation tests with the original difference map for the preceding example. In addition to low contrast regions at the margins of the activation domains and regions of nonspecific activity, some medium and high contrast regions in the difference map (Fig. 3A) were also excluded in Figs. 4F and 5C. As described in the preceding text, this is due to the fact that difference maps ignore the variance of the reflectance values and hence the intensity values represented in them are less reliable.
In sum, these two tests showed that the centers of activation regions have discrimination probabilities that are statistically significantly different from chance.
Reliably localizing activation domains in differential probability maps
It is of interest to accurately and reliably determine both the centers of activation domains as well as their edges. Reliable localization of activation domains will facilitate a better understanding of the relationship between different cortical feature maps as well as the consistent estimation of the number of distinct domains for a specific feature. The preceding analysis demonstrates that in differential probability maps, statistically reliable activation regions can be determined by assessing the significance of the deviation of the discrimination probability for each pixel from chance level. The results shown in Figs. 4 and 5 demonstrate that these two permutation tests can be used to limit inferences about a cortical feature map to the statistically significant regions of the differential probability map.
Relationship of differential probability maps to difference maps
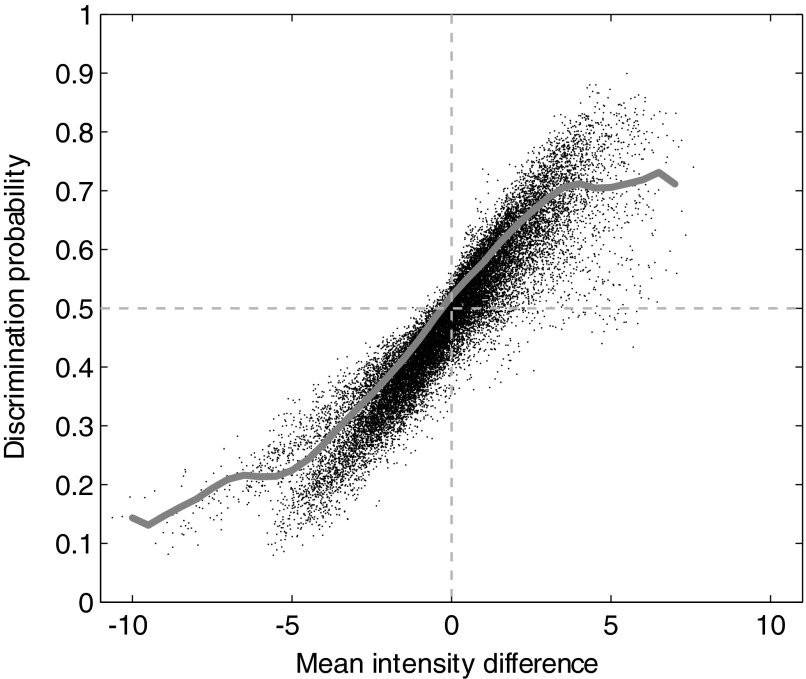
To understand the relationship between difference maps and differential probability maps, we studied the statistical relationship between the difference in mean reflectance and the discrimination probability on a pixel-by-pixel basis. We plotted these two values for the whole image and computed a moving average of 50 adjacent points (Fig. 6). In the middle range, the relationship between the difference in the mean reflectance and the discrimination probability trends toward linearity on the average. There are two abrupt deviations away from linearity at the two extremes, one near a discrimination probability value of ∼0.7 and the other near 0.2. This overall relationship between the difference in mean reflectance and discrimination probability, with the linear region and the deviations from linearity at the extremes, is very similar to the relationship reported between choice probability and the ratio of firing rates for single neurons (Britten et al. 1996). “Choice probability” is estimated as the area under the ROC curve for a pair of firing rate histograms and hence bears a computational relationship to the discrimination probability shown in Fig. 6. Therefore the deviations from linearity seen at the extreme values are not specific to our data or our analysis but may, in fact, reflect the functional relationship between the area under the ROC curve and the difference or ratio of the mean values of the distributions for which the ROC curve is computed.
FIG. 6.
Relationship of discrimination probability to activity difference For each pixel, the difference of the means of the 2 reflectance distributions is shown plotted against the area under the ROC curve for the distribution pair. The moving average (gray line) shows the relationship to be linear only around chance-level discrimination probability (0.5).
Figure 6 also shows that the difference between the mean reflectance values for the two orientations at a pixel is not a consistently good predictor of the discrimination probability for that pixel. In fact, a fairly wide range of values for the difference in mean can yield the same discrimination probability and vice versa. The greater the magnitude of the difference in the mean values, the more likely it is for the corresponding discrimination probability to be variable. This suggests that large deviations in intensity values in the difference map are more likely to be unreliable.
Properties of discrimination probability estimator: consistency, bias, and efficiency
In this section, we examine how sample size influences the estimation of discrimination probability. It is of practical interest to know how the variance of the probability estimate behaves as a function of number of trials (consistency), whether using a small number of trials will bias the probability estimate (bias), and whether better estimation procedures will reduce the number of trials required to achieve an accurate estimate (efficiency). We deal with these three issues in this section.
For consistent estimators, the mean-squared estimation error reduces to zero for a large number of observations (Lehmann 1998). It can be shown from first principles that the traditional estimator for the area under the ROC curve (described in methods) is consistent (see Lehmann 1998; Van Trees 1966). Therefore differential probability maps computed from a large number of reflectance values can be expected to have small estimation errors. But for practical purposes, it is useful to know how the estimation error behaves as a function of trial number within a practical range. To perform these calculations, we randomly drew subsets of 5, 10, 15, 20, and 25 values from the 30 measured reflectance values. For each sample size, we computed the variance of the probability estimates from 100 repeated estimations of the discrimination probability. Both for pixels in activation regions (with mean discrimination probability estimates close to 0 or 1) as well as for those lying along the borders, variance steeply decreased as the sample size increased to 15 and thereafter reached an asymptote around 25 trials (Fig. 7A). Note that discrimination probability is a random variable (see following text) whose variance is lowest near 0 and 1 and highest near 0.5. Because the variance of the estimator of this random variable reaches an asymptote around 25 trials for pixels with discrimination probabilities near 0 and 1 as well as for pixels with probability near 0.5, these results suggest that regardless of what type of feature map is being measured, it suffices to use ∼20–25 outlier-filtered images per stimulus condition to compute a differential probability map.
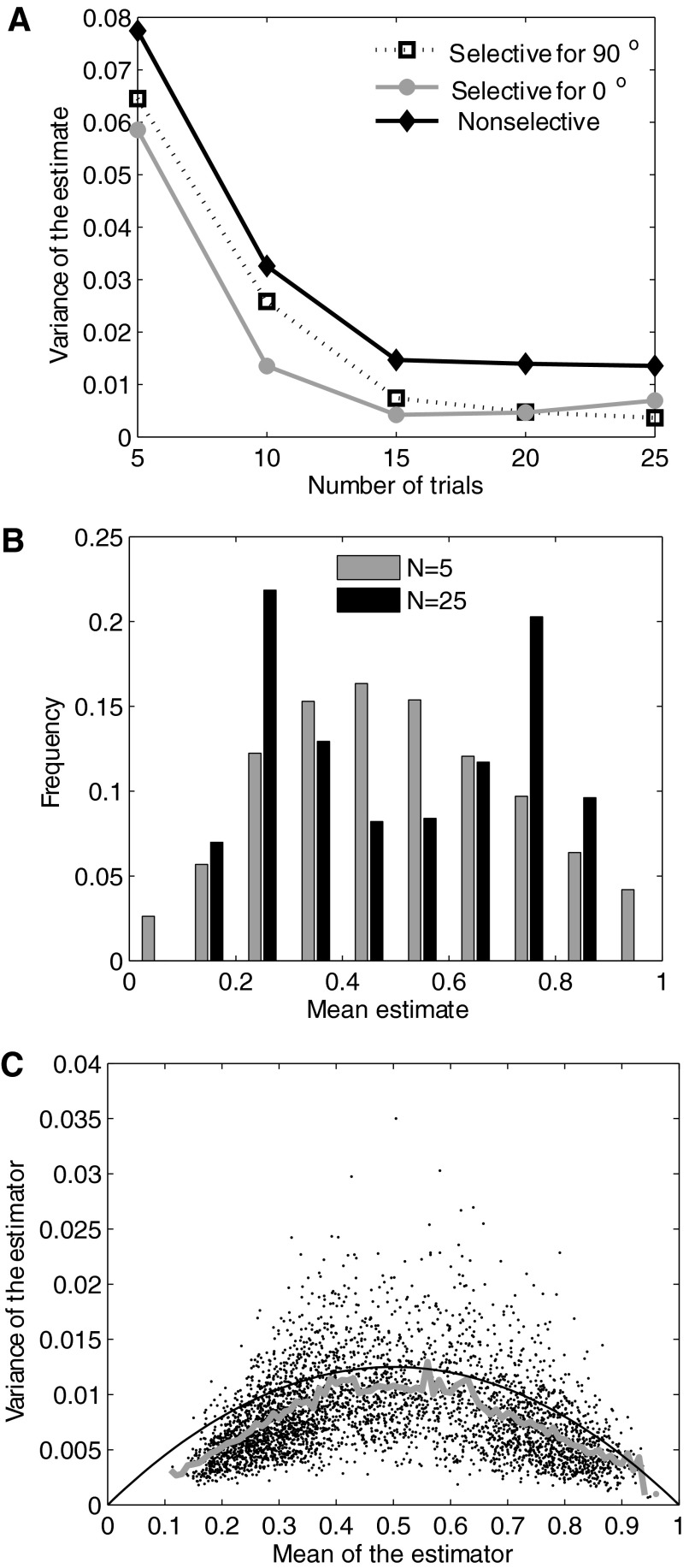
FIG. 7.
Consistency, asymptotic bias, and efficiency of the probability estimator. A: variance of the estimate is plotted against the number of trials for a pixel inside an activation domain for 0° (gray line), for 90° (broken line), and for a pixel not selective for either orientation (black line). Each point represents the variance computed from 100 repeated estimates of the probability using random sampling without replacement of the corresponding number of pairs of reflectance values. B: distribution of the mean probability estimates from the same simulations are shown for 5 and 25 pairs of reflectance values. C: relationship between mean and variance of the probability estimates. The moving average (gray line) is close to the variance of the maximum likelihood estimator for the probability value.
For unbiased estimators, the expected value equals the true value of the estimate and for asymptotically unbiased estimators, the bias (difference between expected and true values) decreases to zero for large sample sizes. We wished to examine if estimates of discrimination probability for optical signals obtained using the ROC method show a bias when the number of trials is small. Figure 7B compares the distribution of probability estimates obtained from 5 and 25 trials, using the Monte Carlo sampling described in the preceding text. Estimates are shown for all pixels in a representative region covering one activation domain for 0° orientation, one for 90°, and the border between the two domains. For the smaller number of trials (5), the distribution tended toward a mean value of 0.5 (chance). As the number of trials increased, the distribution became distinctly bimodal, with one peak near 0.25 representing the activation region for 90° and one near 0.75 representing the 0° domain. Again this trend was observed to hold across several stimulus conditions and animals. Therefore, as the variance of the estimate decreases to a small value for a sample size of ∼20–25, the mean of the estimate also significantly deviates away from chance and approaches the true value. This further supports the thumb rule that 20–25 outlier-filtered images per stimulus condition should suffice to compute a reliable differential probability map.
Estimation accuracy depends both on the number of trials and the estimation method. We wished to examine if an alternative method of estimating discrimination probabilities could achieve the low variance seen in Fig. 7A for 20–25 trials in even fewer trials. An efficient estimator has the lowest variance among all unbiased estimators and, under some conditions, the maximum likelihood (ML) estimator is the efficient estimator (Lehmann 1998; Van Trees 1966). Therefore, a comparison of the variance of the discrimination probability estimates obtained in our study with the variance of the ML estimator for the discrimination probability will show how close our estimation method is to the optimal method. We performed this comparison by computing the variance of the ML estimator as follows. Suppose we have N intensity values corresponding to one stimulus condition and N values to another. These values can be collected into two histograms, and the ROC method can be used to estimate the discrimination probability. Alternatively, one randomly drawn value from the first distribution can be compared with a randomly drawn value from the second. If the first value is greater than the second, then an outcome of 1 can be assigned. If the second value is the greater, then an outcome of 0 can be assigned. This can be repeated N times for each of the N pairs of intensity values drawn without replacement. Thus we have N independent and identically distributed Bernoulli random variables X1, X2,…, XN, each with success (i.e., Xi = 1) probability p equal to the true discrimination probability for the pixel. The ML estimator of p given these N Bernoulli variables is the ML estimator of p in the binomial distribution 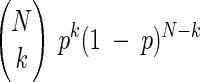 where N is the total number of trials and k is the number of “successes” (i.e., outcomes of 1). This ML estimator is given by p̂=k/N=1/N
where N is the total number of trials and k is the number of “successes” (i.e., outcomes of 1). This ML estimator is given by p̂=k/N=1/N
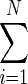 Xi (e.g., see Lehmann 1998; Van Trees 1966). The variance of X̂ is p(1−p)/N. Figure 7C shows a comparison of this variance (continuous black line) with the variance for the discrimination probability estimates obtained from the ROC method with 20 trial images (thick gray line). There is a close match between the two variances at a majority of the mean values of the estimator (plotted on the x axis). This shows that our method of estimating discrimination probabilities for optical images is near-optimal. In other words, no other unbiased estimation procedure can obtain the same estimation accuracy with substantially fewer trials.
Xi (e.g., see Lehmann 1998; Van Trees 1966). The variance of X̂ is p(1−p)/N. Figure 7C shows a comparison of this variance (continuous black line) with the variance for the discrimination probability estimates obtained from the ROC method with 20 trial images (thick gray line). There is a close match between the two variances at a majority of the mean values of the estimator (plotted on the x axis). This shows that our method of estimating discrimination probabilities for optical images is near-optimal. In other words, no other unbiased estimation procedure can obtain the same estimation accuracy with substantially fewer trials.
Differential probability maps for unstimulated cortical regions
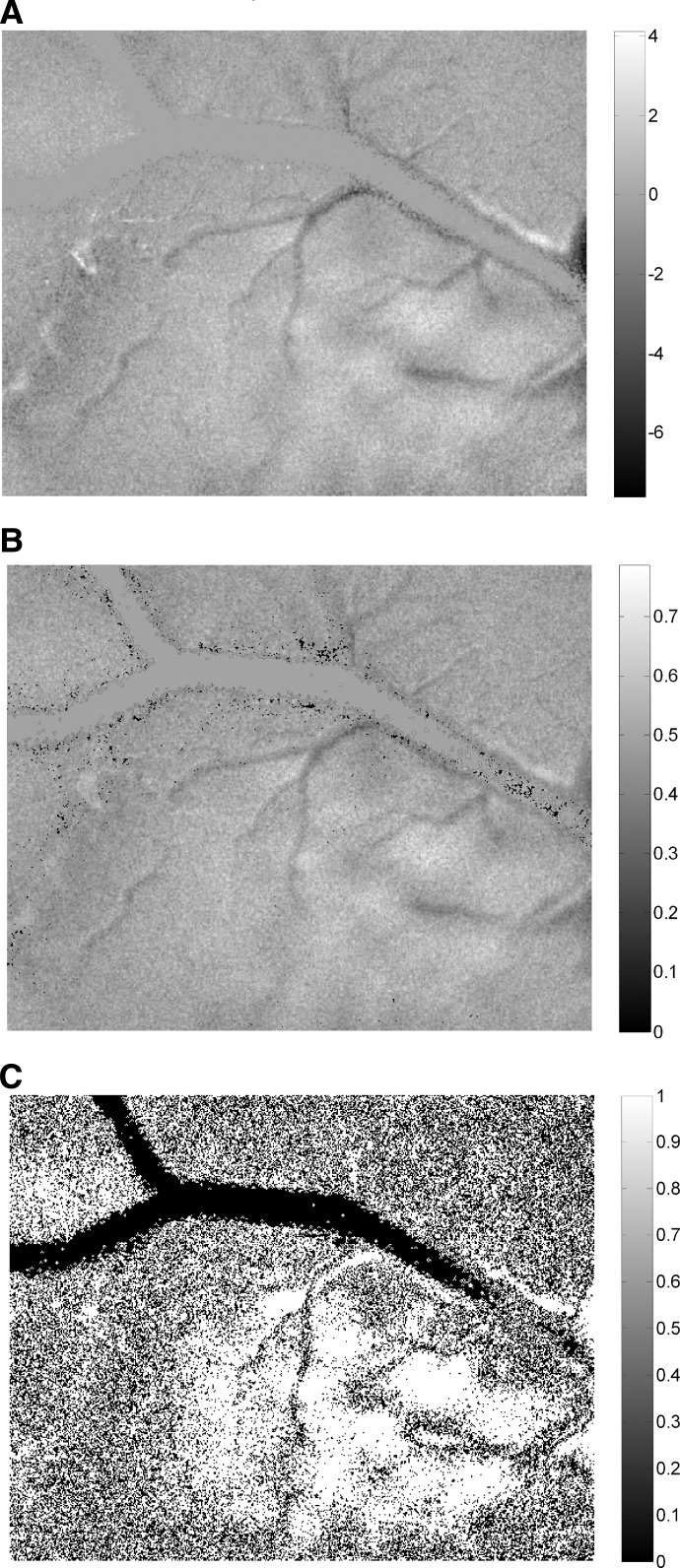
In previous analyses, we examined false positives that occur due to random fluctuations in the optical signal when the cortex is activated by a stimulus. These fluctuations were shown to result in corresponding fluctuations in differential activity and, to a much less extent, in discrimination probability. It was also shown that rigorous statistical analysis can be used to identify false positives in discrimination probability maps. But false positives can also occur in unstimulated regions due to photon scatter, the point spread function of the imaging system, and fluctuations in the physiology of the animal. Therefore, we also tested for false deviations of discrimination probability from chance value that could occur in the absence of sensory stimulation. Instead of full screen stimuli used in the previous experiments, we presented drifting sine and square wave gratings within a 20° circular window centered on the AC. Optically imaged cortical response for such spatially localized stimuli typically shows a region of spiking activity surrounded by a region of subthreshold activation (Das and Gilbert 1995). We therefore imaged a large extent of the cortex to include unstimulated regions beyond the subthreshold surround. Responses were obtained for different orientations presented within this small window. This allowed us to compare results for unstimulated and stimulated regions of the cortex simultaneously while keeping all other nonstimulus dependent factors the same. A comparison of difference map (Fig. 8A) with differential probability map (B) showed activation domains within and around the stimulated region of the cortex and no visually discernible net activity in the unstimulated regions. The permutation test (Fig. 8C) confirmed the presence of a contiguous region of significant net activity in the stimulated part of the cortex but very little net significant activity outside this region. Therefore, systematic changes in noise levels outside of the stimulation region of the cortex influence estimates of discrimination probabilities to a significantly lesser extent than systematic variations of the signal + noise levels inside the stimulated region.
FIG. 8.
Effect of systematic noise in unstimulated regions on probability estimates. A: differential activity map. B: differential probability map. C: results of permutation test showing regions with statistically significant activity (white).
Differential probability maps for spatial frequency
After detailed examination of differential probability maps and errors associated with them, we applied this technique to study the organization of spatiotemporal frequency preference on the cortical surface. First, we consider spatial frequency maps. Previous investigations of spatial frequency maps of the cortex engendered divergent conclusions as discussed in introduction. It remains unclear if spatial frequency has a continuous representation in the form of multiple domains (Everson et al. 1998; Issa et al. 2000; Xu et al. 2007) or a discrete representation in the form of two distinct spatiotemporal frequency domains (Bonhoeffer et al. 1995; Hubener et al. 1997; Shoham et al. 1997; Sirovich and Uglesich 2004). We studied the organization of spatial frequency preference using differential probability maps and compared the results with those obtained for difference maps.
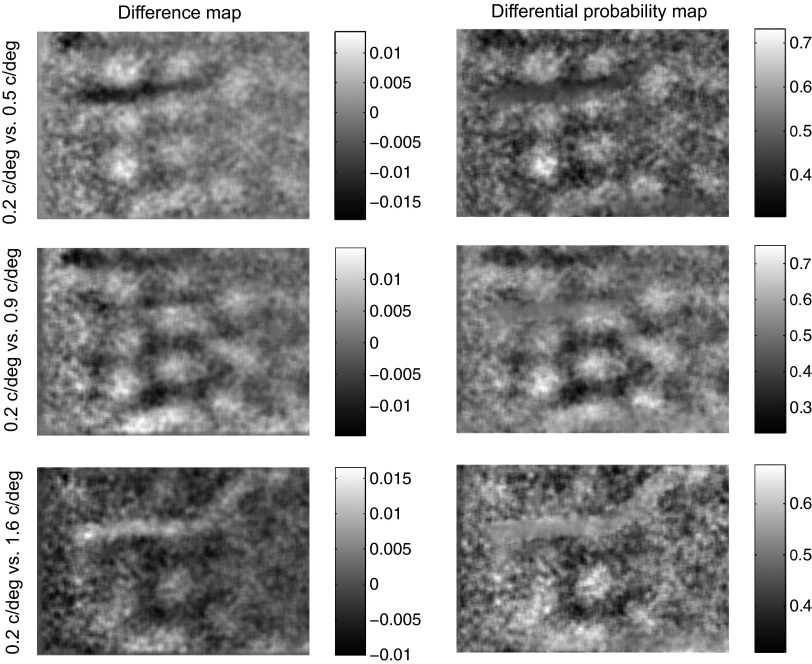
The reflectance of the cortex was imaged for sine wave gratings at four orientations (0, 45, 90, and 135°) and four spatial frequencies (0.2, 0.5, 0.9, and 1.6 cycle/deg) at a single temporal frequency (2 Hz). Reflectance values for each pixel were grouped into two histograms corresponding to two spatial frequencies (e.g., 0.2 vs. 0.5 cycle/deg) regardless of orientation. The difference between the means of these two histograms was represented in the difference map while the area under the ROC curve for this pair of histograms was represented in the differential probability map. The probability with which reflectance for the spatial frequency of 0.2 cycle/deg was lower than the reflectance for other spatial frequencies is represented in Fig. 9. Similar maps for 0.9 and 1.6 cycle/deg are shown in Supplementary Figs. S1 and S2,1 respectively. Corresponding difference maps are shown for comparison in all three figures (contrasts are reversed to facilitate comparison). Overall, the SNR for the differential probability maps was noticeably better than that for difference maps (compare the contrasts of left and right column images in Fig. 9 and Supplementary Figs. S1 and S2). This difference was more pronounced when the SNR in the difference map was low (e.g., last row of Supplementary Fig. S1 and first and last rows of Supplementary Fig. S2).
FIG. 9.
Spatial frequency domains Differential activity maps (left) and probability maps (right) are shown for the test spatial frequency of 0.2 cycle/deg.
Figure 9 shows distinct domains of high (>0.5) and low (<0.5) discrimination probabilities corresponding to pixels where the reflectance was predictably lower and higher, respectively, for 0.2 cycle/deg than for 0.5, 0.9, or 1.6 cycle/deg. The locations of high probability domains were largely independent of the three comparison spatial frequencies (0.5, 0.9, or 1.6 cycle/deg), indicating that the same group of neurons responded better to 0.2 cycle/deg than to any of the other three spatial frequencies at all orientations tested. This suggested that in the imaged part of the visual cortex, neurons were grouped by their preference for the spatial frequency of 0.2 cycle/deg. Similar results were obtained for spatial frequencies of 0.5, 0.9, and 1.6 cycle/deg as well (Supplementary Figs. S1 and S2). Thus the results shown in Fig. 9 and Supplementary Figs. S1 and S2 indicate that neurons that prefer each of these spatial frequencies are likely grouped together on the cortical surface.
These figures also show that there are domains of high discrimination probability for all 6 distinct pairs of these four spatial frequencies. This indicates that the activity patterns produced by each spatial frequency differs from that produced by each of the other spatial frequencies either in spatial distribution or in magnitude or both. If this difference is only in magnitude but not in spatial distribution, then the domains will be at the same spatial locations in all differential probability maps. If the difference in the activity patterns is spatial, then we should see systematic changes in the locations of the high discrimination probability domains. A comparison of Fig. 9 and Supplementary Figs. S1 and S2 shows that there are differences in the locations of these domains, but it is not clear if the domains shift progressively with changing spatial frequency or if there is an abrupt shift in the location as the spatial frequency changes from a low to a high value. Therefore, while the pair wise comparisons facilitated by differential probability maps informed us that there is clustered organization of neurons, they did not clearly indicate if all the 4 tested spatial frequencies had distinct spatial representations. We investigated this issue by computing a composite spatial frequency preference map using ROC analysis, as described in the next section.
Spatial frequency preference map from ROC analysis
How should spatial frequency preference be assigned to a pixel based on its reflectance values? Functional preference maps are often created using a winner-take-all approach in which the stimulus feature which yields the lowest mean reflectance at a pixel is designated as the “preferred feature” of the pixel. As mentioned in the preceding text, this method ignores the variances. A better approach is to assign to each pixel the spatial frequency that can be best discriminated from all other spatial frequencies based on the reflectance values at the pixel. Such an approach, based on discrimination probabilities computed as the area under the ROC curve, would naturally take into account both the mean and the variance of the reflectance distributions.
We used this approach to create a composite spatial frequency map. To assign a spatial frequency preference to a pixel, we required the discrimination of this spatial frequency from all other spatial frequencies to be consistently better than chance. Thus, the spatial frequency represented at a pixel would indicate that the group of neurons at this location had a strong preference for this spatial frequency over all others. The resultant map is shown along with the traditional winner-take-all map in Fig. 10. Both maps were computed from 25 trials for each spatial frequency. Although there is no single objective criterion for comparing these two maps, it is obvious that the spatial frequency preference assignments in the winner-take-all map are quite noisy, resulting in more fragmented domains than those seen in the composite discriminability map. Another notable difference is that the latter map shows more domains for the highest spatial frequency tested (1.6 cycle/deg). As mentioned in the preceding text, the overall activity was lowest at this frequency. Therefore, when normalized to the baseline (or blank screen image, see methods), the reflectance changes due to the other spatial frequencies will dominate the winner-take-all computation. Because the ROC calculations offer better SNR (as demonstrated in the preceding text), the composite discriminability map shows domains even for high spatial frequencies (that evoke overall poor responses). Finally, we note from the composite discriminability map that spatially distinct domains exist for all four spatial frequencies tested.
FIG. 10.
Spatial frequency selectivity map for 2 patches of area V1. A winner-take-all map computed from the mean activities (left) is shown for comparison with a composite spatial frequency selectivity map computed from discrimination probabilities (right).
Differential probability maps for temporal frequency
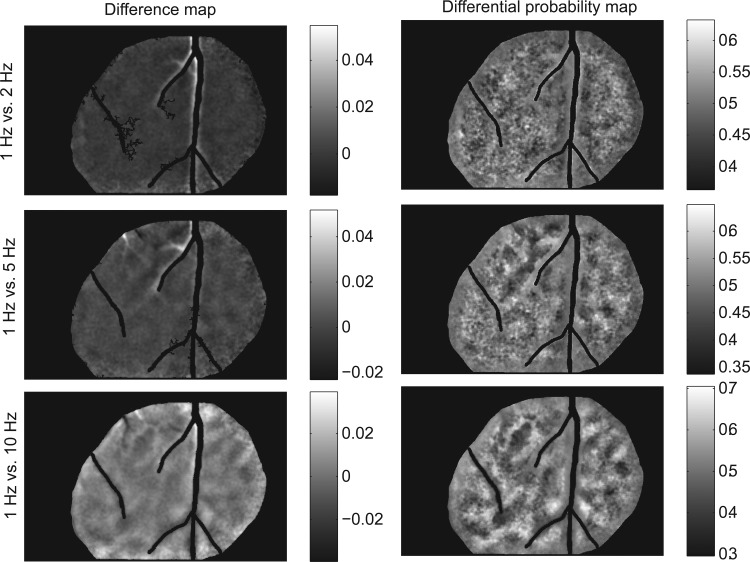
We performed the same analyses as above for temporal frequency as well. Cortical reflectance was imaged for four orientations (0, 45, 90, and 135°) and four temporal frequencies (1, 2, 5, and 10 Hz) at a single spatial frequency (0.5 cycle/deg). Differential probability maps are shown for all pair wise combinations of temporal frequencies in Fig. 11 and Supplementary Figs. S3–S5. These maps show that at the two lowest temporal frequencies tested (1 and 2 Hz), there are no clear domains. But at the highest frequency tested (10 Hz), elongated regions of high discrimination probability can be seen at all comparison frequencies indicating that neurons are likely grouped by their preference for a temporal frequency of 10 Hz. At the intermediate frequency of 5 Hz, stable domains can only be seen at the comparison frequencies of 2 and 10 Hz but not for 1 Hz. These observations are also supported by the composite temporal frequency discriminability map shown in Fig. 12 where domain expression is strongest at 10 Hz.
FIG. 11.
Temporal frequency domains Differential activity maps (left) and probability maps (right) are shown for the test temporal frequency of 1 Hz.
FIG. 12.
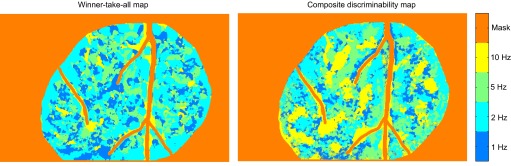
Temporal frequency selectivity map. A winner-take-all map computed from the mean activities (left) is shown for comparison with a composite temporal frequency selectivity map computed from discrimination probabilities.
The TF data described above were collected at the fairly low spatial frequency of 0.5 cycle/deg and with square wave gratings (to elicit better reflectance responses). Even though at the duty cycle we used, higher-order harmonics had low power compared with the fundamental (see methods), to be certain that these results were not an artifact of the stimulus pattern, we also imaged responses for sinusoidal gratings at the same range of spatiotemporal frequencies for a pair of orthogonal orientations. ROC maps for this case as well as four additional cases (imaged as described in the previous paragraph, with square wave gratings) are shown in Supplementary Fig. S6. Overall, all six cases showed the same pattern of results: only at the highest temporal frequencies studied (5 and 10 Hz), domains of TF preference could be prominently seen.
DISCUSSION
Summary
We applied signal detection theory to analyze optical images of intrinsic cortical signals and to derive inferences about the functional organization of the primary visual cortex. We represented optical images as differential probability maps and compared them to the conventional differential activity maps. We found that probability maps have better SNR and fewer false alarms. Probability maps required fewer trials to reach a prescribed SNR level than difference maps. Estimation errors for discrimination probability, such as the variance of the estimate and bias, reduced as the sample size increased and reached an asymptote as the number of trials approached ∼25. We also found that for a given number of trials, the variance of the probability estimates computed using ROC method approached the variance of maximum likelihood estimator for the discrimination probability, thus showing that the ROC method is near-optimal for computing probability maps.
Using permutation tests, we confirmed that domain representation seen in the probability maps was not an artifact of random or limited sample size. We also tested and confirmed that systematic noise in the intrinsic signals from unstimulated regions of the cortex adjacent to stimulated regions cannot give rise to discrimination probability values significantly different from chance. Based on all of these observations we suggest that probability maps offer an efficient, rigorous, and robust method of analyzing optical images and drawing inferences from them.
ROC analysis of single-neuron and optical imaging data
ROC analysis has been successfully used for many decades to analyze single-neuron data, particularly to quantify neural performance on the same scale used to describe behavioral performance in psychophysical studies (e.g., Barlow et al. 1971). But few optical imaging studies have used ROC analysis to quantify and interpret cortical responses in the manner of these electrophysiological studies. We should carefully note that ours is not the first study to apply ROC analysis to optical images. Chen et al. (2006) used the ROC method to delineate a region of interest (ROI), i.e., a large patch of cortex that showed a significant reflectance change when stimuli were presented. ROC analysis was not used in that study to identify and quantify individual activation domains within the activated cortex. In this study, we have used ROC analysis on a pixel-by-pixel basis to represent discrimination probabilities in lieu of the difference between mean activity levels.
Comparison with other methods
REPRESENTATION VERSUS ANALYSIS.
It is important to distinguish representation of image information from the analysis of image data (Rosenfeld and Kak 1982). Our main aim is to represent processed optical imaging data, in a physiologically and behaviorally meaningful manner, on an objective scale. Discrimination probabilities estimated from the ROC analysis admit a direct, physiologically and behaviorally relevant interpretation: they denote the amount of information available to the experimenter, and perhaps the animal itself, to make meaningful discriminations between the stimuli concerned. Representing probability values also constrains experimenters to use a common scale (between 0 and 1) that cannot be arbitrarily transformed.
A number of analyses methods are available for filtering noise, improving contrast, restoring, and deblurring image data (Gonzalez and Woods 2002; Pratt 1991; Rosenfeld and Kak 1982). We should note that our method, which is primarily a method for the objective representation of the end result, is not intended as a substitute for any of these data-analyses methods. In fact, the ROC method can be applied to the end result of other filtering, noise reduction, and contrast enhancing techniques. Several studies have directly evaluated the performance of different filtering and classification techniques on optical images (e.g., Sornborger et al. 2003; Yokoo et al. 2001), and these studies can be used to guide the choice of appropriate preprocessing of the data to be used in the ROC analysis.
SUPPORT VECTOR MACHINES CLASSIFICATION.
Recently, Xiao et al. (2008) have used support vector machines (SVM), a form of nonparametric multivariate classification algorithm (Cristianini and Shawe-Taylor 2000), to estimate orientation preference information in optical images. This method uses SVM to estimate the relative contribution made by each pixel to a binary orientation classification problem that is analogous to the orientation discrimination procedure used to generate each probability map in our study. The root-sum-square of these relative contributions taken over all possible binary classifiers for the number of stimulus conditions used in the experiment is then represented as the orientation preference of the pixel. The authors have shown that this method is effective in suppressing blood vessel artifacts.
This method is in some ways comparable to ours. Procedurally, both methods tackle the multiple hypotheses testing problem (associating each pixel with more than two stimulus conditions) by addressing it as a series of binary hypothesis tests. The end result is a number that is constrained within the interval [0 1] in both cases. This happens naturally in our case because we are representing probability values. In the case of Xiao et al. (2008), the normalization of the weight vectors (orthonormals to the decision lines) results in the final result being confined in this interval.
We implemented this procedure on our data. A comparison of results is presented in Supplementary Fig. S7. We used 25 single-condition images because the variance of the probability estimator reaches an asymptote at this value (Fig. 7A). Under this condition, our implementation of the SVM method showed poor performance compared with the ROC method. Blood vessel artifacts were more prominent in the SVM than in the ROC results. Note that Xiao et al. (2008) used 50 trials in their analysis. We expect the SVM method to perform better and to suppress blood vessel artifacts more completely as the number of single condition images used in the analysis approaches 50.
STUDENT'S t-TEST.
Sometimes, particularly in the fMRI literature, paired Student's t-tests are used on a pixel-by-pixel basis to determine regions of significant activity. A correct application of the t-test (or a nonparametric variation of it, with α-correction for multiple comparisons) can only yield a binary result: accept or reject the null hypothesis depending on whether the P value is below or above the significance level. This will generate a map of binary decisions, i.e., a map consisting only of two (not necessarily contiguous) regions but not a map of probability values that continuously vary between 0 and 1. However, some authors have resorted to using the P value as a measure of the weight of evidence for rejecting the null hypothesis. In this informal approach, maps of P values are used to interpret different regions as “more” or “less” associated with a particular test condition. While this method seems to be popular in the fMRI literature, it is not a recommended way of interpreting P values (see for e.g., Schervish 1996).
Computational burden
Applying ROC analysis on a pixel-by-pixel basis over a large image could be computationally burdensome. It is possible that this concern has been an impediment to a more widespread use of signal-detection methods and tools in optical imaging studies. Although we did not comprehensively capture and analyze computational metrics for the ROC analyses presented in this paper, we tracked CPU time required to compute the area under the ROC curve for all pixels in the 480 × 744 images offered by our optical imager (see methods for details of instrumentation used in our experiments). This analysis was performed using Matlab (ver. R2006a, The Mathworks) on an IBM-compatible PC with two Intel Pentium (4) 3.2-GHz processors. The operations performed for each pixel were as follows: loading two arrays of 25 reflectance values each (for the two stimulus conditions), computing the ROC curve, and estimating the area under this ROC curve using the simple trapezoidal approximation. The mean CPU time for performing these operations on a 480 × 744 image was 182 CPU seconds. The total real-time for loading, analyzing, coloring and plotting the images was ∼4–6 min. Therefore ROC analysis can be performed on optical images in a short time and the computations involved are not prohibitively long or intensive.
Organization of spatial and temporal frequency preferences
We used differential probability maps to understand the organization of spatiotemporal frequency preference in the primary visual cortex of the prosmian primate bush baby. Our main results can be summarized as follows: At the temporal frequency of 2 Hz, we found evidence for distinct activation domains for the entire range of spatial frequencies tested (0.2, 0.5, 0.9, and 1.6 cycle/deg). At the spatial frequency of 0.5 cycle/deg, we found evidence for distinct domains only at the highest temporal frequencies tested (5 and 10 Hz). We did not test for temporal frequency domains at other spatial frequencies.
Previous electrophysiological studies showed that spatial and temporal frequency preferences of V1 neurons in the bush baby are distributed within these ranges and that the mean values of these distributions are ∼0.5 cycle/deg and 2 Hz, respectively (Bonds et al. 1987; De Bruyn et al. 1993). Hence the absence of distinct domains at low temporal frequencies (at our test spatial frequency) is unlikely to be due to sampling issues alone. We therefore infer that over the range of behaviorally relevant spatiotemporal frequencies, domains are more numerous and distinct for spatial frequency preference than for temporal frequency preference. Broadly speaking, these results indicate a tighter clustering of neurons by their spatial frequency preference than by their temporal frequency preference. A similar result was obtained by De Angelis et al. (1999) in cats when the spatial and temporal frequency preferences of nearby pairs of V1 neurons were extracted from the spatiotemporal maps of their receptive fields.
Comparison with other studies
At the temporal frequency of 2 Hz, Shoham et al. (1997) found evidence for two distinct sets of spatial frequency domains, one at the lower range of spatial frequencies they tested (0.1–0.2 cycle/deg) and the other at the higher range (0.6–1.0 cycle/deg). They found very poor activation for the intermediate frequency of 0.4 cycle/deg (at 2 Hz). This was taken as evidence for the existence of only two distinct sets of spatial frequency domains. In contrast to this study and in agreement with Everson et al. (1998), Issa et al. (2000), and Xu et al. (2007), we found that multiple spatial frequencies in the range from 0.1 to 1.2 cycle/deg are represented continuously in distinct domains, at the temporal frequency of 2 Hz. Earlier, Bonhoeffer et al. (1995) found distinct domains at the spatial frequencies of 0.5 and 0.15 cycle/deg, also at the temporal frequency of 2 Hz (Fig. 14 in their paper). Unlike Shoham et al. (1997), Bonhoeffer et al. (1995) did not present activation maps. So it is not clear whether domain representation was confined to 0.15 cycle/deg with poor activation for 0.5 cycle/deg as reported by Shoham et al. (1997) or if both of these spatial frequencies had distinct domains, as found in the current study as well as by Everson et al. (1998), Issa et al. (2000), and Xu et al. (2007). Finally, Sirovich and Uglesich (2004) used an entirely different approach, singular value decomposition, and arrived at a conclusion similar to that of Shoham et al. (1997). Thus while cognizant of the fact that different species were used in the studies discussed in the preceding text, we can conclude that there is no disagreement between any of these studies about the existence of distinct spatial frequency domains at the lowest (0.03–0.2 cycle/deg) and the highest (0.6–2.28 cycle/deg) end of the spatial frequency range used in these studies. There is however disagreement over the intermediate range (∼0.4 cycle/deg). We would like to suggest that the results of a sensitive method like the ROC analysis must be given more weight in resolving this issue because, as shown in detail in this paper, this method offers significantly higher SNR over other methods for detecting domains.
At the spatial frequency of 0.5 cycle/deg, we found evidence for distinct domains at the highest range of temporal frequencies we tested (5–10 Hz) but not at the lowest frequencies tested (1 and 2 Hz). We did not test at other spatial frequencies. Although comparable studies in cats have not been published, it is possible to gain a more complete picture of temporal frequency domains by looking at relevant pieces of information in other studies of spatial frequency domains. For example, Shoham et al. (1997) computed a difference map at 0.6 cycles/deg (close to the 0.5 cycle/deg spatial frequency used in our study) between the temporal frequencies of 0.66 and 2 Hz. This map showed no domains (Fig. 3F in their paper). Despite the species difference, this result is entirely in agreement with our result that there are no domains at the lower end of the temporal frequency range (1–2 Hz) at the spatial frequency of 0.5 cycle/deg. Remarkably, at the spatial frequency of 0.2 cycle/deg, Shoham et al. (1997) did find distinct domains in the difference map computed for the same two temporal frequencies. Therefore it remains to be seen whether and how the pattern of temporal frequency domains changes with spatial frequency in primates. It is intriguing that these two studies found domains for a speed range of 4–20deg/s and both also found that no domains existed for the speed range of 1.6–4°/s despite differences in the experimental conditions and species used. But without additional direct evidence, we cannot conclude that it is speed and not spatiotemporal frequency that is represented as domains in V1.
Khaytin et al. (2008) investigated temporal frequency preference of cortical activity in primate V1 using a large stimulus set and a rich repertoire of clustering analyses on differential activity maps and concluded that local clustering by temporal frequency preference was absent. The current study includes the data analyzed by Khaytin et al. (2008) but has applied ROC analysis instead of relying on differential activity maps. As can be seen from Fig. 11 and Supplementary Figs. S3—S5 (also see Supplementary Fig. S6), the two methods produce different results, with the probability maps exhibiting slightly greater SNR and hence more clearly visible domains at higher temporal frequencies.
In summary, our results show that clustering by orientation preference is stronger than that by spatial frequency preference and clustering by temporal frequency preference is the weakest. The use of a sensitive and rigorous quantitative method will facilitate a better understanding of the overall functional architecture of the primary visual cortex through optical imaging.
GRANTS
This work was supported by National Institutes of Health Grants EY-01778, EY-08126, RR-13947, MH 64913, and HD-15052.
Supplementary Material
Acknowledgments
We thank J. Mavity-Hudson for technical assistance and Dr. Youping Xiao for clarifications on implementing the SVM method.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Barlow et al. 1971.Barlow HB, Levick WR, Yoon M. Responses to single quanta of light in retinal ganglion cells of the cat. Vision Res Suppl 3: 87–101, 1971. [DOI] [PubMed] [Google Scholar]
- Bartfeld and Grinvald 1992.Bartfeld E, Grinvald A. Relationships between orientation-preference pinwheels, cytochrome oxidase blobs, and ocular-dominance columns in primate striate cortex. Proc Natl Acad Sci USA 89: 11905–11909, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basole et al. 2003.Basole A, White LE, Fitzpatrick D. Mapping multiple features in the population response of visual cortex. Nature 423: 986–990, 2003. [DOI] [PubMed] [Google Scholar]
- Blasdel 1992a.Blasdel GG Differential imaging of ocular dominance and orientation selectivity in monkey striate cortex. J Neurosci 12: 3115–3138, 1992a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel 1992b.Blasdel GG Orientation selectivity, preference, and continuity in monkey striate cortex. J Neurosci 12: 3139–3161, 1992b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel and Salama 1986.Blasdel GG, Salama G. Voltage-sensitive dyes reveal a modular organization in monkey striate cortex. Nature 321: 579–585, 1986. [DOI] [PubMed] [Google Scholar]
- Bonds et al. 1987.Bonds AB, Casagrande VA, Norton TT, DeBruyn EJ. Visual resolution and sensitivity in a nocturnal primate (galago) measured with visual evoked potentials. Vision Res 27: 845–857, 1987. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer and Grinvald 1991.Bonhoeffer T, Grinvald A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature 353: 429–431, 1991. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer and Grinvald 1996.Bonhoeffer T and Grinvald A. Optical imaging based on intrinsic signals: the methodology. In: Brain Mapping: The Methods, edited by Toga A, Maziotta JC. Orlando, FL: Academic, 1996, p. 55–97.
- Bonhoeffer et al. 1995.Bonhoeffer T, Kim DS, Malonek D, Shoham D, Grinvald A. Optical imaging of the layout of functional domains in area 17 and across the area 17/18 border in cat visual cortex. Eur J Neurosci 7: 1973–1988, 1995. [DOI] [PubMed] [Google Scholar]
- Bosking et al. 1997.Bosking WH, Zhang Y, Schofield B, Fitzpatrick D. Orientation selectivity and the arrangement of horizontal connections in tree shrew striate cortex. J Neurosci 17: 2112–2127, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitenberg and Braitenberg 1979.Braitenberg V, Braitenberg C. Geometry of orientation columns in the visual cortex. Biol Cybern 33: 179–186, 1979. [DOI] [PubMed] [Google Scholar]
- Britten et al. 1996.Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci 13: 87–100, 1996. [DOI] [PubMed] [Google Scholar]
- Chen et al. 2006.Chen Y, Geisler WS, Seidemann E. Optimal decoding of correlated neural population responses in the primate visual cortex. Nat Neurosci 9: 1412–1420, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman and Li 1996.Coleman TF, Li YY. An interior trust region approach for nonlinear minimization subject to bounds. Siam J Optim 6: 418–445, 1996. [Google Scholar]
- Cook and Maunsell 2002.Cook EP, Maunsell JH. Dynamics of neuronal responses in macaque MT and VIP during motion detection. Nat Neurosci 5: 985–994, 2002. [DOI] [PubMed] [Google Scholar]
- Crair et al. 1997.Crair MC, Ruthazer ES, Gillespie DC, Stryker MP. Ocular dominance peaks at pinwheel center singularities of the orientation map in cat visual cortex. J Neurophysiol 77: 3381–3385, 1997. [DOI] [PubMed] [Google Scholar]
- Cristianini and Shawe-Taylor 2000.Cristianini N, Shawe-Taylor J. An Introduction to Support Vector Machines and Other Kernel-Based Learning Methods. Cambride, UK: Cambridge Univ. Press, 2000.
- Das and Gilbert 1995.Das A, Gilbert CD. Long-range horizontal connections and their role in cortical reorganization revealed by optical recording of cat primary visual cortex. Nature 375: 780–784, 1995. [DOI] [PubMed] [Google Scholar]
- De et al. Valois 1979.De Valois KK, De Valois RL, Yund EW. Responses of striate cortex cells to grating and checkerboard patterns. J Physiol 291: 483–505, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis et al. 1999.DeAngelis GC, Ghose GM, Ohzawa I, Freeman RD. Functional micro-organization of primary visual cortex: receptive field analysis of nearby neurons. J Neurosci 19: 4046–4064, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruyn et al. 1993.DeBruyn EJ, Casagrande VA, Beck PD, Bonds AB. Visual resolution and sensitivity of single cells in the primary visual cortex (V1) of a nocturnal primate (bush baby): correlations with cortical layers and cytochrome oxidase patterns. J Neurophysiol 69: 3–18, 1993. [DOI] [PubMed] [Google Scholar]
- Efron and Tibshirani 1998.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, FL: CRC, 1998.
- Everson et al. 1998.Everson RM, Prashanth AK, Gabbay M, Knight BW, Sirovich L, Kaplan E. Representation of spatial frequency and orientation in the visual cortex. Proc Natl Acad Sci USA 95: 8334–8338, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley et al. 2007.Farley BJ, Yu H, Jin DZ, Sur M. Alteration of visual input results in a coordinated reorganization of multiple visual cortex maps. J Neurosci 27: 10299–10310, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostig et al. 1990.Frostig RD, Lieke EE, Ts'o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci USA 87: 6082–6086, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez and Woods 2002.Gonzalez RC, Woods RE. Digital Image Processing. Englewood Cliffs, NJ: Prentice-Hall, 2002.
- Grinvald et al. 1988.Grinvald A, Frostig RD, Lieke E, Hildesheim R. Optical imaging of neuronal activity. Physiol Rev 68: 1285–1366, 1988. [DOI] [PubMed] [Google Scholar]
- Grinvald et al. 1986.Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature 324: 361–364, 1986. [DOI] [PubMed] [Google Scholar]
- Hubel and Wiesel 1963.Hubel DH, Wiesel TN. Shape and arrangement of columns in cat's striate cortex. J Physiol 165: 559–568, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubener et al. 1997.Hubener M, Shoham D, Grinvald A, Bonhoeffer T. Spatial relationships among three columnar systems in cat area 17. J Neurosci 17: 9270–9284, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa et al. 2000.Issa NP, Trepel C, Stryker MP. Spatial frequency maps in cat visual cortex. J Neurosci 20: 8504–8514, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalatsky and Stryker 2003.Kalatsky VA, Stryker MP. New paradigm for optical imaging: temporally encoded maps of intrinsic signal. Neuron 38: 529–545, 2003. [DOI] [PubMed] [Google Scholar]
- Khaytin et al. 2007.Khaytin I, Chen X, Royal DW, Ruiz O, Jermakowicz WJ, Siegel RM, Casagrande VA. Functional organization of temporal frequency selectivity in primate visual cortex. Cereb Cortex 18: 1828–1842, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann 1998.Lehmann EL Theory of Point Estimation. New York: Springer-Verlag, 1998.
- Maffei and Fiorentini 1973.Maffei L, Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Res 13: 1255–1267, 1973. [DOI] [PubMed] [Google Scholar]
- Maffei and Fiorentini 1977.Maffei L, Fiorentini A. Spatial frequency rows in the straite visual cortex. Vision Res 17: 257–264, 1977. [DOI] [PubMed] [Google Scholar]
- Obermayer and Blasdel 1993.Obermayer K, Blasdel GG. Geometry of orientation and ocular dominance columns in monkey striate cortex. J Neurosci 13: 4114–4129, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermayer et al. 1990.Obermayer K, Ritter H, Schulten K. A principle for the formation of the spatial structure of cortical feature maps. Proc Natl Acad Sci USA 87: 8345–8349, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt 1991.Pratt WK Digital Image Processing. New York: Wiley, 1991.
- Purushothaman and Bradley 2005.Purushothaman G, Bradley DC. Neural population code for fine perceptual decisions in area MT. Nat Neurosci 8: 99–106, 2005. [DOI] [PubMed] [Google Scholar]
- Rao et al. 1997.Rao SC, Toth LJ, Sur M. Optically imaged maps of orientation preference in primary visual cortex of cats and ferrets. J Comp Neurol 387: 358–370, 1997. [DOI] [PubMed] [Google Scholar]
- Rosenfeld and Kak 1982.Rosenfeld A and Kak AC. Digital Picture Processing. New York: Academic, 1982.
- Sakas et al. 1990.Sakas DE, Charnvises K, Borges LF, Zervas NT. Biologically inert synthetic dural substitutes. Appraisal of a medical-grade aliphatic polyurethane and a polysiloxane-carbonate block copolymer. J Neurosurg 73: 936–941, 1990. [DOI] [PubMed] [Google Scholar]
- Schervish 1996.Schervish MJ P values: what they are and what they are not. Am Stat 50: 203–206, 1996. [Google Scholar]
- Shoham et al. 1997.Shoham D, Hubener M, Schulze S, Grinvald A, Bonhoeffer T. Spatio-temporal frequency domains and their relation to cytochrome oxidase staining in cat visual cortex. Nature 385: 529–533, 1997. [DOI] [PubMed] [Google Scholar]
- Sirovich and Uglesich 2004.Sirovich L, Uglesich R. The organization of orientation and spatial frequency in primary visual cortex. Proc Natl Acad Sci USA 101: 16941–16946, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sornborger et al. 2003.Sornborger A, Sailstad C, Kaplan E, Sirovich L. Spatiotemporal analysis of optical imaging data. Neuroimage 18: 610–621, 2003. [DOI] [PubMed] [Google Scholar]
- Swindale 1991.Swindale NV Coverage and the design of striate cortex. Biol Cybern 65: 415–424, 1991. [DOI] [PubMed] [Google Scholar]
- Swindale 2004.Swindale NV How different feature spaces may be represented in cortical maps. Network 15: 217–242, 2004. [PubMed] [Google Scholar]
- Tolhurst and Thompson 1982.Tolhurst DJ, Thompson ID. Organization of neurones preferring similar spatial frequencies in cat striate cortex. Exp Brain Res 48: 217–227, 1982. [DOI] [PubMed] [Google Scholar]
- Tootell et al. 1981.Tootell RB, Silverman MS, De Valois RL. Spatial frequency columns in primary visual cortex. Science 214: 813–815, 1981. [DOI] [PubMed] [Google Scholar]
- Uka and DeAngelis 2004.Uka T, DeAngelis GC. Contribution of area MT to stereoscopic depth perception: choice-related response modulations reflect task strategy. Neuron 42: 297–310, 2004. [DOI] [PubMed] [Google Scholar]
- Van Trees 1966.Van Trees HL Detection, Estimation, and Modulation Theory: Part I. New York, NY: Wiley, 1966.
- Vnek et al. 1999.Vnek N, Ramsden BM, Hung CP, Goldman-Rakic PS, Roe AW. Optical imaging of functional domains in the cortex of the awake and behaving monkey. Proc Natl Acad Sci USA 96: 4057–4060, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao et al. 2008.Xiao YP, Rao R, Cecchi G, Kaplan E. Improved mapping of information distribution across the cortical surface with the support vector machine. Neural Networks 21: 341–348, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. 2007.Xu X, Anderson TJ, Casagrande VA. How do functional maps in primary visual cortex vary with eccentricity?. J Comp Neurol 501: 741–755, 2007. [DOI] [PubMed] [Google Scholar]
- Xu et al. 2004.Xu X, Bosking W, Sary G, Stefansic J, Shima D, Casagrande V. Functional organization of visual cortex in the owl monkey. J Neurosci 24: 6237–6247, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. and Bosking 2005.Xu X, Bosking WH, White LE, Fitzpatrick D, Casagrande VA. Functional organization of visual cortex in the prosimian bush baby revealed by optical imaging of intrinsic signals. J Neurophysiol 94: 2748–2762, 2005. [DOI] [PubMed] [Google Scholar]
- Yokoo et al. 2001.Yokoo T, Knight BW, Sirovich L. An optimization approach to signal extraction from noisy multivariate data. Neuroimage 14: 1309–1326, 2001. [DOI] [PubMed] [Google Scholar]
- Yu et al. 2005.Yu H, Farley BJ, Jin DZ, Sur M. The coordinated mapping of visual space and response features in visual cortex. Neuron 47: 267–280, 2005. [DOI] [PubMed] [Google Scholar]
- Zepeda et al. 2004.Zepeda A, Arias C, Sengpiel F. Optical imaging of intrinsic signals: recent developments in the methodology and its applications. J Neurosci Methods 136: 1–21, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.