Abstract
Under physiological conditions N-methyl-d-aspartate (NMDA) receptor activation requires coincidence of presynaptic glutamate release and postsynaptic depolarization due to the voltage-dependent block of these receptors by extracellular Mg2+. Therefore spontaneous neurotransmission in the absence of action potential firing is not expected to lead to significant NMDA receptor activation. Here we tested this assumption in layer IV neurons in neocortex at their resting membrane potential (approximately −67 mV). In long-duration stable recordings, we averaged a large number of miniature excitatory postsynaptic currents (mEPSCs, >100) before or after application of dl-2 amino 5-phosphonovaleric acid, a specific blocker of NMDA receptors. The difference between the two mEPSC waveforms showed that the NMDA current component comprises ∼20% of the charge transfer during an average mEPSC detected at rest. Importantly, the contribution of the NMDA component was markedly enhanced at membrane potentials expected for the depolarized up states (approximately −50 mV) that cortical neurons show during slow oscillations in vivo. In addition, partial block of the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor component of the mEPSCs did not cause a significant reduction in the NMDA component, indicating that potential AMPA receptor-driven local depolarizations did not drive NMDA receptor activity at rest. Collectively these results indicate that NMDA receptors significantly contribute to signaling at rest in the absence of dendritic depolarizations or concomitant AMPA receptor activity.
INTRODUCTION
The Ca2+ permeability of N-methyl-d-aspartate (NMDA) receptors renders them critical for synaptic activation of several second-messenger-signaling cascades in the postsynaptic neuron. However, the permeability of NMDA receptors at negative membrane potentials is restricted due to their blockade by extracelullar Mg2+ ions (Mayer et al. 1984; Nowak et al. 1984). Thus in physiological settings at rest, the presence of Mg2+ is thought to reduce the conductance of NMDA receptors and limit their impact on neuronal signaling. In contrast, quantitative analyses of NMDA receptor function predict that a substantial amount of NMDA receptor-current remains at the neuronal membrane potentials typically encountered at rest (Jahr and Stevens 1990a,b). In addition, recent work has reported that in the mouse barrel cortex spiny stellate cells contain the NR2C NMDA receptor subunit that confers decreased susceptibility to Mg2+ block, thus enabling NMDA receptor-mediated transmission under physiological conditions (Binshtok et al. 2006; Fleidervish et al. 1998). Furthermore, several studies have documented activation of unitary NMDA receptor-mediated mEPSCs at low levels of Mg2+ (Bekkers and Stevens 1989; Myme et al. 2003; Watt et al. 2000) or at depolarized potentials (more than −40 mV) (McBain and Dingledine 1992). However, it remains unclear if spontaneous neurotransmission under physiological levels of Mg2+ can trigger detectable postsynaptic currents without requiring a noncanonical set of NMDA receptor properties such as those conferred by NR2C subunits. Clarification of the mechanisms that govern NMDA receptor activation at rest has become particularly important as recent studies suggest that synaptic NMDA receptor activation mediated by spontaneous miniature excitatory postsynaptic currents (mEPSCs) at rest can trigger signaling leading to synaptic plasticity within a time window of hours to several days (Chung and Kavalali 2006; Murphy et al. 1994; Slutsky et al. 2004; Sutton et al. 2006).
Here we investigated the contribution of NMDA receptor currents in cortical brain slices from postnatal days 11 to 19 after a developmental time point when the subunit composition of NMDA receptors is thought to be similar to that of adult animals (Burgard and Hablitz 1993a; Monyer et al. 1994). In analogy to evoked EPSCs, most mEPSCs recorded in nominal Mg2+ or at depolarized potentials are composed by a fast non-NMDA component as well as by a slow NMDA component (Burgard and Hablitz 1993b; Koester and Sakmann 1998; Kovalchuk et al. 2000; McBain and Dingledine 1992; Stern et al. 1992; Watt et al. 2000). We found that in neocortical neurons in Layer IV of barrel cortex the NMDA component comprises ≤20 and 30% of the average charge transfer by mEPSCs at −67 and at −52 mV, respectively. In vivo, these cells have been shown to oscillate between these two membrane potentials (Petersen et al. 2003; Steriade et al. 1993). Moreover, partial block of the AMPA receptor component of the mEPSCs did not cause a significant reduction in the NMDA component, indicating that possible AMPA receptor-driven local depolarizations did not drive NMDA receptor activity at rest.
METHODS
Slice preparation
Thalamocortical slices were prepared from postnatal days 11 to 19 Sprague Dawley rats following previously published protocols (Agmon and Connors 1991). Rats were anesthetized (pentobarbital sodium, Nembutal, 150 mg/kg; Abbott Laboratories, Abbott Park, IL) and rapidly decapitated in accordance with the protocol approved by the U.T. Southwestern Institutional Animal Care and Use Committee. Slices, 350 to 400-μm-thick, were cut using a Vibrotome 1000plus (St. Louis, MO) in an ice-cold oxygenated solution. The slicing solution contained (in mM) 234 sucrose, 3 KCl, 1.25 NaH2PO4, 6 MgCl2, 26 NaHCO3, 10 dextrose, and 0.5 CaCl2. Slices were incubated at 32°C for 1 h and kept at room temperature until use.
In the preparation used for this study, L4 neurons mainly connect with other cells within the same barrel and with supra- and infragranular cortical projections within the same column (Feldmeyer et al. 1999). Additionally, a small fraction of synapses (∼15%) may be shared with thalamocortical projections from the ventrobasal complex of the thalamus (Gil and Amitai 1996; White and Rock 1980). In contrast, in tangential preparations, supra- and infragranular projections are lost, and lateral projections between barrels are largely preserved (Binshtok et al. 2006; Fleidervish et al. 1998). Therefore excitatory projections onto L4 neurons are diverse and the total and specific NMDA receptor contribution to miniature neurotransmission may vary among different preparations.
Electrophysiology
Barrels were identified at low magnification under bright field illumination, and the pipette was aligned at the base of one of the barrels flanked by other barrels at each side. Whole cell voltage-clamp recordings were performed from visually identified neurons in Layer IV of the barrel cortex using a higher-magnification objective (×40) and infrared-differential interference contrast optics. The field visualized in the video monitor corresponded to an area of 100 × 100 μm, which roughly fits in the hollow of the barrels. After touching the surface of the brain slice, the pipette was advanced with a vectorial inclination of 45° toward the center of the barrel. Pyramidal-like neurons with an apical dendrite (mainly star pyramids) were targeted for recording, but because cells were not stained for further morphological characterization, we cannot exclude that some recordings were obtained from spiny stellate cells (Fleidervish et al. 1998; Schubert et al. 2003). Cell bodies were ≥30 μm from the slice surface to avoid recording “unhealthy” neurons with likely damaged larger processes.
Pipettes had resistances between 3 and 4.2 MΩ when filled with the internal solution that contained (in mM) 110 K-gluconate, 20 KCl, 10 NaCl, 10 HEPES, 0.6 EGTA, 4 Mg-ATP, 0.3 Na-GTP, 10 lidocaine N-ethyl bromide (QX-314), and buffered to pH 7.3 with KOH (290–300 mosM). The external solution contained (in mM) 126 NaCl, 5 KCl, 1.25 NaH2PO4 26 NaHCO3, 10 dextrose, and 2 CaCl2, saturated with 95% O2-5% CO2. External MgCl2 concentration was varied between 0.5 and 2 mM depending on the experiment. The external solution did not contain added glycine, which is a potent co-agonist for NMDA receptors, in order not to bias our conditions toward larger NMDA currents. Earlier studies suggest that under these conditions extracellular glycine concentration should be in the order of 0.3 μM (Benjamin and Quastel 1972). The junction potential between the internal and external solutions (∼12 mV) was subtracted from all recordings. Most recorded cells had an input resistance of ≥250 MΩ. Experiments were rejected for analysis if the access resistance or the input resistance varied >20% during the recording. Recordings were made with an Axopatch 200A amplifier (Molecular Devices, Union City, CA), and signals were low-pass filtered at 2 kHz and digitized at 10 kHz, and the data were acquired and analyzed using pClamp 9.2 software (Molecular Devices Union City, CA). Excitatory miniature events were recorded in the presence of picrotoxin (PTX 50 μM; Sigma) and tetrodotoxin (TTX, 0.5–1 μM; Calbiochem, San Diego, CA) before and after application of 50 μM dl-2 amino 5-phosphonovaleric acid (AP-5, Sigma, St. Louis, MO).
Statistical analysis
All data are expressed as means ± SE. Statistical differences were established either using paired 2-tailed Student's t-test or repeated-measures one-way ANOVA. The Dunnett's or the Bonferroni's post hoc tests were applied as appropriate.
RESULTS
Long-term stability of mEPSC properties at rest
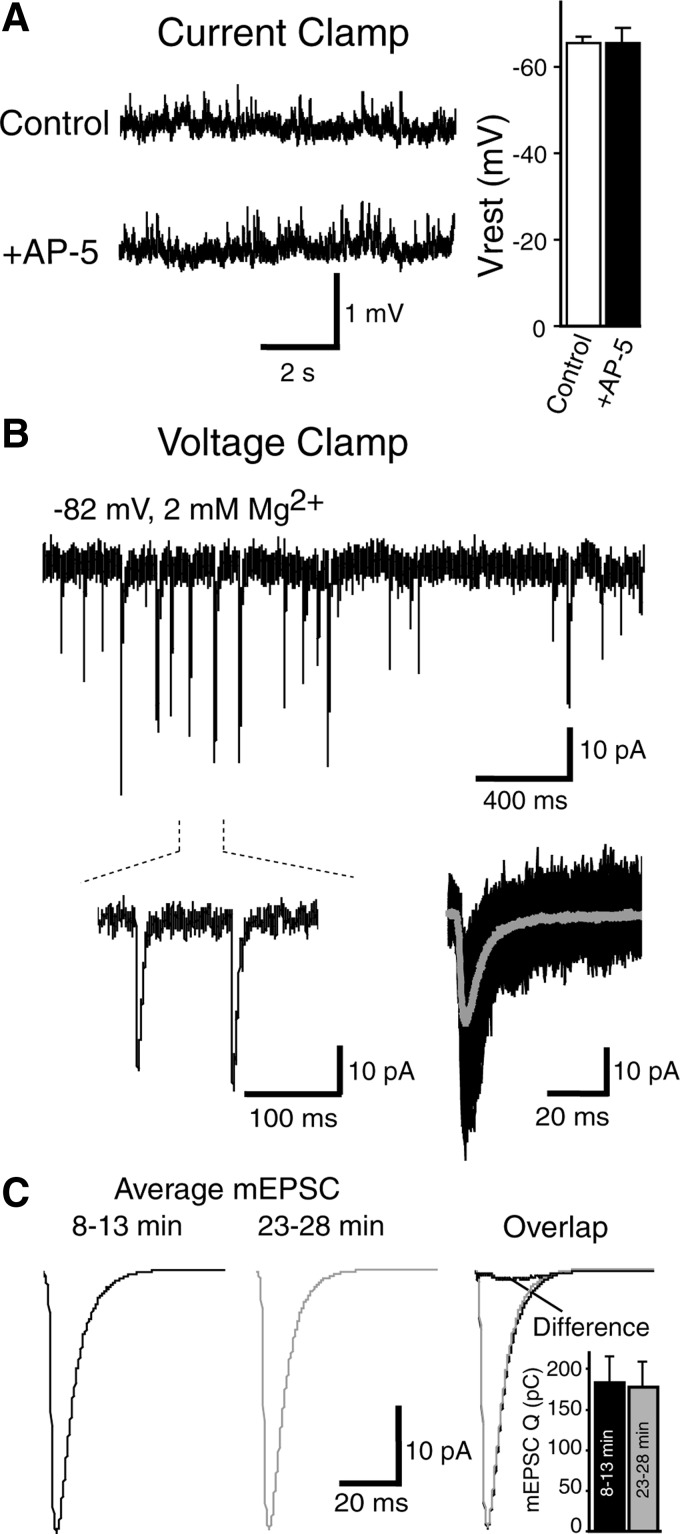
To assess the contribution of NMDA receptor-mediated currents to spontaneous neurotransmission, we first determined the resting membrane potential of layer IV pyramidal cells under our recording conditions at 32°C. Figure 1A shows traces acquired in current-clamp configuration before (control) and after perfusion of 50 μM AP-5, a specific blocker of NMDA receptors. The membrane potential in control conditions ranged between −64 and −70 mV (n = 9). Five-minute application of AP-5 did not alter the baseline membrane potential, suggesting that NMDA receptor activity does not contribute to setting the membrane potential in these cells (Fig. 1A).
FIG. 1.
Long-term stability of miniature excitatory postsynaptic current (mEPSC) properties at rest. A: mEPSPs recorded under current-clamp conditions before (control, top trace) or after addition of dl-2 amino 5-phosphonovaleric acid (AP-5, bottom trace). Right: the average (±SE) resting membrane potential before and after addition of AP-5 (P > 0.5). B: control mEPSCs recorded at −82 mV in 2 mM Mg2+ under voltage-clamp conditions (top trace). Bottom left traces: events extracted from the section indicated by the dotted lines on an expanded time scale. Bottom right: alignment of 100 mEPSCs (black traces) plus average (gray trace) chosen from the same experiment shown above. C: average mEPSCs corresponding to recordings in the control condition (without AP-5). Left and middle: the averages of events respectively chosen during 8–13 and 23–28 min after reaching whole cell configuration. Both traces were overlapped on the right. To test the stability of the signal, the middle trace was subtracted from left trace, and the resultant trace shown (“difference”). Inset: the comparison of charge transfer at times 8–13 min or 23–28 min (n = 7; P > 0.5).
Under physiological conditions at rest, NMDA receptor-mediated current component of an individual mEPSC is expected to be near the baseline noise level (McBain and Dingledine 1992). Therefore to unmask the putative NMDA receptor-mediated current component, we quantified the difference between several mEPSCs averaged before and after AP-5 application during stable recordings. For this analysis, we selected independent events separated by ∼100 ms and avoided events clustered in time (Fig. 1B). This approach is very sensitive to variations in the quality of the recording as well as to changes in the kinetics of mEPSCs throughout the recording. Therefore we first tested the stability of the mEPSC kinetics for long recording durations similar to those we used for the experiments. For this purpose, we recorded mEPSCs in control conditions under voltage clamp for ≤35 min after achieving the whole cell configuration. To avoid possible artifacts due to cell dialysis with the internal solution, control events were selected 8 min following initiation of the whole cell configuration (average mEPSC, 8–13 min; Fig. 1, B, bottom right, and C, left). A second group of events were chosen 20 min after the beginning of whole cell recordings (average mEPSC, 23–28 min; Fig. 1C, middle). We did not detect a difference between the two groups of mEPSCs when overlapped (Fig. 1C, right; n = 7), indicating the adequacy of the experimental conditions for our analysis. In addition, the average charge transfer carried by mEPSCs at times 8–13 or 23–28 min was not significantly different (Fig. 1C, inset).
Contribution of NMDA receptor-mediated currents to mEPSCs under physiological conditions
Next, we assessed the contribution of NMDA receptors to mEPSCs at physiological (1.25 mM) (Heipertz et al. 1979) or high (2 mM) concentrations of extracellular Mg2+. In these experiments, we varied the membrane potential between −42 and −82 mV and recorded traces before and after perfusion of AP-5 (Fig. 2A). To estimate the NMDA receptor-mediated component of mEPSCs, we obtained the difference between the averages of ∼100 mEPSCs recorded under the control condition and after AP-5 application (Fig. 2A). Overall, these Mg2+ concentrations and membrane potential values altered the difference current in the direction consistent with expectations from regulation of NMDA receptor-mediated currents (Jahr and Stevens 1990a,b). The resulting difference current was detectable at potentials as low as −82 mV in the presence of 2 mM Mg2+ and increased with further depolarization or a reduction in Mg2+ concentration (Fig. 2, A and B). Interestingly, AP-5 application accelerated the decaying phase of mEPSCs without significantly altering the peak current amplitudes (Fig. 2A). When normalized to the peak amplitude of the mEPSC before perfusion of AP-5, the peak of the NMDA receptor-mediated component increased from 3.4 ± 0.9% at −82 mV (2 mM Mg2+, n = 10) to 8.6 ± 0.3% at −67 mV (1.25 mM Mg2+, n = 10) and reached 21.0 ± 3.2% at −42 mV (2 mM Mg2+, n = 5; Fig. 2B). Similarly, the relative charge transfer for the NMDA receptor-mediated component at each condition increased from 4.3 ± 4.8% at −82 mV (2 mM Mg2+) to 14.4 ± 9.2% at −82 mV (1.25 mM Mg2+) and reached 21.0 ± 2.8%, at −67 mV (1.25 mM Mg2+; Fig. 2C). As expected, the relative contribution of NMDA receptors to mEPSCs was substantially higher at more depolarized membrane potentials in presence of physiological levels of Mg2+ [31.8 ± 5.2% at −52 mV (1.25 mM Mg2+) and 35.4 ± 6.1% at −42 mV (2 mM Mg2+)].
FIG. 2.
The N-methyl-d-aspartate (NMDA) receptor-mediated component of mEPSCs under physiological conditions. A: panel depicts average mEPSCs recorded before and after 50 μM AP-5 application as well as the difference between the 2 averages from representative experiments at the indicated holding potentials and extracellular Mg2+ concentrations. B: averages of the AP-5-sensitive component at the indicated experimental conditions (numbers of experiments indicated in parenthesis). C: average (left) or relative (right) charge transfer by the NMDA receptor component during mEPSC under different conditions. Statistical significance was assessed with 1-way ANOVA (P < 0.001). The Dunnett's post hoc test was used to compare the no-AP-5 condition, as the control (no-NMDA receptor component was revealed) to all other conditions. Experiments at −67 mV through −42 mV were significantly different from the no-AP-5 control.
Further analysis of experiments conducted at −67 mV (1.25 mM Mg2+) showed that the average peak amplitudes as well as the rise time of mEPSCs were not affected by AP-5 application although the total charge transfer and the decay time of events were reduced (Fig. 3, A–D). This finding suggests that the rising phase of the mEPSC is almost exclusively contributed by the fast AMPA receptor component and NMDA receptors mainly slow the decaying phase. In addition, this analysis revealed that the AP-5 induced reduction in total charge transfer by individual mEPSCs was homogenously distributed across all mEPSC sizes (Fig. 3E). Therefore NMDA receptor activation during a mEPSC does not increase with increasing mEPSC size consistent with the premise that AMPA receptor component of a mEPSC has minimal impact on its NMDA component at rest. In addition, this analysis suggests that most NMDA receptors were co-activated with their AMPA counterparts, as cumulative histograms did not reveal a significant contribution of slow rising mEPSCs solely mediated by NMDA receptors to the overall population of mEPSCs.
FIG. 3.
Changes in the properties of individual mEPSCs after AP-5. A and C: the amplitudes and rise times of mEPSCs were not different before and after application of (n = 10). This suggests that a fast non-NMDA component dominates these 2 parameters at −67 mV (1.25 mM Mg2+). B and D: absolute charge transfer during a mEPSC and mEPSC decay times were significantly reduced in the presence of AP-5 (both, ∼20% difference). E: cumulative distributions of charge transfer by mEPSCs before and after application of AP-5 at −67-mV holding potential. This graph shows that the AP-5 induced reduction in total charge transfer by individual mEPSCs was homogenously distributed across all mEPSC sizes. F and G: cumulative distributions of rise times and decay times of mEPSCs before and after application of AP-5 at −67-mV holding potential. These graphs show that the AP-5 application had a minimal effect on the distribution of rise times, whereas it significantly decreased the decay times of individual mEPSCs. In both cases, however, mEPSCs were affected in a homogenous manner.
Impact of AMPA current inhibition on NMDA receptor-mediated component of mEPSCs
Mature glutamatergic synapses typically possess both AMPA and NMDA receptors (Bekkers and Stevens 1989; Liao et al. 2001; Stern et al. 1992). Therefore it is possible that AMPA receptor activity may augment NMDA receptor activity at rest through electrical means. Such interaction between the activation of the two types of receptors may occur if some dendritic spines comprise electrically isolated compartments due to high spine neck resistance (Bloodgood and Sabatini 2005). Although our recordings were performed under voltage-clamp conditions, we cannot exclude that due to poor space clamp in distal dendrites activation of AMPA receptors may result in sufficient local depolarization to facilitate relief of adjacent NMDA receptors from Mg2+ block (Williams and Mitchell 2008). To examine the interaction between AMPA receptor activation and NMDA receptor component of mEPSCs, we performed the same analysis in the preceding text in a parallel set of experiments in the presence of low concentrations of AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) as well as a more specific antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) as CNQX may interfere with the action of NMDA receptor co-agonist glycine (Lester et al. 1989). These conditions allowed us to select mEPSCs using similar criteria as in the preceding text albeit with reduced amplitudes.
Application of 1.8 μM CNQX or 0.35 μM NBQX in 1.25 mM Mg2+ at −67 mV reduced the average mEPSC amplitude by 60% (Fig. 4, A and E). However, this large reduction in AMPA currents did not have a major effect on the size of the absolute charge transfer mediated by the NMDA receptor components of the mEPSCs (Fig. 4, B, C, F, and G) but increased the relative fraction of the charge transfer mediated by NMDA receptors (Fig. 4, D and H). Taken together, these results suggest that AMPA receptor activation overall does not facilitate NMDA conductance during spontaneous neurotransmission arguing against the premise that NMDA receptor component of mEPSCs is driven by local AMPA-mediated depolarization and Mg2+ unblock.
FIG. 4.
Impact AMPA current inhibition on NMDA receptor-mediated component of mEPSCs. A and E: application of 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX, 1.8 μM) and 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX, 350 nM) caused partial-block of the non-NMDA receptor component of mEPSCs in the presence of AP-5. In these conditions, mEPSCs were reduced by 40 to 60%. B and F: the NMDA receptor mediated component of mEPSCs analyzed in the presence of low concentrations of CNQX or NBQX. Traces depict normalized mEPSCs before (control) or after addition of 50 μM AP-5 (+AP-5). The difference (light gray trace) represents the AP-5-sensitive component. C and G: average charge transfer by NMDA receptors (NMDAR-Q) is unaffected by application of CNQX or NBQX (n = 4; in all cases). D and H: the presence of CNQX or NBQX reduced the amplitudes of the non-NMDA receptor mediated component of mEPSCs and thus increased the relative contribution of NMDA receptors to total charge transfer.
DISCUSSION
In this study, we monitored mEPSCs in neurons of the barrel cortex to assess the contribution of NMDA receptors to spontaneous neurotransmission. We found that spontaneous mEPSCs are sensitive to specific NMDA receptor blocker AP-5 at near physiological conditions (32°C, 1.25–2 mM Mg2+). On average, NMDA receptors mediated 20% of the charge transfer of a mEPSC at −67 mV and nearly 30% of a mEPSC at −52 mV. These membrane potentials represent the two states that neocortical neurons oscillate between in vivo at a frequency of 1 Hz. In addition to the near physiological temperature and physiologically meaningful membrane potentials of these recordings, the concentration of extracellular Mg2+ (1.25 mM) also reflect the Mg2+ concentration in the cerebrospinal fluid, which is expected to be similar to the concentration in the extracellular environment in direct contact with neurons. This value ranges between 1.1 and 1.35 mM in humans (Heipertz et al. 1979) and is around 0.85 mM in rats (Chutkow 1974; Jeong et al. 2006). In addition, our results on the NMDA receptor component of mEPSCs agree well with the earlier findings of Feldmeyer and colleagues on NMDA receptor contribution to single excitatory postsynaptic potentials in barrel cortex layer 4 neurons (Feldmeyer et al. 1999).
Taken together, the results of these experiments lead us to four major conclusions. First, mEPSCs possess a sizable NMDA receptor-mediated component, indicating that NMDA receptors signal at rest under physiological conditions in the absence of dendritic depolarizations. Second, the fraction of current carried by NMDA receptors does not increase with increasing mEPSC size as the inhibition of mEPSCs by AP-5 is uniform across all mEPSC sizes. This finding, together with previous work (McAllister and Stevens 2000), is consistent with the premise that across synapses AMPA and NMDA receptor components scale together. Third, the NMDA receptor component of mEPSCs agrees with earlier estimates of Mg2+ block of canonical NMDA receptors, and therefore it does not necessarily require involvement of NMDA receptor subunits with altered Mg2+ sensitivity. Finally, partial blockade of AMPA receptors does not affect the NMDA receptor-mediated component of mEPSCs arguing against the possibility that the NMDA component is due to relief of Mg2+ block via local depolarization mediated by AMPA receptors. Therefore we propose that the residual NMDA receptor component of spontaneous mEPSCs stems from continual incomplete Mg2+ block of NMDA receptors at the resting membrane potential rather than unblock triggered by concurrent AMPA receptor activity. This proposal is in agreement with earlier predictions of Jahr and Stevens (1990a). An equation they have generated to calculate the relative conductance of NMDA receptors at a particular membrane potential and extracellular Mg2+ concentration estimates that NMDA receptors should be active at 4.3% of their peak conductance at −67 mV in the presence of 1.25 mM Mg2+ (Jahr and Stevens 1990a). Taken together with the relative long decay times of NMDA receptor mediated EPSCs, this estimate is in line with our finding that ≥20% of total charge of mEPSCs is carried by NMDA receptors under the same conditions.
Earlier recordings of mEPSCs in L4 revealed a very large contribution of NMDA receptors even in the presence of blockers of non-NMDA receptors (Binshtok et al. 2006; Fleidervish et al. 1998). However, we could detect clear NMDA receptor-mediated mEPSCs only in one of eight recordings in the presence of 10 μM NBQX to block AMPA currents (data not shown). The most plausible explanation for this discrepancy is that different synapses and NMDA receptor types (i.e., NR2C subunits) were functional in the previous reports due to the tangential preparation used in contrast to the thalamocortical one we employed here. In fact, Binshtok et al. (2006) demonstrated that in the tangential preparation, lateral projections between barrels are preserved while supra- and infragranular projections are lost. Therefore it is likely that NR2C subunits, which are specifically localized to spiny stellate cells, constitutively form part of synapses that are selectively preserved in tangential preparations of the barrel cortex.
Spontaneous glutamate release driven NMDA receptor activation has several implications for neuronal signaling. Earlier studies suggested that synaptic NMDA receptor activation mediated by spontaneous mEPSCs at rest can trigger signaling leading to synaptic plasticity within a time window of hours to several days (Murphy et al. 1994; Slutsky et al. 2004; Sutton et al. 2006). For example, activation of the Ca2+-calmodulin kinase II but not the mitogen-activated protein kinase depends on NMDA receptor-dependent Ca2+ influx induced, in part, by spontaneous neurotransmission (Murphy et al. 1994). NMDA receptor activity at rest has also been shown to regulate the ability of hippocampal synaptic terminals to undergo plasticity (Slutsky et al. 2004). Recent studies by Sutton and colleagues (2006, 2007) showed that spontaneous neurotransmitter release, rather than evoked neurotransmission, is a specific regulator of postsynaptic sensitivity to neurotransmitters by suppressing the dendritic protein translation machinery locally and thereby maintaining receptor composition of synapses. These experiments showed that, unlike the blockade of action potentials, inhibition of either NMDA receptors or AMPA receptors can increase the amplitude of mEPSCs within hours. The frequency of mEPSCs remains unchanged, and the increased mEPSC amplitude is seen even when action potentials are allowed during receptor blockade. Moreover, the authors show that this rapid effect of NMDA receptor blockade on unitary transmission is strictly dependent on protein synthesis. These findings are consistent with our observations, and they strongly suggest that NMDA receptors are active at rest during spontaneous neurotransmission, despite their reduced ion conductance due to Mg2+ block. A recent study by the same investigators showed that regulation of protein translation by spontaneous release events occurs through the eukaryotic elongation factor-2 (eEF2), which distinguishes unitary Ca2+ currents generated by evoked release from currents mediated by spontaneous release and controls protein synthesis accordingly (Sutton et al. 2007). Taken together with the result that NMDA receptor properties makes them susceptible to activation under resting conditions, the findings of Sutton and colleagues (2007) bolster the recent proposal that evoked and spontaneous release may trigger nonoverlapping activation of postsynaptic NMDA receptors (Atasoy et al. 2008).
Our results in neocortical slices corroborate this earlier work and provide an explicit estimate of the size of NMDA receptor contribution to unitary excitatory currents detected in the absence of activity. These findings indicate that mEPSC driven signaling is more widespread than previously thought and reinforce the premise that mEPSCs can drive biochemical signaling in addition to electrical activity. Furthermore, augmentation of spontaneous neurotransmission or NMDA receptor activity by factors such as BDNF (Magby et al. 2006; Tyler and Pozzo-Miller 2003) or Reelin (Chen et al. 2005) can also increase the contribution of this form of signaling to overall neurotransmission. Therefore spontaneous glutamate release may constitute a bone fide pathway for interneuronal signaling independent of pre- and postsynaptic activity.
GRANTS
This work was supported by National Institute of Mental Health Grants MH-068437 and MH-066198 to E. T. Kavalali, who is an Established Investigator of the American Heart Association.
Acknowledgments
We thank Dr. Jesica Raingo for her critical comments on this manuscript.
REFERENCES
- Agmon and Connors 1991.Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41: 365–379, 1991. [DOI] [PubMed] [Google Scholar]
- Atasoy et al. 2008.Atasoy D, Ertunc M, Moulder KL, Blackwell J, Chung C, Su J, Kavalali ET. Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J Neurosci 28: 10151–10166, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers and Stevens 1989.Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature 341: 230–233, 1989. [DOI] [PubMed] [Google Scholar]
- Benjamin and Quastel 1972.Benjamin AM, Quastel JH. Locations of amino acids in brain slices from the rat. Tetrodotoxin-sensitive release of amino acids. Biochem J 128: 631–646, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binshtok et al. 2006.Binshtok AM, Fleidervish IA, Sprengel R, Gutnick MJ. NMDA receptors in layer 4 spiny stellate cells of the mouse barrel cortex contain the NR2C subunit. J Neurosci 26: 708–715, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood and Sabatini 2005.Bloodgood BL, Sabatini BL. Neuronal activity regulates diffusion across the neck of dendritic spines. Science 310: 866–869, 2005. [DOI] [PubMed] [Google Scholar]
- Burgard and Hablitz 1993a.Burgard EC, Hablitz JJ. NMDA receptor-mediated components of miniature excitatory synaptic currents in developing rat neocortex. J Neurophysiol 70: 1841–1852, 1993a. [DOI] [PubMed] [Google Scholar]
- Burgard and Hablitz 1993b.Burgard EC, Hablitz JJ. Developmental changes in NMDA and non-NMDA receptor-mediated synaptic potentials in rat neocortex. J Neurophysiol 69: 230–240, 1993b. [DOI] [PubMed] [Google Scholar]
- Chen et al. 2005.Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, Herz J. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci 25: 8209–8216, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung and Kavalali 2006.Chung C, Kavalali ET. Seeking a function for spontaneous neurotransmission. Nat Neurosci 9: 989–990, 2006. [DOI] [PubMed] [Google Scholar]
- Chutkow 1974.Chutkow JG Metabolism of magnesium in central nervous system. Relationship between concentrations of magnesium in cerebrospinal fluid and brain in magnesium deficiency. Neurology 24: 780–787, 1974. [DOI] [PubMed] [Google Scholar]
- Feldmeyer et al. 1999.Feldmeyer D, Egger V, Lübke J, Sakmann B. Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single “barrel” of developing rat somatosensory cortex. J Physiol 521: 169–190, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleidervish et al. 1998.Fleidervish IA, Binshtok AM, Gutnick MJ. Functionally distinct NMDA receptors mediate horizontal connectivity within Layer 4 of mouse barrel cortex. Neuron 21: 1055–1065, 1998. [DOI] [PubMed] [Google Scholar]
- Gil and Amitai 1996.Gil Z, Amitai Y. Adult thalamocortical transmission involves both NMDA and non-NMDA receptors. J Neurophysiol 76: 2547–2554, 1996. [DOI] [PubMed] [Google Scholar]
- Heipertz et al. 1979.Heipertz R, Eickhoff K, Karstens KH. Magnesium and inorganic phosphate content in CSF related to blood-brain barrier function in neurological disease. J Neurol Sci 40: 87–95, 1979. [DOI] [PubMed] [Google Scholar]
- Jahr and Stevens 1990a.Jahr CE, Stevens CF. Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. J Neurosci 10: 3178–3182, 1990a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr and Stevens 1990b.Jahr CE, Stevens CF. A quantitative description of NMDA receptor-channel kinetic behavior. J Neurosci 10: 1830–1837, 1990b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong et al. 2006.Jeong SM, Hahm KD, Shin JW, Leem JG, Lee C, Han SM. Changes in magnesium concentration in the serum and cerebrospinal fluid of neuropathic rats. Acta Anaesthesiol Scand 50: 211–216, 2006. [DOI] [PubMed] [Google Scholar]
- Koester and Sakmann 1998.Koester HJ, Sakmann B. Calcium dynamics in single spines during coincident pre- and postsynaptic activity depend on relative timing of back-propagating action potentials and subthreshold excitatory postsynaptic potentials. Proc Natl Acad Sci USA 95: 9596–9601, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk et al. 2000.Kovalchuk Y, Eilers J, Lisman J, Konnerth A. NMDA receptor-mediated subthreshold Ca(2+) signals in spines of hippocampal neurons. J Neurosci 20: 1791–1799, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester et al. 1989.Lester RA, Quarum ML, Parker JD, Weber E, Jahr CE. Interaction of 6-cyano-7-nitroquinoxaline-2,3-dione with the N-methyl-d-aspartate receptor-associated glycine binding site. Mol Pharmacol 35: 565–570, 1989. [PubMed] [Google Scholar]
- Liao et al. 2001.Liao D, Scannevin RH, Huganir R. Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J Neurosci 21: 6008–6017, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magby et al. 2006.Magby JP, Bi C, Chen ZY, Lee FS, Plummer MR. Single-cell characterization of retrograde signaling by brain-derived neurotrophic factor. J Neurosci 26: 13531–13536, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer et al. 1984.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309: 261–263, 1984. [DOI] [PubMed] [Google Scholar]
- McAllister and Stevens 2000.McAllister AK, Stevens CF. Nonsaturation of AMPA and NMDA receptors at hippocampal synapses. Proc Natl Acad Sci USA 97: 6173–6178, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain and Dingledine 1992.McBain C, Dingledine R. Dual-component miniature excitatory synaptic currents in rat hippocampal CA3 pyramidal neurons. J Neurophysiol 68: 16–27, 1992. [DOI] [PubMed] [Google Scholar]
- Monyer et al. 1994.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12: 529–540, 1994. [DOI] [PubMed] [Google Scholar]
- Murphy et al. 1994.Murphy TH, Blatter LA, Bhat RV, Fiore RS, Wier WG, Baraban JM. Differential regulation of calcium/calmodulin-dependent protein kinase II and p42 MAP kinase activity by synaptic transmission. J Neurosci 14: 1320–1331, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myme et al. 2003.Myme CI, Sugino K, Turrigiano GG, Nelson SB. The NMDA-to-AMPA ratio at synapses onto layer 2/3 pyramidal neurons is conserved across prefrontal and visual cortices. J Neurophysiol 90: 771–779, 2003. [DOI] [PubMed] [Google Scholar]
- Nowak et al. 1984.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurons. Nature 307: 462–465, 1984. [DOI] [PubMed] [Google Scholar]
- Petersen et al. 2003.Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci USA 100: 13638–13643, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert et al. 2003.Schubert D, Kötter R, Zilles K, Luhmann HJ, Staiger JF. Cell type-specific circuits of cortical layer IV spiny neurons. J Neurosci 23: 2961–2970, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky et al. 2004.Slutsky I, Sadeghpour S, Li B, Liu G. Enhancement of synaptic plasticity through chronically reduced Ca2+ flux during uncorrelated activity. Neuron 44: 835–849, 2004. [DOI] [PubMed] [Google Scholar]
- Steriade et al. 1993.Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci 13: 3266–3283, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern et al. 1992.Stern P, Edwards FA, Sakmann B. Fast and slow components of unitary EPSCs on stellate cells elicited by focal stimulation in slices of rat visual cortex. J Physiol 449: 247–278, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton et al. 2006.Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125: 785–799, 2006. [DOI] [PubMed] [Google Scholar]
- Sutton et al. 2007.Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron 55: 648–661, 2007. [DOI] [PubMed] [Google Scholar]
- Tyler and Pozzo-Miller 2003.Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurons. J Physiol 553: 497–509, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt et al. 2000.Watt AJ, van Rossum MC, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron 26: 659–670, 2000. [DOI] [PubMed] [Google Scholar]
- White and Rock 1980.White EL, Rock MP. Three-dimensional aspects and synaptic relationships of a Golgi-impregnated spiny stellate cell reconstructed from serial thin sections. J Comp Neurol 9: 615–636, 1980. [DOI] [PubMed] [Google Scholar]
- Williams and Mitchell 2008.Williams SR, Mitchell SJ. Direct measurement of somatic voltage clamp errors in central neurons. Nat Neurosci 11: 790–798, 2008. [DOI] [PubMed] [Google Scholar]