Abstract
Coincident spikes have been implicated in vision-related processes such as feature binding, gain modulation, and long-distance communication. The source of these spike-time correlations is unknown. Although several studies have proposed that cortical spikes are correlated based on stimulus structure, others have suggested that spike-time correlations reflect ongoing cortical activity present even in the absence of a coherent visual stimulus. To examine this issue, we collected single-unit recordings from primary visual cortex (V1) of the anesthetized and paralyzed prosimian bush baby using a 100-electrode array. Spike-time correlations for pairs of cells were compared under three conditions: a moving grating at the cells’ preferred orientation, an equiluminant blank screen, and a dark condition with eyes covered. The amplitudes, lags, and widths of cross-correlation histograms (CCHs) were strongly correlated between these conditions although for the blank stimulus and dark condition, the CCHs were broader with peaks lower in amplitude. In both preferred stimulus and blank conditions, the CCH amplitudes were greater when the cells within the pair had overlapping receptive fields and preferred similar orientations rather than nonoverlapping receptive fields and different orientations. These data suggest that spike-time correlations present in evoked activity are generated by mechanisms common to those operating in spontaneous conditions.
INTRODUCTION
Correlated spike times have been implicated in vision related processes, such as feature binding (Engel et al. 1990; Gray et al. 1989), providing stimulus detail (Biederlack et al. 2006; Pillow et al. 2008; Samonds et al. 2004; Zhou et al. 2008), gain modulation (Azouz 2005), and long-distance communication (Fries et al. 2001). Correlated spikes may increase the probability of transmission of salient visual information when they synchronously converge onto their targets. The sources of these correlated spike times remain unclear. Several proposals argue that the spike-time correlations are generated by the spatiotemporal properties of stimuli (Biederlack et al. 2006; Engel et al. 1990; Gray et al. 1989; Zhou et al. 2008). Attentional shifts also have been implicated in correlating spike times in awake animals (Fries et al. 2001), yet several studies also have suggested that spike-time correlations simply reflect network architecture and do not necessarily contain information relevant to stimulus processing (Bair et al. 2001; de la Rocha et al. 2007; Lamme and Spekreijse 1998; Palanca and DeAngelis 2005; Shadlen and Movshon 1999). Understanding the mechanisms underlying spike-time correlations is essential for uncovering their functional relevance.
An important step in reaching this understanding is examining the degree to which these spike-time correlations are inherent in the network and not generated directly by stimulus properties. If spike-time correlations are very similar in stimulus-evoked and spontaneous states, then it is less likely that they carry stimulus information. Examination of the relationship between stimulus-evoked and spontaneous forms of neural activity, such as spike rate, variance, and spike count correlation (Chiu and Weliky 2002; Fiser et al. 2004; Haider et al. 2007; Kenet et al. 2003), suggests that instead of directly representing responses to the attributes of a visual scene, stimulus-evoked responses may reflect the modulation of ongoing cortical activity by the stimulus-dependent input signals. Significant correlations have been detected between stimulus-evoked and spontaneous spike-time correlations in primate visual cortex (Bair et al. 2001; Kohn and Smith 2005; Maldonado et al. 2000, 2008); however, a thorough quantitative analysis of these correlations has not been presented. In addition, it is unclear how spontaneous spike-time correlations are related to the relative orientation preference and receptive field overlap of neurons in a pair, information that may be relevant to understanding the source of these correlations.
We examined the relationship of stimulus-evoked and spontaneous spike-time correlations by recording single unit data from bush baby V1 using a 100-electrode array. V1 is ideal for such studies because in few other cortical areas have connections been as well defined (Angelucci et al. 2002; Malach et al. 1993; see Casagrande and Kaas 1994 for review). The bush baby is also well suited for these studies because its visual system has been intensively studied, the early cortical visual areas are exposed on the brain surface, and the brain is lissencephalic, maximizing the number of neurons that can be recorded simultaneously (Bonds et al. 1987; Collins et al. 2005; Debruyn et al. 1993; Jermakowicz et al. 2006, 2007; Xu et al. 2005). This arrangement allowed for the computation of thousands of cross-correlation histograms (CCHs) for many pairs of V1 neurons. Evoked and spontaneous spike-time correlations were compared by recording spike activity in response to drifting sine wave gratings of a preferred orientation and in response to an equiluminant blank stimulus or to no stimulus, respectively. We compared CCH peak amplitudes, lags, and widths in response to these different conditions. In addition, the influence of receptive field overlap and similarity in orientation tuning preference on the CCH peaks was examined for the two conditions. Our data show that there is a strong relationship between spike-time correlations evoked by a preferred stimulus and those evoked by a blank stimulus or dark condition and they deviated most when both neurons were driven well by the stimulus.
METHODS
Physiological preparation and recording
These experiments were performed on three prosimian bush babies (Otolemur garnetti). All experiments were performed under the guidelines of the American Physiological Society and Vanderbilt University Institutional Animal Care and Use Committee under an approved protocol. Each animal received 1.0 mg of dexamethasone ∼1 h prior to surgery to control for potential inflammation. After induction of anesthesia with ketamine (10 mg/kg), implantation of two venous catheters, and insertion of an exotracheal tube, the animals were placed into a stereotaxic apparatus. Through one of the catheters, 10 mg·kg–1·h–1 of Propofol (Diprivan) was infused continuously to maintain general anesthesia. Through the other catheter, a neuromuscular blockade was initiated with 3.0 mg pancuronium bromide (in 5% dextrose lactated Ringer) and then maintained by infusion of the drug at 0.1–0.3 mg·kg–1·h–1. Throughout the experiment, the animals were artificially ventilated with NO2 (75%), O2 (23.5%), and CO2 (1.5%) at a rate sufficient to maintain the peak end tidal CO2 level at around 4%. At the onset of the procedure, pupils were dilated with 1% atropine eye drops. Then to ensure protection of the cornea and render the retina conjugate with the viewing screen 57 cm distant, contact lenses of appropriate power and with artificial 3-mm pupils were applied to the eyes. The optic discs and areae centralii (ACs) were plotted via a tangent screen onto a plotting table by back reflection of the retinal image from the tapetum using a fiber optic light source.
An 8 × 8-mm craniotomy was performed, and the dura was resected over the region corresponding to V1. A digital picture was taken of the cortical surface and a 10 × 10 cyberkinetics multielectrode array was pneumatically inserted. The array was injected to a depth of ∼600 μm (cortical layers 2 and 3) and then the surface and array were covered with 1% agar to prevent dehydration. To remove noise at each channel, spike sorting was performed using a method widely used with this array (Shoham et al. 2003). This spike sorting method models the distribution of waveforms recorded on each channel as a multivariate t-distribution and uses an expectation-maximization algorithm to distinguish spikes from noise on each electrode.
At the end of each experiment, the animal was killed with an overdose of sodium pentobarbital and perfused transcardially, first with a saline rinse and followed by a fixative consisting of 2% paraformaldehyde in 0.1 M phosphate buffer. The brain then was removed, and the visual cortex was resected and flattened. After soaking overnight in a 30% sucrose solution, the visual cortical tissue was frozen, and 52-μm sections were cut tangentially. Cytochrome oxidase (CO) staining was then performed using methods described previously (Boyd and Matsubara 1996; Xu et al. 2005). For each case, proper placement of the array in V1 was confirmed by observing that the array lay within the CO blob region of the tissue (Supplementary Fig. S1),1 which corresponds to area V1 in the bush baby (Xu et al. 2005). All electrode positions were subsequently reconstructed through serial 1sections (Supplementary Fig. S2) to determine their exact location in V1. Although depth is more difficult to calculate relative to cortical layers in tangential reconstructions, several pieces of information helped us. First, three holes were made at the edge of the sections to align all sections after cutting. The first section was cut thicker (∼150 μm) to align blood vessel patterns with digital photographs taken before the insertion of the array. Second, cortical layers show distinct differences in CO staining in any plane of cut with layer 3B showing the patchy CO pattern known as the CO blobs, layer 3C (4B of Brodmann) staining more lightly and layer 4 (4C of Brodmann) showing a more uniform dark CO staining pattern. For CO, layer 5 stains lightly and layer 6 stains more darkly and shows hints of patches that align with the CO blobs in layer 3B. These laminar differences helped us determine depth. Third, we knew, based on prior measurements, the total depth of cortex and the approximate width of each layer and so could calculate the likely layer of the terminal point of each electrode in the array. Reconstructions showed that no electrode penetrated below layer 4 of cortex and the vast majority were located in layer 3.
Stimulus conditions
A 21-in Sony (Tokyo) Trinitron monitor with a refresh rate set to 120 Hz was used to present the stimuli. Good orientation tuning (see following text) was used as a criterion to select cells for receptive field mapping and was measured with 15 2-s trials of drifting sine wave gratings varying in orientation from 10 to 180° presented at 60% contrast at the preferred spatial (0.5 cycle/°) and temporal (2.0 Hz) frequencies previously reported for bush baby V1 cells (DeBruyn et al. 1993). These stimuli were interleaved with an equiluminant (68 cd/mm2) blank stimulus. To examine preferred spatial and temporal frequencies of individual neurons, we also tested 20 spatial frequencies from 0.1 to 2.0 cycle/° and 20 temporal frequencies from 0.5 to 10.0 Hz. The locations of the receptive fields of individual neurons were plotted with a manually controlled bar of light projected simultaneously on a tangent screen and on a plotting table (Supplementary Fig. S1) (see DeBruyn et al. 1993).
After receptive field plotting, stimuli were presented in the following blocks. First, two blocks each with 30 2-s trials consisting of 18 orientations and one blank (interleaved) were presented. Following these two ∼30-min blocks, the dark condition was presented. For this condition, the animal's eyes were covered with opaque paper, the room lights and monitor turned off and activity was recorded continuously for 10 min. This set of two stimulus blocks and a dark condition then were repeated again in the same order. Finally, at the end of the experiment a fifth stimulus block (identical to block 1) was presented again. Unless otherwise stated, in results, “blank” stimulus refers to the isoluminant gray screen stimulus.
Selection of neuron pairs
Three criteria were used for the initial selection of cells. First, the cell had to produce a vigorous response to hand plotting. Second, the response at the preferred orientation needed to be at least twice that at the nonpreferred orientation. Finally, the preferred orientation and spike waveform (Supplementary Fig. S3) of that cell needed to remain stationary for the duration of the entire recording session (∼3.5 h). These restrictions yielded 135 V1 neurons. From this population we selected only those cells with a minimum spike rate of 2 spike/s in response to the blank stimulus. This fourth criterion yielded a final total of 81 neurons. The average spike rates of these neurons in response to the preferred orientation and blank stimulus did not change throughout each experiment (Supplementary Fig. S4). Spike-time correlations were examined for all possible combinations of these 81 remaining neurons, yielding 1,236 neuron pairs.
In this study, when we discuss the activity of pairs of neurons, “preferred” stimulus is defined as the drifting grating stimulus with an orientation midway between the preferred orientations of each individual neuron in the two-neuron pair. This orientation was presented at a spatial frequency of 0.5 cycle/° and temporal frequency of 2.0 Hz. Although, spatial and temporal frequencies were not tailored to each pair, the frequencies used were similar to the average preferred spatial [0.54 ± 0.06 (SE) cycle/°; 81 neurons] and temporal frequencies (2.22 ± 0.19 Hz; 81 neurons) measured for the population. The average difference between the midpoint of the pairs’ spatial frequencies and the 0.5 cycle/° used for the stimulus was 0.16 ± 0.01 cycle/° (209 pairs). The average difference between the midpoint of the pairs’ temporal frequencies and the 2.0-Hz temporal frequency of the stimulus used was 0.82 ± 0.06 Hz (209 pairs). Additionally, although the stimulus was not at the preferred orientation for either neuron in the pair (unless the neurons had similar orientation tuning), it was important to use the midway orientation because driving both neurons in the pair to a similar extent reduces artifactual shifts in the CCH time lags that may be caused by changes in response onset for each neuron (Maldonado et al. 2000; Nowak et al. 1999). For this study, the average difference in orientation between the preferred orientations of the neurons in each pair and the orientation of the stimulus used to drive the neurons was 24.6 ± 1.1° (mean ± SE; 209 pairs).
Quantifying spike-time correlations
Two different correction methods were used to compute CCHs adjusted to take into account correlations caused by changes in spike rate. The method used for the bulk of the analyses in this paper was the jitter correction method (Harrison et al. 2007; Smith and Kohn 2008). This technique is proposed as a more effective method for removing spike-time correlations caused by the covariation of spike rates because trial-to-trial variations in spike count are taken into account. Correlations caused by spike interactions on scales greater than the size of the jitter window used (50 ms in this case) are effectively removed. First, raw coincidences are computed by plotting all the coincidences between all spikes within a trial for both neurons with millisecond resolution on a 2,000 × 2,000 plot (corresponding to the 2,000-ms stimulus duration), and the coincidences are then summed across trials. The raw CCH is then obtained by integrating across the diagonal of the plot. For the jitter correction method, each trial is divided into 50-ms bins and each spike within each bin is randomly replaced with another spike occurring in that jitter window but from another trial. This preserves the spike count for each trial and also the neurons’ peristimulus time histograms (PSTHs). The CCHs are then re-computed as described for the raw CCH. To obtain the corrected CCH, the averaged jittered CCHs from 100 resamples of the data were subtracted from the raw CCH. The data are presented as coincidences per spike (s-1). Fifty milliseconds was used as the jitter window because most spike-time correlations measured are correlated at scales shorter than this interval (Bair et al. 2001; Samonds and Bonds 2005; Kohn and Smith 2005). In results, the widths of jitter-corrected CCH peaks are compared. By definition, the jitter correction method restricts the overall width of CCH peaks. Because this method was used for all examined CCHs, however, this allowed for a fair comparison of the durations of more tightly correlated spiking events. As an additional control, however, CCHs were also computed with 100-ms jitter windows.
The CCH peaks, as well as the orientation tuning curves (described in the preceding text), were fit with Gaussian curves of the following form
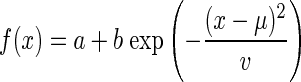 |
where a is the offset, b is the amplitude, μ is the mean, and v is the variance. The equation was fit by finding the values of the parameters that minimize the least squared error between the equation and the correlation data. These best-fit Gaussian curves were used to examine the amplitude, lag (commonly referred to as latency) and width at half-height of the CCHs. The average R2 value of the fits for the preferred and blank stimulus CCHs was 0.68 ± 0.01 (418 CCHs).
Peaks were considered significant if they exceeded the CCH mean by 2 SD, where CCH mean was measured over the range −100 to 100 ms. Using this criterion, 22% of preferred stimulus and 18% of blank stimulus peaks were significant. These significance percentages are consistent with other studies (de Oliveira et al. 1997; Maldonado et al. 2000; Nowak et al. 1999; Samonds et al. 2004). Based on anatomical connectivity, not all neurons in V1 are expected to share common connections; however, as a control to examine the influence of using these significance criteria to limit pairs used in the analysis, we also examined the relationships of preferred and blank stimulus spike-time correlations using all 1,236 pairs (Supplementary Fig. S5). When all neuron pairs, regardless of whether they met our significance criteria, were analyzed in relationship to orientation preference, no significant relationship was found between CCH peak amplitude and relative orientation preference (P > 0.05, 1-way ANOVA).
The second method used to correct the CCHs for spike-time correlations caused by the covariation of spike rates was a modification of the joint-PSTH (JPSTH) method (Aertsen et al. 1989; Zhou et al. 2008). This method removes correlations locked to the stimulus; however, unlike the jitter-corrected CCHs, influences of events correlated over longer time scales are not removed. Raw coincidences are plotted in the same manner as with the jitter correction method; however, the cross-product of each cell's peristimulus time histogram is subtracted from the raw JPSTH and normalized by the SD of the PSTHs to remove correlations caused by chance. The magnitude of spike-time correlation is also represented as the number of coincidences per spike (s-1). Gaussian curves also were fit to these data. Spike-time correlations measured with this shift predictor were included for comparison with previous studies and as a control for comparing preferred and blank stimulus CCH amplitudes. The analysis of these CCHs is predominantly presented in the supplement. CCHs computed with this method had significantly greater stimulus-evoked peak amplitudes than peaks computed with the jitter method (17.2 × 10-3 ± 0.5 × 10-3 s-1 vs. 9.6 × 10-3 ± 0.3 × 10-3 s-1; means ± SE; P < 10-23; Wilcoxon signed-rank test; 209 pairs). Correlations of preferred stimulus and blank CCH properties for correlograms computed with the JPSTH method are shown in Supplementary Fig. S4.
To compute mean spike rates for neuron pairs, we measured the geometric mean spike rate (GMSR) (Bair et al. 2001). This was measured as the square root of the product of each neuron's spike rate. Second-order linear partial correlation analyses were computed to compare correlation coefficients between CCH peak amplitudes of different pairs with GMSR controlled. All variables were z-normalized. Multiple regression analysis was used to compute the residuals of the CCH peak amplitudes controlling for the GMSRs. The partial correlation then represented the correlation of the residuals.
RESULTS
Detection of significant CCH peaks for preferred and blank stimuli
We examined spike-time correlations computed with jitter correction for all combinations of V1 neurons. Similar to previous studies examining spike-time correlations for neurons within a cortical area (Bair et al. 2001; Bruno and Sakmann 2006; Kohn and Smith 2005; Maldonado et al. 2000; Nowak et al. 1995; Samonds et al. 2005), CCH shapes varied greatly among neuron pairs, but the peaks were generally centered around 0-ms lag. Figure 1 shows the evoked and spontaneous CCH peaks from the preferred and blank (isoluminant gray screen) stimuli, respectively, computed for a representative neuron pair. Qualitative examination shows that the CCHs of this pair had peaks at similar lags, but the blank CCHs had lower amplitudes and broader widths at half-height than the preferred stimulus CCHs. In the following text, we describe efforts to quantify these observations for the population.
FIG. 1.
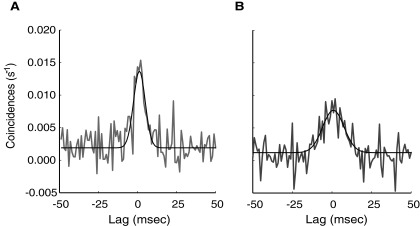
Examples of cross-correlation histograms (CCHs) for preferred and blank stimulus conditions. A: CCH in response to the preferred stimulus. B: CCH from the same neuron pair in response to the blank stimulus. The CCHs were corrected with the jitter correction method. On qualitative examination, it was evident that whereas the lags were similar for these 2 CCHs, they had different amplitudes and widths. For this neuron pair, the blank stimulus CCH was lower in amplitude (0.0079 vs. 0.0134 s-1) and broader in width (17.2 vs. 9.8 ms) than the preferred stimulus CCH. Gray line, CCH; black line, best fit Gaussian curve.
Using our four criteria for selecting cell pairs for analysis (see methods), we examined 1,236 pairs from 81 neurons. CCH peaks were considered significant if a Gaussian curve fit to the peak was >2 SD above the mean of the CCH integrated over lag ranges of −100 to 100 ms (Maldonado et al. 2000; Nowak et al. 1999). Of the V1 pairs, 22% (273/1236) had a significant CCH peak in response to the preferred stimulus with an average difference in peak amplitude and CCH mean of 7.0 × 10-3 ± 0.2 × 10-3 s-1 (Fig. 2A). Eighteen percent (221/1236) of the pairs had significant blank response peaks which, on average, exceeded the CCH mean by 4.6 × 10-3 ±0.1 × 10-3 s-1 (Fig. 2B). Seventeen percent (209/1236) of the neuron pairs had significant peaks in response to both stimulus conditions and these neuron pairs were used for the subsequent analyses.
FIG. 2.
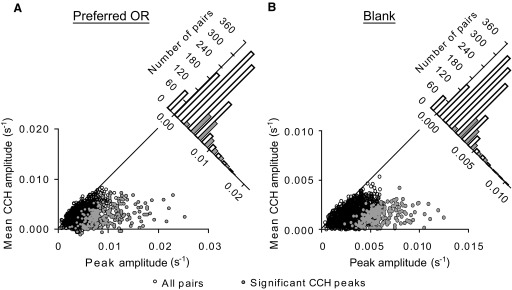
Detection of significant spike-time correlation peaks. A: each point in the plot represents the CCH peak and mean (over range-100 to 100 ms) for one of the 1236 pairs used in the analysis. Twenty-two percent (273/1236) of the pairs had significant CCH peaks with an average difference in peak correlation and CCH mean of 7.0 × 10−3 ± 0.2 × 10−3 s−1 (mean ± SE). Open black circles, all neuron pairs; closed gray circles, pairs with significant CCH peaks. The plot in the top right corner integrates the lower plot along the diagonal. This diagonal plot demonstrates what proportion of pairs had significant spike-time correlations relative to the total number of cell pairs. The plot's x-axis represents peak correlation-mean correlation. White bars, all pairs; gray bars, significant CCH peaks. B: eighteen percent of the pairs (221/1236) had significant CCH peaks in response to the blank stimulus exceeding noise by 4.6 × 10−3 ± 0.1 × 10−3 s−1 (mean ± SE). Same conventions used as in A.
Correlation of CCH peak amplitudes, lags, and widths
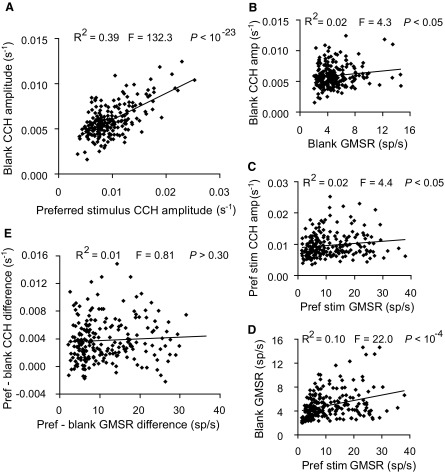
The relationship of spike-time correlation peak amplitudes for the two stimulus conditions was examined first. The CCH peak amplitudes from pairs in response to the preferred and the blank stimuli were strongly correlated (Fig. 3A; regression slope = 0.294; 95% confidence interval = ±0.050; R2 = 0.394; F = 132.3; P < 10-23) with a Pearson correlation coefficient of 0.623. Because studies have demonstrated links between CCH peaks and spike rate (de la Rocha et al. 2007; Kruger and Aiple 1988), we examined whether the correlated changes in GMSR (Bair et al. 2001) for pairs between both conditions could account for the strong correlation observed between the CCH peak amplitudes. Similar to the previous reports, the spike-time correlation peaks were significantly but weakly correlated with the pairs’ GMSRs for both conditions (Fig. 3, B and C; Preferred stimulus: Pearson correlation = 0.143; regression slope = 1 × 10-4; 95% confidence interval = ±1 × 10-4; R2 = 0.021; F = 4.3; P < 0.05; Blank stimulus: Pearson correlation = 0.144; regression slope = 1 × 10-4; 95% confidence interval = ±1 × 10-4; R2 = 0.021; F = 4.4; P < 0.05). In addition, the GMSRs of the cell pairs were significantly correlated between the preferred and blank stimulus conditions (Fig. 3D; Pearson correlation = 0.310; regression slope = 0.091; 95% confidence interval = ±0.038; R2 = 0.096; F = 22.0; P < 10-4).
FIG. 3.
Correlation of preferred stimulus and blank CCH peak amplitudes. A: each point represents the CCH peak amplitudes for 1 of the 209 pairs that had a significant spike-time correlation. The CCH peak amplitudes were strongly correlated for the preferred and blank stimuli (Pearson correlation = 0.623; regression slope = 0.294; 95% confidence interval = ±0.040; R2 = 0.394; F = 132.3; P < 10-23). B: preferred stimulus CCH peak amplitudes were correlated with the geometric mean spike rates (GMSRs) of these pairs (Pearson correlation = 0.143; regression slope = 1.0 × 10-4; 95% confidence interval = ±4 × 10-5; R2 = 0.021; F = 4.3; P < 0.05). C: CCH amplitudes and GMSRs were also correlated for the blank condition (Pearson correlation = 0.144; regression slope = 1 × 10-4; 95% confidence interval = ±1 × 10-4; R2 = 0.021; F = 4.4; P < 0.05). D: similarly the pairs’ GMSRs were significantly correlated between both stimulus conditions (Pearson correlation = 0.310; regression slope = 0.091; 95% confidence interval = ±0.038; R2 = 0.096; F = 22.0; P < 10-4). E: the difference in CCH amplitude for the preferred and blank stimulus CCH amplitudes was not significantly correlated with the difference in GMSRs for the 2 conditions (Pearson correlation = 0.062; regression slope = 2 × 10-5; 95% confidence interval = ±2 × 10-5; R2 = 0.006; F = 0.81; P > 0.30), suggesting that the correlation of CCH amplitudes was independent of changes in spike count.
The degree to which changes in spike rate might account for the relationship observed between the CCH peaks from both conditions was examined next. The purpose was to determine whether the correlation between the preferred and blank stimulus CCH peak amplitudes was secondary to the correlation observed between the GMSRs for the two conditions. First, we observed that the difference between preferred and blank stimulus CCH peak amplitudes was not significantly correlated with the difference in GMSR for these conditions (Fig. 3E; Pearson correlation = 0.062; regression slope = 2 × 10-5; 95% confidence interval = ±2 × 10-5; R2 = 0.006; F = 0.81; P > 0.30). Second, using z-normalized values we statistically controlled for the effects of spike count on the correlation of CCH peaks between conditions using partial correlation analysis. This test suggests that even with differences in GMSR controlled, the CCH peak amplitudes for the two stimulus conditions were correlated with a Pearson correlation of 0.619, only 1.04% lower than without the GMSR controlled. These data, collectively, suggest that spike-time correlation amplitudes were highly correlated between the responses evoked by the preferred and the blank stimuli and that this relationship was largely independent of changes in spike rate.
We next examined whether there were consistent differences in spike-time correlation strength for the two stimulus types (evoked vs. blank). For the 209 neuron pairs, CCHs to the blank stimulus had, on average, 38.5% lower peak amplitudes than CCHs to the preferred stimulus (5.9 × 10-3 ± 0.2 × 10-3 s-1 vs. 9.6 × 10-3 ± 0.3 × 10-3 s-1; P < 10-23; Wilcoxon signed-rank test). For these neuron pairs, the average difference between preferred and blank stimulus CCH amplitudes was 3.7 × 10-3 s-1 ± 0.2 × 10-3 s-1, which was significantly different from 0 (P < 10-23; Wilcoxon signed-rank test). However, a similar trend was observed with the neuron pairs’ GMSRs. The blank stimulus GMSRs were on average 56.4% lower than the preferred stimulus GMSRs (5.01 ± 0.16 spike/s vs. 11.49 ± 0.55 spike/s; P < 10-23; Wilcoxon signed-rank test) with a mean difference of 6.48 spike/s (P < 10-15; Wilcoxon signed-rank test).
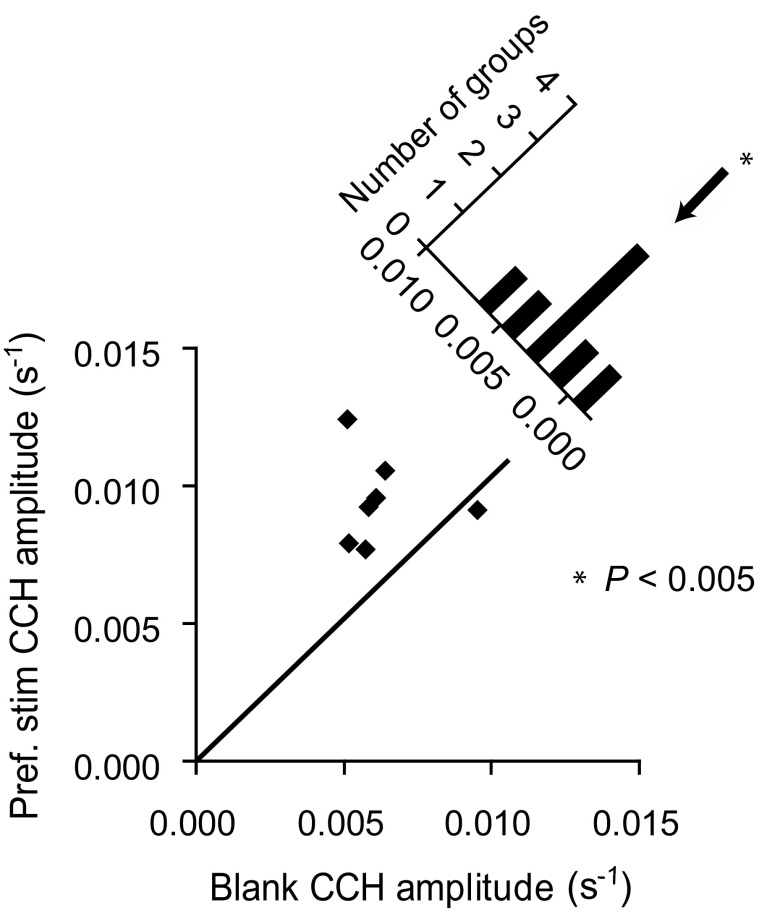
To control for spike rates, the CCHs measured for the two conditions were divided into groups based on similar GMSRs (2–16 spike/s in 2 spikes/s intervals), and the peak amplitudes were compared within each group for the two different stimulus conditions (Fig. 4). Thus in each case preferred and blank stimulus CCHs of similar GMSR were compared. For six of the seven groups tested, the CCH peaks for the blank stimulus had lower mean amplitudes than the CCH peaks for the preferred stimulus. The mean difference between the peak amplitudes for the two conditions was 3.0 × 10-3 ± 0.6 × 10-3 s-1 (P < 0.005; Wilcoxon signed-rank test), which represents the difference in preferred and blank stimulus amplitudes with spike rate controlled. This value was 31% of the average CCH peak amplitude seen for the preferred stimulus.
FIG. 4.
CCH peak amplitudes for preferred vs. blank stimuli with GMSR controlled. The pairs were divided into 7 groups based on GMSR ranging from 2 to 16 spike/s in 2-spike/s intervals. Each point in the bottom plot represents the average CCH amplitude for all preferred stimulus pairs with GMSR at a given range and average CCH amplitude for all blank stimulus pairs with GMSR at the same range. Six of the 7 groups tested had CCH peaks to the blank stimulus that were, on average, lower in mean amplitude than the CCH peaks in response to the preferred stimulus. The diagonal plot displays the distribution of the differences obtained from subtracting the blank CCH amplitudes from the preferred stimulus CCH amplitudes. The x axis of this plot represents preferred stimulus CCH amplitude - blank stimulus CCH amplitude. The mean difference between the preferred and blank stimulus CCH peak amplitudes for these groups was significant, 3.0 × 10-3 ± 0.6 × 10-3 s-1 (P < 0.005; Wilcoxon signed-rank test), which accounted for 31% of the spike-time correlations observed for the preferred stimulus. This analysis included 145 preferred stimulus CCHs and 209 blank stimulus CCHs.
The correlation of CCH peak lags was examined next for both stimulus conditions. Similar to other studies examining spike-time correlations for pairs of neurons in the same visual area, the peaks for both conditions generally occurred with near 0-ms lag (0.41 ± 0.19 ms) (Bair et al. 2001; Gray et al. 1989; Kohn and Smith 2005; Kruger and Aiple 1988; Nowak et al. 1999; Samonds and Bonds 2005). The lags of the CCH peaks obtained for the preferred and blank stimuli were highly correlated (Fig. 5A; Pearson correlation = 0.740; regression slope = 0.68; 95% confidence interval = ±0.08; R2 = 0.553; F = 250.1; P < 10-23). For the neuron pairs examined, the CCH peak lags obtained for the blank stimulus were on average not different from the lags obtained for the preferred stimulus (0.41 ± 0.18 vs. 0.42 ± 0.19 ms; P > 0.50; Student's t-test). The mean difference in lag between the preferred and blank stimulus peaks of each pair was 0.003 ± 0.132, ms not different from 0 ms (P > 0.80; Wilcoxon signed-rank test). Thus the CCH peak lags were strongly correlated with no consistent difference in peak position between the two conditions.
FIG. 5.
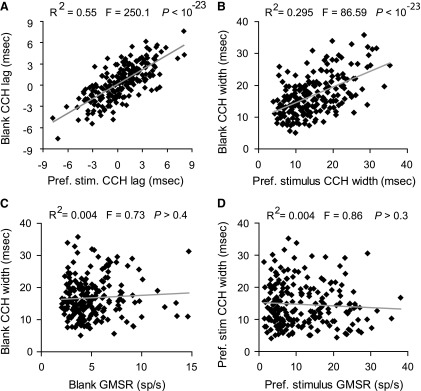
Correlation of CCH peak lags and widths, at half-height, for preferred and blank stimuli. A: the lags of the CCH peaks were strongly correlated between preferred and blank stimulus conditions (Pearson correlation = 0.740; regression slope = 0.68; 95% confidence interval = ±0.08; R2 = 0.553; F = 250.1; P < 10-23). B: CCH widths were strongly correlated between the 2 stimulus conditions (Pearson correlation = 0.543; regression slope = 0.49; 95% confidence interval = ±0.10; R2 = 0.294; F = 86.6; P < 10-23). C: in response to the blank stimulus, spike rate and width at half-height were not significantly correlated (Pearson correlation = 0.059; regression slope = 0.155; 95% confidence interval = ±0.356; R2 = 0.004; F = 0.73; P > 0.4). D: the same was observed for the preferred stimulus (Pearson correlation = −0.064; regression slope = −0.054; 95% confidence interval = ±0.113; R2 = 0.004; F = 0.86; P > 0.3), suggesting that the broader blank CCHs were not an artifact of decreased spike rate.
CCH widths also were strongly correlated for the two conditions (Fig. 5B; Pearson correlation = 0.543; regression slope = 0.49; 95% confidence interval = ±0.10; R2 = 0.294; F = 86.6; P < 10-23). When examined for the 209 neuron pairs, the CCHs were 14.5% wider in response to the blank stimulus compared with those evoked by the preferred stimulus (16.77 ± 0.42 vs. 14.65 ± 0.46 ms; P < 0.001; Wilcoxon signed-rank test). When preferred stimulus CCH widths were subtracted from blank stimulus CCH widths for the same neuron pairs, the average difference was 2.14 ± 0.06 ms, which was significantly different from 0 ms (P < 10-4; Wilcoxon signed-rank test). To test whether this difference could have been due to changes in spike rate, we examined the correlation of CCH width with spike rate for the two stimuli. Figure 5, C and D, shows that CCH widths were not significantly correlated with the spike rates for either condition (blank stimulus: Pearson correlation = 0.059; regression slope = 0.155; 95% confidence interval = ±0.356; R2 = 0.004; F = 0.73; P > 0.4; preferred stimulus: Pearson correlation = −0.064; regression slope = −0.054; 95% confidence interval = ±0.113; R2 = 0.004; F = 0.86; P > 0.3), suggesting that the broader CCHs seen in response to the blank stimulus were not an artifact of changes in spike rate.
As mentioned earlier, use of jitter correction has different effects on events correlated over shorter versus longer time scales. This may differentially influence the computation of CCH widths for preferred and blank stimulus conditions. To test the effect the correction method had on the comparison of CCH widths for the two stimulus conditions, we first compared the widths of CCHs computed using a jitter window of 100 ms instead of 50 ms. Even with this jitter window, the blank CCHs were 9.4% broader than the preferred stimulus CCHs (17.79 ± 0.51 vs. 16.26 ± 0.47 ms; P < 0.01; Wilcoxon signed-rank test). As an additional control, we also compared CCHs measured with shuffle correction. These CCHs also were broader, by 14.6%, for the blank compared with the preferred stimulus condition (Supplementary Fig. S6; 22.84 ± 0.62 vs. 19.93 ± 0.55 ms; P < 0.001; Wilcoxon signed-rank test).
We also asked if the spike-time correlations seen with the blank isoluminant screen might be explained by excitation caused by the luminance (68 cd/mm2) or refresh rate of the screen (120 Hz). Significant spike-time correlations were detected for the dark condition, and these CCH peaks were strongly correlated with the CCHs for the blank stimulus (Supplementary Fig. S7), showing that the spike-time correlations seen between the blank and grating stimuli were not induced by factors specific to the luminance level or refresh rate of the screen. The dark CCHs also were highly correlated with the CCHs for the preferred stimulus (Supplementary Fig. S8).
Relationship to relative orientation preference and receptive field overlap
Several previous studies have suggested that spike-time correlation peaks depend on the relative orientation preference and the receptive field overlap of the neurons in the pair (Engel et al. 1990; Gray et al. 1989; Kohn and Smith 2005; Samonds et al. 2004; Schwartz and Bolz 1991; Ts'o et al. 1986). To further examine the spike-time correlations evoked by preferred and blank stimuli, we next determined whether the correlations for both conditions showed a similar dependence on orientation preference or receptive field overlap between the cell pairs.
To test the effect of orientation tuning on the spike-time correlations, we correlated CCH peak amplitude with the difference in each neuron's orientation preference (Fig. 6A). CCH peak amplitudes showed a significant correlation with similarity in orientation preference for both the preferred (Pearson correlation = 0.403; regression slope = 1 × 10-4; 95% confidence interval = ±5 × 10-5; R2 = 0.162; F = 40.3; P < 1 × 10-4) and blank stimuli (Pearson correlation = 0.239; regression slope = 3 × 10-5; 95% confidence interval = ±2 × 10-4; R2 = 0.060; F = 12.6; P < 0.001). Although the strong spike-time correlations observed among pairs preferring similar orientations in response to the preferred stimulus could have been due to a higher spike rate given our testing method (see methods), this explanation would not account for the same pattern observed for the pairs in response to the blank stimulus. Additionally, the difference in average peak CCH amplitude for the preferred versus blank stimuli was significantly correlated with similarity in orientation preference (Pearson correlation = 0.199; regression slope = 1 × 10-5; 95% confidence interval = ±5 × 10-6; R2 = 0.040; F = 9.3; P < 0.01). Thus preferred stimulus CCH amplitudes deviated from the spontaneous amplitudes most when both neurons were driven well by the stimulus. This difference in CCH peak amplitude was also correlated with similarity in orientation preference even when GMSR was controlled with partial correlation analysis (Pearson correlation = 0.192; 3.5% lower than without GMSR controlled). This result suggests that it is not only the presence of the drifting grating that correlates spike times, but also its orientation relative to the preferred orientations of the neurons in the pair.
FIG. 6.
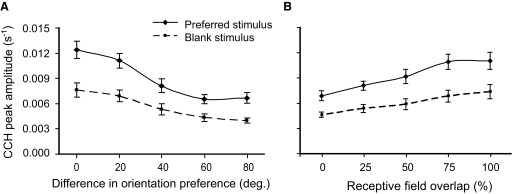
Spike-time correlations for preferred and blank stimuli in relationship to orientation tuning and receptive field overlap. A: CCH peak amplitudes were significantly correlated with similarity in orientation preference for both the preferred (Pearson correlation = 0.403; regression slope = 1 × 10-4; 95% confidence interval = ±5 × 10-5; R2 = 0.162; F = 40.3; P < 1 × 10-4) and blank stimuli (Pearson correlation = 0.239; regression slope = 3 × 10-5; 95% confidence interval = ±2 × 10-4; R2 = 0.060; F = 12.6; P < 0.01). On the x axis, the group labeled 0° contains pairs that differed in preferred orientation 0 and 10°, the group labeled 20° contains pairs differing in preferred orientation by 20 and 30°, etc. B: the relative receptive field overlap of the neurons in the pair was determined by measuring the percentage of each receptive field overlapped by the other field and averaging the 2 percentages for both neurons. The 50% group contains all pairs that had relative receptive field overlap >25% and ≤50%. The same holds for the other groups designated on the x axis. CCH amplitude was significantly correlated with receptive field overlap for both the preferred (Pearson correlation = 0.381; regression slope = 1 × 10-4; 95% confidence interval = ±1 × 10-5; R2 = 0.145; F = 21.1; P < 1 × 10-4) and blank stimuli (Pearson correlation = 0.21; regression slope = 3 × 10-5; 95% confidence interval = ±2 × 10-5; R2 = 0.048; F = 9.1; P < 0.01). —, preferred stimulus; - - -, blank stimulus.
Next, the effect of receptive field overlap on the spike-time correlations for both conditions was examined (Fig. 6B). CCH amplitude showed a significant correlation with receptive field overlap for both the preferred (Pearson correlation = 0.381; regression slope = 1 × 10-4; 95% confidence interval = ±1 × 10-5; R2 = 0.145; F = 21.1; P < 1 × 10-4) and blank stimuli (Pearson correlation = 0.210; regression slope = 3 × 10-5; 95% confidence interval = ±2 × 10-5; R2 = 0.048; F = 9.1; P < 0.01). Thus both evoked and blank spike-time correlations are affected by the degree to which neurons in a pair share receptive field space and orientation preference.
DISCUSSION
In this study we asked whether spike-time correlations seen in pairs of primate V1 neurons that are driven by a moving grating of preferred orientation were similar or different from the correlations seen between these same cell pairs when responding spontaneously to a blank stimulus or to no stimulus. Strong correlations were observed between these conditions in the amplitudes, lags, and widths of their CCHs. These relationships were strongest for cell pairs whose receptive fields overlapped and that preferred a similar orientation. In the following text, we consider the significance of these key findings in light of results published by others.
Comparison with previous studies
Although a number of investigations have detected significant spike-time correlations in spontaneous cortical activity, results reported have been conflicting. In some cases, spike-time correlations were shown to be as strong or stronger for the spontaneous than for the preferred stimulus condition (Bair et al. 2001; de Oliveira et al. 1997). In other cases, investigators have reported the reverse, namely that correlations were weaker in the blank condition (Kohn and Smith 2005; Maldonado et al. 2000, 2008; Nowak et al. 1999). One difference between the current study and others is that we controlled for spike-time correlations caused by spike counts using a more conservative approach, the jitter correction method. This difference is important because the jitter correction method takes into consideration the trial-by-trial covariation in neuron spike counts and therefore is a more reliable correction technique than the more commonly used shuffle corrector (Aertsen et al. 1989). By using the jitter correction we were able to examine spike-time correlations without the influence of loosely-correlated events. Removing such events is important because slow cortical oscillations in activity have been heavily documented in resting and anesthetized brains (Vincent et al. 2007; for review, see Faisal et al. 2008).
Another difference between the current study and previous studies is that we directly compared the blank stimulus responses of cell pairs that had overlapping receptive fields and preferred the same orientations with those that did not. This distinction was not made in other studies (Bair et al. 2001; de Oliveira et al. 1997; Kohn and Smith 2005; Maldonado et al. 2000, 2008; Nowak et al. 1999). Visual cortex spike-time correlations are significantly stronger for preferred compared with nonpreferred stimuli (Gray et al. 1989; Kohn and Smith 2005; Samonds et al. 2004; Zhou et al. 2008). This difference could also account for our finding that spike-time correlations for cell pairs in response to their preferred stimulus were significantly stronger than those evoked by the blank stimulus. Regardless, our study demonstrates that spike-time correlations under blank stimulus conditions show a strong similarity to those under preferred stimulus conditions. So why is this so?
Sources of spike-time correlation
That the underlying circuitry of the visual cortex promotes spike-time correlations is not very surprising considering the important role that these correlations appear to play during normal cortical development. In the developing brain, waves of spontaneous correlated firing have been observed among retinal ganglion cells specific to each eye and this patterned activity helps properly guide the development of these cells’ axons into a laminar pattern within their thalamic targets (Ramoa et al. 1988; Stellwagen and Shatz 2002; Vislay-Meltzer et al. 2006). Cells that fire together tend to wire together, a relationship mediated by the N-methyl-d-aspartate (NMDA) receptor (Constantine-Patton and Cline 1998; Vislay-Meltzer et al. 2006). Disruption of the correlated activity, or of the activity of this receptor, severely perturbs the normal development of the visual cortex (Kleshevnikov et al. 1997; Volgushev et al. 1997; Voronin et al. 1996).
Several features of the cortical circuit have been implicated in correlating spikes. Gap junctions correlate neuronal spike times and their knockout in the mouse reduces this correlation (Christie et al. 2005). Both feedforward (Alonso et al. 1996; Castelo-Branco et al. 1998) and feedback (Sillito et al. 1994) pathways have properties that correlate spike-times among functionally related populations of neurons. In addition, specific subsets of neurons, such as interneurons (Merriam et al. 2005; Nomura et al. 2003), astrocytes (Fellin et al. 2004), and layer 5 “chattering cells” (Gray and McCormick 1996) have been implicated in generating spike-time correlations. Even when retina (Trong and Rieke 2008) and cortex (Reyes et al. 2003) are removed and examined in vitro without the influences of visual stimuli or other brain areas, strong spike-time correlations are detected.
Nevertheless, evoked responses from a preferred stimulus generate greater correlation than is seen under the blank or no-stimulus conditions, suggesting that stimulus properties must be taken into account even when the role of spike counts can be ruled out. Obviously stimuli cannot generate responses without an underlying cortical architecture, which makes it difficult to separate the relative influences of both stimulus and circuitry particularly given the tight coupling in the visual system. Feedforward pathways from the retina are in a good position, however, to modulate early cortical spike-time correlations. Cells in retina, LGN, and V1 have strongly correlated spike times that could be related to properties of the stimulus (Dan et al. 1998; Meister et al. 1995; Pillow et al. 2008; Samonds et al. 2004; Zhou et al. 2008). In addition, correlated spikes among neurons in retina and LGN are more effective at eliciting spikes in their higher area targets than spikes not correlated in time (Alonso et al. 1996; Singer and Bedworth 1973). Thus appropriate stimulus features may add to the correlation of spike times that reflect underlying circuitry.
Functional implications
Neuronal response amplitudes exhibit large trial-to-trial fluctuations (Bair et al. 2001; Kohn and Smith 2005; Zohary et al. 1994; for review see Ermentrout et al. 2008; Faisal et al. 2008). Two processes that have been heavily implicated in this variability are the initial state of a neuron or circuit and noise present in the signal (for review, see Ermentrout et al. 2008; Faisal et al. 2008). Our data support the idea that, like other neuronal response properties (Chiu and Weliky 2002; Fiser et al. 2004; Haider et al. 2007; Kenet et al. 2003), spike-time correlations present during spontaneous cortical activity are strongly related to the spike-time correlations evoked by a preferred stimulus. What remains to be determined, however, is what influence this ongoing activity has on the output of the system. Because the spike-time correlations we observed for the preferred stimulus were similar to, albeit stronger than, the correlations evoked by the blank stimulus, it could be argued that the stimulus-evoked spike-time correlations are simply a reflection of background activity and not involved in coding per se especially given the relative rarity of spike-time correlations.
Alternatively, it can be argued that ongoing cortical activity is required for proper cortical coding. Activating neurons is a metabolically expensive process and maintaining a proper level of neuron activity is essential for normal brain function (Lennie 2003). The brain may have evolved the ability to use noise to keep neurons near their firing thresholds (for review, see Faisal et al. 2008). To the detriment of some of the information in the signals, the ongoing activity would ensure propagation of weaker signals, those that would otherwise not yield downstream responses. In fact, correlated spikes have been suggested to yield supralinear responses in the neurons onto which they converge (Alonso et al. 1996; Azouz 2005; Bruno and Sakmann 2006; Castelo-Branco et al. 1998). Given that our spike times correlated to a significantly greater degree than expected by chance for the blank stimulus, it is likely the spike-time correlations have an impact on the responsiveness of the circuit during normal viewing conditions. If two neurons are tuned to different orientations, for example, our study and others show that it is difficult to generate significant spike-time correlations (Gray et al. 1989; Kohn and Smith 2005; Samonds et al. 2004; Zhou et al. 2008). Even when summed with the spontaneous correlations, these spikes may not have an influence on downstream spiking. Neurons tuned to similar orientations, however, will produce strong spike-time correlations in response to the preferred stimulus and, when summed with the strong correlations already present in spontaneous activity, may have a pronounced effect on downstream neuron responses.
Clearly, much remains to be determined to understand the potential roles of these spike-time correlations in visual processing. Because neurons have specific responses to correlated spike times during development and in adulthood (Alonso et al. 1996; Bruno and Sakmann 2006; Castelo-Branco et al. 1998; Fujisawa et al. 2008; Kleshevnikov et al. 1997; Volgushev et al. 1997; Voronin et al. 1996), it is possible that the brain has evolved sparse coding mechanisms to utilize correlated spikes as information-rich codes that efficiently propagate through the visual hierarchy (Lennie 2003; Pillow et al. 2008; Vinje and Gallant 2000; Weliky et al. 2003). It remains to be determined, however, whether this information can be propagated usefully beyond V1.
GRANTS
The experiments described in this manuscript were made possible by support from National Institutes of Health Grants EY-01778 to V. A. Casagrande and EY-08126 to A. B. Bonds. Core facilities were funded by NIH Grants HD-155052 and EY-014680-03. Students were supported by NIH Training Grants EY-007135 to W. J. Jermakowicz and GM-07347 to I. Khaytin.
Supplementary Material
Acknowledgments
We are grateful to J. Mavity-Hudson for expert assistance with histological procedures and preparation of figures, and M. Feurtado for excellent assistance with animal care and anesthesia. In addition, the assistance of Drs. Z. Zhou and M. R. Bernard was invaluable during the recording sessions. We also are grateful to Dr. M. Wallace, R. Marion, and R. Fan for helpful comments on earlier versions of this manuscript.
Present address of Dr. X. Chen: Department of Molecular and Cell Biology, U.C. Berkeley, 145 LSA, Berkeley, CA 94720.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Aertsen et al. 1989.Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity.” J Neurophysiol 61: 900–917, 1989. [DOI] [PubMed] [Google Scholar]
- Alonso et al. 1996.Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature 383: 815–819, 1996. [DOI] [PubMed] [Google Scholar]
- Angelucci et al. 2002.Angelucci A, Levitt JB, Walton EJ, Hupe JM, Bullier J, Lund JS. Circuits for local and global signal integration in primary visual cortex. J Neurosci 22: 8633–8646, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz 2005.Azouz R Dynamic spatiotemporal synaptic integration in cortical neurons neuronal gain, revisited. J Neurophysiol 94: 2785–2796, 2005. [DOI] [PubMed] [Google Scholar]
- Azouz and Gray 2003.Azouz R, Gray CM. Adaptive coincidence detection and dynamic gain control in visual cortical neurons in vivo. Neuron 37: 513–523, 2003. [DOI] [PubMed] [Google Scholar]
- Bair and Koch 1996.Bair W, Koch C. Temporal precision of spike trains in extrastriate cortex of the behaving macaque monkey. Neural Comput 8: 1185–1202, 1996. [DOI] [PubMed] [Google Scholar]
- Bair et al. 2001.Bair W, Zohary E, Newsome WT. Correlated firing in macaque visual area MT: time scales and relationship to behavior. J Neurosci 21: 1676–1697, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederlack et al. 2006.Biederlack J, Castelo-Branco M, Neuenschwander S, Wheeler DW, Singer W, Nikolic D. Brightness induction: rate enhancement and neuronal synchronization as complementary codes. Neuron 52: 1073–1083, 2006. [DOI] [PubMed] [Google Scholar]
- Bonds et al. 1987.Bonds AB, Casagrande VA, Norton TT, Debruyn EJ. Visual resolution and sensitivity in a nocturnal primate (galago) measured with visual evoked potentials. Vision Res 27: 845–857, 1987. [DOI] [PubMed] [Google Scholar]
- Boyd and Matsubara 1996.Boyd JD, Matsubara JA. Laminar and columnar patterns of geniculocortical projections in the cat: relationship to cytochrome oxidase. J Comp Neurol 365: 659–682, 1996. [DOI] [PubMed] [Google Scholar]
- Bruno and Sakmann 2006.Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science 312: 1622–1627, 2006. [DOI] [PubMed] [Google Scholar]
- Casagrande and Kaas 1994.Casagrande VA, Kaas JH. The afferent, intrinsic and efferent connections of primary visual cortex in primates. In: Cerebral Cortex, New York: Plenum, p. 201–259, 1994.
- Castelo-Branco et al. 1998.Castelo-Branco M, Neuenschwander S, Singer W. Synchronization of visual responses between the cortex, lateral geniculate nucleus, and retina in the anesthetized cat. J Neurosci 18: 6395–6410, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu and Weliky 2002.Chiu C, Weliky M. Relationship of correlated spontaneous activity to functional ocular dominance columns in the developing visual cortex. Neuron 35: 1123–1134, 2002. [DOI] [PubMed] [Google Scholar]
- Christie et al. 2005.Christie JM, Bark C, Hormuzdi SG, Helbig I, Monyer H, Westbrook GL. Connexin36 mediates spike synchrony in olfactory bulb glomeruli. Neuron 46: 761–772, 2005. [DOI] [PubMed] [Google Scholar]
- Collins et al. 2005.Collins CE, Xu X, Khaytin I, Kaskan PM, Casagrande VA, Kaas JH. Optical imaging of visually evoked responses in the middle temporal area after deactivation of primary visual cortex in adult primates. Proc Natl Acad Sci USA 102: 5594–5599, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton and Cline 1998.Constantine-Paton M, Cline HT. LTP and activity-dependent synaptogenesis: the more alike they are, the more different they become. Curr Opin Neurobiol 8: 139–148, 1998. [DOI] [PubMed] [Google Scholar]
- Dan et al. 1998.Dan Y, Alonso JM, Usrey WM, Reid RC. Coding of visual information by precisely correlated spikes in the lateral geniculate nucleus. Nat Neurosci 1: 501–507, 1998. [DOI] [PubMed] [Google Scholar]
- de la Rocha et al. 2007.de la Rocha J, Doiron B, Shea-Brown E, Josic K, Reyes A. Correlation between neural spike trains increases with firing rate. Nature 448: 802–806, 2007. [DOI] [PubMed] [Google Scholar]
- de Oliveira et al. 1997.de Oliveira SC, Thiele A, Hoffmann KP. Synchronization of neuronal activity during stimulus expectation in a direction discrimination task. J Neurosci 17: 9248–9260, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyn et al. 1993.Debruyn EJ, Casagrande VA, Beck PD, Bonds AB. Visual resolution and sensitivity of single cells in the primary visual cortex (V1) of a nocturnal primate (bush baby): correlations with cortical layers and cytochrome oxidase patterns. J Neurophysiol 69: 3–18, 1993. [DOI] [PubMed] [Google Scholar]
- Engel et al. 1990.Engel AK, Konig P, Gray CM, Singer W. Stimulus-dependent neuronal oscillations in cat visual cortex: inter-columnar interaction as determined by cross-correlation analysis. Eur J Neurosci 2: 588–606, 1990. [DOI] [PubMed] [Google Scholar]
- Ermentrout et al. 2008.Ermentrout GB, Galan RF, Urban NN. Reliability, synchrony and noise. Trends Neurosci 31: 428–434, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisal et al. 2008.Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci 9: 292–303, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin et al. 2004.Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43: 729–743, 2004. [DOI] [PubMed] [Google Scholar]
- Fiser et al. 2004.Fiser J, Chiu C, Weliky M. Small modulation of ongoing cortical dynamics by sensory input during natural vision. Nature 431: 573–578, 2004. [DOI] [PubMed] [Google Scholar]
- Fries et al. 2001.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291: 1560–1563, 2001. [DOI] [PubMed] [Google Scholar]
- Fujisawa et al. 2008.Fujisawa S, Amarasingham A, Harrison MT, Buzsaki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat Neurosci 11: 823–833, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray et al. 1989.Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338: 334–337, 1989. [DOI] [PubMed] [Google Scholar]
- Gray and McCormick 1996.Gray CM, McCormick DA. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science 274: 109–113, 1996. [DOI] [PubMed] [Google Scholar]
- Haider et al. 2007.Haider B, Duque A, Hasenstaub AR, Yu Y, McCormick DA. Enhancement of visual responsiveness by spontaneous local network activity in vivo. J Neurophysiol 97: 4186–4202, 2007. [DOI] [PubMed] [Google Scholar]
- Harrison et al. 2007.Harrison M. T, Amarasingham A, Geman S. Jitter methods for investigating spike train dependencies. Comput Syst Neurosci Abstr III-17, 2007.
- Jermakowicz et al. 2006.Jermakowicz WJ, Chen X, Khaytin I, Zhou Z, Bernard MR, Bonds AB, Casagrande VA. Is local neuronal synchrony better at discriminating stimulus spatial frequency in primary visual cortex (V1) than firing rate? Soc Neurosci 734. 12/J 14, 2006.
- Jermakowicz et al. 2007.Jermakowicz WJ, Chen X, Khaytin I, Zhou Z, Bernard MR, Bonds AB and Casagrande VA. Is Synchrony a reasonable coding strategy for visual areas beyond V1 in primates? J Vis 7: 325a, 2007. [Google Scholar]
- Kenet et al. 2003.Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature 425: 954–956, 2003. [DOI] [PubMed] [Google Scholar]
- Kleschevnikov et al. 1997.Kleschevnikov AM, Sokolov MV, Kuhnt U, Dawe GS, Stephenson JD, Voronin LL. Changes in paired-pulse facilitation correlate with induction of long-term potentiation in area CA1 of rat hippocampal slices. Neuroscience 76: 829–843, 1997. [DOI] [PubMed] [Google Scholar]
- Kohn and Smith 2005.Kohn A, Smith MA. Stimulus dependence of neuronal correlation in primary visual cortex of the macaque. J Neurosci 25: 3661–3673, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger and Aiple 1988.Kruger J, Aiple F. Multimicroelectrode investigation of monkey striate cortex: spike train correlations in the infragranular layers. J Neurophysiol 60: 798–828, 1988. [DOI] [PubMed] [Google Scholar]
- Lamme and Spekreijse 1998.Lamme VA, Spekreijse H. Neuronal synchrony does not represent texture segregation. Nature 396: 362–366, 1998. [DOI] [PubMed] [Google Scholar]
- Lennie 2003.Lennie P The cost of cortical computation. Curr Biol 13: 493–497, 2003. [DOI] [PubMed] [Google Scholar]
- Malach et al. 1993.Malach R, Amir Y, Harel M, Grinvald A. Relationship between intrinsic connections and functional architecture revealed by optical imaging and in vivo targeted biocytin injections in primate striate cortex. Proc Natl Acad Sci USA 90: 10469–10473, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado et al. 2008.Maldonado P, Babul C, Singer W, Rodriguez E, Berger D, Grun S. Synchronization of neuronal responses in primary visual cortex of monkeys viewing natural images. J Neurophysiol 100: 1523–1532, 2008. [DOI] [PubMed] [Google Scholar]
- Maldonado et al. 2000.Maldonado PE, Friedman-Hill S, Gray CM. Dynamics of striate cortical activity in the alert macaque: II. Fast time scale synchronization. Cereb Cortex 10: 1117–1131, 2000. [DOI] [PubMed] [Google Scholar]
- Meister et al. 1995.Meister M, Lagnado L, Baylor DA. Concerted signaling by retinal ganglion cells. Science 270: 1207–1210, 1995. [DOI] [PubMed] [Google Scholar]
- Merriam et al. 2005.Merriam EB, Netoff TI, Banks MI. Bistable network behavior of layer I interneurons in auditory cortex. J Neurosci 25: 6175–6186, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura et al. 2003.Nomura M, Fukai T, Aoyagi T. Synchrony of fast-spiking interneurons interconnected by GABAergic and electrical synapses. Neural Comput 15: 2179–2198, 2003. [DOI] [PubMed] [Google Scholar]
- Nowak et al. 1999.Nowak LG, Munk MH, James AC, Girard P, Bullier J. Cross-correlation study of the temporal interactions between areas V1 and V2 of the macaque monkey. J Neurophysiol 81: 1057–1074, 1999. [DOI] [PubMed] [Google Scholar]
- Nowak et al. 1995.Nowak LG, Munk MH, Nelson JI, James AC, Bullier J. Structural basis of cortical synchronization. I. Three types of interhemispheric coupling. J Neurophysiol 74: 2379–2400, 1995. [DOI] [PubMed] [Google Scholar]
- Palanca and DeAngelis 2005.Palanca BJ, DeAngelis GC. Does neuronal synchrony underlie visual feature grouping? Neuron 46: 333–346, 2005. [DOI] [PubMed] [Google Scholar]
- Pillow et al. 2008.Pillow JW, Shlens J, Paninski L, Sher A, Litke AM, Chichilnisky EJ, Simoncelli EP. Spatio-temporal correlations and visual signalling in a complete neuronal population. Nature 454: 995–999, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoa et al. 1988.Ramoa AS, Campbell G, Shatz CJ. Dendritic growth and remodeling of cat retinal ganglion cells during fetal and postnatal development. J Neurosci 8: 4239–4261, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes 2003.Reyes AD Synchrony-dependent propagation of firing rate in iteratively constructed networks in vitro. Nat Neurosci 6: 593–599, 2003. [DOI] [PubMed] [Google Scholar]
- Samonds et al. 2004.Samonds JM, Allison JD, Brown HA, Bonds AB. Cooperative synchronized assemblies enhance orientation discrimination. Proc Natl Acad Sci USA 101: 6722–6727, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samonds and Bonds 2005.Samonds JM, Bonds AB. Gamma oscillation maintains stimulus structure-dependent synchronization in cat visual cortex. J Neurophysiol 93: 223–236, 2005. [DOI] [PubMed] [Google Scholar]
- Schwarz and Bolz 1991.Schwarz C, Bolz J. Functional specificity of a long-range horizontal connection in cat visual cortex: a cross-correlation study. J Neurosci 11: 2995–3007, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen and Movshon 1999.Shadlen MN, Movshon JA. Synchrony unbound: a critical evaluation of the temporal binding hypothesis. Neuron 24: 67–25, 1999. [DOI] [PubMed] [Google Scholar]
- Shoham et al. 2003.Shoham S, Fellows MR, Normann RA. Robust, automatic spike sorting using mixtures of multivariate t-distributions. J Neurosci Methods 127: 111–122, 2003. [DOI] [PubMed] [Google Scholar]
- Sillito et al. 1994.Sillito AM, Jones HE, Gerstein GL, West DC. Feature-linked synchronization of thalamic relay cell firing induced by feedback from the visual cortex. Nature 369: 479–482, 1994. [DOI] [PubMed] [Google Scholar]
- Singer and Bedworth 1973.Singer W, Bedworth N. Inhibitory interaction between X and Y units in the cat lateral geniculate nucleus. Brain Res 49: 291–307, 1973. [DOI] [PubMed] [Google Scholar]
- Smith and Kohn 2008.Smith MA, Kohn A. Spatial and temporal scales of neuronal correlation in primary visual cortex. J Neurosci 28: 12591–12603, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Softky and Koch 1993.Softky WR, Koch C. The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J Neurosci 13: 334–350, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen and Shatz 2002.Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron 33: 357–367, 2002. [DOI] [PubMed] [Google Scholar]
- Trong and Rieke 2008.Trong PK, Rieke F. Origin of correlated activity between parasol retinal ganglion cells. Nat Neurosci 11: 1343–1351, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ts'o et al. 1986.Ts'o DY, Gilbert CD, Wiesel TN. Relationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysis. J Neurosci 6: 1160–1170, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent et al. 2007.Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anesthetized monkey brain. Nature 447: 83–86, 2007. [DOI] [PubMed] [Google Scholar]
- Vinje and Gallant 2000.Vinje WE, Gallant JL. Sparse coding and decorrelation in primary visual cortex during natural vision. Science 287: 1273–1276, 2000. [DOI] [PubMed] [Google Scholar]
- Vislay-Meltzer et al. 2006.Vislay-Meltzer RL, Kampff AR, Engert F. Spatiotemporal specificity of neuronal activity directs the modification of receptive fields in the developing retinotectal system. Neuron 50: 101–114, 2006. [DOI] [PubMed] [Google Scholar]
- Volgushev et al. 1997.Volgushev M, Voronin LL, Chistiakova M, Singer W. Relations between long-term synaptic modifications and paired-pulse interactions in the rat neocortex. Eur J Neurosci 9: 1656–1665, 1997. [DOI] [PubMed] [Google Scholar]
- Voronin et al. 1996.Voronin LL, Volgushev M, Chistiakova M, Kuhnt U, Singer W. Involvement of silent synapses in the induction of long-term potentiation and long-term depression in neocortical and hippocampal neurons. Neuroscience 74: 323–330, 1996. [DOI] [PubMed] [Google Scholar]
- Weliky et al. 2003.Weliky M, Fiser J, Hunt RH, Wagner DN. Coding of natural scenes in primary visual cortex. Neuron 37: 703–718, 2003. [DOI] [PubMed] [Google Scholar]
- Xu et al. 2005.Xu X, Bosking WH, White LE, Fitzpatrick D, Casagrande VA. Functional organization of visual cortex in the prosimian bush baby revealed by optical imaging of intrinsic signals. J Neurophysiol 94: 2748–2762, 2005. [DOI] [PubMed] [Google Scholar]
- Zhou et al. 2008.Zhou Z, Bernard MR, Bonds AB. Deconstruction of spatial integrity in visual stimulus detected by modulation of synchronized activity in cat visual cortex. J Neurosci 28: 3759–3768, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary et al. 1994.Zohary E, Shadlen MN, Newsome WT. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature 370: 140–143, 1994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.