Abstract
Brain function depends on coordinated interactions in spatially distributed neuronal populations. Thanks to recent technological advances, it is now possible to monitor the activity of large groups of neurons. Although significant progress has been made in analyzing neuronal interactions across large samples of simultaneously recorded cells, most of the available approaches do not allow direct visualization of multidimensional correlations in the time domain. This study describes a novel analysis technique, termed four-dimensional spike-triggered joint histogram (4-d STJH) that permits the study of co-modulations of unit activity across four simultaneously recorded brain regions while preserving the time domain. To illustrate how this technique works, we recorded simultaneously from basolateral amygdala (BLA) and medial prefrontal (mPFC), as well as perirhinal and entorhinal neurons in animals learning an appetitive trace-conditioning task. Using the 4d-STJH, we show that coincident activity in the BLA and mPFC modulates the interactions between perirhinal and entorhinal neurons in a manner that cannot be explained by a linear combination of the individual BLA and mPFC-related modulations. We conclude with a discussion of the strengths and limitations of 4-d STJH and offer recommendations regarding optimal conditions for its use.
INTRODUCTION
Recent advances in neuronal recording techniques have highlighted the complex computations taking place in neuronal circuits (Buzsaki 2004). Although some studies have examined interactions between two brain regions (Buschman and Miller 2007; Castelo-Branco et al. 2000; Hoffman and McNaughton 2002; Pesaran et al. 2008), much less is known about how three or more interact. To take a specific example, memory formation involves many areas of the medial temporal lobe and neocortex (Frankland and Bontempi 2005; Murray et al. 2007; Squire et al. 2004; Wiltgen et al. 2004). Among them, the rhinal cortices process high-order sensory information during learning of associations between objects and then transfer information between the hippocampus and neocortex during memory consolidation (Sutherland and McNaughton 2000; Wiltgen et al. 2004). Moreover, other structures such as the medial prefrontal cortex (mPFC) and basolateral amygdala (BLA) contribute to memory consolidation, the BLA facilitating memory for emotionally arousing events (Frankland and Bontempi 2005; McGaugh 2004). Although this suggests that all these areas act cooperatively during memory formation, much of the evidence comes from studies that explored one or two areas at a time.
However, recording simultaneously from multiple components of distributed networks is only one aspect of the problem. Another challenge is to develop methods to analyze and identify co-modulations between the neurons under study. This requires estimation of the high-order probability distribution, and the required amount of data grows exponentially with the number of neurons. There have been few attempts to address this concern in local networks. Recent approaches identified synchronous activity in groups of neurons (Harris et al. 2003; Schnitzer and Meister 2003) and characterized the contribution of different orders of correlations (Schneidman et al. 2006). The advantage of these methods is that they require no or few assumptions on the structure of the correlations and they are less constrained by the number of dimensions (i.e., number of neurons). On the other hand, these methods typically lose the temporal structure of the correlations (i.e., whether this or that neuron fired before another one) and do not guide hypotheses by prior knowledge about the network under study. The latter property is probably due to the fact that in local networks, one cannot associate specific functions to particular neurons. The present paper introduces a new method, termed four-dimensional spike-triggered joint histogram (4-d STJH), which builds on and extends previous methods that preserve the temporal shape of the correlations (Aertsen et al. 1989; Czanner et al. 2005; Prut et al. 1998; Vaadia et al. 1995). Furthermore, this method uses anatomical, physiological, and functional knowledge about the network under study to constrain hypotheses and study co-modulations of unit activity across four simultaneously recorded brain regions.
Because the BLA and mPFC are interconnected and project to the rhinal cortices (Apergis-Schoute et al. 2006; Pitkanen et al. 2000; Price 1999), coordinated activity in the BLA and mPFC likely modulates rhinal interactions. In the specific example used here, we hypothesized that the BLA and mPFC affect interactions between perirhinal and entorhinal neurons and thus modulate information transfer between the neocortex and hippocampus. Consistent with this, simultaneous recordings of perirhinal and entorhinal neurons, as well as either amygdala (Paz et al. 2006) or mPFC neurons (Paz et al. 2007) during the acquisition of a trace conditioning task have revealed that the BLA facilitates impulse transfer from the perirhinal to the entorhinal cortex early in learning, and the mPFC from the entorhinal to perirhinal cortex late in learning. However, there is no reason to assume that the combined influence of the BLA and mPFC on the rhinal cortices can be inferred from the individual modulations produced by each structure independently. We therefore examined this question by recording simultaneously from all four areas and used the 4d-STJH to analyze their co-activity in behaving animals learning a trace-conditioning task.
METHODS
Surgery, histology, and recording methods
All procedures were approved by the Institutional Animal Care and Use Committee of Rutgers University, in compliance with the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services). Three adult cats were preanesthetized with ketamine and xylazine (15 and 2 mg/kg im) and artificially ventilated with a mixture of ambient air, oxygen, and isoflurane. Atropine (0.05 mg/kg im) was administered. In sterile conditions, an incision was performed on the midline of the scalp, and the skull muscles were retracted. Then, a reference screw was inserted in the skull overlying the cerebellum and silver-ball electrodes were inserted in the supraorbital cavity to monitor eye movements. After trepanation and opening of the dura mater, an array of high-impedance tungsten microelectrodes (10–12 MΩ; Frederic Haer, Bowdoin, ME) was inserted in the mPFC (Fig. 1A) with an oblique, lateromedial approach, under stereotaxic guidance. Then a second microelectrode array was lowered until the electrodes reached the BLA and deep rhinal layers (Fig. 1B). Finally, four screws were cemented to the skull. These screws were later used to fix the cat's head in a stereotaxic position without pain or pressure. At the end of the surgery, the animals were administered penicillin (20,000 UI/kg im) and an analgesic (Ketophen, 2 mg/kg sc, daily for 3 days). Recording sessions began 8 days after the surgery.
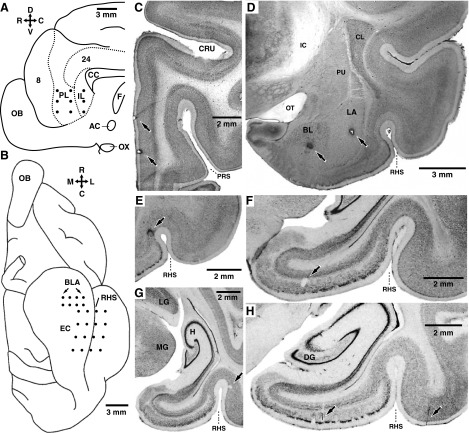
FIG. 1.
Recording method and histological identification of recording sites. A and B: schemes showing a view of the midline (A) or ventral aspect (B) of the cat brain, respectively. •, intended position of microelectrodes in the medial prefrontal (mPFC, A) or basolateral amygdala (BLA), entorhinal (EC), and perirhinal cortex (B). C–H: histological verification of recording sites. Coronal brain sections showing the location of electrolytic lesions (↑) performed at the end of the experiments to mark the position of the microelectrode tips in the mPFC (C), BLA (D), perirhinal cortex (E, G, and H), and entorhinal cortex, (F and H). The orientation of A and B is indicated by crosses where R, C, D, V, M, and L stand for rostral, caudal, dorsal, ventral, medial, and lateral, respectively. AC, anterior commissure; CC, corpus callosum; CL, claustrum; CRU, cruciate sulcus, BL, basolateral nucleus of the amygdala; DG, dentate gyrus; F, fornix; H, hippocampus; IC, internal capsule; IL, infralimbic area; LA, lateral nucleus of the amygdala; LG, lateral geniculate nucleus; MG, medial geniculate nucleus; OB, olfactory bulb; OT, optic tract; OX, optic chiasm; PL, prelimbic area; PRS, presylvian sulcus; PU, putamen; RHS, rhinal sulcus.
At the end of the experiments, the animals were given an overdose of sodium pentobarbital (50 mg/kg iv) and perfused fixed. The recording sites were marked with electrolytic lesions (0.5 mA, 5–10 s). The brains were later sectioned on a vibrating microtome (at 100 μm) and stained with cresyl violet to verify the position of recording electrodes (Fig. 1, C–H). Microelectrode tracks were reconstructed by combining micrometer readings with the histology.
Neuronal activity was sampled at 100-μm intervals (MEA, Multichannel Systems, Reutlingen, Germany). Each time the electrodes were moved to a new site (once a day), 30 min elapsed before data were acquired to ensure mechanical stability. It is extremely unlikely that time-dependent effects reported in this study are due to a change in the exact cortical layers sampled by our electrodes on different days because these effects were seen to occur at the same time in all animals even though histological controls revealed that the starting point of the electrode tracks varied by as much as 0.5 mm between animals, due to the imprecision of the stereotaxic technique. The signals picked up by the electrodes were observed on an oscilloscope, digitized at 25 kHz, and stored on a hard disk. Spike sorting was performed off-line using a custom made software that features a clustering algorithm based on principal-component analysis and K-means.
Behavioral paradigm
The learning task was an appetitive trace-conditioning paradigm in which a visual conditioned stimulus (CS, 1.5 s) was followed by a 1.5-s delay period, after which a liquid reward was administered (Fig. 2A). The delay period between the end of the CS and the reward is thought to render this task hippocampal-dependent (McEchron et al. 2003; Munera et al. 2001; Savage et al. 2004; Shors 2004; Solomon et al. 1986). Although our task is similar to other hippocampal-dependent trace-conditioning tasks, the hippocampal dependence of our task has not been directly tested yet.
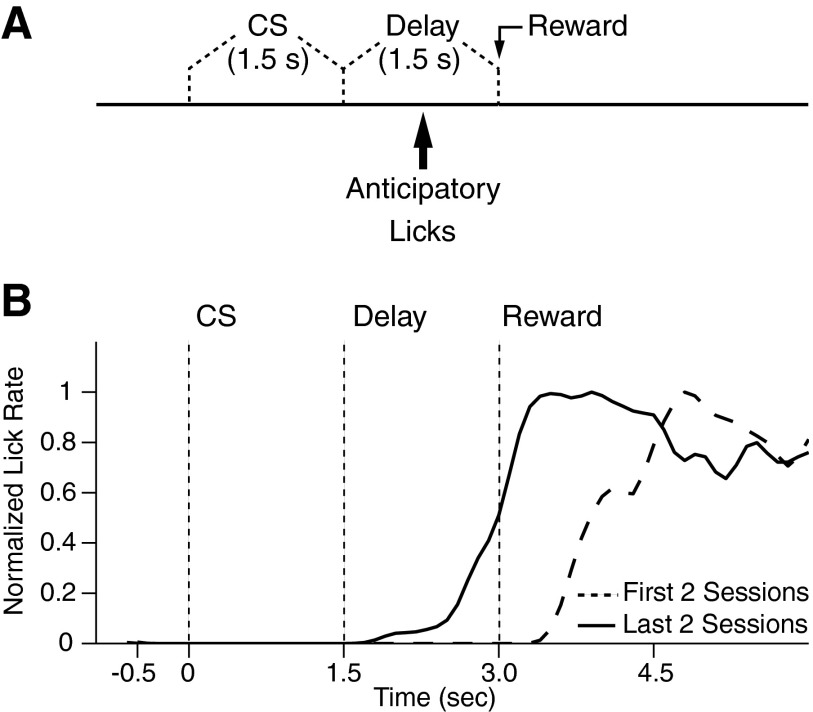
FIG. 2.
Trace conditioning task. A: relative timing of the visual CS, delay period, and reward delivery in the trace conditioning task. B: average lick frequency (y axis) as a function of time (x axis) during trace conditioning trials of the 1st 2 (- - -) and last 2 (—) training sessions in one cat. To facilitate comparison between licking behavior at early and late stages of training, the data were normalized to the peak licking frequency. All cats showed significant increase in anticipatory licking behavior during the late sessions (t-test, P < 0.05 for all cats).
In the present study, trials occurred at random intervals of 30–90 s. The animals performed 40–100 trials per daily session for 8–9 consecutive days. The liquid reward (2 ml/trial) was a preferred food [Gerber's (Fremont, MI) pureed baby food “sweet potatoes and turkey”]. The animals were only fed during recording sessions, and their weight was monitored daily to maintain body weight within 10% of its initial value. Behavior was monitored by means of a switch detecting when the tongue of the animals contacted the receptacle where the food reward was administered. The visual CS was a global change in the illumination (from black to white) of a 12-in liquid crystal display (LCD) screen placed 1 ft in from of the animals. Detection of the visual CS did not necessitate that the animals maintain a fixed gaze at the center of the LCD screen because it encompassed most of their visual field. Because the animals were hungry, they were highly aroused and remained awake at all times (as assessed by EEG recordings) with their eyes opened.
Analysis
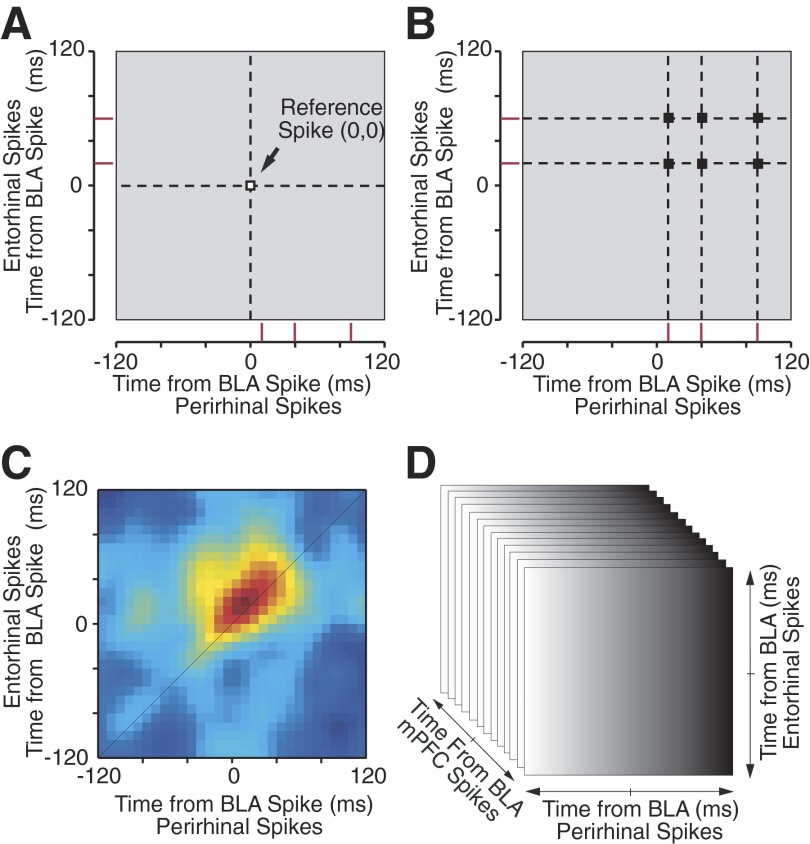
To analyze correlated neuronal activity among four brain regions, we extended a recently introduced analysis method, the STJH. The name STJH was originally suggested by Czanner et al. (2005) for a modification of the joint peristimulus time histogram method (JPSTH) (Aertsen et al. 1989) where, instead of computing correlations with respect to external stimuli as in the JPSTH (Vaadia et al. 1995), spikes from a third neuron are used as temporal references (Prut et al. 1998) to study correlations between two other neurons. In the initial implementation of the STJH (Paz et al. 2006), the method was used to study how activity in a reference region (the BLA) affected correlated activity between perirhinal and entorhinal neurons. The STJH was computed by taking ±120-ms segments of rhinal activity around spikes at the reference site (time 0; Fig. 3A) and by plotting the spikes of the perirhinal cell on the x-axis and of the entorhinal cell on the y axis (red ticks in Fig. 3, A and B). Around each reference spike, the bins of the STJH that contained a coincidence of rhinal spikes were incremented by one (Fig. 3B). Repeating this process for each reference spike gradually produced the raw STJH where the counts of coincident activity were color-coded (Fig. 3C).
FIG. 3.
Computation and display of correlation cubes. To examine the temporal relationship between the activity of BLA, mPFC, perirhinal, and entorhinal neurons, we used a modification of the spike-triggered joint histogram (STJH) analysis. A–C: the original STJH used to analyze the relationship among the activity of BLA, perirhinal, and entorhinal neurons. A: the STJH was computed by isolating ±120-ms segments of perirhinal (x axis) and entorhinal (y axis) activity (red ticks) around spikes at the BLA reference site (time 0, 0). B: around each reference spike, the bins of the STJH that contained a coincidence of rhinal spikes (black squares at intercept of dashed lines) were incremented by 1. C: repeating this process for each action potential in the BLA spike train gradually produced the raw STJH where the counts of coincident activity were color-coded. D: to take into consideration the activity of a fourth simultaneously recorded region (mPFC), a z axis is added to the STJH. Such STJHs can no longer be depicted in a single graph. To solve this problem, the cube is simply divided in as many matrices as bins along the z axis.
In the present study, however, the activity of four simultaneously recorded regions is considered. This requires the computation of correlation cubes that can no longer be depicted in a single graph (Fig. 3D). To solve this problem, the cube is divided in as many matrices as bins along the extra axis, as shown in Fig. 4. Thus each time a BLA spike was encountered, we extracted segments of mPFC (z axis), perirhinal (x axis), and entorhinal (y axis) spiking in 10-ms bins from 120 ms before the time of the BLA spike to 120 ms after it. We then looked for the occurrence of spikes in all three regions within this ±120-ms window. When such coincident firing in all three regions was observed, we incremented the appropriate bin in the cube by one. For example, if mPFC neurons fired 30 ms before the BLA cells, perirhinal neurons 40 ms after, and entorhinal cells 60 ms later, then we incremented the bin (−30, 40, 60) by one. Repeating this process for all BLA spikes and normalizing by z-score transformation, produced a correlation cube of all possible delays. For this report, we tried using either BLA or mPFC spikes as references (time 0, the cube origin), but both approaches produced similar results. This is expected given that changing the spikes used as time 0 (BLA vs. mPFC) does not modify what events figure in the correlation cube, only the frame of reference. Indeed, because for a spike to be included the 4d-STJH, there must be coincident firing of all four cells within ±120 ms of each other, all that changes when using a different reference event is the pictorial representation of the correlation cube. The same information is displayed differently.
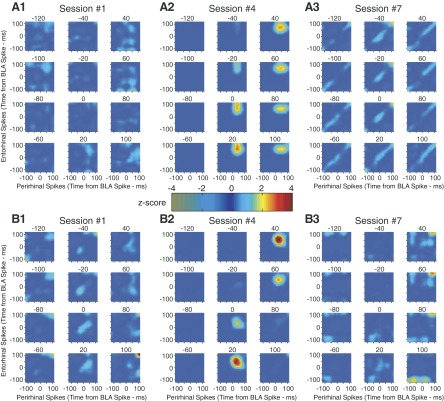
FIG. 4.
Impact of joint BLA-mPFC activity on rhinal correlations. Each group of 12 matrices is a correlation cube derived from 1 recording session but sliced in 20-ms bins along the z axis (time of mPFC spikes). Thus each matrix shows correlations between perirhinal spikes (x axis) and entorhinal spikes (y axis) at different delays (10-ms bins) relative to BLA spikes (at time 0, 0) and relative to mPFC spikes. For 2 animals (A, 1–3, and B, 1–3), we show the 1st (A1 and B1), 4th (A2 and B2), and 7th (A3 and B3) training sessions.
The bin duration (10 ms) we selected is a compromise between the requirement to have a sufficient number of spikes per bin while preserving the temporal resolution of the analyses. The duration of the time window included in the 4d-STJH is based on the effective period of temporal PSP summation in neurons.
For presentation purposes only, cubes were smoothed with a three-dimensional (3-D) Gaussian with variance of 10 ms, and sliced along its z axis (the mPFC one) in 20-ms slices. Thus each analysis results in as many matrices as 20-ms bins along the mPFC (z) axis. Within each of these matrices, the BLA spikes are still at time (0,0), the perirhinal time is plotted along the x axis, and the entorhinal time along the y axis, and bins are of 10 ms (as in Fig. 4).
RESULTS
Database
In three head-restrained cats, we recorded neuronal activity using four groups of eight microelectrodes chronically implanted in the mPFC and BLA as well as perirhinal and entorhinal cortices, respectively (Fig. 1, A and B). This report includes only neurons that were histologically confirmed to be located in the regions of interest (Fig. 1, C–H). The cats were trained on an appetitive trace-conditioning paradigm in which a visual CS (1.5 s) was followed by a 1.5-s delay period, after which a liquid reward was administered (Fig. 2A). The animals acquired the association over a period of 8–9 days as evidenced by the gradual emergence of anticipatory licking during the delay period (Fig. 2B).
Computing the 4d-STJH
It should be realized that in order for a spike to be included the 4d-STJH, there must be coincident firing of all four cells within ±120 ms of each other. As a result, only a minority of action potentials is included in the 4d-STJH. This is especially problematic for brain regions, such as the BLA, where neurons have low firing rates (Gaudreau and Pare 1996). To address the requirement for large amounts of data, we used very long periods of activity (∼1.5 h) that included the trace conditioning trials and intertrial intervals such that the incidence of coincident firing among the four regions was sufficient. Additionally, because we are interested in inter- rather than intraregional interactions, within each region considered separately, we collapsed the individual spike trains recorded by the eight different electrodes to form a single vector of spikes. Thus, our analyses were performed on four composite spike vectors per session, one per recorded region (median number of spikes per session: 115,346, minimum: 62,000). For each session, we then constructed correlation cubes (Fig. 3) where BLA spikes are the reference events at the center of each cube, and each bin shows the normalized coincident spike counts generated in the other three areas within ±120 ms of BLA spikes (x, perirhinal; y, entorhinal; and z, mPFC axes, bins of 10 ms; see methods).
Figure 4 shows the result of these analyses for two sets of three sessions (panels 1–3) obtained at different stages of training in two animals (Fig. 4, A and B). The reason for illustrating these particular sessions will become clear below, when we examine time-dependent changes in rhinal correlations driven by BLA-mPFC activity (Fig. 5). Note that for each session shown in Fig. 4, the mPFC (z) axis was sliced in 12 20-ms bins to allow a 2-D representation of the correlation cubes.
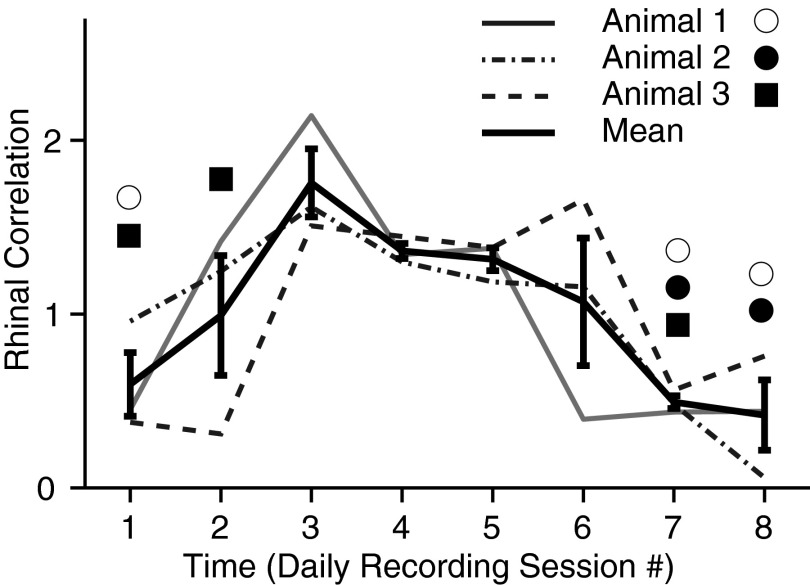
FIG. 5.
Different animals show similar time-dependent shifts in magnitude of mPFC-BLA based rhinal correlations. The graph plots magnitude of perirhinal-entorhinal correlations (y axis) as a function of daily recording sessions (x axis). The magnitude of perirhinal-entorhinal correlations was estimated by computing a ratio of the counts of coincident rhinal (peri- and entorhinal) spikes after vs. before joint BLA and mPFC spiking. There are 4 superimposed curves, one for each the 3 animals. The 4th (thick) curve is the average ± SE of the 3 subjects. Symbols mark sessions where the correlation cubes did not reach significance, coded by cat as indicated in the legend.
Testing the statistical significance of multi-dimensional correlations
Our statistical analyses aimed to answer the following question: does joint activity in the BLA and mPFC give rise to specific kinds of correlations between perirhinal and entorhinal neurons? To answer this question, each correlation cube was tested against three null hypotheses: that rhinal correlations 1) caused by coincident BLA-mPFC activity are similar to the correlations produced by BLA or mPFC activity considered separately, 2) are a result of perirhinal and entorhinal responding independently to BLA-mPFC activity, and 3) occur with no timing preference relative to coincident BLA-mPFC activity. A recording session was considered as one where joint mPFC-BLA activity significantly modulated correlations between perirhinal and entorhinal neurons, if all three null hypotheses were rejected for the cube of that session.
To test null hypothesis 1, we separately randomized the BLA or mPFC spike times (see Supplementary Fig. S11 ). This was done locally within each segment of ±120 ms so that the number of spikes in each segment remained constant. The randomization of spike times we used did not preserve interspike intervals. However, due to the fact that in ≥94% of instances where correlated firing was seen in all four structures, only one spike per region was generated within the ±120-ms time windows, this should not be a concern. After randomization of spikes times, the correlation cube was then reconstructed. This process was repeated 100 times to produce 100 shuffled matrices, creating a distribution per bin.
To test null hypothesis 2, we used the “shift-predictor” approach and randomly shuffled the segments of perirhinal activity in relation to the segments of entorhinal activity (keeping the BLA and mPFC constant) and recalculated the correlation cube. This process was repeated 100 times to produce 100 shuffled matrices, creating a distribution per bin.
To test for significance when evaluating null hypotheses 1 and 2, we used two methods. First, we compared the counts in a specific bin of the original cube to the distribution of counts of the corresponding bin in the shuffled matrices (t-test, P < 0.05). We then counted the number of bins that were significantly different. The null hypothesis was rejected if the number of significant bins was much higher than expected from chance (total number of bins times 0.01, binomial test). In a second approach, we recalculated the cubes with a decreased resolution of 100 ms bins with segments of −100 to +100 ms. The null hypothesis was rejected if any of its 8 (2 ^ 3) bins was significantly different (t-test, P < 0.05, Bonferroni corrected for the number of bins, 8) from the distribution of that same bin from in the shuffled matrices. This approach was selected for two reasons. First, it was adopted to eliminate the requirement for the investigator to arbitrarily define specific regions of interest within the correlation cubes. Second, with a Bonferonni correction of the significance level, not reducing the temporal resolution of the correlation cubes would force the investigator to use extremely stringent significance levels, greatly increasing the risk of type 1 errors.
Null hypothesis 3 is that rhinal correlations occur with no timing preference relative to coincident BLA and mPFC activity. In other words, if BLA and mPFC activity contribute to rhinal correlations, then the correlations should emerge preferentially after the joint activity rather than before it. To test this, we compared the counts of coincident rhinal (perirhinal and entorhinal) spikes after joint BLA and mPFC spiking, to counts of coincident rhinal spikes just before the joint BLA and mPFC spiking (2-tailed paired t-test, P < 0.05).
Using this approach, we could reject all three null hypotheses in 17 of the 25 sessions (P < 0.01, binomial test). In other words, in these 17 sessions, joint mPFC-BLA activity was associated with significant perirhinal-entorhinal correlations. In Fig. 4 for instance, the correlation cubes of session 4 in cats 1 (Fig. 4A2) and 3 (Fig. 4B2) were deemed significant whereas the other sessions were not. Interestingly, in both animals, the correlated rhinal activity largely occurred after BLA and mPFC spikes. The timing of rhinal activity with respect to BLA firing can be appreciated by considering the time of entorhinal and perirhinal firing on the y and x axes of Fig. 4, A2 and B2, respectively. Perirhinal and entorhinal neurons tended to fire 30–50 and 40–70 ms after BLA activity, respectively. The relative timing of mPFC activity with respect to BLA firing can be appreciated by considering which of the 12 matrices in Fig. 4, A2 and B2, show elevated counts. In both animals, little correlated rhinal activity is seen in the –120 to –20 ms matrices. Most of the correlated activity is seen in the +20 to +40 matrices. Overall, these results suggest that the prevalent pattern of activity involved the following sequence of events: BLA, mPFC, perirhinal, and then entorhinal firing.
To determine how the incidence of correlated firing by neurons in all four structures compared with that expected by chance, we shuffled all four spike trains 100 times and recomputed the 4d-STJH each time for all significant sessions. This analysis revealed that the incidence of correlated firing in all four structures was 38 ± 7% higher than in the randomized distributions. To determine how the incidence of correlated firing in all four structures compared with that expected given the frequency of mPFC- or BLA-rhinal correlations, we repeated the same test but shuffling only the BLA or mPFC spike trains, respectively. This analysis revealed that the incidence of correlated firing in all four structures was higher by 13 ± 2 and 8 ± 1% than expected from mPFC- or BLA-rhinal correlations, respectively. These values represent the deviations from the mean chance levels.
Because the cats were being trained on a trace conditioning task during the period analyzed and because our hypothesis predicts that joint mPFC-BLA activity affects memory formation by enhancing rhinal interactions, there was reason to expect that the impact of joint-mPFC-BLA activity on rhinal correlations would fluctuate as a function of time during training. To evaluate this prediction, Fig. 5 plots fluctuations in the magnitude of perirhinal-entorhinal correlations (y axis) as a function of time during training on the trace-conditioning task (y axis, in days). The figure shows the average of the three cats as well as the data obtained in each cat separately. A consistent pattern of time-dependent fluctuations in rhinal correlations was seen across subjects. Indeed, in all cats, the rhinal correlations peaked during the third daily recording session, stayed elevated through sessions 4 and 5, and then decayed back to (or below) starting levels by session 7. This is in contrast to the timing of the modulations produced independently by the BLA (Paz et al. 2006) and mPFC (Paz et al. 2007) that, respectively, peak at the first and last training sessions, suggesting that BLA and mPFC influences interact nonlinearly to modulate rhinal interactions (see following text). Here, it should be noted that sessions 3–5 correspond to the learning phase where the rate of anticipatory licking rate reaches an asymptotic level in the trace conditioning task.
Testing for nonlinearity
Because both the mPFC and BLA project to the rhinal cortices (Apergis-Schoute et al. 2006; Pitkanen et al. 2000; Price 1999), one could reason that the correlations revealed by the 4d-STJH reflect a simple combination of the two independent influences. Yet the existence of reciprocal excitatory connections between the BLA and mPFC suggests that this intuitive reasoning might be incorrect. The simplest possibility would be that rhinal correlations following joint mPFC-BLA activity are a sum of the individual ones. Indeed if BLA projections to the rhinal cortices enhance rhinal correlations, and mPFC inputs do the same, then joint mPFC-BLA activity might enhance correlations in an additive fashion.
To test whether the combined influence of the BLA and mPFC on the rhinal cortices is a linear function of the individual modulations produced by each structure independently, three approaches were used. In the first, for each session, we calculated BLA-only based correlations (Fig. 6, A2 and B2; STJHs ignoring mPFC activity) and mPFC-only based correlations (A3 and B3; STJHs ignoring BLA activity). In fact, this corresponds exactly to the approach we used previously to compute 3d-STJHs (Paz et al. 2006, 2007). To allow comparison of the 3d-STJHs to the 4d-STJH, we collapsed the correlation cubes along the mPFC axis (Fig. 6, A1 and B1). The 3d- and 4d-STJHs were then normalized to the correlations observed before time 0. Next we compared the normalized distribution of bin values in the collapsed 4d-STJH to that of the mPFC-based 3d-STJH, the BLA-based 3d-STJH, or the sum of the two (Fig. 6, A4 and B4). In 13 of the 25 sessions, the correlations following coincident mPFC-BLA activity were significantly different from those linked to the activity of the BLA alone, the mPFC alone, and their sum (Mann-Whitney tests, P < 0.05 for all comparisons). This proportion was significantly higher than expected by chance (P < 0.01, binomial test).
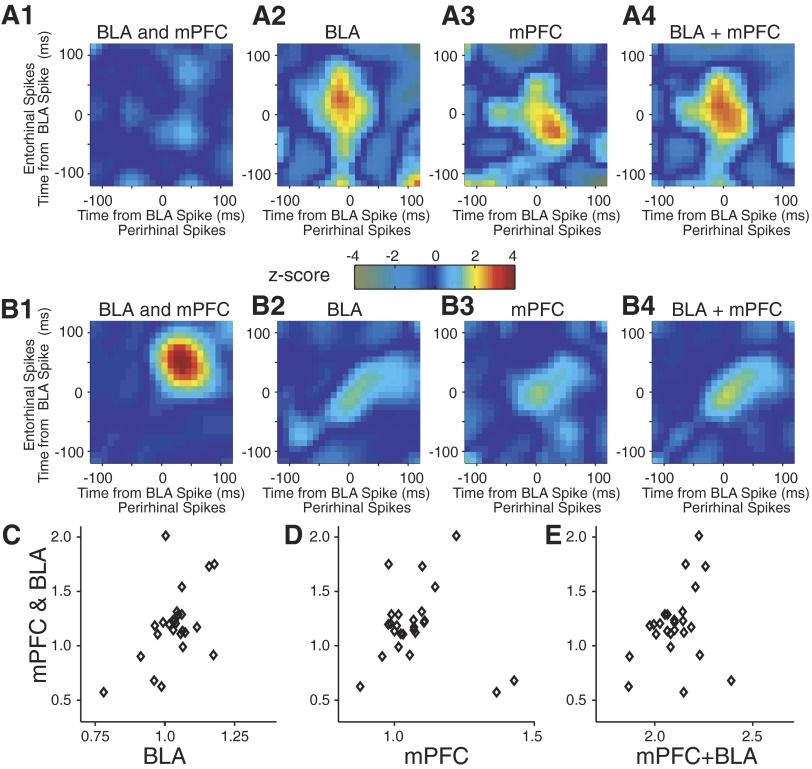
FIG. 6.
Rhinal correlations are modulated nonlinearly by joint BLA-mPFC activity. A and B: data from 2 recording sessions shown in Fig. 4, A1 and B2, respectively. From left to right, we show rhinal correlations around joint BLA-mPFC activity (1); BLA activity alone (2); mPFC activity alone (3); and summation of 2 and 3 (4). The plots in A1 and B1 were obtained by collapsing the mPFC axis. C–E: correlation cubes were re-calculated in 100-ms bins and the correlations following time (0, 0, 0) were normalized to the average of the bins before the reference time. A linear fit over sessions was then performed. No relationship between rhinal correlations following joint BLA-mPFC activity (y axis) and rhinal correlations following BLA alone (x axis; C, P > 0.1, linear regression), mPFC alone (D, P > 0.2), and their sum (E, P > 0.3).
In a second approach, we re-calculated correlation cubes in 100-ms bins and normalized the correlations following time (0, 0, 0) to the average of the bins before the reference time. Next, we performed a linear fit over sessions to test whether the rhinal correlations produced by joint mPFC-BLA activity are a linear function of the individual ones (Fig. 6, C and D) or their sum (Fig. 6E). Rhinal correlations that followed coincident mPFC-BLA activity were not significantly correlated with those linked to the activity of the BLA alone (Fig. 6C; P > 0.1), mPFC alone (D; P > 0.2), or their sum (E; P > 0.1).
Finally, in a third approach, we considered the possibility of a more complex linear relationship of the form: CorrmPFCandBLA = a*CorrmPFC + b*CorrBLA + c. This model was fitted in each session separately with a multiple linear regression using the 10-ms bins as observations. If there is such a complex interaction between mPFC versus BLA modulation of rhinal correlations, then similar coefficients should emerge in the different sessions. However, a visual inspection of plots with a range of coefficients failed to reveal any consistent relationship.
DISCUSSION
The present report describes a new analysis method, the 4d-STJH, which allows the study of co-modulations of unit activity across four simultaneously recorded brain regions while preserving the temporal shape of the correlations. To illustrate how the 4d-STJH is used, we recorded simultaneously from BLA and mPFC as well as perirhinal and entorhinal neurons in behaving animals learning an appetitive trace-conditioning task thought to be dependent on the hippocampus. On the basis of previous work on the connectivity of the recorded structures as well as lesion, pharmaco-behavioral, and single-unit recording studies, we hypothesized that joint mPFC-BLA activity would facilitate rhinal interactions in a manner that cannot be predicted by a simple linear combination of the influence exerted by each structure independently. While testing this hypothesis, we illustrated various approaches that can be used to assess statistical significance of the relations observed with the 4d-STJH. In the following account, we consider the significance of the interactions revealed by the 4d-STJH in the BLA-mPFC-rhinal network and then discuss the advantages and limitations of this correlation technique.
Cellular interactions revealed by the 4d-STJH in the BLA-mPFC-rhinal network
Our analyses indicate that joint BLA-mPFC activity facilitates rhinal interactions during learning. The prevalent pattern of activity observed in the 4d-STJH involved the following sequence of events: BLA, mPFC, perirhinal, and then entorhinal firing. However, this pattern of activity was not present at initial stages of learning. It emerged later, during daily training sessions 3–5, when behavioral improvements reached an asymptotic level, and vanished thereafter. The temporal profile of facilitated rhinal interactions revealed in the 4d-STJH contrasts with that seen when we considered BLA (Paz et al. 2006) or mPFC activity (Paz et al. 2007), independently. Indeed 3d-STJHs revealed that the facilitation of rhinal interactions by BLA or mPFC activity emerged at early and late stages of training, respectively. Moreover, these analyses indicated that BLA activity facilitates perirhinal to entorhinal communication, whereas mPFC activity enhances rhinal interactions in the opposite direction (Paz et al. 2006, 2007).
The contrasting temporal profile and directionality of the effects disclosed in the 4d-STJH indicate that they do not reflect a simple additive combination of the influence exerted by the BLA and mPFC independently. Rather these findings suggest that the 4d-STJH isolates a distinct type of interactions. At present, the mechanisms explaining the delayed emergence and subsequent disappearance of these interactions are unclear. Previous inactivation and lesion studies revealed that the BLA and mPFC have a different but partially overlapping temporal profile of involvement in memory consolidation (Frankland and Bontempi 2005; McGaugh 2004). The BLA is mainly involved at early stages of training, whereas mPFC involvement becomes critical at late stages of training. The time-dependent interactions disclosed in our 4d-STJH analyses may be related to the partial overlap between the temporal profile of involvement of the BLA and mPFC in memory consolidation. In addition, it is likely that as learning progresses, task-related synaptic inputs to the mPFC and/or BLA undergo activity-dependent changes in efficacy, and these plastic events alter how BLA and mPFC neurons interact with each other and with rhinal neurons. Presumably, the time dependence of the interactions disclosed in the 4d-STJH is a function of this network level plasticity.
Irrespective of the underlying mechanisms, our results suggest that besides the dissociable roles attributed to the BLA and mPFC in memory consolidation, their joint activity is of high importance. In principle, the enhancement of rhinal interactions by joint mPFC-BLA activity should facilitate the neocortical-hippocampal dialogue (Sutherland and McNaughton 2000; Wiltgen et al. 2004) and/or play an active role in learning and memory formation in the rhinal cortices themselves (Leutgeb et al. 2005; Murray et al. 2007).
Advantages and limitations of the 4d-STJH
The main disadvantage of the 4d-STJH is the requirement for large amounts of data. Indeed because the 4d-STJH only considers action potentials generated nearly simultaneously (within ±120 ms) in four different neurons, only a minute portion of the activity is included in the analysis. As a result, long activity periods are required, especially for neurons with low firing rates. Thus the 4d-STJH can rarely be used to analyze trial-based activity with the exception of tasks where subjects can perform hundreds of trials in one session and where the recorded cells have high spontaneous firing rates. In addition, the 4d-STJH is ill suited for the study of four-way neuronal interactions where two or more of the areas under study are linked by inhibitory connections. Indeed because inhibitory interactions can only be seen on a background of activity, disclosing such interactions with the 4d STJH further increases the requirements for large amounts of data obtained from neurons with high spontaneous firing rates.
Because the neuronal populations recorded here had low firing rates and because we were mostly interested in inter-regional (by opposition to intraregional) interactions, the spike trains generated by multiple neurons within each structure were collapsed into four composite spike vectors. However, in favorable cases where the recorded cells have high spontaneous firing rates, the 4d-STJH could be used to analyze multiple cell quadruplets separately. In such cases, if information regarding the identity of the recorded cells was available (local-circuit vs. projection cells or cells with different projection sites), one could test whether the observed multidimensional correlations are expressed differentially as a function of cell identity.
However, the main advantage of the 4d-STJH is that it allows direct examination of the temporal structure of the correlated activity. Although by definition causality can never be established in correlative studies, observing a systematic temporal bias in firing sequences allows one to identify specific coding schemes and exclude others. For instance, if some neurons systematically fire after others, the first cannot be responsible for the firing of the latter. Of course, a fifth population, not recorded in a given study, might drive the correlations but this could be tested in a separate experiment using reversible inactivations with local pharmacological manipulations.
Comparison with other analysis methods
The 4d-STJH constitutes the first instance where high-order (>3) correlations are used to study inter-regional neuronal correlations. In previous reports that studied three simultaneously recorded areas (Paz et al. 2006, 2007), we adapted the JPSTH method (Aertsen et al. 1989; Vaadia et al. 1995) and used spikes in a particular structure as references instead of a behavioral event or sensory stimulus (Prut et al. 1998) to compute correlations between two other neurons. A similar qualitative technique (the snowflake plot), was suggested by Perkel et al. (1975) and studied more rigorously by Czanner et al. (2005). However, this technique was not used frequently, probably because its triangular coordinates are difficult to understand. Usually these techniques and others that measure high-order correlations are concerned with narrow time scales (ms scale) because they look for correlations that exceed firing-rate modulations and are exhibited in a local network. Here because we applied the technique to inter-regional correlations, where correlations have a lower time scale, we adapted the hypotheses accordingly. Similarly, we are less concerned with factors that limit the interpretation of standard JPSTH, such as onset fluctuations (Brody 1999) or stimulus variability (Ben-Shaul et al. 2001).
Other techniques that measure high-order correlations (e.g., Gutig et al. 2003; Harris et al. 2003; Martignon et al. 2000; Schneidman et al. 2006; Schnitzer et al. 2003) can provide important information about the existence of different orders of correlation in a population of neurons and involve no a priori assumptions regarding the structure of the correlations. However, they lose the temporal shape of the correlations. To deal with the requirement for large volumes of data, we had to adopt a hypothesis-driven approach—that one or two areas (in this case amygdala and mPFC) modulate correlations in the other two areas (rhinal cortices), and our significance testing was designed accordingly. Thus our technique is very helpful to visualize and measure correlations, but the tradeoff is that some hypothesis on the nature of the correlations in the network must be made.
Conclusions
Using the 4-d STJH, we showed that coincident activity in the BLA and mPFC modulates the interactions between perirhinal and entorhinal neurons in a manner that cannot be explained by a linear combination of the individual BLA and mPFC-related modulations. Because the 4d-STJH requires large amounts of data, it is best adapted to the study of neuronal populations with high firing rates. For the analysis of trial-based activity, the 4d-STJH can only be used in tasks where hundreds of trials are available. On the other hand, the 4d-STJH has a unique strength, the possibility of directly observing the temporal structure of the correlations. Overall, these considerations suggest that the 4d-STJH constitutes a useful addition to the array of analysis techniques currently available to study multidimensional neuronal correlations.
GRANTS
This material is based on work supported by National Institute of Mental Health Grant RO1 MH-073610 to D. Paré and National Research Service Award Fellowship 5F32MH076640 to E. P. Bauer.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Aertsen et al. 1989.Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity.” J Neurophysiol 61: 900–917, 1989. [DOI] [PubMed] [Google Scholar]
- Apergis-Schoute et al. 2006.Apergis-Schoute J, Pinto A, Pare D. Ultrastructural organization of medial prefrontal inputs to the rhinal cortices. Eur J Neurosci 24: 135–144, 2006. [DOI] [PubMed] [Google Scholar]
- Ben-Shaul et al. 2001.Ben-Shaul Y, Bergman H, Ritov Y, Abeles M. Trial to trial variability in either stimulus or action causes apparent correlation and synchrony in neuronal activity. J Neurosci Methods 111: 99–110, 2001. [DOI] [PubMed] [Google Scholar]
- Buschman and Miller 2007.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315: 1860–1862, 2007. [DOI] [PubMed] [Google Scholar]
- Brody 1999.Brody CD Disambiguating different covariation types. Neural Comput 11: 1527–35, 1999. [DOI] [PubMed] [Google Scholar]
- Buzsaki 2004.Buzsaki G Large-scale recording of neuronal ensembles.Nat Neurosci 7: 446–451, 2004. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco et al. 2000.Castelo-Branco M, Goebel R, Neuenschwander S, Singer W. Neural synchrony correlates with surface segregation rules. Nature 405: 685–689, 2000. [DOI] [PubMed] [Google Scholar]
- Czanner et al. 2005.Czanner G, Grün S, Iyengar S. Theory of the snowflake plot and its relations to higher-order analysis methods. Neural Comput 17: 1456–79, 2005. [DOI] [PubMed] [Google Scholar]
- Frankland and Bontempi 2005.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci 6: 119–130, 2005. [DOI] [PubMed] [Google Scholar]
- Gaudreau and Pare 1996.Gaudreau H, Pare D. Projection neurons of the lateral amygdaloid nucleus are virtually silent throughout the sleep-waking cycle. J Neurophysiol 75: 1301–1305, 1996. [DOI] [PubMed] [Google Scholar]
- Harris et al. 2003.Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsaki G. Organization of cell assemblies in the hippocampus. Nature 424: 552–556, 2003. [DOI] [PubMed] [Google Scholar]
- Gütig et al. 2003.Gütig R, Aertsen A, Rotter S. Analysis of higher-order neuronal interactions based on conditional inference. Biol Cybern 88: 352–359, 2003. [DOI] [PubMed] [Google Scholar]
- Hoffman and McNaughton 2002.Hoffman KL, McNaughton BL. Coordinated reactivation of distributed memory traces in primate neocortex. Science 297: 2070–2073, 2002. [DOI] [PubMed] [Google Scholar]
- Leutgeb et al. 2005.Leutgeb S, Leutgeb JK, Moser MB, Moser EI. Place cells, spatial maps and the population code for memory. Curr Opin Neurobiol 15: 738–746, 2005. [DOI] [PubMed] [Google Scholar]
- Martignon et al. 2000.Martignon L, Deco G, Laskey K, Diamond M, Freiwald W, Vaadia E. Neural coding: higher-order temporal patterns in the neurostatistics of cell assemblies. Neural Comput 12: 2621–53, 2000. [DOI] [PubMed] [Google Scholar]
- McEchron et al. 2003.McEchron MD, Tseng W, Disterhoft JF. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. J Neurosci 23: 1535–1547, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh 2004.McGaugh JL The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27: 1–28, 2004. [DOI] [PubMed] [Google Scholar]
- Munera et al. 2001.Munera A, Gruart A, Munoz MD, Fernandez-Mas R, Delgado-Garcia JM. Hippocampal pyramidal cell activity encodes conditioned stimulus predictive value during classical conditioning in alert cats. J Neurophysiol 86: 2571–2582, 2001. [DOI] [PubMed] [Google Scholar]
- Murray et al. 2007.Murray EA, Bussey TJ, Saksida LM. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annu Rev Neurosci 30: 99–122, 2007. [DOI] [PubMed] [Google Scholar]
- Paz et al. 2007.Paz R, Bauer EP, Pare D. Learning-related facilitation of rhinal interactions by medial prefrontal inputs. J Neurosci 27: 6542–6551, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz et al. 2006.Paz R, Pelletier JG, Bauer EP, Pare D. Emotional enhancement of memory via amygdala-driven facilitation of rhinal interactions. Nat Neurosci 9: 1321–1329, 2006. [DOI] [PubMed] [Google Scholar]
- Perkel et al. 1975.Perkel DH, Gerstein GL, Smith MS, Tatton WG. Nerve-impulse patterns: a quantitative display technique for three neurons. Brain Res 100: 271–296, 1975. [DOI] [PubMed] [Google Scholar]
- Pesaran et al. 2008.Pesaran B, Nelson MJ, Andersen RA. Free choice activates a decision circuit between frontal and parietal cortex. Nature 453: 406–409, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen et al. 2000.Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann NY Acad Sci 911: 369–391, 2000. [DOI] [PubMed] [Google Scholar]
- Price 1999.Price JL Prefrontal cortical networks related to visceral function and mood. Ann NY Acad Sci 877: 383–396, 1999. [DOI] [PubMed] [Google Scholar]
- Prut et al. 1998.Prut Y, Vaadia E, Bergman H, Haalman I, Slovin H, Abeles M. Spatiotemporal structure of cortical activity: properties and behavioral relevance. J Neurophysiol 79: 2857–2874, 1998. [DOI] [PubMed] [Google Scholar]
- Savage et al. 2004.Savage LM, Buzzetti RA, Ramirez DR. The effects of hippocampal lesions on learning, memory, and reward expectancies. Neurobiol Learn Mem 82: 109–119, 2004. [DOI] [PubMed] [Google Scholar]
- Schneidman et al. 2006.Schneidman E, Berry MJ 2nd, Segev R, Bialek W. Weak pairwise correlations imply strongly correlated network states in a neural population. Nature 440: 1007–1012, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer and Meister 2003.Schnitzer MJ, Meister M. Multineuronal firing patterns in the signal from eye to brain. Neuron 37: 499–511, 2003. [DOI] [PubMed] [Google Scholar]
- Shors 2004.Shors TJ Memory traces of trace memories: neurogenesis, synaptogenesis and awareness. Trends Neurosci 27: 250–256, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon et al. 1986.Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci 100: 729–744, 1986. [DOI] [PubMed] [Google Scholar]
- Squire et al. 2004.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci 27: 279–306, 2004. [DOI] [PubMed] [Google Scholar]
- Sutherland and McNaughton 2000.Sutherland GR, McNaughton B. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol 10: 180–186, 2000. [DOI] [PubMed] [Google Scholar]
- Vaadia et al. 1995.Vaadia E, Haalman I, Abeles M, Bergman H, Prut Y, Slovin H, Aertsen A. Dynamics of neuronal interactions in monkey cortex in relation to behavioral events. Nature 373: 515–518, 1995. [DOI] [PubMed] [Google Scholar]
- Wiltgen et al. 2004.Wiltgen BJ, Brown RA, Talton LE, Silva AJ. New circuits for old memories: the role of the neocortex in consolidation. Neuron 44: 101–108, 2004. [DOI] [PubMed] [Google Scholar]