Abstract
Estimation of creatinine clearance requires knowledge of creatinine generation which can vary in different groups of patients. Since the main source of creatinine is muscle we used dual-energy X-ray absorptiometry to measure the mass of muscle in a cohort of adult men and women in Rochester, Minnesota. Serum and 24 h urinary creatinines were measured directly. The urinary creatinine was estimated using equations based on age and gender and muscle mass in order to calculate creatinine clearance. Among 664 subjects with a mean age of 55±20 years, 51% of whom were women, the model fit for urinary creatinine estimated with age and gender (R2 = 0.359) was similar to that estimated with measured muscle mass (R2 = 0.359). The likelihood of chronic kidney disease (creatinine clearance of less than 60 ml/min per 1.73m2) in older subjects was highest with equations that used age, and likelihood of CKD in women was highest with equations that used gender. The outcomes of mortality and cardiovascular disease had stronger associations with decreased creatinine clearance calculated with age and gender than by the clearance calculated with muscle mass. This could be explained by age being a potent predictor of mortality and cardiovascular disease independent of urinary creatinine, muscle mass, and gender. Our study shows that the likelihood of chronic kidney disease in the elderly and in women and the risk of adverse outcomes may be inflated by equations that use patient demographics to estimate creatinine generation.
Keywords: chronic kidney disease, creatinine, creatinine clearance, dietary protein, glomerular filtration rate, muscle mass
Chronic kidney disease (CKD) has been defined by a glomerular filtration rate (GFR) or creatinine clearance (CrCl) less than 60 ml/min per 1.73m2, with no requirement for corroborating evidence of kidney damage below this threshold even if GFR is estimated rather than directly measured.1 CrCl is calculated from the rate of creatinine generation divided by the concentration of serum creatinine (SCr). Intrinsic creatinine generation can be directly measured with a 24-h collection of urinary creatinine (UCr), but timing errors, incomplete collection, exercise, diet, and stress lead to a substantial within-person variability.2-5 To address the imprecision and inconvenience of directly measuring UCr, equations were developed to estimate UCr using easily measured and precise surrogates. The Cockcroft-Gault equation was developed to estimate creatinine generation using age, sex, and weight.6 Later, the Modification of Diet in Renal Disease (MDRD) equation was developed to estimate creatinine generation using age, sex, and race.7,8 Owing to the increased risk of mortality and cardiovascular disease with CKD,9,10 automated reporting of estimated CrCl (eCrCl) or GFR by these equations has been advocated whenever SCr levels are evaluated.1,11
Relatively few studies have examined how the choice of surrogates for creatinine generation influences associations with CKD as classified with equations. Verhave et al.12 found that the association with cardiovascular risk factors differed between urinary CrCl, the Cockcroft-Gault equation, and the MDRD equation. When comparing existing equations, it is uncertain if differences are due to the surrogates for creatinine generation vs other factors (estimating CrCl vs GFR, assay calibration,13,14 or source population14-16). Demographic variables are used in equations because they are convenient and correlate with muscle mass, the primary source of creatinine generation. However, this correlation is imperfect, and the use of demographics may contribute to the poor performance of equations for classifying CKD compared with directly measured GFR.17 Further, demographics may associate with CKD, or with adverse outcomes of CKD, independent of muscle mass or creatinine generation. This study assessed whether equations based on age and sex lead to bias in corresponding risk estimates compared with equations based on a direct measure of muscle mass. The development of novel estimating equations, using the same assays and the same source population, ensured that differences in the associations with each equation were fully explained by the choice of surrogates for creatinine generation.
RESULTS
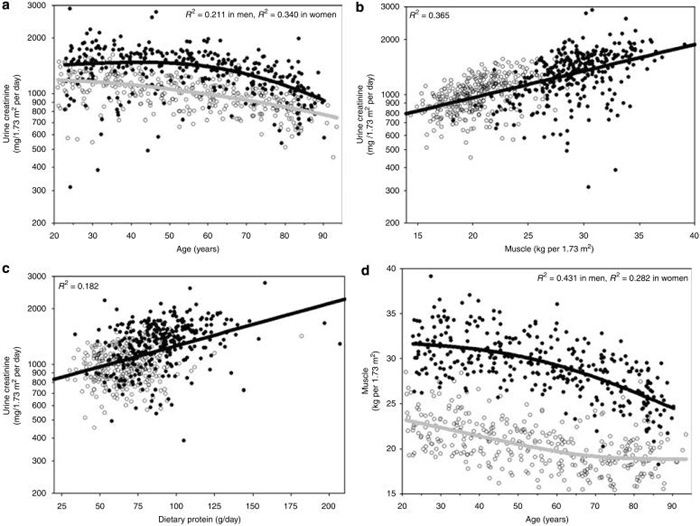
The study sample (n = 664) had a mean±s.d. age of 55±20 years; 51% were women. The mean±s.d. UCr was 1171±340 mg/1.73m2 per day; Scr was 0.81±0.24 mg/100 ml; muscle mass was 25±5 kg/1.73m2; and dietary protein was 77±23 g/day. There were four subjects missing UCr levels and 19 subjects missing dietary protein measures. Figure 1a shows UCr to be lower in women than in men and lower with older age, particularly after 55 years. Figure 1b shows that UCr is lower with decreased muscle mass, and Figure 1c shows a lower UCr with decreased dietary protein intake. Finally, Figure 1d confirms that, as expected, the relation between muscle mass and demographics is similar to that seen with UCr and demographics (Figure 1a).
Figure 1. Comparison between surrogates for creatinine generation rate.
Estimation of 24-h urinary creatinine by age (a), dual-energy X-ray absorptiometry skeletal muscle mass (b), and dietary protein (c) among an age-stratified sample of 660 Rochester, Minnesota residents. (d) The relationship between muscle mass and age. Men—solid circles; women—open circles. Curves represent a smoother regression (λ = 100,000) for men (black) and women (gray) in (a) and (d), and linear regression in (b) and (c).
Models were developed to estimate UCr with age, sex, muscle mass, and dietary protein (Table 1). An age spline transformation with a knot at 55 years led to a higher model fit (R2) than linear age (0.174 vs 0.150, P=0.03). A model based on age and sex had a similar fit to one based on muscle mass (0.359 vs 0.359, P = 0.86), but a model based on muscle mass and age had a higher fit than a model based on age and sex (0.424 vs 0.359, P = 0.0005). Sex was not a significant independent predictor (P = 0.43) in a model with age and muscle mass. Dietary protein was a significant independent predictor (P = 0.002) in a model with muscle mass and age, but this did not improve model fit on cross-validation (Table 1). Models that estimate UCr, using coefficients from the full sample, were converted into equations for eCrCl by dividing by SCr (in mg/100 ml):
| (1) |
| (2) |
| (3) |
Table 1.
Linear regression models estimating log (UCr/1.73 m2) with surrogates for creatinine generation among an age-stratified sample of 660 Rochester, Minnesota residents
| Model variables | Mean model R2 |
|---|---|
| Age | 0.150 |
| Age splinea | 0.174 |
| Sex | 0.190 |
| Age spline, sexa | 0.359b |
| Dietary protein | 0.153 |
| Muscle mass | 0.359b |
| Muscle mass, dietary proteinc | 0.358 |
| Age spline, muscle massa | 0.424b |
| Age spline, muscle massa, dietary proteinc | 0.420 |
UCr, urinary creatinine.
The R2 is the mean from 10 replications of a random 10% (n=66) validation set using coefficients estimated with the remaining 90% (n=594) training set.
If age ≤55 years, then age spline = 0; if age >55 years, then age spline = age -55 years.
Models chosen for final equations.
In persons missing dietary protein (n=20), estimate based on similar model without a dietary protein variable.
Table 2 compares measured urinary CrCl with eCrCl by each equation. The mean CrCl varied slightly between methods (103-105 ml/min per 1.73m2) as expected with logarithmic regression models. The interquartile range for the difference between urinary CrCl and eCrCl was lowest with eCrClAge,Muscle (22 ml/min per 1.73m2). The prevalence of CKD was 8.9% with urinary CrCl, 3.3% with eCrClMuscle, and 5.1% with eCrClAge,Sex. The increased likelihood of CKD with age was higher with equations that used age than with equations that did not use age. The likelihood of CKD in women compared with men was lower with urinary CrCl and eCrClAge,Muscle, but was not lower when muscle mass was replaced by sex in the equation (eCrClAge,Sex). The increased likelihood of CKD with decreasing muscle mass was higher in the equations that used muscle mass, than in the equations that did not use muscle mass.
Table 2.
Comparison of creatinine clearance (CrCl) among an age-stratified sample of 664 Rochester, Minnesota residents across methods used to measure or estimate the rate of creatinine generation
| Urinary CrCl | Estimated CrCl (muscle mass) | Estimated CrCl (age and sex) | Estimated CrCl (age and muscle mass) | |
|---|---|---|---|---|
| Correlation, R | Reference | 0.71 | 0.71 | 0.75 |
| Mean±s.d. | 105±32 | 103±23 | 103±26 | 104±26 |
| Interquartile range of estimated CrCl—urinary CrCl, ml/min per 1.73m2 | Reference | 27 | 26 | 22 |
| Prevalence of mild reduction, 60-89 ml/min per 1.73m2 | 22% (n=148) | 25% (n=164) | 25% (n=163) | 24% (n=158) |
| Prevalence of chronic kidney disease, <60 ml/min per 1.73m2 | 8.9% (n=59) | 3.3% (n=22) | 5.1% (n=34) | 5.7% (n=38) |
| Likelihood of chronic kidney disease with predictors that can also be used as surrogates for low creatinine generation | Odds ratio (95% confidence interval) | |||
| Age per s.d. (20 years) increase | 3.6 (2.5, 5.5) | 7.4 (3.4, 20) | 13 (6.0, 36) | 19 (8.2, 57) |
| Female vs male | 0.5 (0.3, 0.8) | 0.8 (0.3, 1.9) | 0.9 (0.4, 1.7) | 0.5 (0.2, 1.0) |
| Muscle mass per s.d. (5.3 kg/1.73m2) decline | 1.3 (1.0, 1.7) | 2.0 (1.3, 3.5) | 1.7 (1.1, 2.5) | 1.8 (1.2, 2.6) |
The mean follow-up time to death or last clinic visit was 12.2 years. During follow-up, 26% (175/664) of the subjects died, whereas the incidence of diagnosed cardiovascular disease was 31% (162/525). This included coronary artery disease in 19.4% (112/576), heart failure in 13.4% (86/643), stroke in 12.7% (80/632), and peripheral arterial disease in 15.3% (93/607). Table 3 compares associations with outcomes between each method of determining CrCl. With the exception of heart failure, outcomes were more strongly associated with eCrCl than urinary CrCl. There were stronger associations between outcomes and eCrCl based on equations that used age (eCrClAge,Sex and eCrClAge,Muscle) than with the equation that did not use age (eCrClMuscle). This finding was present irrespective of whether eCrCl was treated as a continuous (per -30 ml/min per 1.73m2) or a categorical (<60, 60-89, 90 ml/min per 1.73m2) variable.
Table 3.
Risk of adverse outcomes with creatinine clearance (CrCl) among an age-stratified sample of 664 Rochester, Minnesota residents across methods used to measure or estimate creatinine generation
| Urinary CrCl |
eCrClMuscle |
eCrClAge,Sex |
eCrClAge,Muscle |
|
|---|---|---|---|---|
| Hazard ratios (95% confidence interval) |
||||
| Adverse outcome | per 30 ml/min per 1.73m2 decline | |||
| Mortality | 2.7 (2.3, 3.2) | 2.9 (2.3, 3.5) | 3.6 (2.9, 4.4) | 3.5 (2.9, 4.2) |
| Cardiovascular disease | 1.7 (1.4, 1.9) | 1.8 (1.3, 2.4) | 2.1 (1.7, 2.5) | 2.1 (1.7, 2.5) |
| Coronary artery disease | 1.4 (1.2, 1.7) | 1.5 (1.1, 1.9) | 1.8 (1.4, 2.3) | 1.8 (1.4, 2.2) |
| Heart failure | 2.3 (1.9, 2.9) | 2.2 (1.6, 3.0) | 3.1 (2.3, 4.0) | 2.8 (2.2, 3.7) |
| Stroke | 1.9 (1.5, 2.4) | 2.1 (1.5, 2.8) | 2.1 (1.6, 2.8) | 2.2 (1.7, 3.0) |
| Peripheral vascular disease | 1.7 (1.4, 2.1) | 1.8 (1.3, 2.3) | 2.0 (1.5, 2.5) | 2.0 (1.6, 2.6) |
| 60-89 vs ≥90 ml/min per 1.73m2 | ||||
| Mortality | 4.2 (3.0, 5.9) | 3.3 (2.4, 4.6) | 4.8 (3.4, 6.6) | 4.0 (2.9, 5.6) |
| Cardiovascular disease | 1.9 (1.3, 2.7) | 2.0 (1.4, 2.8) | 3.1 (2.2, 4.3) | 3.1 (2.2, 4.3) |
| Coronary artery disease | 1.5 (0.9, 2.3) | 1.3 (0.8, 2.0) | 1.9 (1.2, 2.9) | 1.9 (1.3, 2.9) |
| Heart failure | 3.4 (2.1, 5.5) | 1.9 (1.2, 3.1) | 4.2 (2.6, 6.7) | 3.7 (2.3, 6.0) |
| Stroke | 2.4 (1.4, 3.9) | 2.6 (1.6, 4.1) | 2.9 (1.8, 4.6) | 2.9 (1.8, 4.7) |
| Peripheral vascular disease | 2.0 (1.3, 3.2) | 2.0 (1.3, 3.1) | 3.0 (1.9, 4.6) | 3.5 (2.3, 5.4) |
| <60 vs ≥90 ml/min per 1.73m2 | ||||
| Mortality | 8.8 (5.9, 13) | 11 (6.4, 18) | 16 (9.8, 25) | 18 (11, 27) |
| Cardiovascular disease | 3.1 (1.8, 5.0) | 7.0 (3.3, 13) | 8.1 (4.2, 14) | 7.5 (3.8, 14) |
| Coronary artery disease | 1.9 (0.9, 3.5) | 4.2 (1.6, 8.8) | 4.8 (2.2, 9.2) | 4.8 (2.1, 9.4) |
| Heart failure | 6.3 (3.4, 11) | 11 (5.6, 21) | 13 (6.8, 25) | 15 (7.6, 27) |
| Stroke | 3.9 (1.9, 7.4) | 5.0 (1.5, 12) | 7.9 (3.3, 16) | 7.9 (3.4, 16) |
| Peripheral vascular disease | 3.6 (1.9, 6.5) | 4.7 (1.8, 10) | 6.7 (3.0, 13) | 9.3 (4.4, 18) |
eCrCl, estimated creatinine clearance.
As eCrCl equations differed only by the surrogates used to estimate creatinine generation, differences in Table 3 were further explored by assessing whether the surrogates themselves were predictors of outcomes independent of their correlation with creatinine generation. Table 4 compares the risk (per s.d.) of each outcome with individual surrogates, adjusting for all other surrogates for creatinine generation. Age was a strong predictor of each outcome (hazard ratios (HRs) 2.8-5.7; P<0.0001 for all) independent of UCr, muscle mass, and sex. Decreased UCr was also an independent predictor of mortality (HR = 1.6; P=0.0009). Decreased muscle mass was weakly associated with a lower risk of heart failure (HR = 0.5; P = 0.02), and female sex was weakly associated with lower mortality (HR = 0.5; P=0.04) independent of other surrogates. Results were similar with further adjustment for dietary protein and SCr.
Table 4.
Adjusteda risk of adverse outcomes for surrogates of a decreasing rate of creatinine generation among an age-stratified sample of 664 Rochester, Minnesota residents
| Urinary creatinine per - s.d. |
Muscle mass per - s.d. |
Age per s.d. (20 years) |
Sex (female) |
|
|---|---|---|---|---|
| Adverse outcomes | Hazard ratios (95% confidence interval) | |||
| Mortality | 1.6 (1.2, 2.0) | 0.9 (0.6, 1.3) | 5.5 (4.1, 7.5) | 0.5 (0.3, 1.0) |
| Cardiovascular disease | 1.2 (1.0, 1.6) | 0.7 (0.5, 1.0) | 3.6 (2.8, 4.6) | 1.1 (0.6, 2.2) |
| Coronary artery disease | 0.9 (0.7, 1.2) | 0.7 (0.4, 1.1) | 3.5 (2.6, 4.8) | 1.1 (0.5, 2.2) |
| Heart failure | 1.4 (1.0, 2.0) | 0.5 (0.3, 0.9) | 5.7 (3.8, 8.6) | 1.3 (0.5, 3.1) |
| Stroke | 1.2 (0.8, 1.8) | 1.1 (0.7, 2.0) | 2.9 (2.1, 4.2) | 0.8 (0.3, 2.1) |
| Peripheral vascular disease | 1.2 (0.9, 1.6) | 1.0 (0.6, 1.6) | 2.8 (2.0, 3.9) | 0.5 (0.2, 1.1) |
Adjusted for all other surrogates; results similar with further adjustment for dietary protein intake and reciprocal serum creatinine.
DISCUSSION
Two types of analyses are prominent in the literature on renal function equations: the first focuses on the development and validation of equations against directly measured GFR or urinary CrCl, whereas the second focuses on epidemiological associations with these equations. By combining these two types of analyses, this study shows that the choice of surrogates for creatinine generation in equations can influence our understanding of risk factors for and outcomes of CKD. In particular, we found that the use of age and sex variables in equations that estimated creatinine clearance distorted the likelihood of CKD by age and sex and exaggerated the risk of mortality and cardiovascular disease with CKD. This is important because a primary argument for using equations over SCr alone to classify CKD has been that equations correct for age and sex differences in muscle mass. Unfortunately, the apparent likelihood of CKD is inflated in women with equations that use sex and inflated in the elderly with equations that use age. Equations that employ demographics cannot uncouple the association of CKD with age and sex from the estimation of creatinine generation with age and sex. Further, the risk of mortality and cardiovascular disease with CKD is inflated by equations that use age, because the residual variability in age that does not correlate with creatinine generation is a strong predictor of these outcomes.
In the first part of the analysis, muscle mass and dietary protein were assessed as novel surrogates for creatinine generation. Muscle mass was the individual surrogate with the highest model fit for predicting UCr. This is not surprising as creatinine, which is formed from creatine, is the end product of muscle catabolism, and almost 98% of the creatine pool is stored in muscle.18 The `creatinine equivalence' (kg muscle mass/g UCr) in this study was 21 kg/g, consistent with a range of 17-22 kg/g reported in other studies.5 Dietary protein,19 and cooked meat in particular,20 also increases UCr. In this study, dietary protein independently predicted UCr but did not improve model fit on cross-validation. However, dietary protein has an impact on creatinine that varies with diet, and a 7-day diet history may not capture the influence of dietary protein on creatinine during a 24-h urine collection or at the moment of a blood draw for SCr.
The comparison with directly measured muscle mass provides a novel insight into the limitation of demographics as surrogates for creatinine generation. Although it is well known that the decline in muscle mass with age contributes to the decline in UCr with age,5,21 age also predicts a decline in UCr independent of muscle mass. The addition of age as an equation variable with muscle mass improved the prediction of UCr by 6.5% over muscle mass alone. A potential explanation is that diffuse fat infiltration into muscle, or `myosteatosis' with aging,22 may not be fully distinguishable from muscle itself by dual-energy X-ray absorptiometry (DXA). On the other hand, as sex was not a predictor of UCr independent of muscle mass, differences in creatinine generation between the sexes could be fully explained by differences in muscle mass.
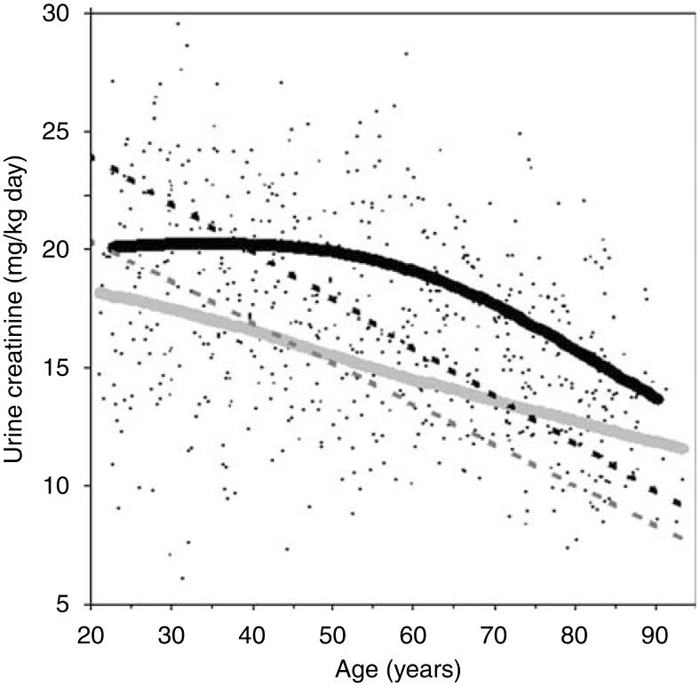
How does an equation developed in the general population compare with the MDRD and Cockcroft-Gault equations that were developed in select clinical populations? The Cockcroft-Gualt equation was actually derived in a similar manner to this study, by regressing UCr per kg body weight onto age.6 Figure 2 shows that over the age of 40 years, UCr/kg is higher in the general population than is estimated in hospital medical wards with the Cockcroft-Gault equation. A higher creatinine-generation rate in the general population than CKD populations may also explain why GFR estimates are higher in normal persons than in CKD patients at the same SCr level.23 Direct comparison of eCrClAgeSex with the MDRD equation is limited because UCr was not used to derive the MDRD equation. As the MDRD equation was derived using clinically diagnosed CKD patients, the demographic coefficients in that equation do not model risk of CKD but rather primarily model creatinine generation. The 26% lower creatinine generation in women compared with men with the MDRD equation is similar to the 23% difference estimated by eCrClAgeSex. By using logarithmic age to estimate creatinine generation, the MDRD equation models the steepest decline with age in young adults, whereas this study found that the steepest decline with age occurs in older adults (Figure 1a). Douville et al.24 also found the steepest decline with age in older adults.
Figure 2.
Estimation of urinary creatinine per kg per day by age among 660 Rochester, Minnesota residents in this study (solid smoother curves) compared with that estimated by the Cockcroft-Gault equation (dashed lines) in men (black) and women (light gray).
In the second part of the analysis, the impact of using demographics in equations on the epidemiology of CKD was assessed by comparison with urinary CrCl and equations that used muscle mass. The prevalence of CKD was highest with urinary CrCl (8.9%), followed by eCrClAgeSex (5.1%), and the lowest with eCrClMusc (3.3%). When comparing the prevalence of CKD between CrCl methods, it is important to consider the within-person variability of the surrogates for creatinine generation. The coefficient of variation for UCr is relatively high at 15%,3 but trivial for DXA muscle mass at 0.6%25 and effectively 0% for demographics. The imprecision of measured UCr compared with estimated UCr explains the higher prevalence of CKD with urinary CrCl than with eCrCl. The higher prevalence of CKD for equations that used age, compared with equations that did not use age, was due to the age-stratified study sample; because of the uniform age distribution, the very elderly were over-represented compared with other surrogates for low creatinine generation (that is, there were equal numbers of subjects in the older and middle-age groups, but fewer subjects with low muscle mass compared with average muscle mass). The imprecision of UCr and the limited twofold range of UCr in the general population (10-90%: 779-1629 mg/1.73m2) also lowers the signal-to-noise ratio and limits the model fit (R2) for estimating logarithmic UCr (Table 1).
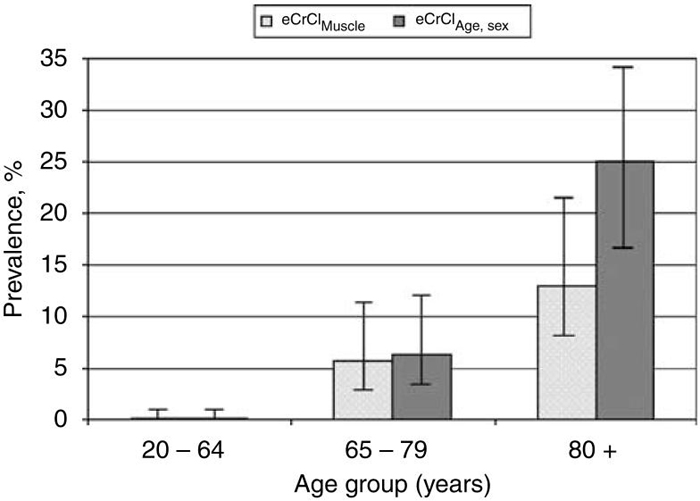
When evaluating age as a risk factor for CKD, associations were strongest with equations that used age as a surrogate for creatinine generation. The problem is that equations cannot uncouple age as a surrogate for creatinine generation from age as a risk factor for a low CrCl, and nothing is a stronger predictor of age than age itself. Figure 3 shows that an equation based on age and sex doubles the prevalence of CKD in the elderly compared with an equation based on muscle mass. This same problem is true for sex, because equations cannot uncouple sex as a surrogate for creatinine generation from sex as a risk factor for low CrCl, thus inflating CKD in women. Unfortunately, this masks the important finding that men actually have a higher likelihood of CKD than women, as shown by urinary CrCl and an equation that used muscle mass and age.
Figure 3. The prevalence of chronic kidney disease (creatinine clearance less than 60 ml/min per 1.73m2) with 95% confidence intervals across different age groups among 664 Rochester, Minnesota residents.
The light gray column represents estimated creatinine clearance with an equation based on dual-energy X-ray absorptiometry skeletal muscle mass, and the dark gray column represents estimated creatinine clearance with an equation based on age and sex. In the elderly (age>80 years), prevalence of CKD is 25% (95% CI: 17-34%) with an equation based on age and sex compared with 13% (95% CI: 8.0-22%) with an equation based on muscle mass. CKD, chronic kidney disease.
As expected, this study found that a reduced CrCl was a strong predictor of mortality and cardiovascular disease. The novel insight provided, however, is that these associations are stronger with equations that include age than equations that do not include age (Table 3). This was not explained by a better estimation of creatinine generation with age, because eCrClAge,Sex and eCrClMuscle, both had a similar R2 of 0.36 for estimating UCr. Instead, it was the residual variation in age that does not correlate with creatinine generation that is a potent predictor of adverse outcomes (Table 4). Female sex was independently protective against mortality, consistent with the increased longevity in women compared with men, but this did not translate into substantive difference in eCrClAge,Sex compared with eCrClAge,Musc with respect to mortality risk. Interestingly, a decreased UCr was associated with mortality independent of muscle mass, age, and sex. It is possible that `cardiac cachexia' or other illnesses with negative creatinine balance could explain this finding.
There are several potential limitations to consider with these findings. First, the equations in this study estimated CrCl not GFR. The missing factor, tubular secretion of creatinine (CrCl/GFR), is primarily affected by GFR26 and by medications (for example, cimetidine and trimethoprim). Nonetheless, these findings are still relevant because age, sex, muscle mass and dietary protein are surrogates for creatinine generation regardless of whether an equation estimates GFR or CrCl. Rowe et al.27 found age to have no substantive association with the tubular secretion of creatinine (CrCl/GFR). Second, it might be argued that only the age-sex-adjusted association between eCrCl and outcomes is worth investigation. With age and sex adjustment, there is no advantage to evaluating associations with eCrClage,sex over evaluating these same associations with reciprocal SCr (1/SCr), obviating the purpose for an equation. The impact of using demographics in equations on CKD associations can only be determined in analyses that do not adjust for demographics. Finally, this study could not evaluate ethnicity or race as a surrogate for creatinine generation because the study population was almost exclusively non-Hispanic white. By analogy, a black race variable used to model increased creatinine generation (that is, the MDRD equation) would be expected to underestimate CKD in blacks, and this hypothesis is supported by other data.28
In conclusion, it has been widely reported that CKD is common and under-recognized, particularly in the elderly and women,29-31 but the use of demographics in equations that classify CKD may be part of the problem. Bias from the sex variable in the MDRD equation contributes to a higher risk in women than men for estimated GFR<60 ml/min per 1.73m2,32 contrary to the lower risk in women than men for albuminuria,30 decline in estimated GFR over time,33 or end-stage renal disease.34 To the extent adverse outcomes with CKD are biased by equations that use age, targeted interventions to treat CKD and prevent adverse outcomes will be difficult to evaluate for efficacy. In the screening setting, the evaluation of SCr levels with respect to the upper limit of normal (1.3 mg/100 ml in men and 1.1 mg/100 ml in women) may be a more defensible approach as these thresholds also reflect an age-appropriate low GFR.35-38 Uncertainty regarding CKD with borderline SCr levels is appropriate and may require further investigations (for example, urinalysis, 24-h urinary protein and CrCl, and renal ultrasound). Equations may be more appropriate for staging disease severity after a clinical diagnosis of CKD has been made.
MATERIALS AND METHODS
Setting and participants
As detailed elsewhere,25 an age- and sex-stratified random sample of the Rochester, Minnesota population was identified using the medical record linkage system of the Rochester Epidemiology Project.39 After excluding dementia, death before contact, severe debility, pregnancy, and radiation workers, 899 men and 812 women were invited, of which 348 men (39%) and 351 women (43%) participated in a study visit between 1991 and 1996. After excluding subjects with missing DXA scans and SCr levels and restricting the sample to non-Hispanic white ethnicity, there were 326 men and 338 women in the final sample. The sample was limited to non-Hispanic whites, because race confounds the relationship between SCr and GFR,7,15 and there was inadequate minority representation to evaluate this factor. An institutional review board approved the study, and the protocol was consistent with the principles of the Declaration of Helsinki.
Measures of creatinine
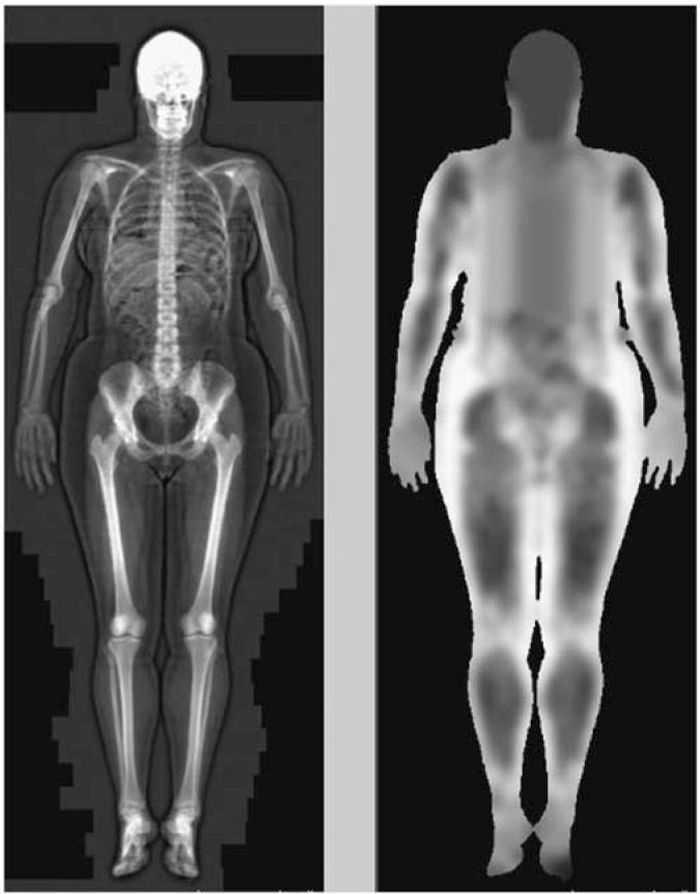
Serum creatinine and UCr levels (from a home 24-h collection) were determined with a rate-Jaffe assay (Multistat III plus; Instrumentation Laboratories, Lexington, MA, USA). SCr was calibrated to standardized levels (SCrStandardized=0.95 × SCrStudy-0.10), based on the difference in mean SCr between this study and the third National Health and Nutrition Examination Survey.13,40,41 Skeletal muscle mass was measured with a whole-body DXA scan (Hologic QDR 2000 instrument; Hologic, Waltham, MA, USA) using version 5.67 software. DXA exploits the attenuation of two photon energies to determine the mass of mineral (that is, bone), fat, and lean (that is, non-mineral, non-fat) in the body. Figure 4 shows the results of a DXA scan, which is a two-dimensional (area-based) measure of body composition as opposed to the three-dimensional (volume-based) measures seen with computed tomography. The software used skeletal landmarks to distinguish the extremities from the trunk. Wang et al.42 found that lean mass in the extremities represents 75% of the total body skeletal muscle mass. Thus, total skeletal muscle mass (kg) was determined from the lean body mass of all four extremities multiplied by 1.33. With repeated measures, DXA lean body mass had a coefficient of variation of 0.6%.25 Dietary protein intake (g/day) was calculated by professional dietitians from 7-day diet histories. Surrogates for creatinine generation were measured on the day of the study visit (DXA scan) or immediately before the study visit (24-h UCr and 7-day diet history).
Figure 4. Dual-energy X-ray absorptiometry scan of a 30-year-old woman.
Images generated from the scan can be optimized to contrast skeleton (white) from soft tissue (black) as shown in the left panel or to contrast lean mass (black) from fat (white) as shown in the right panel.
Outcomes
Diagnostic codes (manually or automatically coded from the final diagnoses in clinical notes) since 1935 are indexed and linked among nearly all local providers through the Rochester Epidemiology Project.39 Outcomes were identified through March 2008 using International Classification of Diseases-9 codes and equivalent Hospital Adaptation of the International Classification of Diseases-8 codes. Coronary artery disease (410-413), heart failure (402.91, 428.0, 428.1, and 428.9), stroke (430, 431, 432.9, 433, 434, 435.0, 435.1, and 435.8), and peripheral arterial disease, including aortic aneurism (440-444, 557.0, 557.1, 557.9, and 593.81), were evaluated as individual outcomes and as a composite outcome of cardiovascular disease. For estimating incident disease (after the study visit), subjects with prevalent disease (before the study visit) were excluded. Mortality was determined from Minnesota State death certificates.
Statistical analysis
As the goal was to estimate CrCl in ml/min per 1.73m2 of body surface area, 24-h UCr was normalized to mg/1.73m2 by multiplying by 1.73/body surface area. Body surface area was calculated from height and weight with the DuBois equation.43 Muscle mass was also normalized to kg per 1.73m2. Logarithmic 24-h UCr was regressed on each surrogate for creatinine generation (age, sex, muscle mass, and dietary protein). A linear spline with a knot at 55 years was also considered for age. Multivariable linear regression models with various combinations of age, sex, muscle mass, and dietary protein were developed to estimate log UCr. Model fit (R2) was determined in a random 10% (n = 66) validation set using coefficients estimated with the remaining 90% (n=594) training set. This cross-validation was replicated for 10 different random 10%/90% splits, and the mean R2 was compared between models with paired t-tests. CrCl (ml/min per 1.73m2) was calculated by dividing UCr (measured or estimated) by SCr × 14.4 (min × 100 ml)/(days ml). The prevalence of a mild reduction in CrCl (60-89 ml/min per 1.73m2) and of CKD (<60 ml/min per 1.73m2)1 was compared between urinary and estimated CrCl.
Logistic regression compared the likelihood of CKD with surrogates for decreasing creatinine generation: age, female sex, and decreasing muscle mass across each method of estimating CrCl. Cox proportional hazards models calculated the HR for morbidity and mortality outcomes by each method of CrCl. Censoring occurred for death (except when predicting mortality), last clinical contact date, and March 2008. As CrCl equations differed only by the choice of creatinine-generation surrogates, each surrogate was evaluated as a predictor for outcomes independent of other surrogates. All statistical analyses were performed with JMP, version 7.0.1 (SAS Institute Inc., Cary, NC, USA).
ACKNOWLEDGMENTS
This project was supported by research grants (DK78229, AR27065, AR30582, and RR00585) from the National Institutes of Health, US Public Health Service.
Footnotes
DISCLOSURE All the authors declared no competing interests.
REFERENCES
- 1.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Int Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [comment][erratum appears in Ann Intern Med. 2003 Oct 7;139(7):605] [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis. 2002;39:S1–S246. [PubMed] [Google Scholar]
- 3.James GD, Sealey JE, Alderman M, et al. A longitudinal study of urinary creatinine and creatinine clearance in normal subjects. Race, sex, and age differences. Am J Hypertens. 1988;1:124–131. doi: 10.1093/ajh/1.2.124. [DOI] [PubMed] [Google Scholar]
- 4.Petri M, Bockenstedt L, Colman J, et al. Serial assessment of glomerular filtration rate in lupus nephropathy. Kidney Int. 1988;34:832–839. doi: 10.1038/ki.1988.257. [DOI] [PubMed] [Google Scholar]
- 5.Heymsfield SB, Arteaga C, McManus C, et al. Measurement of muscle mass in humans: validity of the 24-h urinary creatinine method. Am J Clin Nutr. 1983;37:478–494. doi: 10.1093/ajcn/37.3.478. [DOI] [PubMed] [Google Scholar]
- 6.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 9.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 10.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 12.Verhave JC, Gansevoort RT, Hillege HL, et al. Drawbacks of the use of indirect estimates of renal function to evaluate the effect of risk factors on renal function. J Am Soc Nephrol. 2004;15:1316–1322. [PubMed] [Google Scholar]
- 13.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 14.Stevens LA, Manzi J, Levey AS, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 2007;50:21–35. doi: 10.1053/j.ajkd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Poggio ED, Wang X, Greene T, et al. Performance of the modification of diet in renal disease and Cockcroft-gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005;16:459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 16.Rule AD. Understanding estimated glomerular filtration rate: implications for identifying chronic kidney disease. Curr Opin Nephrol Hypertens. 2007;16:242–249. doi: 10.1097/MNH.0b013e328057de8b. [DOI] [PubMed] [Google Scholar]
- 17.Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 18.Stevens LA, Levey AS. Measurement of kidney function. Med Clin North Am. 2005;89:457–473. doi: 10.1016/j.mcna.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Lykken GI, Jacob RA, Munoz JM, et al. A mathematical model of creatine metabolism in normal males—comparison between theory and experiment. Am J Clin Nutr. 1980;33:2674–2685. doi: 10.1093/ajcn/33.12.2674. [DOI] [PubMed] [Google Scholar]
- 20.Jacobsen FK, Christensen CK, Mogensen CE, et al. Pronounced increase in serum creatinine concentration after eating cooked meat. BMJ. 1979;1:1049–1050. doi: 10.1136/bmj.1.6170.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomlinson BE, Walton JN, Rebeiz JJ. The effects of ageing and of cachexia upon skeletal muscle. A histopathological study. J Neurol Sci. 1969;9:321–346. doi: 10.1016/0022-510x(69)90079-3. [DOI] [PubMed] [Google Scholar]
- 22.Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med. 2008;24:455–469. doi: 10.1016/j.cger.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Int Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 24.Douville P, Martel AR, Talbot J, et al. Impact of age on glomerular filtration estimates. Nephrol Dial Transplant. 2008;24:97–103. doi: 10.1093/ndt/gfn473. [DOI] [PubMed] [Google Scholar]
- 25.Melton LJ, III, Khosla S, Riggs BL. Epidemiology of sarcopenia. Mayo Clin Proc. 2000;75(Suppl):S10–S12. discussion S12-S13. [PubMed] [Google Scholar]
- 26.Bauer JH, Brooks CS, Burch RN. Clinical appraisal of creatinine clearance as a measurement of glomerular filtration rate. Am J Kidney Dis. 1982;2:337–346. doi: 10.1016/s0272-6386(82)80091-7. [DOI] [PubMed] [Google Scholar]
- 27.Rowe JW, Andres R, Tobin JD, et al. The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol. 1976;31:155–163. doi: 10.1093/geronj/31.2.155. [DOI] [PubMed] [Google Scholar]
- 28.Foley RN, Wang C, Ishani A, et al. NHANES III: influence of race on GFR thresholds and detection of metabolic abnormalities. J Am Soc Nephrol. 2007;18:2575–2582. doi: 10.1681/ASN.2006121411. [DOI] [PubMed] [Google Scholar]
- 29.Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 30.Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and Trends among U.S. adults, 1999-2000. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 31.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 32.Bang H, Vupputuri S, Shoham DA, et al. Screening for occult renal disease (SCORED): a simple prediction model for chronic kidney disease. Arch Intern Med. 2007;167:374–381. doi: 10.1001/archinte.167.4.374. [DOI] [PubMed] [Google Scholar]
- 33.Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69:375–382. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- 34.Excerpts from the USRDS 2003 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Am J Kidney Dis. 2003;42:S1–S230. US Renal Data System. [PubMed] [Google Scholar]
- 35.Poggio ED, Rule AD. Can we do better than a single estimated GFR threshold when screening for chronic kidney disease? Kidney Int. 2007;72:534–536. doi: 10.1038/sj.ki.5002452. [DOI] [PubMed] [Google Scholar]
- 36.Wetzels JF, Kiemeney LA, Swinkels DW, et al. Age- and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int. 2007;72:632–637. doi: 10.1038/sj.ki.5002374. [DOI] [PubMed] [Google Scholar]
- 37.Rule AD, Larson TS. Do we need another equation to estimate GFR from serum creatinine in renal allograft recipients? Nephrol Dial Transplant. 2008;23:2427–2428. doi: 10.1093/ndt/gfn119. author reply 2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rule AD, Gussak HM, Pond GR, et al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43:112–119. doi: 10.1053/j.ajkd.2003.09.026. [erratum appears in Am J Kidney Dis. 2004 Dec;44(6):1126] [DOI] [PubMed] [Google Scholar]
- 39.Melton LJ., III History of the rochester epidemiology project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 40.Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988-1994) Arch Intern Med. 2001;161:1207–1216. doi: 10.1001/archinte.161.9.1207. [DOI] [PubMed] [Google Scholar]
- 41.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 42.Wang ZM, Visser M, Ma R, et al. Skeletal muscle mass: evaluation of neutron activation and dual-energy X-ray absorptiometry methods. J Appl Physiol. 1996;80:824–831. doi: 10.1152/jappl.1996.80.3.824. [DOI] [PubMed] [Google Scholar]
- 43.DuBois D, DuBois E. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]