Abstract
Many hospital antimicrobial stewardship programs restrict the availability of selected drugs by requiring prior approval. Carbapenems may be among the restricted drugs, but it is unclear if hospitals that restrict availability actually use fewer carbapenems than hospitals that do not restrict use. Nor is it clear if restriction is related to resistance. We evaluated the relationship between carbapenem restriction and the volume of carbapenem use and both the incidence rate and proportion of carbapenem-resistant Pseudomonas aeruginosa isolates from 2002 through 2006 in a retrospective, longitudinal, multicenter analysis among a consortium of academic health centers. Carbapenem use was measured from billing records as days of therapy per 1,000 patient days. Hospital antibiograms were used to determine both the incidence rate and proportion of carbapenem-resistant P. aeruginosa isolates. A survey inquired about restriction policies for antibiotics, including carbapenems. General linear mixed models were used to examine study outcomes. Among 22 hospitals with sufficient data for analysis, overall carbapenem use increased significantly over the 5 years of study (P < 0.0001), although overall carbapenem resistance in P. aeruginosa did not change. Hospitals that restricted carbapenems (n = 8; 36%) used significantly fewer carbapenems (P = 0.04) and reported lower incidence rates of carbapenem-resistant P. aeruginosa (P = 0.01) for all study years. Fluoroquinolone use was a potential confounder of these relationships, but hospitals that restricted carbapenems actually used fewer fluoroquinolones than those that did not. Restriction of carbapenems is associated with both lower use and lower incidence rates of carbapenem resistance in P. aeruginosa.
Hospital antimicrobial stewardship programs (ASPs) attempt to improve antibacterial prescribing, commonly using formulary restrictions and by requiring preauthorization (7). Carbapenems are restricted in some hospitals for treatment of gram-negative bacterial infections resistant to first-line drugs, although carbapenem resistance among Pseudomonas aeruginosa and Enterobacteriaceae is increasing (3, 14, 18-20).
Individual hospitals have reported improvement in bacterial susceptibilities to carbapenems after implementing ASPs that restricted carbapenem use (1, 11, 22). However, these “before and after” study designs have been criticized, and it is not known if the results are generalizable (6). There are no multihospital investigations that have assessed the effect of carbapenem restriction on carbapenem use and carbapenem-resistant P. aeruginosa over multiple years. In this study we evaluated the association between carbapenem restriction in academic health centers and the volume of carbapenem use and both the incidence rate and proportion of carbapenem-resistant P. aeruginosa from 2002 through 2006.
(This study was presented in part at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, September 2007.)
MATERIALS AND METHODS
Data source.
The University HealthSystem Consortium (UHC) consists of 102 academic hospitals in the United States. Some UHC members also participate in the Clinical Resource Manager (CRM) database from which medication utilization data for this study was obtained. The number of hospitals subscribing to the CRM database that also agreed to contribute data to this investigation increased from 23 hospitals in 2002 to 35 hospitals in 2006. Additional details of the UHC network are described in a recent publication that examined trends in adult antibacterial drug use within the network (12).
Assessment of antibacterial restriction and antibiogram construction.
A 12-question survey requesting information regarding the hospital's antibacterial stewardship program policies between the years 2002 and 2006 was sent via e-mail in 2006 and 2007 to a pharmacist or physician infectious diseases specialist at each hospital for whom we had contact information (n = 30). One survey question asked if a prior approval restriction policy for select antibacterials was used and if so, for which drugs. Other questions inquired about the methods used to construct the hospital's antibiogram, including whether duplicate patient isolates were removed when compiling resistance rates. We also asked if there had been any changes in the ASP policies over these years. In this investigation, hospitals that stated that they restricted carbapenem use over the study period were contrasted to hospitals that did not.
Assessment of resistance.
Antibiograms were requested for years 2002 to 2006. Only antibiograms that included a full calendar year of susceptibility data from all clinical sources were used. From these we estimated two measures of imipenem/meropenem-resistant P. aeruginosa: (i) the proportion of resistant isolates (percent resistant) and (ii) the incidence rate of resistance (number of resistant isolates/1,000 discharges). Schwaber et al. have argued that measuring incidence rates of resistance is the more appropriate measure for assessing the relationship between antibacterial use and resistance (17).
Assessment of antibacterial use.
Yearly carbapenem use (meropenem and imipenem) with adult patients discharged between 1 January 2002 through 31 December 2006 was obtained from billing records as previously described; antibacterial use was validated at one of these hospitals (12). We did not measure use of ertapenem. Carbapenem use was expressed as days of therapy per 1,000 patient days (DOT/1,000 PD). If a patient received a single dose of an antibacterial drug on a given day, whether or not multiple doses are usually administered, it was registered as 1 DOT. The advantages of DOT as a measure of antibacterial use compared with the defined daily dose (DDD) have recently been reported (15).
Because fluoroquinolone use has been reported to be a risk factor for carbapenem resistance in P. aeruginosa (5, 8, 13, 14, 20), we also measured total fluoroquinolone use in hospitals where carbapenems were restricted and compared this to total fluoroquinolone use in hospitals that did not restrict carbapenems. The fluoroquinolones in this analysis included ciprofloxacin, levofloxacin, moxifloxacin, and gatifloxacin, and their use was measured as previously described (12).
Statistical analysis.
The following three different general linear mixed models were used to examine three outcomes over the study period among hospitals that did and did not restrict carbapenems: (i) carbapenem use and both the (ii) proportion and (iii) incidence rates of carbapenem-resistant isolates for P. aeruginosa. Use of the mixed model enabled examination of relationships across time, with missing data points (10). We also used linear regression to evaluate univariate relationships between carbapenem and fluoroquinolone use and incidence rates of carbapenem-resistant P. aeruginosa. A P value of <0.05 was considered statistically significant. The statistical software used was JMP (version 7.0; SAS Institute, Cary, NC). The Institutional Review Board at Virginia Commonwealth University approved this investigation.
RESULTS
A total of 25 hospitals responded to the ASP policy survey; of these, 22 hospitals provided data regarding carbapenem use and carbapenem resistance (both proportion and number of resistant isolates) for at least 2 years. Of 110 possible data points (22 hospitals and 5 years of data), there were 94 (85%) carbapenem use data points and 89 (81%) data points for incidence rates of resistance. Hospitals represented the following regions: Mid-Continent (5 hospitals); Midwestern (4 hospitals); Mid-Atlantic (4 hospitals); Southeastern (5 hospitals); Western (3 hospitals); and New England (1 hospital). Mean bed size was 427 (±139), and mean age for all patients in all hospitals was 50 (±4.0) years.
Of these 22 hospitals, 8 (36%) stated that they restricted carbapenem use by requiring prior approval (1 hospital required approval after 48 h); the remaining 14 did not. None of the hospitals reported changes in their carbapenem restriction policy over the 5 years of the study. Most hospitals used both meropenem and imipenem. For example in 2004, 15 of 20 reporting hospitals used more imipenem than meropenem, and 3 of these used imipenem exclusively. For the same year, 5 hospitals used more meropenem than imipenem, and 2 of these used meropenem exclusively.
Fifteen hospitals removed duplicate isolates when compiling susceptibility data, three did not; information regarding duplicate isolate removal was not available for four.
Overall carbapenem use in these 22 hospitals increased significantly over time, from a mean of 23.3 DOT/1,000 PD in 2002 to 21.3 (2003), 24.7 (2004), 27.8 (2005), and 31.4 DOT/1,000 PD in 2006 (P < 0.0001). (These 22 hospitals are not identical to the 22 hospitals reported in reference 12.) The overall proportion of carbapenem-resistant P. aeruginosa isolates did not change significantly over time; it increased from a mean of 18% in year 2002 to 19% (2003), 20% (2004), 23% (2005), and 22% in 2006 (P = 0.21). In addition, the overall incidence rate of resistant P. aeruginosa isolates did not change significantly over time; it changed from a mean of 7.9/1,000 discharges in 2002 to 6.7 (2003), 8.2 (2004), 6.2 (2004), 6.2 (2005), and 5.2/1,000 discharges in 2006 (P = 0.50). There were significant linear relationships between total carbapenem use and incidence rates of carbapenem-resistant P. aeruginosa in 2002 (adjusted R2 = 0.47, P = 0.0038), 2003 (adjusted R2 = 0.37, P = 0.003), and 2004 (adjusted R2 = 0.18, P = 0.033); the relationship was not significant thereafter. The linear relationships between carbapenem use and the proportion of resistant P. aeruginosa isolates were positive for all years, but were not statistically significant (data not shown).
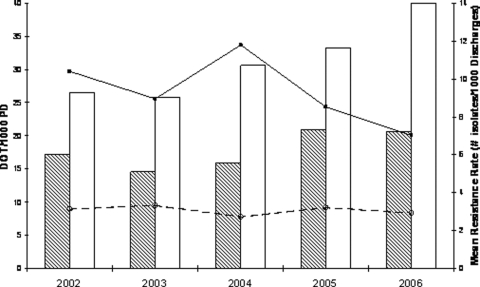
Hospitals that restricted carbapenems used significantly fewer carbapenems than hospitals that did not restrict for all 5 years (P = 0.04) (Fig. 1). In addition, the incidence rates of carbapenem-resistant P. aeruginosa in hospitals that restricted carbapenems were significantly lower for all 5 years compared with those that did not restrict (P = 0.01) (Fig. 1). Whereas the mean proportion of resistant P. aeruginosa isolates was consistently lower for all 5 study years in hospitals that restricted carbapenems than that of hospitals that did not restrict, these differences were not statistically significant (data not shown).
FIG. 1.
Mean carbapenem use (DOT/1,000 PD) was significantly lower in hospitals that restricted (shaded bars) versus did not restrict (open bars) carbapenems (P = 0.04). Incidence rates of carbapenem-resistant P. aeruginosa (number of isolates/1,000 discharges) were lower for hospitals that restricted (dashed line) versus did not restrict (solid line) carbapenems (P = 0.01).
The mean total fluoroquinolone use in hospitals that restricted carbapenems was consistently lower, by approximately 20 DOT/1,000 PD, depending upon the year, than that of hospitals that did not restrict carbapenems (data not shown). There was no significant linear relationship between total fluoroquinolone use and incidence rates of carbapenem-resistant P. aeruginosa for any of the 5 years of study.
When excluding the three hospitals that did not remove duplicate isolates when compiling susceptibility analyses and the four hospitals for which the duplicate isolate policy was unknown, results of the mixed model analyses did not change.
DISCUSSION
The National Nosocomial Infections Surveillance (NNIS) system reported that the percentage of P. aeruginosa isolates resistant to imipenem rose 15% from the time period 1998 to 2002 compared to the final study year, 2003 (3). The causes of this are not known but may in part be related to differences in levels of carbapenem use in NNIS hospitals during this period. Some hospitals restrict the use of carbapenems as an antimicrobial stewardship strategy, as seen in this investigation, yet few studies have examined the effect of restriction on carbapenem use or resistance, and to our knowledge there are no previous multicenter investigations of this issue. Bantar et al. (1) developed an intervention program to optimize hospital antibacterial use and demonstrated a statistically significant decrease in carbapenem use over a 2-year period (from 13.5 to 6.2 DDD/1,000 PD; P = 0.03). This was associated with a significant decrease in the proportion of imipenem-resistant P. aeruginosa isolates, from 19% to 0%. White and colleagues (22) studied the effect of an antimicrobial control program on antimicrobial expenditures and susceptibilities. Monthly expenditures for imipenem during the program decreased by 40%, and the proportion of P. aeruginosa isolates susceptible to imipenem increased significantly, from 83% to 95% for inpatients and from 65% to 83% for intensive care unit patients. Martin et al. (11) described an antimicrobial stewardship program that restricted the use of carbapenems and found that carbapenem susceptibilities for P. aeruginosa improved over 5 years, from 86% for the first year of the study to 91% in the last year. Carbapenem use was variable throughout the study period and actually increased from the first study year to the final year.
The present study examined a relatively large cohort of academic health centers over a 5-year period and observed that hospitals that restricted the use of carbapenems used significantly fewer of these agents and had significantly lower incidence rates of resistance among P. aeruginosa isolates. There was not a statistically significant difference in the proportions of carbapenem-resistant P. aeruginosa isolates between hospitals that restricted carbapenems and those that did not, although hospitals that restrict carbapenems had lower proportions of resistance for each study year. The lack of significance in the analysis of the proportion of resistance may in part be due to the relatively few hospitals that restricted carbapenems and insufficient statistical power. On the other hand, the method or measuring resistance is taking on increasing importance. Schwaber has argued that the use of proportions of resistant organisms to assess the impact of antibacterial use can be misleading since proportions are dependent on the susceptible and resistant bacterial populations, whereas the incidence rate of resistance depends only upon the resistant population (17). Antibacterial use likely leads to a decrease in the absolute number of susceptible organisms, which results in an increase in the proportion of isolates that are resistant. This does not necessarily translate to an increase in the absolute number of resistant isolates or the burden of resistance. Cook assessed the impact of ciprofloxacin restriction on both proportion and incidence rates of resistant nosocomial isolates of P. aeruginosa (4). He found that ciprofloxacin use declined by 57% and that both incidence rates and the proportion of ciprofloxacin-resistant P. aeruginosa isolates declined significantly. However, imipenem/meropenem use increased over the same time period, as did the incidence rates and proportions of carbapenem-resistant P. aeruginosa isolates. Rogues reported a stronger relationship between antibiotic use (as DDD/1,000 PD) among 47 French hospitals and incidence rates of methicillin-resistant Staphylococcus aureus (MRSA) and drug-resistant P. aeruginosa than between antibiotic use and proportions of these resistant organisms (16). Burton also reported that the incidence rates of MRSA bacteremia in patients with central lines from 1,684 U.S. intensive care units monitored by the Centers for Disease Control and Prevention decreased by nearly 50% over the 10-year period 1997 to 2007 (2). When the same data were expressed as a proportion (percent), there was an apparent increase of 25% in MRSA bloodstream infections. The association between antibiotic use and incidence rates versus proportion of resistant organisms deserves additional investigation.
There are limitations to this study. First, we examined aggregated hospital-level data; this “ecologic bias” does not necessarily reflect antibacterial use and resistance patterns at the patient level (9). While our results cannot prove a causal relationship between carbapenem restriction policies, lower carbapenem use, and lower incidence rates of carbapenem-resistant P. aeruginosa, the association was relatively large, statistically significant, and persistent over time. Turnidge has argued that when these aggregate-level “correlations” are statistically significant, they are likely of importance and suggest that a reduction in consumption of the correlated antibiotic class will reduce resistance (21). It is also noteworthy that simple univariate associations between carbapenem use and incidence rates of carbapenem resistance were relatively strong and significant in the early years of this investigation, but the strength of these associations subsided with time. This shift emphasizes the need to examine these relationships for more than a single year or single point in time. Second, we did not measure hospital infection control measures, and it is possible that hospitals that restrict carbapenems also have more stringent infection control practices, leading to lower incidence rates of carbapenem resistance. Third, while we were able to separate carbapenem use with adults from use with patients less than 18 years of age, we were not able to separate clinical isolates of P. aeruginosa by patient age. Consequently, the microbiological end points in this investigation are a mix of adult and pediatric data, potentially weakening the association between adult antibiotic use and resistant P. aeruginosa in adults. Finally, carbapenem resistance in P. aeruginosa is more complex than simply carbapenem use and cross-transmission, and many additional risk factors have been reported from case-control investigations (8, 13). Many of these carbapenem-resistant isolates are likely to be multidrug resistant (5), and the use of other antimicrobials-in particular the fluoroquinolones-has been found to select for carbapenem resistance (5, 13, 14). Consequently, hospitals in this investigation that restricted the use of carbapenems may use relatively larger amounts of other, nonrestricted drugs, possibly increasing rates of resistance to those agents or even to the restricted agent. This phenomenon has been referred to as “squeezing the balloon” (14). However, we did not find fluoroquinolone use in hospitals that restricted carbapenems to be higher than that in hospitals that did not restrict carbapenems, nor did we find significant linear relationships between fluoroquinolone use and incidence rates of carbapenem-resistant P. aeruginosa. We did not attempt to incorporate the use of other antibacterial classes in the present assessment, and it is not yet possible to model all of these complex, competing, and possibly confounding relationships.
These data suggest that restricting carbapenems may be an effective element in an overall strategy for addressing the problem of carbapenem resistance in P. aeruginosa. Of equal or greater concern is the emergence of carbapenemase production among Enterobacteriaceae, although it is unclear at this point what role carbapenem restriction may have in delaying emergence or in the control of these organisms (18, 20). There are few antibacterial agents under development for gram-negative organisms, and preserving the effectiveness of available antibacterials is a necessary strategy in the management of antibacterial resistance (19).
Acknowledgments
We thank UHC CRM member participants, including pharmacists, microbiologists, and infectious disease physicians for their willingness to provide the data necessary to conduct this study.
This work was supported by Virginia Commonwealth University A. D. Williams Trust Funds.
Potential conflicts of interest include the following: R.E.P. received research funding from Merck and Co., ViroPharma Inc., and Bayer Pharma (grant support assumed by Schering-Plough) and is on the Schering-Plough Speakers Bureau; and A.L.P. received an honorarium from Schering-Plough for an oral presentation and grant support from ViroPharma, Inc. M.O. has no potential conflicts to report.
Footnotes
Published ahead of print on 9 March 2009.
REFERENCES
- 1.Bantar, C., B. Sartori, E. Vesco, C. Heft, M. Saúl, F. Salamone, and M. E. Oliva. 2003. A hospital wide intervention program to optimize the quality of antibiotic use: impact on proscribing practice, antibiotic consumption, cost savings, and bacterial resistance. Clin. Infect. Dis. 37:180-186. [DOI] [PubMed] [Google Scholar]
- 2.Burton, D. C., J. R. Edwards, T. C. Horan, J. A. Jernigan, and S. K. Fridkin. 2009. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997-2007. JAMA 301:727-736. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2004. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued August 2004. Am. J. Infect. Control 32:470-485. [DOI] [PubMed] [Google Scholar]
- 4.Cook, P. P., T. D. Das, M. Gooch, and P. G. Catrou. 2008. Effect of a program to reduce hospital ciprofloxacin use on nosocomial Pseudomonas aeruginosa susceptibility to quinolones and other antimicrobial agents. Infect. Control Hosp. Epidemiol. 29:716-722. [DOI] [PubMed] [Google Scholar]
- 5.D'Agata, E. M. C., M. A. Cataldo, R. Cauda, and E. Tacconelli. 2006. The importance of addressing multidrug resistance and not assuming single-drug resistance in case-control studies. Infect. Control Hosp. Epidemiol. 27:670-674. [DOI] [PubMed] [Google Scholar]
- 6.Davey, P., E. Brown, L. Fenelon, R. Finch, I. Gould, A. Holmes, C. Ramsay, E. Taylor, M. Wilcox, and P. Wiffen. 2006. Systematic review of antimicrobial drug prescribing in hospitals. Emerg. Infect. Dis. 12:211-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dellit, T. H., R. C. Owens, J. E. McGowan, D. N. Gerding, R. A. Weinstein, J. P. Burke, W. C. Huskings, D. L. Paterson, N. O. Fishman, C. F. Carpenter, P. J. Brennan, M. Billeter, and T. M. Hooton. 2007. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 44:159-177. [DOI] [PubMed] [Google Scholar]
- 8.Falagas, M. E., and P. Kopterides. 2006. Risk factors for the isolation of multi-drug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa: a systematic review of the literature. J. Hosp. Infect. 64:7-15. [DOI] [PubMed] [Google Scholar]
- 9.Harbarth, S., A. D. Harris, Y. Carmeli, and M. H. Samore. 2001. Parallel analysis of individual and aggregated data on antibiotic exposure and resistance in Gram-negative bacilli. Clin. Infect. Dis. 33:1462-1468. [DOI] [PubMed] [Google Scholar]
- 10.Krueger, C., and L. Tian. 2004. A comparison of the general linear mixed model and repeated measures ANOVA using a dataset with multiple missing data points. Biol. Res. Nurs. 6:151-157. [DOI] [PubMed] [Google Scholar]
- 11.Martin, C., I. Ofotokun, R. Rapp, K. Empey, J. Armistead, C. Pomeroy, A. Hoven, and M. Evans. 2005. Results of an antimicrobial control program at a university hospital. Am. J. Health Syst. Pharm. 62:732-738. [DOI] [PubMed] [Google Scholar]
- 12.Pakyz, A. L., C. MacDougall, M. Oinonen, and R. E. Polk. 2008. Trends in antibacterial use in U.S. academic health centers: 2002 to 2006. Arch. Int. Med. 168:1-7. [DOI] [PubMed] [Google Scholar]
- 13.Paramythiotou, E., J. C. Lucet, J. F. Timsit, D. Vanjak, C. Paugam-Burtz, J. L. Trouillet, S. Belloc, N. Kassis, A. Karabinis, and A. Andremont. 2004. Acquisition of multidrug-resistant Pseudomonas aeruginosa in patients in intensive care units: role of antibiotics with antipseudomonal activity. Clin. Infect. Dis. 38:670-677. [DOI] [PubMed] [Google Scholar]
- 14.Peterson, L. R. 2005. Squeezing the antibiotic balloon: the impact of antimicrobial classes on emerging resistance. Clin. Microbiol. Infect. 11(Suppl. 5):4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polk, R. E., J. Letcavage, A. Mahoney, and C. A. MacDougall. 2007. Adult antibiotic usage in 130 US hospitals: comparison of defined daily dose (DDD) to duration of therapy (DOT)/1000 patient days (PD). Clin. Infect. Dis. 44:664-670. [DOI] [PubMed] [Google Scholar]
- 16.Rogues, A. M., C. Dumartin, B. Amadéo, A. G. Venier, N. Marty, P. Paerneix, and J. P. Gachie. 2007. Relationship between rates of antimicrobial consumption and the incidence of antimicrobial resistance in Staphylococcus and Pseudomonas aeruginosa isolates from 47 French hospitals. Infect. Control Hosp. Epidemiol. 28:1389-1395. [DOI] [PubMed] [Google Scholar]
- 17.Schwaber, M. J., T. De-Medina, and Y. Carmeli. 2004. Epidemiological interpretation of antibiotic resistance studies-what are we missing? Nat. Rev. 2:979-983. [DOI] [PubMed] [Google Scholar]
- 18.Schwaber, M., and Y. Carmeli. 2008. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA 300:2911-2912. [DOI] [PubMed] [Google Scholar]
- 19.Spellberg, B., R. Guidos, D. Gilbert, J. Bradley, H. W. Bucher, W. M. Scheld, J. G. Bartlett, and J. Edwards, Jr. 2008. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 46:155-164. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan, A., and J. B. Patel. 2008. Klebsiella pneumoniae carbapenemase-producing organisms: an ounce of prevention really is worth a pound of cure. Infect. Control Hosp. Epidemiol. 29:1107-1109. [DOI] [PubMed] [Google Scholar]
- 21.Turnidge, J., and K. Christiansen. 2005. Antibiotic use and resistance-proving the obvious. Lancet 365:548-549. [DOI] [PubMed] [Google Scholar]
- 22.White, A. C., Jr., R. L. Atmar, J. Wilson, T. R. Cate, C. E. Stager, and S. B. Greenberg. 1997. Effects of requiring prior authorization for selected antimicrobials: expenditures, susceptibilities, and clinical outcomes. Clin. Infect. Dis. 25:230-239. [DOI] [PubMed] [Google Scholar]