Abstract
Lopinavir (LPV) exposure is reduced during the third trimester of pregnancy. We report the pharmacokinetics of standard LPV-ritonavir dosing (400/100 mg twice daily) in the immediate and early postpartum period when initiated during labor. In 16 human immunodeficiency virus-infected Thai women, the median (range) LPV area under the concentration-time curve and maximum and minimum concentrations in plasma were 99.7 (66.1 to 180.5) μg·h/ml, 11.2 (8.0 to 17.5) μg/ml, and 4.6 (1.7 to 12.5) μg/ml, respectively, at 41 (12 to 74) h after delivery. All of the women attained adequate LPV levels through 30 days postpartum. No serious adverse events were reported.
The addition of a short-course intrapartum/postpartum antiretroviral treatment, such as zidovudine (ZDV)-lamivudine for 3 or 7 days (4) or single-dose tenofovir-emtricitabine (1), can reduce the incidence of nonnucleoside reverse transcriptase inhibitor resistance in human immunodeficiency virus (HIV)-infected pregnant women who receive a single intrapartum dose of nevirapine (SD-NVP) for the prevention of mother-to-child transmission of HIV, but the optimal choice and duration of such treatment remain unknown.
International Maternal Pediatric Adolescent AIDS Clinical Trial (IMPAACT) P1032 (NCT00109590; www.clinicaltrials.gov), conducted in Thailand, is a phase II, three-arm, randomized, open-label study investigating the efficacy of three different short-course intrapartum/postpartum antiretroviral regimens to reduce the incidence of nevirapine resistance following SD-NVP administration. One of the three regimens is a triple-drug combination of ZDV, didanosine enteric coated (ddI-EC), and lopinavir (LPV) coformulated with low-dose ritonavir (LPV/r) initiated intrapartum and continued for 30 days postpartum.
LPV/r is a potent antiretroviral drug with a short plasma half-life and a high genetic barrier for the selection of viral resistance, making it an ideal choice for inclusion in a short-course intrapartum/postpartum antiretroviral regimen. However, studies with pregnant U.S. women have shown a significant reduction in LPV plasma exposure with standard dosing (400/100 mg twice daily) during the third trimester (6) and it is unknown if these changes continue to affect LPV exposure in the immediate postpartum period. Due to this uncertainty, a pharmacokinetic (PK) substudy was performed to assess if standard LPV dosing provides adequate exposure during the first 30 days postpartum. At the start of the P1032 trial, women randomized to receive ZDV/ddI-EC plus LPV/r during delivery and for 30 days postpartum were scheduled for LPV/r PK assessment postpartum until evaluable PK results were available for 12 women.
Study subjects received 300 mg ZDV at the onset of labor, once every 3 h during labor, and then twice daily for 30 days postpartum and 250 mg ddI-EC (400 mg if their body weight was ≥60 kg) once daily and 400/100 mg LPV/r (soft-gel capsules) twice daily at the onset of labor, during labor, and for 30 days postpartum. Within 72 h after delivery, a predose blood sample was drawn prior to the scheduled LPV/r dose, after which LPV/r was administered with food and blood samples were collected at 1, 2, 4, 6, 8, and 12 h after dosing. At day 30 postpartum, sampling was restricted to predose and 2 and 4 h postdose. Blood samples were centrifuged, and the plasma was removed, aliquoted, and frozen at −70°C within 1 h of collection. LPV/r concentrations in plasma were determined in real time by a high-performance liquid chromatography assay (2). PK parameters, including the area under the concentration-time curve (AUC), the maximum drug concentration in plasma (Cmax), the time to Cmax (Tmax), and the minimum drug concentration in plasma (Cmin), were calculated with WinNonLin (version 5.2; Pharsight) by noncompartmental methods.
The target LPV AUC was ≥52 μg · h/ml, the 10th percentile for the LPV AUC in nonpregnant adults (Kaletra Package Insert, Abbott Laboratories, Abbott Park, IL) (5). If 3 or more of the first 6 women or 4 or more of the 12 women failed to meet the target, we would have 95% confidence that the true rate of PK failure exceeds 10% (i.e., the lower limit of the 90% confidence interval will exceed 10%). If either of these stopping criteria were met, PK sampling would be repeated with 12 additional women at increased LPV/r doses of 533/133 mg. Adverse events were graded by using the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (version 1.0, dated December 2004).
Eighteen women were scheduled for LPV/r PK sampling. No PK sampling was performed for two women, one of whom did not receive SD-NVP prior to delivery and the other of whom immediately discontinued all study drugs after receiving a drug (methergine) prohibited by the protocol during labor. Sixteen women completed both LPV/r PK sampling visits, but evaluable PK results were not available for two women at day 30 postpartum due to suspected poor adherence to the drug regimen. Two additional women were included beyond the target enrollment of 12 women because they had already been scheduled for LPV/r PK assessment when evaluable data on 12 women at both PK time points became available.
At study entry, the 16 women who had LPV/r PK sampling performed had a median age (range) of 27 (18 to 38) years, an HIV type 1 RNA viral load of 3,369 (<400 to 61,750) copies/ml, and a CD4 cell count of 464 (292 to 844)/mm3. Their median weight was 58 (44 to 84) kg within 72 h after delivery and 53 (41 to 79) kg at day 30 postpartum.
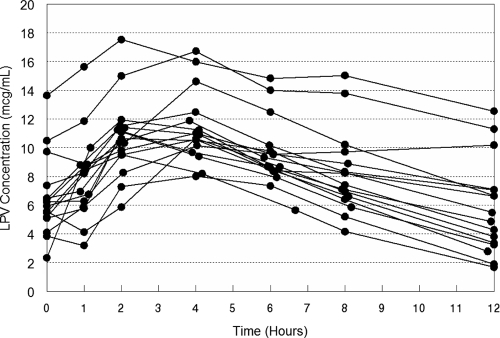
Intensive PK sampling was started at 47 (22 to 80) h after LPV/r initiation (during labor) and at 41 (12 to 74) h after delivery. Individual plasma LPV concentration-time curves are shown in Fig. 1, and LPV/r PK parameters are presented in Table 1. All of the women had an LPV AUC above the target within 72 h postpartum. The median Tmax for both LPV and RTV was 3.9 h. Ritonavir PK parameters in the immediate postpartum period were similar to those in HIV-infected adults at steady state. At day 30 postpartum, predose LPV/r levels were significantly higher than within 72 h postpartum (Table 1). No serious adverse events were reported.
FIG. 1.
Individual plasma LPV concentration-time curves within 72 h postpartum for 16 HIV-infected Thai women who initiated LPV/r (400/100 mg twice daily) intrapartum.
TABLE 1.
LPV and ritonavir PK parameters and concentrations in serum after initiation of 400 and 100 mg, respectively, intrapartuma
| Drug and parameter | Within 72 h postpartum (n = 16) | At day 30 postpartum (n = 14) | Within-subject geometric mean 72-h/day 30 ratio (90% CI)f |
|---|---|---|---|
| LPV | |||
| AUC0-12 (μg·h/ml) | 99.7 (66.3-180.5) | ||
| Cmax (μg/ml) | 11.17 (8.01-17.52) | ||
| Tmax (h) | 3.9 (1.9-4.0) | ||
| Cpredose (μg/ml) | 6.08 (2.34-13.64) | 9.17 (5.28-14.97) | 0.66 (0.53-0.81)b |
| C2 h (μg/ml) | 10.50 (5.87-17.53) | 11.72 (7.42-19.88) | 0.85 (0.70-1.04) |
| C4 h (μg/ml) | 10.78 (8.01-16.72) | 12.96 (8.78-21.37) | 0.81 (0.71-0.94)c |
| C12 h (μg/ml) | 5.18 (1.68-12.54) | ||
| Ritonavir | |||
| AUC0-12 (μg·h/ml) | 4.27 (1.54-7.89) | ||
| Cmax (μg/ml) | 0.60 (0.33-1.33) | ||
| Tmax (h) | 3.9 (1.9-4.0) | ||
| Cpredose (μg/ml) | 0.16 (<0.05-1.33) | 0.29 (0.09-0.66) | 0.54 (0.39-0.74)d |
| C2 h (μg/ml) | 0.45 (0.14-1.33) | 0.61 (0.15-1.45) | 0.76 (0.60-0.98)e |
| C4 h (μg/ml) | 0.58 (0.18-1.33) | 0.81 (0.21-3.11) | 0.68 (0.50-0.93)e |
| C12 h (μg/ml) | 0.10 (<0.05-0.26) |
Reported values are medians and ranges. Cpredose, C2 h, C4 h, and C12 h are the predose and 2-, 4-, and 12-h postdose drug concentrations in serum, respectively.
Geometric mean significantly lower (P = 0.009, Wilcoxon signed-rank test).
Geometric mean significantly lower (P = 0.048, Wilcoxon signed-rank test).
Geometric mean significantly lower (P = 0.038, Wilcoxon signed-rank test).
Geometric mean significantly lower (P = 0.030, Wilcoxon signed-rank test).
CI, confidence interval.
Given the approximately 50% reduction in LPV exposure during the third trimester compared to that of nonpregnant women (6), it is perhaps surprising that standard LPV/r dosing resulted in adequate LPV exposure for all of the women in this study. However, two factors may account for this difference. First, two retrospective analyses of therapeutic drug monitoring databases have reported an inverse correlation between body weight and LPV concentrations (3, 7). Second, the timing of LPV initiation is different, i.e., antepartum versus intrapartum, and it is unclear how long the physiological changes affecting LPV exposure during pregnancy persist postpartum. Thus, the lower body weight and the timing of LPV/r treatment initiation in the study reported here may facilitate higher LPV concentrations.
To maximize the efficacy of short-course intrapartum/postpartum antiretroviral treatments to prevent the selection of nonnucleoside reverse transcriptase inhibitor mutations postpartum, it is critical that adequate drug concentrations be rapidly attained and maintained throughout. Standard LPV/r dosing initiated intrapartum and continued for 30 days postpartum provided adequate LPV drug exposure in HIV-infected Thai women.
Acknowledgments
We thank all of the women who participated in IMPAACT P1032 and the study staff conducting the protocol at the sites.
Overall support for the IMPAACT group was provided by the National Institute of Allergy and Infectious Diseases (U01 AI068632) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. This work was supported by grants U01AI069399, U01AI069399, and U01AI069512 from the National Institute of Allergy and Infectious Diseases. Pharmaceutical support was provided by Abbott Laboratories, GlaxoSmithKline, and Boehringer Ingelheim. LPV and ritonavir for the antiretroviral drug assay were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
The content of this report is solely our responsibility and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
The IMPAACT P1032 team members were chairs Russell Van Dyke and Gonzague Jourdain; vice chair Robert Maupin, Jr.; DAIDS medical officer Elizabeth Smith; NICHD medical officer Heather Watts; clinical trial specialists Jennifer Gardella and Lara Akinsanya; statisticians David E. Shapiro and Paula Britto; field representatives Maureen Shannon and Pra-ornsuda Sukrakanchana; data manager Bonnie Zimmer; virologists Lisa Frenkel, Nicole Ngo-Giang-Huong, and Walter A. Scott; DAIDS pharmacist Paul Tran; pharmacologists Mark Mirochnick and Tim R. Cressey; immunologist Ann Melvin; laboratory technologist Bill Kabat; laboratory data managers Travis Behm and Courtney Ashton; liaison to PICL Ruth Dickover; investigators Kenneth McIntosh and Anuvat Roongpisuthipong; community constituency group representative Vinnie Dipoalo; Marisol Martinez of Abbott Laboratories; Wendy Snowden of GlaxoSmithKline; and Carolyn Conner of Boehringer Ingelheim Pharmaceuticals. Members from the Research Institute for Health Sciences (RIHES), Chiang Mai University, Chiang Mai, were Thanyawee Puthanakit, Virat Sirisanthana, Linda Aurpibul, Chintana Khamrong, Nataporn Kosachunhanan, and Kittipong Rungruengthanakit. Those from Siriraj Hospital, Bangkok, were Nirun Vanprapar, Kulkanya Chokephaibulkit, Anuvat Roongpisuthipong, Wasana Prasitseubsai, Wimon Anansakunwatt, Pilaipan Puthavathana, Nongluck Seetapun, Nantaka Kongstan, and Pirom Noisumdaeng. Those from PHPT-IRD174, Chiang Mai, were Marc Lallemant, Gonzague Jourdain, Nicole Ngo-Giang-Huong, Tim R. Cressey, Pra-ornsuda Sukrakanchana, Kanchana Than-in-at, Dujrudee Chinwong, Nusara Krapunpongsakul, Wannipa Yenjai, Renoo Wongsrisai, Janjira Thonglo, Tiwacha Timakahan, Yardpiron Taworn, and Suriyan Tanasri. Those from Chiang Rai Prachanukoh Hospital, Chiang Rai, were Patcharee Kantipong, Jullapong Achalapong, Angkana Sophon, Yupawan Thaweesombat, and Chaiporn Kumluang. Those from Chonburi Hospital, Chonburi, Thailand, were Chureeratana Bowonwatanuwong, Nantasak Chotivanich, Ladda Argadamnuy, Kessarin Chaisiri, Prakit Yothipitak, and Somrat Matchua. Those from Prapokklao Hospital, Chantaburi, Thailand, were Prapap Yuthavisuthi, Ubon Chanasit, Pisut Greetanukroh, Suteerat Srisupaluk, and Paleerutch Kerdprasert. Those from Bhumibol Adulyadej Hospital, Bangkok, Thailand, were Napawadee Impoolsup, Sinart Promma, Paleerutch Kerdprasert, Marina Thitathan, and Titima Taweewattanapan.
Footnotes
Published ahead of print on 23 February 2009.
Substudy of the International Maternal Pediatric Adolescent AIDS Clinical Trial (IMPAACT) group P1032 study.
REFERENCES
- 1.Chi, B. H., M. Sinkala, F. Mbewe, R. A. Cantrell, G. Kruse, N. Chintu, G. M. Aldrovandi, E. M. Stringer, C. Kankasa, J. T. Safrit, and J. S. Stringer. 2007. Single-dose tenofovir and emtricitabine for reduction of viral resistance to non-nucleoside reverse transcriptase inhibitor drugs in women given intrapartum nevirapine for perinatal HIV prevention: an open-label randomised trial. Lancet 370:1698-1705. [DOI] [PubMed] [Google Scholar]
- 2.Droste, J. A., C. P. Verweij-Van Wissen, and D. M. Burger. 2003. Simultaneous determination of the HIV drugs indinavir, amprenavir, saquinavir, ritonavir, lopinavir, nelfinavir, the nelfinavir hydroxymetabolite M8, and nevirapine in human plasma by reversed-phase high-performance liquid chromatography. Ther. Drug Monit. 25:393-399. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons, S., D. Back, and S. Khoo. 2007. Variability in lopinavir concentrations in the clinical setting and factors affecting concentrations, abstr. 37. Eighth International Workshop on Clinical Pharmacology of HIV Infection, Budapest, Hungary, April 2007.
- 4.McIntyre, J., N. Martinson, Investigators for the Trial 1413, V. Boltz, S. Palmer, J. Coffin, J. Mellors, M. Hopley, T. Kimura, P. Robinson, and D. Mayers. 2004. Addition of short course combivir (CBV) to single dose viramune (sdNVP) for prevention of mother-to-child transmission (MTCT) of HIV-1 can significantly decrease the subsequent development of maternal NNRTI-resistant virus, abstr. LbOrB09. XV International AIDS Conference, Bangkok, Thailand, 11 to 16 July 2004.
- 5.Murphy, R. L., S. Brun, C. Hicks, J. J. Eron, R. Gulick, M. King, A. C. White, Jr., C. Benson, M. Thompson, H. A. Kessler, S. Hammer, R. Bertz, A. Hsu, A. Japour, and E. Sun. 2001. ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naive adults with HIV-1 infection: 48-week results. AIDS 15:F1-F9. [DOI] [PubMed] [Google Scholar]
- 6.Stek, A. M., M. Mirochnick, E. Capparelli, B. M. Best, C. Hu, S. K. Burchett, C. Elgie, D. T. Holland, E. Smith, R. Tuomala, A. Cotter, and J. S. Read. 2006. Reduced lopinavir exposure during pregnancy. AIDS 20:1931-1939. [DOI] [PubMed] [Google Scholar]
- 7.van der Leur, M. R., D. M. Burger, C. J. la Porte, and P. P. Koopmans. 2006. A retrospective TDM database analysis of interpatient variability in the pharmacokinetics of lopinavir in HIV-infected adults. Ther. Drug Monit. 28:650-653. [DOI] [PubMed] [Google Scholar]