Abstract
The efficacy of vancomycin against methicillin-resistant Staphylococcus aureus (MRSA)-related infections has been called into question by recent findings of higher rates of failure of vancomycin treatment of infections caused by strains with high MICs. Continuous infusion may be the best way to maximize the time-dependent activity of vancomycin. The aim of this study was to create dosing nomograms in relation to different creatinine clearance (CLCr) estimates for use in daily clinical practice to target the steady-state concentrations (Csss) of vancomycin during continuous infusion at 15 to 20 mg/liter (after the administration of an initial loading dose of 15 mg/kg of body weight over 2 h). The correlation between vancomycin clearance (CLv) and CLCr was retrospectively assessed in a cohort of critically ill patients (group 1, n = 70) to create a formula for dosage calculation to target Css at 15 mg/liter. The performance of this formula was prospectively validated in a similar cohort (group 2, n = 63) by comparison of the observed and the predicted Csss. A significant relationship between CLv and CLCr was observed in group 1 (P < 0.001). The application of the calculated formula to vancomycin dosing in group 2 {infusion rate (g/24 h) = [0.029 × CLCr (ml/min) + 0.94] × target Css × (24/1,000)} led to a significant correlation between the observed and the predicted Csss (r = 0.80, P < 0.001). Two dosing nomograms based on CLCr were created to target the vancomycin Css at 15 and 20 mg/liter in critically ill patients. These nomograms could be helpful in improving the vancomycin treatment of MRSA infections, especially in the presence of borderline-susceptible pathogens and/or of pathophysiological conditions which may enhance the clearance of vancomycin, while potentially avoiding the increased risk of nephrotoxicity observed with the use of high intermittent doses of vancomycin.
After more than five decades of widespread clinical use, vancomycin may still have a major role in the treatment of multidrug-resistant gram-positive bacterial infections (18, 23), even though its clinical efficacy for the treatment of deep-seated infections is often called into question because of it poor penetration into tissue and its relatively weak antibacterial activity (5, 33).
The recent findings of higher failure rates when vancomycin was used for the treatment of methicillin-resistant Staphylococcus aureus (MRSA)-related bacteremia due to strains with MICs higher than 1 mg/liter (14, 34) have added further worries.
In an attempt to improve the efficacy of vancomycin in this context of borderline susceptibility, several authors suggested the rapid achievement and maintenance of trough (minimum) plasma concentrations (Cmin) of 15 to 20 mg/liter as a possible solution (2, 10).
Administration of the conventional dose of vancomycin (15 mg/kg of body weight every 12 h) has clearly been shown to fail to reliably achieve a Cmin of >15 mg/liter in the majority of cases (13, 24). Therefore, innovative approaches were advocated: whereas some authors believe that the use of higher intermittent daily dosages could be helpful (10, 32), others are confident that alternative dosing regimens aimed at optimizing the pharmacodynamics of vancomycin may be worthwhile (7, 24, 26, 28, 29).
Since 2001 we have been administering the standard vancomycin dosage (30 mg/kg/daily, eventually adjusted according to the patient's renal function) by continuous infusion at our university teaching hospital with the intent of improving the pharmacodynamics of vancomycin for the treatment of documented or suspected MRSA infections in critically ill patients. The target of the continuous infusion is a steady-state plasma concentration (Css) of 15 mg/liter and is achieved by therapeutic drug monitoring (TDM).
The aims of this study were to assess retrospectively the correlation between vancomycin clearance (CLv) and creatinine clearance (CLCr) estimates with a large cohort of critically ill patients who received vancomycin by continuous infusion in order to create a formula for calculation of the daily dosage needed to target Css at about 15 mg/liter, to validate prospectively the performance of this formula with a similar cohort by comparison of the observed Css with the Csss predicted by the formula itself, and to create dosing nomograms to be used in daily clinical practice to target the vancomycin Css at 15 and/or 20 mg/liter.
MATERIALS AND METHODS
Study design.
Critically ill patients who were treated with vancomycin by continuous infusion because of suspected or documented MRSA infections and who had TDM for the vancomycin Css between January 2002 and June 2003 were included in the retrospective cohort (group 1). In order to avoid underexposure during the first hours of therapy, an initial loading dose of 15 mg/kg was administered over 2 h, irrespective of the patient's renal function, with the continuous infusion starting immediately afterwards. Data collected from the TDM program were used to estimate CLv for individuals by means of the following formula:
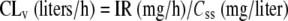 |
(1) |
where IR is the rate of continuous infusion of vancomycin. Plasma vancomycin concentrations were assessed by means of a fluorescence polarization immunoassay (AxSYM; Abbott, Rome, Italy). To increase the statistical power, whenever feasible, in patients who presented pathophysiological changes that affected the pharmacokinetics of vancomycin during their hospitalization, assessment of the individual CLv was repeated after achievement of the new steady state.
Considering that vancomycin is mainly eliminated by glomerular filtration (17), the correlation between individual CLv and CLCr estimates according to the formula of Cockcroft and Gault (3) was assessed by linear regression. In order to avoid the potential inaccuracy of CLCr estimates for patients who had been bedridden for a long time (21), only patients with recent hospital admission (<14 days) were included in this analysis.
The resulting CLv was used, according to equation 1, for calculation of the daily vancomycin dosage needed by continuous infusion to target Css at about 15 mg/liter in relation to the CLCr estimates.
The performance of this formula was evaluated prospectively with all consecutive critically ill patients treated with continuous-infusion vancomycin therapy between July 2003 and January 2004 (group 2). Validation of the formula was performed by determining by linear regression analysis the similarity between the observed and the predicted vancomycin Csss.
The validated formula was subsequently used to create two user-friendly dosing nomograms to be used for critically ill patients receiving vancomycin by continuous infusion to easily target the Css at 15 and 20 mg/liter in daily clinical practice.
Statistical analysis.
Descriptive data inside each group were expressed as the mean ± standard deviation (SD) or the median and range, according to normal and nonnormal distributions, respectively. Categorical variables were compared by the χ2 test with Yate's correction or Fisher's exact test, when necessary, and continuous variables were compared by using the Student t test. A P value of <0.05 was required to achieve statistical significance.
RESULTS
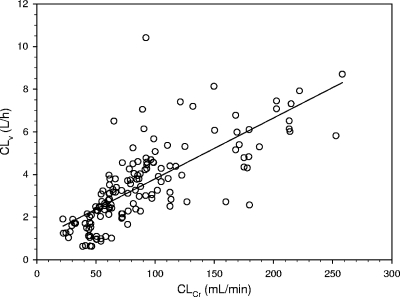
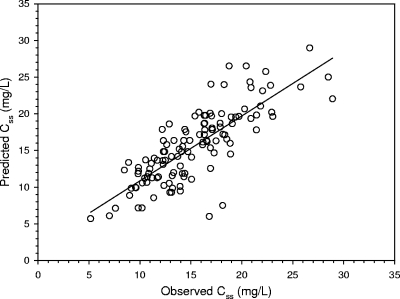
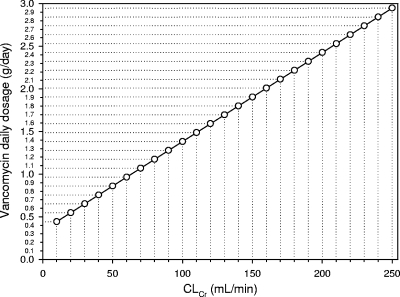
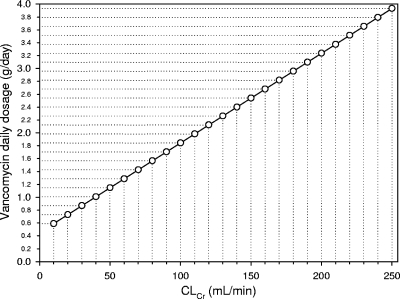
Seventy patients represented the retrospective cohort included in group 1, whereas 63 patients were prospectively included in group 2. Their characteristics are shown in Table 1. Figure 1 shows the relationship between CLv and CLCr observed in group 1, which was highly significant {[CLv (liters/h) = 0.029 × CLCr (ml/min) + 0.94]; r = 0.75, P < 0.001}. From this correlation, according to equation 1, the following formula was created for calculation of the rate of vancomycin continuous infusion needed as a function of the CLCr estimates to achieve the appropriate target Css in group 2: IR (g/24 h) = [0.029 × CLCr (ml/min) + 0.94] × target Css × (24/1,000). Figure 2 shows the highly significant relationship between the observed and the predicted Csss of vancomycin in group 2 (r = 0.80, P < 0.001). Figures 3 and 4 depict simple user-friendly nomograms based on different CLCr estimates for calculation of the daily dosage of vancomycin that must be administered by continuous infusion to critically ill patients for achievement of target Csss of 15 and 20 mg/liter, respectively.
TABLE 1.
Patient characteristics
| Characteristic | Group 1 | Group 2 | P valuea |
|---|---|---|---|
| No. of patients | 70 | 63 | |
| Age (yr)b | 58.9 ± 17.8 | 60.8 ± 16.1 | 0.53 |
| Gender (no. of M/no. of F)c | 57/13 | 45/18 | 0.25d |
| Body wt (kg)b | 76.7 ± 17.7 | 72.1 ± 11.6 | 0.08 |
| Height (cm)b | 172.7 ± 6.9 | 170.8 ± 7.0 | 0.13 |
| BMIe (kg/m2)b | 25.8 ± 6.3 | 24.7 ± 3.5 | 0.22 |
| CLCr (ml/min)b,f,g | 95.9 ± 57.1 | 97.1 ± 53.5 | 0.90 |
| Vancomycin Cssb,f (mg/liter) | 15.5 ± 4.8 | 15.8 ± 5.0 | 0.69 |
| No. (%) of patients with the following reason for hospital admission: | |||
| ICUh ward | 47 (67.1) | 31 (49.3) | 0.09d |
| Surgical ward | 16 (22.9) | 11 (17.4) | 0.44d |
| Medical ward | 7 (10.0) | 21 (33.3) | 0.01d |
| Main reason for vancomycin | |||
| Hospital acquired pneumonia | 22 (31.4) | 16 (25.4) | 0.28d |
| Empirical use for severe sepsis | 20 (28.6) | 23 (36.5) | 0.21d |
| Bloodstream infections | 13 (18.6) | 12 (19.1) | 0.56d |
| Cardiosurgical infections | 10 (14.3) | 8 (12.7) | 0.80d |
| Intra-abdominal infections | 5 (7.1) | 4 (6.3) | 0.56d |
Statistical significance was assessed by means of unpaired t test, unless otherwise specified.
Values are expressed as means ± standard deviations; steady-state concentrations.
M, males; F, females.
χ2 test.
BMI, body mass index.
At time of first TDM.
CLCr was estimated by the formula of Cockcroft and Gault (3).
ICU, intensive care unit.
FIG. 1.
Relationship between individual CLv and CLCr estimated by means of the formula of Cockcroft and Gault (3) in group 1 (n = 70 patients and 151 samples): CLv (liters/h) = 0.029 × CLCr (ml/min) + 0.94 (r = 0.75, P < 0.001).
FIG. 2.
Relationship between the predicted and the observed vancomycin Csss in group 2 (n = 63 patients and 120 samples). (r = 0.80, P < 0.001).
FIG. 3.
Nomogram based on CLCr estimates (3) for calculation of the vancomycin daily dosage administered by continuous infusion which is needed for achievement of the target Css of 15 mg/liter in critically ill patients.
FIG. 4.
Nomogram based on CLCr estimates (3) for calculation of the vancomycin daily dosage administered by continuous infusion which is needed for achievement of the target Css of 20 mg/liter in critically ill patients.
DISCUSSION
The results of this study confirm that vancomycin elimination in critically ill patients recently admitted to hospital may be effectively predicted on the basis of CLCr estimates. The results also confirm the reliability of the proposed formula for calculation of the vancomycin dosage to be used to achieve the target Css during continuous infusion in this population.
In recent years, several authors have suggested that the rapid achievement and maintenance of a Cmin of 15 to 20 mg/liter may be worthwhile to improve the efficacy of vancomycin (2, 10). Indeed, administration of the standard vancomycin dosage by the conventional twice-daily regimen may frequently cause only suboptimal Cmins in patients with normal renal function. Among 780 patients who received the standard daily dose administered as two to four daily doses, the Cmin at 36 to 48 h after the start of therapy was <10 mg/liter in 45.1% of the cases (13). Importantly, an even lower Cmin may be expected during conventional vancomycin therapy in critically ill patients who present with pathophysiological and/or iatrogenic conditions that may cause an enlargement of the extracellular space and/or an increase in the renal clearance of hydrophilic antimicrobials (21, 25). In an old study carried out with febrile neutropenic patients with acute leukemia empirically treated with vancomycin at a mean daily dose of 15 mg/kg every 12 h, the Cmin on day 3 was <5 mg/liter in as many as 56% of the cases (22).
Further support for the need for a more aggressive dosing regimen for the appropriate treatment of MRSA infections with vancomycin in critically ill patients may come from the recent findings of vancomycin MIC creep in MRSA (8, 35, 36) and of either a significantly longer duration of infection (19) or a significantly higher mortality rate (14, 34) associated with MRSA bacteremia due to strains with vancomycin MICs >1 mg/liter. Shifts in vancomycin MICs against populations of S. aureus strains toward values of ≥1 mg/liter over time have been noted in several studies in the past decade (8). However, it appears that the rate of vancomycin MIC creep varies greatly between different centers, perhaps also because of, among other reasons, the different selective pressures present (8). This may explain why MIC creep has not been noted in settings with low rates of vancomycin usage (1). Interestingly, the development of staphylococcal resistance to vancomycin has also been associated with prolonged exposure to low serum concentrations of the drug (30). In settings with a high incidence of MRSA-related infections, a policy of the restriction of vancomycin use is very difficult to support, unless the rates of prescription of newer antimicrobials with activity against gram-positive organisms is increased. However, in these settings it may be speculated that vancomycin MIC creep in MRSA isolates and the clinical failure of vancomycin treatment of MRSA infection may be prevented, or at least reduced, by avoiding the occurrence of suboptimal exposure at the infection site due to inappropriate dosing.
A possible solution could be the administration of larger intermittent daily dosages with the intent of achieving a Cmin of 15 to 20 mg/liter in all patients, but the unacceptable increase in the risk of nephrotoxicity recently documented in patients with very high Cmins during intermittent dosing with high doses (10), especially when the dose is greater than 4 g per day (15), advises against this choice.
Conversely, we believe that in an era in which the efficacy of vancomycin is doubtful and the use of higher intermittent vancomycin daily doses should be avoided, the application of continuous infusion with a target Css of 15 to 20 mg/liter may be helpful in maximizing the pharmacodynamics of vancomycin against MRSA while avoiding the risk of increased nephrotoxicity (24).
Vancomycin exhibits time-dependent antibacterial activity and no in vitro concentration-dependent killing effect against staphylococci and a short to moderate postantibiotic effect against gram-positive cocci (16). Given these pharmacodynamic characteristics, one would predict that the duration of time that the levels in serum exceed the MIC (T > MIC) would be the pharmacodynamic parameter that most strongly correlated with its efficacy. The early attainment and maintenance of a Cmin above the MIC should represent the goal of therapy, since this approach may ensure the presence of suprainhibitory concentrations for the entire dosing interval (T > MIC, 100%) (24). Indeed, consideration of only this assumption may lead to inappropriate dosing strategies (30), considering that an area under the concentration-time curve (AUC)/MIC ratio of 350 to 400 was associated with successful outcomes in patients with MRSA pneumonia (20). Therefore, this pharmacodynamic parameter may be the best predictor of the clinical efficacy of vancomycin and must be taken into account when a vancomycin dosage is chosen. However, further studies seem to be warranted (30), considering that conflicting results were observed in another recent study (6).
While awaiting conclusive evidence, continuous infusion may be suggested as the best way to maximize the time-dependent activity of vancomycin in daily clinical practice, since, with the same daily dose, it may retain higher and more sustained concentrations with equal exposure in terms of the AUC. Accordingly, it may be hypothesized that targeting of the vancomycin Css at 15 to 20 mg/liter by also allowing a sufficiently high AUC may represent a valid approach for optimization of both of the pharmacodynamic efficacy targets of vancomycin (24). It is worth noting that by ensuring that the plasma concentrations persistently exceed 15 mg/liter, namely, a value at least four to five times above the MIC breakpoint for MRSA, this strategy may reduce the selective pressure due to very low Cmins (30) and may avert the development of MRSA resistance even in cases of long-term treatment (31). At the same time, it may represent an easy approach for clinicians to guarantee the recommended AUC/MIC ratio of >350 against all pathogens with vancomycin MICs ≤1 mg/liter.
One objection may be that Soriano et al. (34) noted a higher mortality rate among patients with MRSA bacteremia due to strains with MICs of >1 mg/liter, even though all patients were treated to achieve a goal Cmin of ≥10 mg/ml. However, initial suboptimal exposure could not be excluded in that study since, as correctly argued by Porath and Brooks (27), they did not report what percentage of patients actually achieved this target Cmin at the first measurement, nor did they report the mean time to attainment of the target Cmin. Indeed, the prompt achievement and maintenance of the optimal exposure at the infection site by the timely administration of broad-spectrum antimicrobial agents is currently considered mandatory in critically ill patients with severe sepsis and/or septic shock, as recommended by the Surviving Sepsis Campaign guidelines (4). Continuous infusion coupled with loading may be helpful in this context, since it was shown to ensure the achievement of the targeted serum vancomycin levels more rapidly than intermittent administration (37). Of note, one must be reminded that an initial loading dose (15 mg/kg over 2 h) must always be administered, irrespective of the patient's renal function, and that continuous infusion must be started starting immediately afterwards in order to rapidly achieve therapeutically effective concentrations and to avoid the risk of underexposure in the first hours of treatment (23, 24).
The performance of the formula proposed in this article was prospectively evaluated with a cohort of patients with characteristics similar to those of the retrospective cohort, and this enabled accurate validation with a highly significant correlation between the observed and the predicted Csss.
Accordingly, two simple user-friendly nomograms based on CLCr estimates were created to help clinicians in daily clinical practice with the calculation of the continuous-infusion dosage needed to achieve a target vancomycin Css of 15 or 20 mg/liter.
The application of these dosing nomograms is easy and could be effective in improving vancomycin exposure, especially during the treatment of deep-seated infections. Additionally, this choice may be helpful in avoiding the potentially increased risk of nephrotoxicity, considering that for antimicrobials with time-dependent activities the total daily doses usually needed to achieve optimal exposures by the use of continuous infusion may be lower than those needed to achieve optimal exposures by the use of intermittent dosing. For example, it was estimated that dosages of less than 4 g/daily by continuous infusion may ensure a Css of 20 mg/liter even in patients with very high CLCr estimates of 250 ml/min. Interestingly, a recent retrospective study with outpatients receiving vancomycin by continuous infusion showed that a vancomycin Css only more than 1.4-fold greater than this value, namely, 28 mg/liter, was independently associated with a markedly increased risk of nephrotoxicity (12). Indeed, during our study, only two patients had values that temporarily exceeded this value, but none developed renal insufficiency thanks to the rapid adjustment of the dosage.
For these reasons, we and other authors call for more intensive studies which may determine the relative clinical efficacy of this approach (24, 29).
We recognize that our study may suffer from a relevant limitation. CLCr estimates may be safely applied only to critically ill patients recently admitted to hospital. A recent study carried out with 359 patients in an intensive unit confirmed that estimates of CLCr obtained by use of the formula of Cockcroft and Gault (3) highly correlated (r2 = 0.8357) with the CLCr measured at 24 h, the day after admission (9). Conversely, in patients with a long history of hospital admission (>1 month), CLCr estimates may lead to the overestimation of renal function, so that the direct measurement of CLCr should be performed to accurately assess the glomerular filtration rate and to prevent vancomycin overexposure. An inaccuracy of CLCr estimates was encountered whenever the daily output of creatinine from muscles is impaired by the degree of muscle loss which may occur when a patient is bedridden for a long time (9, 11). Another limitation may be represented by the fact that these nomograms are not valid for functionally anephric patients on dialysis and perhaps also for patients with very low CLCr estimates of <10 ml/min.
In conclusion, we are confident that the two nomograms provided here could help clinicians improve the vancomycin treatment of MRSA infections in critically ill patients, especially in the presence of borderline-susceptible pathogens and/or pathophysiological conditions which may enhance the CLv. The wise use of vancomycin should occur not only through restricted use but also through better use, and the development of validated nomograms that maintain Cmins at four to five times the MIC has been suggested as a reasonable solution (30). A prospective assessment of the clinical efficacy, safety, and tolerability of this approach is ongoing.
Acknowledgments
No financial support was received for this study.
Federico Pea has been has been on the speakers bureaus of Pfizer and Novartis. Pierluigi Viale has been a consultant to, has been on the speakers bureau of, and has received grant support from Pfizer and Novartis. Federica Pavan was a postgraduate fellow at the Institute of Clinical Pharmacology and Toxicology, University of Udine, at the time of the study, and is currently employed at GlaxoSmithKline, Verona, Italy. None of the other authors has a potential conflict of interest to report.
Footnotes
Published ahead of print on 17 February 2009.
REFERENCES
- 1.Alos, J. I., A. Garcia-Canas, P. Garcia-Hierro, and F. Rodriguez-Salvanes. 2008. Vancomycin MICs did not creep in Staphylococcus aureus isolates from 2002 to 2006 in a setting with low vancomycin usage. J. Antimicrob. Chemother. 62:773-775. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society and Infectious Diseases Society of America. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388-416. [DOI] [PubMed] [Google Scholar]
- 3.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 4.Dellinger, R. P., M. M. Levy, J. M. Carlet, J. Bion, M. M. Parker, R. Jaeschke, K. Reinhart, D. C. Angus, C. Brun-Buisson, R. Beale, T. Calandra, J. F. Dhainaut, H. Gerlach, M. Harvey, J. J. Marini, J. Marshall, M. Ranieri, G. Ramsay, J. Sevransky, B. T. Thompson, S. Townsend, J. S. Vender, J. L. Zimmerman, and J. L. Vincent. 2008. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 34:17-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deresinski, S. 2007. Counterpoint: vancomycin and Staphylococcus aureus—an antibiotic enters obsolescence. Clin. Infect. Dis. 44:1543-1548. [DOI] [PubMed] [Google Scholar]
- 6.Drew, R. H., I. Lu, M. Joyce, D. K. J. Benjamin, and V. G. Fowler. 2004. Lack of relationship between predicted area under the time-concentration curve/minimum inhibitory concentration and outcome in vancomycin-treated patients with Staphylococcus aureus bacteremia, abstr. A-1493. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 7.Gonzalez-Ruiz, A., and J. Richardson. 2008. Are glycopeptides still appropriate and convenient for empiric use? J. Chemother. 20:531-541. [DOI] [PubMed] [Google Scholar]
- 8.Gould, I. M. 2008. Clinical relevance of increasing glycopeptide MICs against Staphylococcus aureus. Int. J. Antimicrob. Agents 31(Suppl. 2):1-9. [DOI] [PubMed] [Google Scholar]
- 9.Herrera-Gutierrez, M. E., G. Seller-Perez, E. Banderas-Bravo, J. Munoz-Bono, M. Lebron-Gallardo, and J. F. Fernandez-Ortega. 2007. Replacement of 24-h creatinine clearance by 2-h creatinine clearance in intensive care unit patients: a single-center study. Intensive Care Med. 33:1900-1906. [DOI] [PubMed] [Google Scholar]
- 10.Hidayat, L. K., D. I. Hsu, R. Quist, K. A. Shriner, and A. Wong-Beringer. 2006. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch. Intern. Med. 166:2138-2144. [DOI] [PubMed] [Google Scholar]
- 11.Hoste, E. A., J. Damen, R. C. Vanholder, N. H. Lameire, J. R. Delanghe, K. Van den Hauwe, and F. A. Colardyn. 2005. Assessment of renal function in recently admitted critically ill patients with normal serum creatinine. Nephrol. Dial. Transplant. 20:747-753. [DOI] [PubMed] [Google Scholar]
- 12.Ingram, P. R., D. C. Lye, P. A. Tambyah, W. P. Goh, V. H. Tam, and D. A. Fisher. 2008. Risk factors for nephrotoxicity associated with continuous vancomycin infusion in outpatient parenteral antibiotic therapy. J. Antimicrob. Chemother. 62:168-171. [DOI] [PubMed] [Google Scholar]
- 13.Kitzis, M. D., and F. W. Goldstein. 2006. Monitoring of vancomycin serum levels for the treatment of staphylococcal infections. Clin. Microbiol. Infect. 12:92-95. [DOI] [PubMed] [Google Scholar]
- 14.Lodise, T. P., J. Graves, A. Evans, E. Graffunder, M. Helmecke, B. M. Lomaestro, and K. Stellrecht. 2008. Relationship between vancomycin MIC and failure among patients with MRSA bacteremia treated with vancomycin. Antimicrob. Agents Chemother. 52:3315-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodise, T. P., B. Lomaestro, J. Graves, and G. L. Drusano. 2008. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob. Agents Chemother. 52:1330-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowdin, E., I. Odenholt, and O. Cars. 1998. In vitro studies of pharmacodynamic properties of vancomycin against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 42:2739-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matzke, G. R., G. G. Zhanel, and D. R. Guay. 1986. Clinical pharmacokinetics of vancomycin. Clin. Pharmacokinet. 11:257-282. [DOI] [PubMed] [Google Scholar]
- 18.Mohr, J. F., and B. E. Murray. 2007. Point: vancomycin is not obsolete for the treatment of infection caused by methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 44:1536-1542. [DOI] [PubMed] [Google Scholar]
- 19.Moise, P. A., G. Sakoulas, A. Forrest, and J. J. Schentag. 2007. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 51:2582-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moise-Broder, P. A., A. Forrest, M. C. Birmingham, and J. J. Schentag. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 43:925-942. [DOI] [PubMed] [Google Scholar]
- 21.Pea, F., M. Bertolissi, A. Di Silvestre, D. Poz, F. Giordano, and M. Furlanut. 2002. TDM coupled with Bayesian forecasting should be considered an invaluable tool for optimizing vancomycin daily exposure in unstable critically ill patients. Int. J. Antimicrob. Agents 20:326-332. [DOI] [PubMed] [Google Scholar]
- 22.Pea, F., D. Poz, M. Baraldo, and M. Furlanut. 2000. Optimisation of vancomycin regimen in neutropenic haematological patients with normal renal function: multiple daily doses may be preferable. Clin. Drug Investig. 19:213-218. [Google Scholar]
- 23.Pea, F., and P. Viale. 2007. Pharmacodynamics of antibiotics to treat multidrug-resistant gram-positive hospital infections. Expert Rev. Anti-Infect. Ther. 5:255-270. [DOI] [PubMed] [Google Scholar]
- 24.Pea, F., and P. Viale. 2008. Should the currently recommended twice-daily dosing still be considered the most appropriate regimen for treating MRSA ventilator-associated pneumonia with vancomycin? Clin. Pharmacokinet. 47:147-152. [DOI] [PubMed] [Google Scholar]
- 25.Pea, F., P. Viale, and M. Furlanut. 2005. Antimicrobial therapy in critically ill patients: a review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin. Pharmacokinet. 44:1009-1034. [DOI] [PubMed] [Google Scholar]
- 26.Plan, O., G. Cambonie, E. Barbotte, P. Meyer, C. Devine, C. Milesi, O. Pidoux, M. Badr, and J. C. Picaud. 2008. Continuous-infusion vancomycin therapy for preterm neonates with suspected or documented gram-positive infections: a new dosage schedule. Arch. Dis. Child Fetal Neonatal Ed. 93:F418-F421. [DOI] [PubMed] [Google Scholar]
- 27.Porath, A. D., and G. D. Brooks. 2008. Vancomycin minimum inhibitory concentration as a predictor of mortality in methicillin-resistant Staphylococcus aureus bacteremia: a second look. Clin. Infect. Dis. 46:1483-1484. [DOI] [PubMed] [Google Scholar]
- 28.Rello, J., J. Sole-Violan, M. Sa-Borges, J. Garnacho-Montero, E. Munoz, G. Sirgo, M. Olona, and E. Diaz. 2005. Pneumonia caused by oxacillin-resistant Staphylococcus aureus treated with glycopeptides. Crit. Care Med. 33:1983-1987. [DOI] [PubMed] [Google Scholar]
- 29.Roberts, J. A., J. Lipman, S. Blot, and J. Rello. 2008. Better outcomes through continuous infusion of time-dependent antibiotics to critically ill patients? Curr. Opin. Crit. Care 14:390-396. [DOI] [PubMed] [Google Scholar]
- 30.Rybak, M. J. 2006. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 42(Suppl. 1):S35-S39. [DOI] [PubMed] [Google Scholar]
- 31.Sakoulas, G., H. S. Gold, R. A. Cohen, L. Venkataraman, R. C. Moellering, and G. M. Eliopoulos. 2006. Effects of prolonged vancomycin administration on methicillin-resistant Staphylococcus aureus (MRSA) in a patient with recurrent bacteraemia. J. Antimicrob. Chemother. 57:699-704. [DOI] [PubMed] [Google Scholar]
- 32.Scheetz, M. H., R. G. Wunderink, M. J. Postelnick, and G. A. Noskin. 2006. Potential impact of vancomycin pulmonary distribution on treatment outcomes in patients with methicillin-resistant Staphylococcus aureus pneumonia. Pharmacotherapy 26:539-550. [DOI] [PubMed] [Google Scholar]
- 33.Shorr, A. F., A. Combes, M. H. Kollef, and J. Chastre. 2006. Methicillin-resistant Staphylococcus aureus prolongs intensive care unit stay in ventilator-associated pneumonia, despite initially appropriate antibiotic therapy. Crit. Care Med. 34:700-706. [DOI] [PubMed] [Google Scholar]
- 34.Soriano, A., F. Marco, J. A. Martinez, E. Pisos, M. Almela, V. P. Dimova, D. Alamo, M. Ortega, J. Lopez, and J. Mensa. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46:193-200. [DOI] [PubMed] [Google Scholar]
- 35.Steinkraus, G., R. White, and L. Friedrich. 2007. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001-05. J. Antimicrob. Chemother. 60:788-794. [DOI] [PubMed] [Google Scholar]
- 36.Wang, G., J. F. Hindler, K. W. Ward, and D. A. Bruckner. 2006. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J. Clin. Microbiol. 44:3883-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wysocki, M., F. Delatour, F. Faurisson, A. Rauss, Y. Pean, B. Misset, F. Thomas, J. F. Timsit, T. Similowski, H. Mentec, L. Mier, and D. Dreyfuss. 2001. Continuous versus intermittent infusion of vancomycin in severe staphylococcal infections: prospective multicenter randomized study. Antimicrob. Agents Chemother. 45:2460-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]