Abstract
Infective endocarditis (IE) is the fourth leading cause of life-threatening infection in the United States and imposes significant morbidity and mortality. The American Heart Association guidelines for the diagnosis and treatment of IE do not address continuous-infusion (CI) oxacillin. This retrospective study compares outcomes between CI oxacillin and intermittent-infusion (II) oxacillin in the treatment of IE caused by methicillin-susceptible Staphylococcus aureus (MSSA). A total of 709 medical records were reviewed for inpatients with definitive IE treated between 1 January 2000 and 31 December 2007. Continuous data were analyzed by Student's t test or the Wilcoxon rank sum test. The chi-square test or Fisher's exact test was used to compare nominal data. A multivariate logistic model was constructed. One hundred seven patients met eligibility criteria for inclusion into the study. Seventy-eight patients received CI oxacillin, whereas 28 received II oxacillin. CI and II groups were similar with respect to 30-day mortality (8% versus 10%, P = 0.7) and length of stay (20 versus 25 days, P = 0.4) but differed in 30-day microbiological cure (94% versus 79%, P = 0.03). Sixty-three patients received synergistic gentamicin, whereas 44 did not. The gentamicin and no-gentamicin groups were similar with respect to 30-day mortality (11% versus 4%, P = 0.2) and 30-day microbiological cure (90% versus 89%, P = 0.8); however, times to defervescence (4 versus 2 days, P = 0.02) were significantly different. CI oxacillin is an effective alternative to II oxacillin for the treatment of IE caused by MSSA and may improve microbiological cure. This convenient and pharmacodynamically optimized dosing regimen for oxacillin deserves consideration for patients with IE caused by MSSA.
Infective endocarditis (IE) is the fourth leading cause of life-threatening infection in the United States and imposes significant morbidity and mortality (2). Staphylococcus aureus now exceeds viridans group streptococci as the leading cause of IE in much of the developed world, including the United States, and IE caused by S. aureus has been shown to be an independent predictor of increased mortality (4, 9). Overall, mortality from IE remains as high as 40% in some series, and complications of IE, such as congestive heart failure, extracardiac infections, and thromboembolic events, occur more often with IE caused by S. aureus than with IE caused by other pathogens (1, 9, 11, 15-17). The American Heart Association (AHA) has recently issued evidence-based guidelines for the diagnosis and treatment of patients with IE, which recommend intermittent-infusion (II) oxacillin at 12 g daily divided every 4 to 6 h for up to 6 weeks for treatment of IE caused by methicillin-susceptible S. aureus (MSSA) (1). Although data for continuous-infusion (CI) oxacillin are lacking, oxacillin exhibits time-dependent bactericidal activity, has a short postantibiotic effect against MSSA, and has been promoted as an alternative to II oxacillin, which maximizes bactericidal activity (5, 22). Unfortunately, due to the lack of clinical data, the AHA guidelines do not address the use of CI oxacillin for IE caused by MSSA.
In addition, experience at our institution suggests that in non-intensive care unit (ICU) settings, dosing schedules of every 4 h potentially result in more missed doses than CI (unpublished data). As a result, we implemented CI as a therapeutic option approximately 7 years ago. These observations, coupled with the unacceptably high mortality rate associated with IE caused by S. aureus, prompted us to address the use of CI oxacillin to treat IE caused by MSSA.
The objective of this retrospective study was to compare microbiological cures, 30-day mortalities, times to clearance of bacteremia, times to defervescence, lengths of hospital stay, and durations of antibiotic therapy between CI and II oxacillin in the treatment of IE caused by MSSA.
MATERIALS AND METHODS
Study design and participants.
This retrospective cohort study performed at University Hospital, University Health System, San Antonio, TX, assessed the use of CI versus II oxacillin for the treatment of IE caused by MSSA. The two groups were comprised of patients who received either 2 g II oxacillin intravenously over 30 min every 4 h or 12 g CI oxacillin intravenously over 24 h once daily. The study was approved by both the hospital's research department and the institutional review board of the University of Texas Health Science Center at San Antonio.
Inclusion criteria.
Data from patients admitted to University Hospital between 1 January 2000 and 31 December 2007 were included. Patients were identified using the International Classification of Diseases, Ninth Revision, Clinical Modification codes for endocarditis (18a). Patients 18 years and older, with absolute neutrophil counts of ≥1,000 cells/mm3, who met modified Duke's criteria for IE and bacteremia (≥2 positive cultures from blood samples drawn 12 h apart, all of 3 blood samples positive, or a majority of ≥4 separate cultures of blood positive) with MSSA were eligible for inclusion in the study. Definite IE caused by MSSA was defined, per AHA evidence-based guidelines and modified Duke's criteria, as having two major, one major and three minor, or five minor criteria (6, 14). Inclusion was determined by review of microbiological data, echocardiographic imaging, physical exam, and vital signs contained in the patient's electronic medical record. Patients also must have received oxacillin for treatment using one of the two dosing regimens described above or, for patients who had previously existing hepatic dysfunction or who developed hepatic dysfunction (12), a reduced daily dose of 8 g, the maximum manufacturer-recommended dose.
Exclusion criteria.
Patients were excluded if they failed to meet modified Duke's criteria for IE caused by MSSA, had IE due to an unidentifiable organism or an organism other than MSSA, or received IE treatment with a drug other than oxacillin (use of nafcillin was not evaluated). Other reasons for exclusion included incomplete medical records without follow up, such as for patients who transferred to University Hospital during treatment or who transferred to an outside hospital from University Hospital prior to completion of their therapy. Patients who were switched from one method of drug administration to another (II to CI or CI to II) during treatment were also deemed ineligible for inclusion. Finally, patients who died in the hospital were excluded from analyses of length of stay and duration of antibiotic therapy.
Data.
Data were collected from patients' electronic medical records and documented on standardized data collection sheets. Patient demographics, baseline laboratory values, preexisting and predisposing conditions, signs and symptoms on admission, microbiology results, anti-infective therapy, evidence of fever (temperature of >38°C), lengths of hospital and ICU stays, and mortality data were collected.
Outcomes.
The primary outcome was microbiological cure at 30 days, defined as no positive cultures within 30 days of the end of treatment by use of the method of last observation carried forward. Secondary endpoints included 30-day mortality, time to clearance of bacteremia (defined as the number of days from the first positive culture until the day prior to the first known negative culture), time to defervescence, hospital and ICU length of stay, and duration of antibiotic therapy. In addition, both primary and secondary outcomes as well as rates of acute kidney injury, defined as an increase in serum creatinine of ≥0.5 mg/dl or a 50% increase from baseline, were compared for patients who did and did not receive synergistic gentamicin (18, 21).
Data analysis.
Data were analyzed using JMP 7.0 (SAS Institute Inc., Cary, NC). Descriptive statistics were used to summarize patient demographics and outcomes. The Shapiro-Wilk W test was used to assess continuous variables for normality. Normally distributed continuous data were analyzed by Student's t test, while non-normally distributed continuous data were analyzed by the Wilcoxon rank sum test. The chi-square test or Fisher's exact test was used to compare nominal data. If the antibiotic regimen was associated with any of the study endpoints in univariate analysis (P ≤ 0.05), then the antibiotic regimen was entered into a separate stepwise regression model together with age, white blood cell (WBC) count, chills, hemodialysis, synergistic gentamicin, and days on gentamicin therapy to investigate whether the antibiotic regimen was independently associated with the study endpoint. Criteria for model entry and attrition were P values of ≤0.10 and ≤0.05, respectively. Variables were entered in a forward stepwise fashion, and the log-likelihood ratio test was used to determine which variables improved the fit of the logistic regression model. Interaction terms were considered if more than one variable remained in the final regression model. An alpha level of ≤0.05 was used to determine statistical significance for all comparisons.
RESULTS
Of the 709 patient charts reviewed, 528 were excluded because of bacteremia due to another organism or because an organism was not identified, 68 were excluded due to not meeting modified Duke's criteria, and 6 were excluded because the patients had not received treatment with oxacillin. Comparisons of patient characteristics are presented in Table 1. The 107 adult patients had a median age of 41 (range, 18 to 85) years. Most patients were Hispanic (71%) and male (72%). Eighty percent had a history of intravenous drug use (IDU). Despite the high percentage of intravenous drug users in the study, the infected heart valves were evenly distributed between the left and right sides; 35% on the left side, 37% on the right side, 7% on both sides, and 21% having no identified vegetation either because echocardiography was negative or because the patients did not receive an echocardiogram, having already been diagnosed with IE by modified Duke's criteria (e.g., one major and three minor criteria). See Table 2 for a complete breakdown of site of infection by method of administration.
TABLE 1.
Comparison of patients treated with CI and II for IE caused by MSSA
| Characteristic | Valuea for patients treated with:
|
P value | ||
|---|---|---|---|---|
| CI or II (overall) | CI (n = 78) | II (n = 29) | ||
| Median age, yr (range) | 41 (18-85) | 40 (18-85) | 45 (19-84) | 0.1 |
| Male gender | 77 (72) | 58 (74) | 19 (66) | 0.4 |
| Race or ethnic group | ||||
| White | 27 (25) | 16 (21) | 11 (38) | 0.07 |
| Black | 3 (3) | 1 (1) | 2 (7) | 0.2 |
| Hispanic | 76 (71) | 60 (77) | 16 (55) | 0.03 |
| Asian | 1 (0.9) | 1 (1) | 0 (0) | 1 |
| Median body mass index (range)b | 24 (11-72) | 24 (11-72) | 24 (19-32) | 0.8 |
| Median creatinine clearance (range)c | 85 (15-262) | 85 (15-248) | 84 (19-262) | 0.8 |
| Risk factors/comorbidities | ||||
| CABGd | 4 (4) | 2 (3) | 2 (7) | 0.3 |
| Hemodialysis dependent | 7 (7) | 7 (9) | 0 (0) | 0.09 |
| Diabetes mellitus | 25 (23) | 19 (24) | 6 (21) | 0.8 |
| IDU | 86 (80) | 63 (81) | 23 (79) | 1 |
| Preexisting mechanical valve | 8 (8) | 6 (8) | 2 (7) | 1 |
| History of infective endocarditis | 20 (19) | 14 (18) | 6 (21) | 0.8 |
| HIVe positive | 8 (8) | 4 (5) | 4 (14) | 0.2 |
| Diagnostic criteria | ||||
| Two major criteria | 85 (79) | 62 (79) | 23 (79) | 1 |
| One major criterion | 22 (21) | 16 (21) | 6 (21) | 1 |
| Presenting signs/symptoms | ||||
| Fever/temp of >38°Cf | 91 (85) | 65 (83) | 26 (90) | 0.5 |
| Chills | 60 (56) | 48 (62) | 12 (41) | 0.06 |
| Serum creatinine (mg/dl) | 1.3 | 1.3 | 1.2 | 0.9 |
| WBC count (×1,000 cells per μl) | 14.5 | 15 | 13 | 0.1 |
| Janeway's lesions | 8 (8) | 7 (9) | 1 (3) | 0.3 |
| Septic emboli | 31 (29) | 25 (32) | 6 (21) | 0.2 |
| Pulmonary emboli | 59 (55) | 43 (55) | 16 (55) | 1 |
| Osler's nodes | 8 (8) | 7 (9) | 1 (3) | 0.3 |
| Splinter hemorrhage | 7 (7) | 5 (6) | 2 (7) | 0.9 |
| Poor dentition | 35 (33) | 26 (33) | 9 (31) | 0.8 |
Values are numbers (percentages) unless indicated otherwise.
The body mass index was calculated as follows: weight (kg)/height (m2).
Creatinine clearance was determined by the Cockcroft-Gault method.
CABG, coronary artery bypass graft.
HIV, human immunodeficiency virus.
The mean temperatures were 38.3°C for all patients treated with CI or II, 38.4°C for the patients treated with CI, and 38.3°C for the patients treated with II (P = 0.6).
TABLE 2.
Sites of IE infection
| Site of infection | No. (%) of patients treated with:
|
P value | ||
|---|---|---|---|---|
| CI or II (overall) | CI (n = 78) | II (n = 29) | ||
| Left side | 37 (35) | 27 (35) | 10 (35) | 0.3 |
| Right side | 40 (37) | 29 (37) | 11 (38) | 0.4 |
| Both sides | 7 (7) | 3 (4) | 4 (14) | 1.0 |
| Nonea | 23 (21) | 19 (24) | 4 (14) | 0.06 |
Patients either did not demonstrate evidence of vegetation on the echocardiogram or did not receive an echocardiogram, having already met definitive IE criteria. The site of infection was determined by either a transthoracic echocardiogram or a transesophageal echocardiogram.
Overall, microbiological cure, 30-day mortality, and median time to clearance of bacteremia were 90.7% (97/107 patients), 8.4% (9/107 patients), and 7 (1 to 42) days, respectively (see Table 3 for complete results).
TABLE 3.
Subgroup analysis of synergistic gentamicin use overall
| Outcome | No. (%) of patients or daysa
|
P value | OR (95% CI)b | |
|---|---|---|---|---|
| With gentamicin (n = 63) | Without gentamicin (n = 44) | |||
| 30-Day mortality | 7 (11) | 2 (5) | 0.2 | 2.63 (0.5-13.3) |
| Hospital LOSc | 20 | 21 | 0.8 | |
| 30-Day microbiological cure | 57 (91) | 39 (89) | 0.8 | 1.22 (0.4-4.3) |
| Clearance of bacteremia | 7 | 9 | 0.9 | |
| Time to defervescence | 4 | 2 | 0.02 | |
Results represent the numbers of days (median values for clearance of bacteremia), except results for 30-day mortality and 30-day microbiological cure, which represent the numbers (percentages) of patients.
OR, odds ratio; 95% CI, 95% confidence interval.
LOS, length of stay.
CI versus II oxacillin.
Seventy-eight patients received CI oxacillin, whereas 29 received II oxacillin (Table 4). CI and II groups were similar with respect to 30-day mortality (8% versus 10%, respectively; P = 0.7) and length of hospital stay (20 versus 25 days, respectively; P = 0.4) but differed for microbiological cure at 30 days (94% versus 79%, respectively; P = 0.03).
TABLE 4.
Primary and secondary outcomes for patients receiving CI versus II oxacillin
| Outcome or parameter | No. (%) of patients or daysa
|
P value | OR (95% CI)b | ||
|---|---|---|---|---|---|
| CI or II (overall) | CI (n = 78) | II (n = 29) | |||
| 30-Day microbiological cure | 97 (91) | 73 (94) | 23 (79) | 0.03 | 3.8 (1.06-13.6) |
| Hospital LOSc | 21 | 20 | 25 | 0.4 | |
| 30-Day mortality | 9 (8) | 6 (8) | 3 (10) | 0.7 | 0.7 (0.2-3.1) |
| Clearance of bacteremia | 7 | 7 | 7 | 0.5 | |
| Time to defervescence | 3 | 3 | 3 | 0.8 | |
| Duration of therapy | 42 | 42 | 39 | 0.6 | |
| Concurrent administration of gentamicind | 63 (59) | 40 (51) | 23 (79) | 0.009 | 0.3 (0.1-0.8) |
Results represent the numbers of days (median values for clearance of bacteremia and time to defervescence), except results for 30-day microbiological cure, 30-day mortality, and concurrent administration of gentamicin, which represent the numbers (percentages) of patients.
OR, odds ratio; 95% CI, 95% confidence interval.
LOS, length of stay.
Gentamicin was administered at 1 mg/kg of body weight for every 8-h dosing period.
Synergistic gentamicin versus no gentamicin.
Because synergistic gentamicin is an optional treatment modality per guidelines, a subgroup analysis was performed to detect whether the use of synergistic gentamicin influenced mortality, hospital length of stay, microbiological cure at 30 days, clearance of bacteremia, or time to defervescence. The intended length of gentamicin therapy was physician specific and was not controlled in this study. The median number of days of gentamicin therapy was 4, with a range of 1 to 14 days. Overall, the gentamicin versus no-gentamicin groups were similar with respect to outcomes tested; however, the median times to defervescence were statistically different between the two groups, favoring the group that did not receive gentamicin (4 versus 2 days, P = 0.02). Results of the subgroup analysis are included in Table 5.
TABLE 5.
Subgroup analysis of synergistic gentamicin use per method of administration
| Groupa | Outcome | No. (%) of patients or daysb
|
P value | OR (95% CI)c | |
|---|---|---|---|---|---|
| With gentamicin | Without gentamicin | ||||
| CI-gent (n = 41) vs CI-no gent (n = 37) | 30-Day mortality | 5 (12) | 1 (3) | 0.2 | 2.0 (0.3-11.6) |
| Hospital LOSd | 18 | 21 | 0.2 | ||
| 30-Day microbiological cure | 39 (95) | 34 (92) | 0.7 | 4.5 (0.5-43) | |
| Clearance of bacteremia | 6 | 9 | 0.8 | ||
| Time to defervescence | 4 | 3 | 0.05 | ||
| Duration of gentamicin treatment | 3 | 0 | <0.0001 | ||
| II-gent (n = 23) vs II-no gent (n = 6) | 30-Day mortality | 3 (13) | 0 (0) | 1 | |
| Hospital LOS | 30 | 17 | 0.4 | ||
| 30-Day microbiological cure | 18 (78) | 5 (83) | 1 | 0.7 (0.1-7.7) | |
| Clearance of bacteremia | 7 | 8 | 0.9 | ||
| Time to defervescence | 5 | 1 | 0.1 | ||
| Duration of gentamicin treatment | 5 | 0 | 0.0002 | ||
CI-gent, CI oxacillin with gentamicin; CI-no gent, CI oxacillin without gentamicin; II-gent, II oxacillin with gentamicin; II-no gent, II oxacillin without gentamicin.
Results represent the numbers of days (median values for clearance of bacteremia, time to defervescence, and duration of gentamicin treatment), except results for 30-day mortality and 30-day microbiological cure, which represent the numbers (percentages) of patients.
OR, odds ratio; 95% CI, 95% confidence interval.
LOS, length of stay.
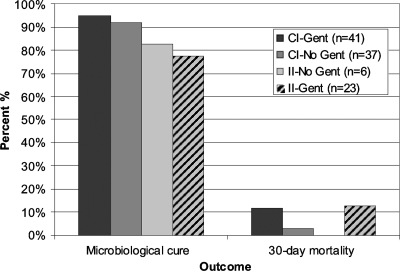
When the results for use of synergistic gentamicin were compared within groups (CI oxacillin with gentamicin versus CI oxacillin without gentamicin and II oxacillin with gentamicin versus II oxacillin without gentamicin), no statistically significant differences in the primary or secondary outcomes were noted. These results are shown graphically in Fig. 1. Rates of acute kidney injury were also compared for those patients receiving synergistic gentamicin and those that did not. Patients receiving hemodialysis and those having preexisting chronic kidney disease were excluded from analyses of acute kidney injury (n = 7). Rates of acute kidney injury were similar between patients who received synergistic gentamicin (n = 60) and those who did not receive synergistic gentamicin (n = 40) (18% versus 7%, respectively; P = 0.1).
FIG. 1.
Outcomes of oxacillin therapy for patients with IE caused by MSSA. CI-Gent, CI oxacillin with gentamicin; CI-No Gent, CI oxacillin without gentamicin; II-Gent, II oxacillin with gentamicin; II-No Gent, II oxacillin without gentamicin.
Multivariable analysis.
Patient characteristics identified as being statistically different between the two treatment groups (CI versus II oxacillin) were included in a multivariable regression model. Results of the model are included in Table 6. The antibiotic regimen (i.e., CI versus II) was the only independent factor responsible for microbiological cure at 30 days after a stepwise logistic regression analysis (P = 0.04). Neither antibiotic regimen (i.e., CI versus II) nor any of the clinically relevant factors (i.e., age, WBC count, chills, hemodialysis, synergistic gentamicin, or days on gentamicin therapy) were independently responsible for time to defervescence after a stepwise logistic regression analysis.
TABLE 6.
Results of multivariable logistic fit for microbiological cure
| Variable | P value |
|---|---|
| CI | 0.01 |
| Synergistic gentamicin treatment | 0.32 |
| Days on gentamicin therapy | 0.13 |
| Hemodialysis | 0.52 |
| Chills | 0.45 |
| WBC count | 0.48 |
| Age | 0.89 |
Comparative safety profiles.
The safety of the study drug was also examined. There were two patients in each arm of the analysis who experienced transaminitis (CI group, n = 2; II group, n = 2), defined as normal aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels increasing to greater than three times the upper limits of normal or, if AST/ALT levels were elevated at the start of therapy, doubling from baseline.
All of the patients experiencing transaminitis were included in the analysis of all outcomes. Each of the four patients had at least one risk factor for developing transaminitis at the start of therapy. Risk factors included hepatitis C infection, a history of ethanol abuse, and a history of cirrhosis. Two patients, both in the CI group, had normal AST/ALT levels at the start of therapy, while the other two patients in whom transaminitis occurred were in the II group and had elevated AST/ALT levels at the start of therapy. One patient in each treatment group was switched from oxacillin to another antibiotic after receiving at least 31 days of CI oxacillin and 35 days of II oxacillin. The two patients who switched therapies completed 42 days of treatment on vancomycin and cefazolin, respectively. All four patients remained culture negative at 30 days, and none of the four died within 30 days of completing therapy. The transaminitis resolved (returned to start-of-therapy baseline) for all patients.
DISCUSSION
The results of this study suggest an improved microbiological cure in patients with IE caused by MSSA treated with CI oxacillin compared to that in patients treated with II oxacillin. Outcomes between treatment groups were similar with respect to 30-day mortality, length of hospital stay, median time to clearance of bacteremia, median time to defervescence, and duration of antibiotic therapy. These findings demonstrate that CI oxacillin is an effective alternative to II oxacillin for the treatment of IE caused by MSSA. The CI method of administration was found to be statistically different from the II method for the primary outcome of microbiological cure at 30 days. Furthermore, the statistical superiority of CI versus II was preserved in a stepwise logistic regression model. Although statistically different for the primary outcome, these results do not indicate a clinical superiority of CI over II, especially since no difference in mortality between the two groups was seen.
These results are important considering that S. aureus has become the primary cause of IE in many parts of the world (4, 9). While methicillin-resistant S. aureus has become a concern, MSSA still accounts for the majority of cases of IE caused by S. aureus in many recent series (4, 8, 9, 19, 20). Also, other studies have shown that CI of a β-lactam reduces pharmacy preparation time, reduces nursing administration time, and maximizes antimicrobial pharmacodynamics (7). However, difficulties with CI administration do exist, namely, difficulty associated with the requirement for continuous venous access. This can be potentially problematic in patients with limited venous access or in critical-care settings, where patients require the administration of multiple intravenous agents.
A subgroup analysis of concurrent, synergistic gentamicin use illustrated no benefit concerning microbiological cure and an association with higher rates of mortality and acute kidney injury. Our analysis was consistent with previously reported data suggesting that synergistic gentamicin reduces the total number of days that individuals are bacteremic by about 1 day (13). Hastening the time to clearance of bacteremia did not improve overall mortality, and in fact, this investigation highlights a disturbing trend of higher mortality, in both arms and overall, for patients receiving synergistic gentamicin. Further exploration of this practice is warranted.
The decision to use synergistic gentamicin is one of the major limitations of this retrospective investigation. By nature, retrospective studies are more prone to bias than prospective studies. It may be that clinicians treated patients who appeared clinically worse with gentamicin but not those who seemed less ill.
This study population had high rates of IDU and previous endocarditis but was balanced with regard to site of infection (right versus left side). This is surprising considering that previous reports describe mainly right-sided disease in patients that were intravenous drug users (1, 4, 9). Our population had a higher-than-expected incidence of left-sided disease, which is important because these patients tend to have higher failure rates than patients with right-sided IE; however, this did not appear to affect our outcomes (8).
Safety analyses were supportive of the treatment guideline recommendation that synergistic gentamicin be regarded as an optional treatment, as our study found no clinical benefit and numerically high but not statistically different rates of acute kidney injury in the gentamicin-treated group compared to the no-gentamicin group (1).
Hepatotoxicity, although a known side effect of oxacillin, was infrequent, with only 4% (4/107) of patients experiencing transaminitis (3, 10). All cases were limited and reversible, with positive outcomes (microbiological cure and patient survival) in all instances. Although two patients were switched to different drugs to complete therapy, this was unlikely to have affected the patient outcomes.
Limitations.
There are several limitations to this study. First, this was a single-center analysis with a limited number of patients in the II arm of the study. Second, this analysis was conducted retrospectively; however, a prospective study of this nature requires prohibitive amounts of both time and money. As a result of the retrospective study design, we were unable to control for bias between the two treatment groups and also within a treatment group, such as is seen with the duration of gentamicin therapy in patients that received gentamicin. Also, this study had a relatively large population of intravenous drug users, which in addition to limiting the external validity of the study might account for some microbiological failure. Another limitation is that it is very difficult to assess compliance with home health antibiotic therapy. Despite these limitations, this is, to our knowledge, the first and only study to address the suitability of CI oxacillin for the treatment of IE.
Conclusion.
CI oxacillin offers a pharmacodynamically optimized treatment regimen for the management of patients with IE caused by MSSA. These results indicate that CI oxacillin is a suitable alternative to II oxacillin and possibly results in better microbiological outcomes. These results are significant when considering the costs associated with prolonged antibiotic therapy. Other evaluations of CI β-lactam antibiotics have found that CI reduces pharmacy preparation time, reduces nursing administration time, maximizes antimicrobial pharmacodynamics, and costs less to prepare and administer (3). More data, including prospective randomized trials, comparing the two methods of oxacillin administration for IE and other serious infections caused by MSSA would be of value to further validate these findings.
Acknowledgments
We thank James H. Jorgensen and Michael W. Ellis for their critical review of this study as well as their helpful comments and suggestions.
No financial support was provided for this study.
We declare no conflicts of interest.
Footnotes
Published ahead of print on 2 March 2009.
REFERENCES
- 1.Baddour, L. M., W. R. Wilson, A. S. Bayer, V. G. Fowler, Jr., A. F. Bolger, M. E. Levison, P. Ferrieri, M. A. Gerber, L. Y. Tani, M. H. Gewitz, D. C. Tong, J. M. Steckelberg, R. S. Baltimore, S. T. Shulman, J. C. Burns, D. A. Falace, J. W. Newburger, T. J. Pallasch, M. Takahashi, and K. A. Taubert. 2005. Infective endocarditis: diagnosis, antimicrobial therapy and management of complications. Circulation 111:e394-e434. [DOI] [PubMed] [Google Scholar]
- 2.Bayer, A. S., A. F. Bolger, K. A. Taubert, W. Wilson, J. Stekelberg, A. W. Karchmer, M. Levison, H. F. Chambers, A. S. Dajani, M. H. Gewitz, J. W. Newburger, M. A. Gerber, S. T. Shluman, T. J. Pallasch, T. W. Gage, and P. Ferrieri. 1998. Diagnosis and management of infective endocarditis and its complications. Circulation 98:2936-2948. [DOI] [PubMed] [Google Scholar]
- 3.Betts, R. F., S. W. Chapman, and R. L. Penn (ed.). 2002. A practical approach to infectious disease, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 4.Cabell, C. H., J. G. Jollis, G. E. Peterson, G. R. Corey, D. J. Anderson, D. J. Sexton, C. W. Woods, B. Reller, T. Ryan, and V. G. Fowler, Jr. 2002. Changing patient characteristics and the effect on mortality in endocarditis. Arch. Intern. Med. 162:90-94. [DOI] [PubMed] [Google Scholar]
- 5.Craig, W. A., and S. C. Ebert. 1992. Continuous infusion of β-lactam antibiotics. Antimicrob. Agents Chemother. 36:2577-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durack, D. T., A. S. Lukes, and D. K. Bright. 1994. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am. J. Med. 96:200-209. [DOI] [PubMed] [Google Scholar]
- 7.Florea, N. R., S. Kotapati, J. L. Kuti, E. C. Ceissler, C. H. Nightingale, and D. P. Nicolau. 2003. Cost analysis of continuous versus intermittent infusion of pipercillin-tazobactam: a time-motion study. Am. J. Health Syst. Pharm. 60:2321-2327. [DOI] [PubMed] [Google Scholar]
- 8.Fowler, V. G., Jr., H. W. Boucher, R. Corey, E. Abrutyn, A. W. Karchmer, M. E. Rupp, D. P. Levine, H. F. Chambers, F. P. Rally, G. A. Vigliani, C. H. Cabell, A. S. Link, I. DeMeyer, S. G. Filler, M. Zervos, P. Cook, J. Parsonnet, J. M. Bernstein, C. S. Price, G. N. Forrest, G. Fatkenheuer, M. Gareca, S. J. Rehm, H. R. Brodt, A. Tice, and S. E. Cosgrove. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653-665. [DOI] [PubMed] [Google Scholar]
- 9.Fowler, V. G., Jr., J. M. Miro, B. Hoen, C. H. Cabell, E. Abrutyn, E. Rubinstein, G. R. Corey, D. Spelman, S. F. Bradley, B. Barsic, P. A. Pappas, K. J. Anstrom, D. Wray, C. Q. Fortes, I. Anguera, E. Athan, P. Jones, J. T. M. van der Meer, T. S. J. Elliott, D. P. Levine, and A. S. Bayer. 2005. Staphylococcus aureus endocarditis throughout the world: a consequence of medical progress. JAMA 293:3012-3021. [DOI] [PubMed] [Google Scholar]
- 10.Hardman, J. G., L. E. Limbird, and A. G. Gilman (ed.). 2001. Goodman & Gilman's the pharmacological basis of therapeutics, 10th ed. McGraw-Hill, New York, NY.
- 11.Hoen, B., F. Alla, C. Selton-Suty, I. Beguinot, A. Bouvet, S. Briancon, J. P. Casalta, N. Danchin, F. Delahaye, J. Etienne, V. Le Moing, C. Leport, J. L. Mainardi, R. Ruimy, and F. Vandenesch. 2002. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA 288:75-81. [DOI] [PubMed] [Google Scholar]
- 12.Kalso, R. K. (ed.). 3 February 2009, accession date. Drugdex system (electronic version). Thomson Micromedex, Greenwood Village, CO.
- 13.Korzeniowski, O., and M. A. Sande. 1982. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parental drugs and in nonaddicts: a prospective study. Ann. Intern. Med. 97:496-503. [DOI] [PubMed] [Google Scholar]
- 14.Li, J. S., D. J. Sexton, N. Mick, R. Nettles, V. G. Fowler, Jr., T. Ryan, T. Bashore, and G. R. Corey. 2000. Proposed modifications to the Duke criteria for the diagnosis infective endocarditis. Clin. Infect. Dis. 30:633-638. [DOI] [PubMed] [Google Scholar]
- 15.Miro, J. M., I. Anguera, C. H. Cabell, A. Y. Chen, J. A. Stafford, G. R. Corey, L. Olaison, S. Eykyn, B. Hoen, E. Abrutyn, D. Raoult, A. Bayer, and V. G. Fowler, Jr. 2005. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin. Infect. Dis. 41:507-514. [DOI] [PubMed] [Google Scholar]
- 16.Mourvillier, B., J. L. Trouillet, J. F. Timsit, J. Baudot, J. Chastre, B. Regnier, C. Gibert, and M. Wolff. 2004. Infective endocarditis in the intensive care unit: clinical spectrum and prognostic factors in 228 consecutive patients. Intensive Care Med. 30:2046-2052. [DOI] [PubMed] [Google Scholar]
- 17.Mylonakis, E., and S. B. Calderwood. 2001. Infective endocarditis in adults. N. Engl. J. Med. 345:1318-1330. [DOI] [PubMed] [Google Scholar]
- 18.Pannu, N., and M. K. Nadim. 2008. An overview of drug-induced acute kidney injury. Crit. Care Med. 36:S216-S223. [DOI] [PubMed] [Google Scholar]
- 18a.PMIC. 2008. ICD-9-CM: international classification of diseases, 9th revision; clinical modification, 6th ed, vol. 1, 2, 3. Practice Management Information Corporation, Los Angeles, CA.
- 19.Remadi, J. P., G. Habib, G. Nadji, A. Brahim, F. Thuny, J. P. Casalta, M. Peltier, and C. Tribouilloy. 2007. Predictors of death and impact of surgery in Staphylococcus aureus infective endocarditis. Ann. Thorac. Surg. 83:1295-1302. [DOI] [PubMed] [Google Scholar]
- 20.Ruotsalainen, E., K. Sammalkorpi, J. Laine, K. Huotari, S. Sarna, V. Valtonen, and A. Jarvinen. 2006. Clinical manifestations and outcome in Staphylococcus aureus endocarditis among injection drug users and nonaddicts: a prospective study of 74 patients. BMC Infect. Dis. 6:137-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rybak, M. J., B. J. Abate, S. L. Kang, M. J. Ruffing, S. A. Lerner, and G. L. Drusano. 1999. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob. Agents Chemother. 43:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turnidge, J. D. 1998. The pharmacodynamics of β-lactams. Clin. Infect. Dis. 27:10-22. [DOI] [PubMed] [Google Scholar]