Abstract
Vancoresmycin is a novel tetramic acid antibiotic, probably interfering with functions of the cytoplasmic membrane. To investigate its mode of action, mutants of Streptococcus pneumoniae exhibiting reduced susceptibility to vancoresmycin were isolated at a low frequency. Four of them were further examined and showed similar pleiotropic phenotypes, including reduced growth rate, early autolysis, and chain formation. In one mutant, the level of transcripts from a single locus encoding the potential ABC transporter Spr0812-Spr0813 was increased sixfold. The corresponding DNA sequence revealed a nonsense mutation (C1744T) in spr0813, leading to the formation of a truncated permease lacking 2 of the 10 predicted transmembrane helices. As demonstrated by deletion and transformation analysis and reconstructing the spr0813C1744T mutation in the wild-type background, this nucleotide exchange was sufficient to cause reduced susceptibility to vancoresmycin and higher amounts of spr0812-spr0813 mRNA. Mapping and reporter assays of the cognate promoter Pabc showed that spr0812 and spr0813 are cotranscribed with a preceding small gene and that the spr0813C1744T mutation does not affect the activity of Pabc. Due to the similarity of Spr0812-Spr0813 to bacitracin transporters of Streptococcus mutans and Bacillus spp., the bacitracin susceptibility of spr0813 mutants was examined. Both the spr0813C1744T nonsense mutation and the deletion of the transporter genes led to a clearly increased sensitivity to bacitracin. Thus, the intact transporter is required for intrinsic resistance to bacitracin, whereas reduced vancoresmycin susceptibility is mediated by the truncated permease.
Antibiotic-resistant bacterial pathogens are of growing concern worldwide. Thus, extensive and ongoing efforts are required to screen for new drugs active against clinically relevant bacteria such as Streptococcus pneumoniae, one of the major human pathogens. Derivatives of tetramic acid (2,4-pyrrolidinedione) represent one group of chemical compounds with a high potential of antibacterial activity (28); however, there is currently no drug of this type in the market. A number of tetramic acid derivatives with antibacterial activity have been detected by testing natural or or chemically synthesized products (see reference 37 [and references therein] and references 24 and 31).
The antimicrobial mechanism of action for most of these compounds is not well characterized. Notable exceptions are reutericyclin (produced by Lactobacillus reuteri), which acts as a proton ionophore, dissipating the transmembrane proton potential (4, 5), and several submicromolar inhibitors of undecaprenyl pyrophosphate synthase that were recently designed on the basis of a pharmacophore hypothesis (24). This points to the cytoplasmic membrane as the cellular target of tetramic acid antibiotics and is consistent with the observation that their activity can be improved by introducing more lipophilic side chains at the N-substituted position (37). Partitioning of such compounds into the cytoplasmic membrane is hampered by the asymmetrical gram-negative outer membrane (20), which is the likely reason for their selective activity against gram-positive bacteria (4, 10).
Vancoresmycin (Var) is a natural tetramic acid derivative produced by a strain of the actinomycete Amycolatopsis sp. It exhibits potent antibiotic activity against gram-positive bacteria (including S. pneumoniae and vancomycin-resistant Enterococcus spp.), whereas gram-negative bacteria and fungi are not inhibited. At the 3 position of the tetramic acid core, Var carries a C-45 long partially unsaturated and highly oxygenated alkyl chain replaced by an aminoglycoside (10). Since this structure does not show obvious similarities to the activity-related pharmacophores of reutericyclin or undecaprenyl pyrophosphate synthase inhibitors, there is no reliable clue to the mode of action of Var. We addressed this issue by the isolation and transcription profiling of mutants of S. pneumoniae with reduced sensitivity to this antibiotic.
Although the mode of action of Var was not determined, a truncated ABC transporter with protein homology to bacitracin transporters from other species was identified that could confer reduced Var susceptibility and that also appears to be involved in bacitracin resistance.
MATERIALS AND METHODS
Bacterial strains, plasmids, oligonucleotides, growth conditions, and transformation.
S. pneumoniae strains and plasmids used in this work are listed in Table 1. PCR primers were synthesized at Operon Biotechnologies and are listed in Table 2. Primers used for sequencing and confirming the correct integration of DNA sections delivered to the Streptococcus pneumoniae genome are not listed. S. pneumoniae was grown in C-medium (15) supplemented with 0.2% yeast extract at 37°C without aeration or on D agar supplemented with 3% defibrinated sheep blood (Oxoid). Growth of S. pneumoniae in liquid cultures was monitored by nephelometry. MICs of antibiotics were determined by agar dilution using Var concentrations in the range of 0.3 to 0.8 μg/ml (0.05 μg/ml intervals) and bacitracin concentrations in the range of 0.5 to 6 μg/ml (0.2 μg/ml intervals). Antibiotic resistance genes used for chromosomal integrations in S. pneumoniae were selected with 80 μg/ml spectinomycin (Spc [aad9]), 200 μg/ml streptomycin (Str [rpsL]), 100 or 20 μg/ml kanamycin (Kan [aphIII, with or without promoter]), and 3 μg/ml tetracyclin (Tet [tetM]), respectively. Various concentrations of Var were prepared from a stock solution (0.5 mg/ml) in methanol and kept at −20°C. Transformation of S. pneumoniae was performed using naturally competent cells as previously described (18).
TABLE 1.
S. pneumoniae strains and plasmids
| Strain or plasmid | Relevant properties | Source or reference |
|---|---|---|
| Strains | ||
| R6 | Wild type | 22 |
| VarA | R6, Varr | This work |
| VarE | R6, Varr | This work |
| VarF | R6, Varr | This work |
| VarG | R6, spr0813C1744T, Varr | This work |
| VarGt | R6 transformed with DNA containing spr0813C1744T, selected on Var, Varr | This work |
| VarGc | R6 transformed with DNA containing spr0813C1744T::aphIII, selected on Kan, Kanr Varr | This work |
| VarAΔcopY | VarA, rpsL, ΔcopY, Strr Varr | This work |
| VarEΔcopY | VarE, rpsL, ΔcopY, Strr Varr | This work |
| VarFΔcopY | VarF, rpsL, ΔcopY, Strr Varr | This work |
| VarAΔcyl | VarA, Δcyl::aad9, Spcr Varr | This work |
| VarEΔcyl | VarE, Δcyl::aad9, Spcr Varr | This work |
| VarFΔcyl | VarF, Δcyl::aad9, Spcr Varr | This work |
| R6Δabc | R6, Δspr0811a-spr0813::aphIII, Kanr | This work |
| VarGΔabc | VarG, Δspr0811a-spr0813::aphIII, Kanr | This work |
| R6-Pabc | R6, bgaA::tetM-PabclacZ, Tetr | This work |
| VarG-Pabc | VarG, bgaA::tetM-PabclacZ, Tetr Varr | This work |
| VarGt-Pabc | VarGt, bgaA::tetM-PabclacZ, Tetr Varr | This work |
| VarGc-Pabc | VarGc, bgaA::tetM-PabclacZ, Tetr Varr | This work |
| RP100 | R6, bgaA::tetM-lacZ, Tetr | 7 |
| RP204 | R6, bgaA::tetM-PvegMlacZ, Tetr | 7 |
| Plasmids | ||
| pUC19 | ColE1, PlaclacZ′, bla | 36 |
| pdel17 | pUC19 with Δcyl::aad9 flanked by fragments of S. pneumoniae DNA in SmaI site | This work |
| pCR2.1spc | ColE1, bla, neo, aad9 | 18 |
| pPP2 | ColE1, integrative promoter probe vector for S. pneumoniae, htrA′-′lacZ reporter gene, bla, tetM | 7 |
| pPP2Pabc | pPP2, PabclacZ | This work |
TABLE 2.
Primers
| Primer | Sequencea |
|---|---|
| 17xxspec_for | GTTACATTTGCATTGCTAGGATCGATTTTCGTTCGTGAATAC |
| 17xxspec_rev | GAGCCTTCAACACAGATTACCATATGCAAGGGTTTATTG |
| 274-lacZ | GGGAAGGGCGATCGGTGCGGG |
| abcB-down_r | ACGGCAGATAATGGAACGGAATTCAATCGC |
| abcB-down_rr | TTCTGAGGAGCACATCTATTATGCGCACCC |
| abcB-downSal_f | tagtgggtcgaCCAAATGTAAAAAAAGATACCTCGACTTCAAAATCGAGG SalI |
| abcB-up_f | CCTTCTTCATAGCTGTTTTACTGGTTATCTTTGGG |
| abcB-up_ff | TGTTTAATGCAGGGATTACCGTTTTCCTCC |
| abcB-upBam_r | cgcggatccTTTA*ATCTAAACCGACTTTCTGCAAGATAATAAAGCGC BamHI |
| aph3Bam_f | cgcggatccAAAGAGGAAGGAAATAATAAATGGCTAAAATGAGAATATCACC BamHI |
| aph3Sal_r | tagtgggtcgaCGAACCAATTGGTTAATTGTAGGCATCTACATTCTCC SalI |
| aphIII_fwd | ATGGCTAAAATGAGAATATCACCGG |
| aphIII_rev | CTAAAACAATTCATCCAGTAAAATATAATA |
| bgaA_rev | GCTAAACCTGCTGCTACTGCTGC |
| cop_up1 | CTCTAATTCCCTTAAAGTGGGAACTAGAG |
| cop_up2 | TTGACTGGACCAGAACCATGACCGAGTTGAG |
| copY_down1 | GAGTTCCCAGCGCAACCAAGGTATCC |
| copY_down2 | CCTGCAACTAACATAATAGGCGTTGTTGC |
| copY_fwd+JanusLin | ACGTCCAAAAGCATAAGGAAAGGGGCCCAAGTAAGATGTAATTGTATGTAAAGGAGACG |
| copY_rev+JanusLin | CGGATCCGATCCATTTCCTCTGGAATAGGCCTGCCATTCTGCATCTGAAATCTGCAT |
| ko-TR_aphIII_fwd | TATTATATTTTACTGGATGAATTGTTTTAGAAAAAGATACCTCGACTTCAAAATCGAGG |
| L17xx_for | TCCAGCATTTCCTACAAGAATAAGTAGAGG |
| L17xx_rev | ATTCACGAACGAAAATCGATCCTAGCAATGCAAATGTAAC |
| Mini-copY_fwd | ATGCAGATTTCAGATGCAGAATGGCAGGAAGTAAGATGTAATTGTATGTAA |
| Mini-copY_rev | TTACATACAATTACATCTTACTTCCTGCCATTCTGCATCTGAAATCTGCAT |
| Proorf1_Kanlink_rev | CCGGTGATATTCTCATTTTAGCCATGATGTTACTCCTTTGTTCTTTATGAGTC |
| Pspr0812_fwd1 | gacgcatgcCTTATATTTTACAACTTTAAAAATAGG SphI |
| Pspr0812_rev1 | cgcggatccCTTTATGAGTCTAGTTTACATCAAAAAAAG BamHI |
| R17xx_for | ACAATAAACCCTTGCATATGGTAATCTGTGTTGAAGGCTC |
| R17xx_rev | CAGAAGAGTTGATTGACCAGCACTATCAGG |
| RACE-PCR_5 | GATATGCGCGAATTCCTG |
| RTgyrAf | TATCACAGCAGTACGTGATGAG |
| RTgyrAr | GGATAGCGAGCATATTGAAACC |
| RTspr0812_fwd5 | AAGTCGTGGTCAGGTTTAC |
| RTspr0812_rev7 | GCGGAAGCAAGATATTGTC |
| RTspr0813_fwd1 | AAACCGCAAACTCTACTATCC |
| RTspr0813_rev1 | GACGACAAACATACCAAATCC |
| spr0812_down3 | TGCTTGTCTTGACGGATCGAAAGAGTAG |
| spr0812_down4 | TTCTTTCCATCACGGCAGATAATGGAAC |
| spr0812_rev5 | GCGGTGTCAGTTCCATTCAAGTAAACC |
| spr0812_rev6 | GATTTACCAGAACCAGACTCACCC |
| spr0812_up1 | ACAGGCTGTAATTTAGTCGGCAATGTGAAG |
| spr0812_up2 | ATTAATAATATTTCGCCAGCTTCATCC |
The nucleotides printed in lowercase letters were added to introduce the underlined cleavage sites for the restriction enzymes indicated next to the sequences. The position of the C1744T exchange introduced into spr0813 by the use of the mutagenic primer abcB-upBam_r is indicated by an asterisk.
For cloning in the pPP2 vector, Escherichia coli DH5α [φ80dlacZΔM15Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1] (35) was used as an intermediate host. E. coli was cultivated in LB media (30) and transformed by using chemically competent cells (8).
RNA extraction.
Total RNA was extracted from S. pneumoniae by a modified hot phenol procedure as described previously (19). For each strain, cells harvested from two independent 100-ml cultures at a density of 80 nephelometric turbidity units were used. After final precipitation, washing, and drying of the nucleic acids, they were redissolved in 300 μl of diethylpyrocarbonate-treated water. DNA was then digested by the addition of 24 U of RNase-free DNase (NEB) in 33 μl of 10× DNase buffer (NEB) and incubation for 10 min at 37°C. The RNA was further purified using a Qiagen RNeasy minikit according to the manufacturer's instructions.
Microarray-based transcriptome analysis.
The microarray used (obtained from MWG Biotech AG) carried 50-mer oligonucleotide probes for all S. pneumoniae R6 annotated genes (11), each spotted in duplicate onto Schott Nexterion E slides.
Reverse transcription of RNA into labeled cDNA, prehybridization, hybridization, slide washing, scanning, and analysis of the data were performed exactly as described by McKessar and Hakenbeck (19). Only genes which showed reproducible changes in the transcript amount that were greater than threefold were considered further.
DNA manipulations.
Plasmid isolation and routine DNA manipulations were carried out by standard methods (30). Chromosomal DNA was isolated from S. pneumoniae as described earlier (12), and PCR products and DNA recovered after restriction endonuclease digestions were purified using the a JETquick spin column technique kit (Genomed) or a NucleoSpin Extract II kit (Macherey-Nagel).
Restriction enzymes and T4 DNA ligase were purchased from Roche Applied Science and used according to the manufacturer's instructions. PCRs were performed using either Goldstar Red Taq polymerase (Eurogentec) or iProof high-fidelity DNA polymerase (Bio-Rad) according to the manufacturer's instructions. Nucleotide sequencing was performed using an ABI Prism BigDye Terminator ready-reaction cycle sequencing kit (version 3.1; Perkin Elmer-ABI). Nucleotide sequences were analyzed by using CloneManager and Chromas software.
Construction of delivery cassettes and plasmids.
An in-frame deletion (ΔcopY) in copY was constructed via a two-step process in which the central part of the gene was first replaced with the Janus cassette (33), conferring a Kanr Strs phenotype in an Strr background. In the second step, the Janus cassette was deleted, thus restoring the original Strr phenotype. Two “integration fragments” flanking the central part (346 bp) of copY were amplified from chromosomal DNA of S. pneumoniae R6 by using primer pair cop_up1 and copYrev_JanusLinker and primer pair copYfwd_JanusLink and copY_down1 to obtain two PCR products (1,253 and 982 bp), each overlapping with one end of the Janus cassette by 29 bp and 28 bp, respectively. These fragments were mixed with the Janus cassette (1,359 bp), and after annealing, the desired product consisting of the Janus cassette flanked by the two “integration fragments” was amplified by using the nested primers cop_up2 and copY_down2. This product was used to transform Strr derivatives of VarA, VarE, and VarF, which were obtained by transformation of these strains with chromosomal S. pneumoniae DNA carrying the AmiA9 resistance marker (29). In the resulting Kanr Strs transformants, the correct position of the Janus cassette was confirmed by DNA extraction and PCR with appropriate primers. Two PCR products (1,247 bp and 981 bp) were generated separately using primer pair cop_up1 and Mini-copY_rev and primer pair Mini-copY_fwd and copY_down1 to obtain overlapping fragments flanking the desired copY deletion. These products were then mixed and subjected to further PCR amplification with the nested primers cop_up2 and copY_down2 to obtain a product containing the deletion and the flanking DNA regions. This product was used to transform the derivatives of VarA, VarE, and VarF carrying the integrated Janus cassette. DNA from transformants displaying a Kans Strr phenotype was amplified by PCR and sequenced to confirm the deletion in the resulting strains VarAΔcopY, VarEΔcopY, and VarFΔcopY.
The spr1764-spr1773 region (cylM gene cluster) of S. pneumoniae R6 was exchanged for the Spcr marker aad9 (Δcyl::aad9) with the help of plasmid pdel17, which contains the aad9 gene between two “integration fragments” corresponding to the flanking regions of the desired deletion. The “integration fragments” were amplified from chromosomal strain R6 DNA by using primer pair L17xx_for and L17xx_rev and primer pair R17xx_for and R17xx_rev to obtain two PCR products (996 bp and 820 bp), each overlapping by 20 bp with one end of the aad9 cassette. These fragments were joined with the aad9 cassette (1,198 bp; obtained by amplification from the pCR2.1spc plasmid with the primer pair 17xxspec_for and 17xxspec_rev) in two consecutive rounds of overlapped PCRs. The resulting product was ligated with SmaI-linearized pUC19 vector, and the desired plasmid, pdel17, was isolated after transformation of E. coli DH5α. pdel17 was used to transform S. pneumoniae VarA, VarE, and VarF. DNA from Spcr transformants was amplified by PCR and sequenced to confirm replacement of spr1764-spr1773 with aad9 in the resulting strains VarAΔcyl, VarEΔcyl, and VarFΔcyl.
To delete the spr0811a-spr0813 genes (encoding a putative ABC transporter), they were replaced with the promoterless Kanr aphIII gene (Δspr0811a-spr0813::aphIII). Two “integration fragments” flanking the desired deletion were amplified from chromosomal R6 DNA by using primer pair spr0812_up1 and Proorf1_Kanlink_rev and primer pair ko-TR_aphIII_fwd and spr0812_down3 to obtain PCR products (752 bp and 1,161 bp), each overlapping with one end of the aphIII gene by 22 bp and 30 bp, respectively. These fragments were mixed with the aphIII DNA (795 bp; obtained by amplification from the Janus cassette with the primer pair aphIII_fwd and aphIII_rev), and the desired product containing the aphIII gene flanked by the two “integration fragments” was amplified by using the nested primers spr0812_up2 and spr0812_down4. This product was used to transform S. pneumoniae VarG and R6. DNA from Kanr transformants was amplified by PCR and sequenced to confirm replacement of spr0811a-spr0813 with aphIII in the resulting strains VarGΔabc and R6Δabc.
A derivative of R6 carrying the C1744T nonsense mutation in spr0813 was constructed by introducing this nucleotide exchange together with the Kanr marker aphIII (spr0813C1744T::aphIII). Two “integration fragments” (1,071 bp and 1,030 bp) flanking a 232-bp 3′-terminal part of spr0813 were amplified from chromosomal R6 DNA by using primer pair abcB-up_f and abcB-upBam_r (introducing the C1744T exchange) and primer pair abcB-downSal_f and abcB-down_r, and the promoterless aphIII gene (904 bp) was amplified from the Janus cassette by using the primer pair aph3Bam_f and aph3Sal_r. The three resulting DNA fragments were restricted with BamHI and SalI as appropriate and ligated, and the desired product (2,886 bp) was amplified from the ligation mixture by using the nested primers abcB-up_ff and abcB-down_rr. This product was used to transform S. pneumoniae R6. DNA from Kanr transformants was amplified by PCR and sequenced to confirm the presence of the spr0813C1744T mutation in the resulting VarGc strain.
To assay the promoter of the spr0811a-spr0813 genes (Pabc), a PabclacZ reporter fusion was constructed. A 176-bp fragment carrying Pabc was amplified from chromosomal DNA of S. pneumoniae R6 by using the primer pair Pspr0812_fwd1 and Pspr0812_rev1. The PCR products were cleaved with SphI and BamHI and ligated with the SphI- and BamHI-digested promoter probe pPP2 vector, and the desired plasmid, pPP2Pabc, was isolated after transformation of E. coli DH5α. pPP2Pabc was used to transform S. pneumoniae R6, VarG, VarGt, and VarGc. DNA from Tetr transformants was amplified by PCR and sequenced to confirm the presence of the PabclacZ fusion in the resulting R6-Pabc, VarG-Pabc, VarGt-Pabc, and VarGc-Pabc strains.
Realtime RT-PCR.
Quantification of RNA levels by real-time reverse transcriptase PCR (RT-PCR) was carried out as previously described (19). For each strain, RNA prepared from two independent cultures was used, and each sample was measured in duplicate. Primer pairs were designed for the amplification of products of 149 to 154 bp in length. As an unregulated control, the gyrase gyrA gene was probed with the primer pair RtgyrAf and RTgyrAr. For detection of mRNA transcribed from spr0812 and spr0813, primer pair Rtspr0812_fwd5 and RTspr0812_rev7 and primer pair Rtspr0813_fwd1 and RTspr0813_rev1 were used.
Determination of transcriptional start site.
The start point of spr0811a-spr0813 transcription was determined by 5′ rapid amplification of cDNA ends (RACE) as described previously (13). The primer spr0812_rev5 was used for reverse transcription of RNA ligated to the RNA adapter, and the nested primer spr0812_rev6 was used for amplification of cDNA. The PCR products were analyzed using a 2% agarose gel, and the nucleotide sequence of the resulting fragment was determined.
Determination of β-galactosidase activity.
Preparation of cell extracts from cultures of S. pneumoniae, grown to a density of 80 to 90 nephelometric turbidity units in C-medium, and determination of specific β-galactosidase activities were performed as described by Halfmann et al. (7).
RESULTS
Spontaneous mutants of S. pneumoniae show reduced susceptibility to Var.
The MIC of Var for the S. pneumoniae R6 strain was 0.4 μg/ml. This concentration of the antibiotic reduced the number of colony-forming cells about 2,000-fold. In the presence of 0.5 μg of Var/ml, colonies were obtained with a frequency of 2 × 10−7; at 0.6 μg/ml, no colonies at all were recovered.
Four mutants (VarA, VarE, VarF, and VarG), isolated from agar plates containing 0.5 μg of Var/ml after 24 h of incubation, were examined in more detail. They had distinct phenotypes with respect to MIC and growth properties (Table 3), indicating that reduced susceptibility to Var may be acquired by different mutational pathways. All mutants showed a tendency for chain formation during growth in liquid medium. The growth rates of the mutants were not severely affected; however, after the stationary phase was entered, autolysis occurred earlier than in cultures of the parent R6 strain.
TABLE 3.
Properties of strains exhibiting reduced Var susceptibility
| Strain | Growth
|
MIC [μg/ml]d
|
|||
|---|---|---|---|---|---|
| Rate (min−1)a | Time of autolysis (h)b | Chain formationc | Var | Bacitracin | |
| R6 | 0.022 | 6.0 | − | 0.4 | 4.8 |
| VarA | 0.022 | 3.9 | ++ | 0.7 | 5.2 |
| VarE | 0.021 | 3.0 | + | 0.7 | 5.2 |
| VarF | 0.020 | 3.4 | ++ | 0.65 | 5.2 |
| VarG | 0.018 | 4.0 | ++ | 0.5 | 1.0 |
| VarGt | 0.019 | NDe | ND | 0.5 | 1.0 |
| VarGc | ND | ND | ND | 0.5 | 1.0 |
| R6Δabc | 0.022 | ND | ND | 0.4 | 1.0 |
| VarGΔabc | 0.019 | ND | ND | 0.4 | 1.0 |
Bacterial growth was monitored by nephelometry and is expressed in nephelometry units (NU). Growth rates were calculated as lnNUt2 − lnNUt1/t2 − t1, where t1 and t2 are limits of a time interval during exponential growth.
Values represent the time between the start of the stationary phase and the onset of autolysis. The time point of entering the stationary phase was determined from semilogarithmically blotted growth curves as the intersection of two lines obtained by elongating the linear sections of the blots representing exponential and stationary growth.
−, mainly diplococci; +, mainly short chains of 4 to 8 cells; ++, mainly long chains of more than 10 cells.
Mean values calculated from the results of three independent experiments.
ND, not determined.
Transcription profiles of the VarA, VarE, and VarF mutants did not reveal primary resistance determinants.
To identify genetic determinants involved in susceptibility of S. pneumoniae to Var, global transcription patterns of the mutants were compared to those of the parent R6 strain by using oligonucleotide microarrays of the S. pneumoniae R6 genome (19). For each strain, data sets from at least four hybridizations were used for normalization and statistical analysis. Only data which showed P values below 10−4 in a paired t test (1) and relative changes greater than threefold are listed in Table 4.
TABLE 4.
Differentially expressed genes in strains exhibiting reduced Var susceptibility
| Locus | Gene | Predicted function(s) and description | Change (fold) in transcript amt in mutant
|
|||
|---|---|---|---|---|---|---|
| VarA | VarE | VarF | VarG | |||
| spr0030 | NAa | Unknown | 0.32 | |||
| spr0446 | hsdS | Type I restriction modification enzyme specificity protein | 3.76 | |||
| spr0448 | hsdS | Type I restriction modification enzyme specificity protein | 0.29 | |||
| spr0466 | blpB | Transport protein, C-terminal fragment | 3.18 | |||
| spr0467 | blpB | Transport protein, N-terminal fragment | 3.61 | |||
| spr0468 | blpA | Bacteriocin transport-processing ATP-binding protein | 3.70 | |||
| spr0639 | copY | Copper transport transcriptional repressor | 5.24 | 3.15 | 3.99 | |
| spr0812 | NA | ABC transporter ATP-binding protein | 8.90 | |||
| spr0881 | coiA | Competence protein, possible transcription factor | 0.32 | |||
| spr1548 | NA | Unknown | 0.30 | |||
| spr1549 | NA | Unknown | 0.31 | |||
| spr1628 | pilD | Type IV prepilin peptidase | 0.26 | |||
| spr1758 | cinA | Competence damage-inducible protein | 0.29 | |||
| spr1764 | NA | Unknown | 6.67 | |||
| spr1765 | NA | Unknown | 4.15 | 4.08 | ||
| spr1766 | NA | Unknown | 7.11 | 7.50 | 8.40 | |
| spr1767 | cylM | Cytolysin subunit modifying protein | 3.47 | 4.49 | 5.87 | |
| spr1768 | NA | Unknown | 3.94 | 6.32 | 3.38 | |
| spr1769 | NA | Unknown | 3.50 | 5.24 | ||
| spr1770 | cylB | Toxin secretion ABC transporter ATP-binding-permease protein | 4.73 | 7.17 | 5.46 | |
| spr1773 | NA | ABC transporter ATP-binding protein | 3.22 | 3.94 | 3.30 | |
| spr1859 | NA | Competence protein | 0.28 | |||
| spr1863 | cglB | Competence protein, type II secretion system protein | 0.24 | |||
| spr1864 | cglA | Competence protein, type II secretion system ATPase | 0.24 | |||
NA, not available.
Strains VarA, VarE, and VarF showed partly overlapping profiles of differentially expressed genes, sharing some of the most prominent increases in mRNA levels observed. These changes concerned copY, encoding a transcriptional repressor involved in the control of the intracellular copper concentration (26), and a major part of the spr1764-spr1773 locus, which contains homologs of the enterococcal cylM gene cluster, specifying the production, processing, and secretion of and immunity against cytolysin (32). Mutant VarA in addition showed increased amounts of transcripts from the blpBA genes (spr0466-spr0468), which encode an ABC transporter involved in bacteriocin production of S. pneumoniae (16). Simultaneous affection of transcription of the cylM cluster and the blpAB genes has previously been observed in a variety of other S. pneumoniae mutants with altered cell wall biochemistry and might be related to some kind of general stress response (18).
To test the relevance of increased expression of the loci retrieved by transcriptome analysis for the susceptibility to Var, they were individually deleted in the respective mutants. In the case of copY, it appeared that this gene may be transcriptionally coupled with the downstream genes ctpA and spxB. To minimize polar effects on the expression of these genes, copY was deleted by constructing an in-frame minigene (ΔcopY). In the case of the spr1764-spr1773 locus, a deletion mutant (Δcyl) was constructed by replacing the entire DNA region with an Spc resistance cassette. The two deletions were individually introduced into each of the VarA, VarE, and VarF mutants, and the MICs of Var were established. Neither ΔcopY nor Δcyl had any effect on Var susceptibility, suggesting that enhanced expression of the respective genes in the VarA, VarE, and VarF mutants was not the primary cause of their reduced susceptibility but was rather an indirect effect of yet-unidentified determinants.
Reduced Var susceptibility is associated with increased expression of an ABC transporter in the VarG mutant.
The transcription profile of VarG was unique in that it showed only one single signal beyond the threefold threshold (Table 4). This signal was not observed with the other mutants and revealed an 8.9-fold-increased level of mRNA transcribed from spr0812, encoding the ATP-binding component of a putative ABC transporter.
The spr0812 gene appeared to constitute an operon together with the preceding (not annotated) short open reading frame spr0811a (108 bp) and the downstream gene spr0813, which has the potential to encode a membrane-spanning permease of unknown specificity (Fig. 1). In order to investigate whether the products of this putative operon are involved in reduced Var susceptibility, a deletion of all three genes (Δspr0811a-spr0813) was introduced into strain VarG by replacing them with the Kan resistance gene aphIII. In the resulting mutant, VarGΔabc, Var susceptibility was increased to the level seen with the wild-type R6 strain (Table 3). Introducing the same deletion into the R6 strain (R6Δabc), in contrast, did not affect its Var MIC. Moreover, transformation of R6 with a PCR product amplified from the spr0811a-spr0813 region of the VarG mutant yielded transformants resistant to 0.5 μg of Var/ml (VarGt) at a high frequency.
FIG. 1.
Genetic organization of the S. pneumoniae R6 spr0811a-spr0813 region and derivatives. spr0811a has not been annotated (11), but its expression has been experimentally demonstrated (not shown). Wide horizontal arrows indicate the directions and lengths of spr0811a (dotted), spr0812 (hatched), spr0813 (black), the Kanr marker aphIII (gray), and the flanking genes spr0811 and spr0814 (empty). The positions of the Pabc promoter and of putative ρ-independent terminators (T [ΔG > 12 kcal/mol]) are given by an angled arrow and vertical arrows, respectively. Replacement of spr0811a-spr0813 with aphIII is shown as a wide box (giving the extend of the deletion) containing a gray arrow. Insertion of aphIII is indicated with dotted lines. Relevant genotypic features are given at the right side of the figure.
These observations clearly showed (i) that the sequence of the spr0811a-spr0813 region of the VarG mutant was different from that of the wild type and (ii) that this difference was necessary to confer the reduced-susceptibility phenotype.
Reduced Var susceptibility depends on C-terminal truncation of the permease Spr0813.
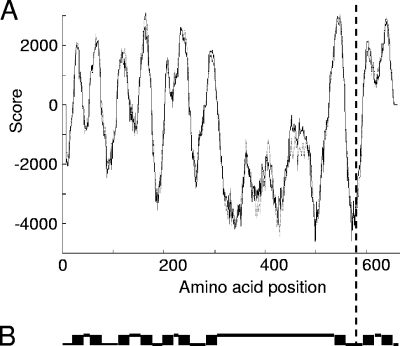
The nucleotide sequence of the spr0811a-spr0813 region of the VarG mutant, including the distance to the preceding, divergently oriented gene spr0811 (Fig. 1), was established. The sequence was identical to that of the parent R6 strain, except for one single nucleotide exchange (C to T) at the first position of codon 582 in spr0813, generating the nonsense triplet TAA. The truncated reading frame (spr0813C1744T) encodes a fragment of the putative permease Spr0813 in which 81 C-terminal amino acids are missing. From topology predictions, it appeared that this fragment (Spr0813Q582*) has only 8 of the 10 transmembrane helices calculated for the wild-type protein (Fig. 2).
FIG. 2.
Transmembrane topology prediction for Spr0813. The dashed vertical line indicates the C terminus of the truncated permease Spr0813Q582*. (A) Probabilities for transmembrane helices were calculated with the Tmpred program (9). The solid curve shows the strongly preferred prediction (N terminus inside); the dotted curve shows the less likely prediction (N terminus outside). (B) Transmembrane helices (black boxes) were predicted with the [TMHMM] program (14). Lower lines represent protein sections located inside the membrane; upper lines represent sections located outside.
In order to exclude the possibility that secondary mutations at unknown sites which may contribute to the reduced susceptibility phenotype had been acquired during the selection of VarG and VarGt, the nonsense mutation in spr0813 was introduced into the wild type without Var selection. This was achieved by transforming R6 with a synthetic cassette in which the truncated gene spr0813C1744T was linked to the aphIII resistance marker and by selection of the resulting strain construct, VarGc, in the presence of Kan. VarGc showed the same Var MIC as the original VarG mutant and the VarGt transformant. Thus, the C-terminally truncated protein Spr0813Q582* was sufficient to confer reduced Var susceptibility to S. pneumoniae.
Spr0813 truncation does not affect the activity of the Pabc promoter.
The spr0811a-spr0813 locus had initially been noticed due to the increased level of spr0812 mRNA in the transcriptome of VarG (Table 4). To confirm this observation, transcript amounts of spr0812 and spr0813 were quantified by real-time RT-PCR. In the VarG mutant as well as in the VarGt transformant and the VarGc construct, the mRNA levels of the spr0812 and spr0813 genes were about sixfold higher than in the R6 strain (Table 5). To decide whether this was the consequence of enhanced transcription of the spr0811a-spr0813 region, the 5′ end of the corresponding mRNA was mapped by RACE and located to a position 23 bp upstream of the spr0811a locus. The inferred promoter, Pabc (Fig. 1), was used to drive the expression of the lacZ reporter gene after single-copy integration at the bgaA locus of R6, VarG, and VarGt (7). As shown in Table 5, the activity of the Pabc promoter was rather weak compared with that of the PvegM reference promoter. Surprisingly, no significant activity changes of Pabc were detected in VarG and VarGt as a consequence of the spr0813C1744T mutation. Therefore, the higher amounts of spr0812-spr0813 transcripts observed in these mutants may have been due to increased mRNA stability and/or increased activity of one or more additional promoters which have not yet been identified.
TABLE 5.
Transcription of spr0811a-spr0813 in strains exhibiting reduced Var susceptibility
| Strain | Amt of transcripta
|
Strainb | Activity of Pabc (U of β-galactosidase)c | |
|---|---|---|---|---|
| spr0812 | spr0813 | |||
| R6 | 1 | 1 | R6-Pabc | 7 |
| VarG | 6.4 | 6.6 | VarG-Pabc | 6 |
| VarGt | 6.3 | 6.5 | VarGt-Pabc | 6 |
| VarGc | 5.8 | 5.9 | R6Δabc-Pabc | 6 |
| VarGΔabc-Pabc | 5 | |||
| RP100d | 0 | |||
| RP204e | 1,140 | |||
Values were determined by quantitative RT-PCR. Relative transcript amounts with respect to the wild-type R6 strain results are shown. Transcripts were detected with primer pairs specific for spr0812 and spr0813, respectively.
All strains were deficient in endogenous β-galactosidase due to disruption of the bgaA gene.
U, units expressed as nanomoles of nitrophenol produced per minute per milligram of protein. Mean values obtained from the results of experiments using two independent cultures are given.
Negative control, containing promoterless lacZ.
Positive control, containing PvegMlacZ.
Spr0812 and Spr0813 are also involved in susceptibility to bacitracin.
The protein products predicted for spr0812 and spr0813 showed remarkable degrees of sequence identity with the ABC transporters MbrA (56%) and MbrB (30%) of S. mutans, BceA (48%) and BceB (24%) of Bacillus subtilis, and BcrA (47%) and BcrB (24%) of B. licheniformis. For each of these transporters, it has been demonstrated that they are directly involved in resistance to the nonribosomal peptide antibiotic bacitracin (21, 25, 34). Therefore, the bacitracin susceptibility of the S. pneumoniae spr0813C1744T nonsense mutants was tested. All of them (VarG, VarGt, and VarGc) showed a strongly reduced bacitracin MIC (1 μg/ml) compared with the value of 4.8 μg/ml for the parent R6 strain (Table 3). Deletion of the complete spr0811a-spr0813 locus (Δspr0811a-spr0813) in VarG did not lead to a further decrease of bacitracin MICs for those strains, whereas the same deletion reduced the bacitracin resistance of the wild-type R6 strain to the level seen with the spr0813C1744T nonsense mutants. This indicated that truncation of Spr0813 was sufficient to completely abolish the contribution of the Spr0812-Spr0813 transporter to bacitracin resistance of S. pneumoniae. It thus appeared that the intact transporter was required for resistance to bacitracin, whereas the truncated Spr0983Q582* permease mediated reduced susceptibility to Var.
DISCUSSION
In this study, mutants of S. pneumoniae that exhibit reduced susceptibility to the tetramic acid antibiotic Var were characterized. Mutants could be isolated at a rather low frequency, and their drug MICs (0.5 to 0.7 μg/ml) were only moderately increased compared to that seen with the parental R6 strain (0.4 μg/ml). This indicated that Var might not interact with one specific cellular target at which single point mutations would be expected to lead to a more distinct resistance phenotype. Due to its suggested partitioning into the cytoplasmic membrane (37), the antibiotic probably interferes with a variety of functions that depend on the integrity of the cell envelope. In agreement with this view, transcription profiling revealed that genes differentially expressed in the mutants investigated here mostly encode products which are associated with or depend on the cytoplasmic membrane for activity: the cylM gene cluster encoding the production of a homologue of the potent membrane-active cytolysin of Enterococcus spp., a bacteriocin transporter, a regulator of copper transport, and proteins involved in genetic competence (see Table 4). It is therefore not surprising that all four mutants were resistant at only low levels and showed pleiotropic phenotypes such as a reduced growth rate, a shorter stationary phase followed by early lysis, and a tendency for chain formation.
From these properties of the mutants, it appeared that there are different routes by which mechanisms protective against Var may emerge. One of these routes relies on modification of the ABC transporter Spr0812-Spr0813, which is also involved in the susceptibility of S. pneumoniae to the structurally unrelated antibiotic bacitracin. Since Var, like bacitracin, possibly displays its antimicrobial activity by interference with essential functions of the cytoplasmic membrane, both drugs may be accessible to the Spr0813 permease, the substrate-specific component of the transporter. C-terminal truncation of the permease (in strain VarG) and deletion of the complete transporter gene locus (in strain VarGΔabc), however, had opposing effects on the susceptibility of the bacteria to Var, whereas resistance to bacitracin was abolished by both mutations.
In B. licheniformis and B. subtilis, it seems that BcrAB and BceAB act by transporting the bacitracin molecule itself, thus directly removing the antibiotic from its membrane target (25, 27). The issue of whether these transport systems mediate bacitracin efflux or influx, however, is still a matter of debate (3, 27). Based on very similar transmembrane topology predictions for BceB and Spr0983 (9 or 10 transmembrane helices, with a large hydrophilic domain between helices 7 and 8), the Spr0812-Spr0813 transporter of S. pneumoniae may also use bacitracin itself as a substrate, and the C-terminal pair of predicted transmembrane domains in Spr0813 (Fig. 2) may be indispensable to this function. The absence of these transmembrane domains, in contrast, was the primary cause of reduced susceptibility to Var. As one plausible explanation of this finding, truncation of the Spr0813 permease may lead to altered substrate specificity so that Var itself can be transported. Alternatively, the mutated transporter could export an unknown substance inactivating the antibiotic outside the cell. Apart from that, the possibility remains that the (sixfold-) elevated level of spr0812-spr0813 transcripts in spr0813C1744T mutants leads to overexpression of the transporter, which in turn may either exclusively or partly (together with the spr0813C1744T mutation) account for the reduced-susceptibility phenotype. Consistent with this possibility, it has been reported that mutations affecting the specificity of an enzyme can be compensated for by overexpression, which in turn permits a broader range of substrates to be used (2).
The spr0813C1744T mutation had no detectable effect on the activity of the Pabc promoter, indicating that higher amounts of spr0812-spr0813 mRNA may be due to increased stability of the transcripts or increased activity of unidentified promoters. The interrelation between spr0813 truncation and these effects, however, remains unclear.
It is also not known whether the 36-amino-acid peptide encoded by spr0811a is functionally associated with the Spr0812 and Spr0813 transporters. As verified by assaying a translational spr0811a::lacZ fusion, this peptide is in fact expressed in S. pneumoniae (not shown). BLAST searches, however did not reveal any hints to possible functions of Spr0811a, and no open reading frames with the potential to encode similar products are present upstream of the genes of the related bacitracin transporters BcrAB and BceAB of Bacillus spp. or MbrAB of S. mutans.
In the case of the BceAB system of B. subtilis, it was recently shown that it is not only active as a bacteriocin detoxification pump but is also crucial for bacitracin perception by the histidine kinase BceS (27). BceS and the cognate response regulator BceR are encoded immediately upstream of bceAB and mediate induction of these genes in the presence of bacitracin (21). Similarly, the genes for the homologous ABC transporter MbrAB of S. mutans are clustered with genes for the two-component system MbrCD. In contrast, no two-component system is encoded in the vicinity of spr0812-spr0813 in S. pneumoniae. Of all proteins predicted for S. pneumoniae, the Rr01 response regulator and the Hk01 sensor kinase of the so-far-uncharacterized 01 two-component system (23) show the highest identities with BceR (43%) and BceS (28%) of B. subtilis and MbrC (41%) and MbrD (32%) of S. mutans, respectively. As with BceS and MbrD, the N-terminal input domain of S. pneumoniae Hk01 indeed shows the typical architecture (two deduced transmembrane helices, with no extracytoplasmic linker in between) of intramembrane-sensing histidine kinases (17) belonging to the phylogenetically conserved HPK3i subgroup (6). In contrast to the members of this subgroup, which is characterized by the location of the respective genes adjacent to those encoding functionally linked ABC transporters, the 01 two-component system of S. pneumoniae, however, is encoded 0.64 Mb away from the spr0812-spr0813 transporter genes. Further studies will be necessary to establish whether there is a regulatory link between these two loci.
Acknowledgments
Financial support for this work was provided by the Forschungsschwerpunkt Biotechnologie of the Technische Universität Kaiserslautern and INTAFAR project LSHM-CT-2004-512138 of the European Community.
We thank Gerhard Seibert at Sanofi-Aventis GmbH for supplying the antibiotic Var, Carsten Volz for the gift of plasmid pdel17, Alexander Halfmann for the gift of the pPP2 promoter test vector, and Donald A. Morrison for the gift of the Janus cassette. We are grateful to Martin Blettinger for constructing and characterizing the mutant VarGc.
Footnotes
Published ahead of print on 9 March 2009.
REFERENCES
- 1.Baldi, P., and A. D. Long. 2001. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics 17:509-519. [DOI] [PubMed] [Google Scholar]
- 2.Berg, C. M., M. D. Wang, N. B. Vartak, and L. Liu. 1988. Acquisition of new metabolic capabilities: multicopy suppression by cloned transaminase genes in Escherichia coli K-12. Gene 65:195-202. [DOI] [PubMed] [Google Scholar]
- 3.Bernard, R., A. Guiseppi, M. Chippaux, M. Foglino, and F. Denizot. 2007. Resistance to bacitracin in Bacillus subtilis: unexpected requirement of the BceAB ABC transporter in the control of expression of its own structural genes. J. Bacteriol. 189:8636-8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gänzle, M. G. 2004. Reutericyclin: biological activity, mode of action, and potential applications. Appl. Microbiol. Biotechnol. 64:326-332. [DOI] [PubMed] [Google Scholar]
- 5.Gänzle, M. G., and R. F. Vogel. 2003. Studies on the mode of action of reutericyclin. Appl. Environ. Microbiol. 69:1305-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grebe, T. W., and J. B. Stock. 1999. The histidine protein kinase superfamily. Adv. Microb. Physiol. 41:139-227. [DOI] [PubMed] [Google Scholar]
- 7.Halfmann, A., R. Hakenbeck, and R. Bruckner. 2007. A new integrative reporter plasmid for Streptococcus pneumoniae. FEMS Microbiol. Lett. 268:217-224. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann, K., and W. Stoffel. 1993. A database of membrane spanning protein segments. Biol. Chem. Hoppe-Seyler 374:166. [Google Scholar]
- 10.Hopmann, C., M. Kurz, M. Brönstrup, J. Wink, and D. LeBeller. 2002. Isolation and structure elucidation of vancoresmycin—a new antibiotic from Amycolatopsis sp. ST 101170. Tetrahedron Lett. 43:435-438. [Google Scholar]
- 11.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kharat, A. S., and A. Tomasz. 2003. Inactivation of the srtA gene affects localization of surface proteins and decreases adhesion of Streptococcus pneumoniae to human pharyngeal cells in vitro. Infect. Immun. 71:2758-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovács, M., A. Halfmann, I. Fedtke, M. Heintz, A. Peschel, W. Vollmer, R. Hakenbeck, and R. Bruckner. 2006. A functional dlt operon, encoding proteins required for incorporation of d-alanine in teichoic acids in gram-positive bacteria, confers resistance to cationic antimicrobial peptides in Streptococcus pneumoniae. J. Bacteriol. 188:5797-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krogh, A., B. Larsson, H. G. von, and E. L. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567-580. [DOI] [PubMed] [Google Scholar]
- 15.Lacks, S., and R. D. Hotchkiss. 1960. A study of the genetic material determining an enzyme in Pneumococcus. Biochim. Biophys. Acta 39:508-518. [DOI] [PubMed] [Google Scholar]
- 16.Lux, T., M. Nuhn, R. Hakenbeck, and P. Reichmann. 2007. Diversity of bacteriocins and activity spectrum in Streptococcus pneumoniae. J. Bacteriol. 189:7741-7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mascher, T., J. D. Helmann, and G. Unden. 2006. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 70:910-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mascher, T., D. Zahner, M. Merai, N. Balmelle, A. B. de Saizieu, and R. Hakenbeck. 2003. The Streptococcus pneumoniae cia regulon: CiaR target sites and transcription profile analysis. J. Bacteriol. 185:60-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKessar, S. J., and R. Hakenbeck. 2007. The two-component regulatory system TCS08 is involved in cellobiose metabolism of Streptococcus pneumoniae R6. J. Bacteriol. 189:1342-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikaido, H. 1994. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264:382-388. [DOI] [PubMed] [Google Scholar]
- 21.Ohki, R., Giyanto, K. Tateno, W. Masuyama, S. Moriya, K. Kobayashi, and N. Ogasawara. 2003. The BceRS two-component regulatory system induces expression of the bacitracin transporter, BceAB, in Bacillus subtilis. Mol. Microbiol. 49:1135-1144. [DOI] [PubMed] [Google Scholar]
- 22.Ottolenghi, E., and R. D. Hotchkiss. 1962. Release of genetic transforming agent from pneumococcal cultures during growth and disintegration. J. Exp. Med. 116:491-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paterson, G. K., C. E. Blue, and T. J. Mitchell. 2006. Role of two-component systems in the virulence of Streptococcus pneumoniae. J. Med. Microbiol. 55:355-363. [DOI] [PubMed] [Google Scholar]
- 24.Peukert, S., Y. Sun, R. Zhang, B. Hurley, M. Sabio, X. Shen, C. Gray, J. Dzink-Fox, J. Tao, R. Cebula, and S. Wattanasin. 2008. Design and structure-activity relationships of potent and selective inhibitors of undecaprenyl pyrophosphate synthase (UPPS): tetramic, tetronic acids and dihydropyridin-2-ones. Bioorg. Med. Chem. Lett. 18:1840-1844. [DOI] [PubMed] [Google Scholar]
- 25.Podlesek, Z., A. Comino, B. Herzog-Velikonja, D. Zgur-Bertok, R. Komel, and M. Grabnar. 1995. Bacillus licheniformis bacitracin-resistance ABC transporter: relationship to mammalian multidrug resistance. Mol. Microbiol. 16:969-976. [DOI] [PubMed] [Google Scholar]
- 26.Reyes, A., A. Leiva, V. Cambiazo, M. A. Mendez, and M. Gonzalez. 2006. Cop-like operon: structure and organization in species of the Lactobacillale order. Biol. Res. 39:87-93. [DOI] [PubMed] [Google Scholar]
- 27.Rietkötter, E., D. Hoyer, and T. Mascher. 2008. Bacitracin sensing in Bacillus subtilis. Mol. Microbiol. 68:768-785. [DOI] [PubMed] [Google Scholar]
- 28.Royles, B. J. L. 1995. Naturally occurring tetramic acids: structure, isolation, and synthesis. Chem. Rev. 95:1981-2001. [Google Scholar]
- 29.Salles, C., L. Creancier, J. P. Claverys, and V. Mejean. 1992. The high level streptomycin resistance gene from Streptococcus pneumoniae is a homologue of the ribosomal protein S12 gene from Escherichia coli. Nucleic Acids Res. 20:6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Schobert, R., and A. Schlenk. 2008. Tetramic and tetronic acids: an update on new derivatives and biological aspects. Bioorg. Med. Chem. 16:4203-4221. [DOI] [PubMed] [Google Scholar]
- 32.Shankar, N., P. Coburn, C. Pillar, W. Haas, and M. Gilmore. 2004. Enterococcal cytolysin: activities and association with other virulence traits in a pathogenicity island. Int. J. Med. Microbiol. 293:609-618. [DOI] [PubMed] [Google Scholar]
- 33.Sung, C. K., H. Li, J. P. Claverys, and D. A. Morrison. 2001. An rpsL cassette, janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67:5190-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuda, H., Y. Yamashita, Y. Shibata, Y. Nakano, and T. Koga. 2002. Genes involved in bacitracin resistance in Streptococcus mutans. Antimicrob. Agents Chemother. 46:3756-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodcock, D. M., P. J. Crowther, J. Doherty, S. Jefferson, E. DeCruz, M. Noyer-Weidner, S. S. Smith, M. Z. Michael, and M. W. Graham. 1989. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 17:3469-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 37.Yendapally, R., J. G. Hurdle, E. I. Carson, R. B. Lee, and R. E. Lee. 2008. N-substituted 3-acetyltetramic acid derivatives as antibacterial agents. J. Med. Chem. 51:1487-1491. [DOI] [PubMed] [Google Scholar]