Abstract
VIM-1-producing Klebsiella pneumoniae (VPKP) is an emerging pathogen. A prospective observational study was conducted to evaluate the importance of VIM production on outcome of patients with K. pneumoniae bloodstream infections (BSIs). Consecutive patients with K. pneumoniae BSIs were identified and followed up until patient discharge or death. A total of 162 patients were included in the analysis; 67 (41.4%) were infected with VPKP, and 95 were infected with non-VPKP. Fourteen of the patients infected with VPKP were carbapenem resistant (Carbr) (MIC > 4 μg/ml), whereas none of the non-VPKP exhibited carbapenem resistance. The patients infected with a Carbr organism were more likely (odds ratio, 4.08; 95% confidence interval [CI], 1.29 to 12.85; P = 0.02) to receive inappropriate empirical therapy. The all-cause 14-day mortality rates were 15.8% (15 of 95) for patients infected with VIM-negative organisms, 18.9% (10 of 53) for those infected with VIM-positive carbapenem-susceptible organisms, and 42.9% (6 of 14) for those infected with VIM-positive Carbr organisms (P = 0.044). In Cox regression analysis, age (hazard ratio [HR], 1.03; 95% CI, 1.01 to 1.06; P = 0.021), rapidly fatal underlying disease (HR, 2.84; 95% CI, 1.26 to 6.39; P = 0.012), and carbapenem resistance (HR, 2.83; 95% CI, 1.08 to 7.41; P = 0.035) were independent predictors of death. After adjustment for inappropriate empirical or definitive therapy, the effect of carbapenem resistance on outcome was reduced to a level of nonsignificance. In patients with K. pneumoniae BSIs, carbapenem resistance, advanced, age, and severity of underlying disease were independent predictors of outcome, whereas VIM production had no effect on mortality. The higher mortality associated with carbapenem resistance was probably mediated by the failure to provide effective therapy.
Over the last few years, the reliance on carbapenems has been challenged owing to the wide spread of acquired metallo-β-lactamases [MBLs] (2, 11, 20, 25, 31). Two dominant groups of acquired MBLs have been recognized: the IMP and VIM types. This class of enzymes is characterized by the ability to hydrolyze carbapenems and all available β-lactams with the exception of aztreonam. Moreover, since the MBL genes are linked to other resistance determinants within the same integron or plasmid, MBL-producing organisms are commonly multidrug resistant, further compromising our therapeutic options (8, 26, 28).
MBLs have spread throughout the world with an overall trend moving from Pseudomonas aeruginosa into Enterobacteriaceae (9, 10, 14, 16, 18, 22, 23, 28). Recently, there have been several reports on the emergence of VIM-producing Klebsiella pneumoniae (VPKP), mainly in Southern Europe (16, 20). In Greece, VPKP isolates are endemic in various hospitals and cause life-threatening infections (5, 8, 29). Based on previous reports (30) and on the National Surveillance System for Antibiotic Resistance Database (www.mednet.gr/whonet/top.htm), the dissemination of such isolates appears to have occurred in a relatively short period of time in this region, probably between 2002 and 2003.
Although there have been several reports on the emergence and spread of MBL-producing organisms (3, 4, 8, 13, 16), our understanding of what MBL-production entails in terms of clinical impact and antimicrobial chemotherapy for infections caused by K. pneumoniae is limited. Given the clinical importance of VPKP, a prospective observational study was conducted to investigate: (i) the microbiologic characteristics of VPKP causing bloodstream infections (BSIs) and (ii) the effect of VIM-production on the outcome of such infections. The microbiologic data of this survey have been reported elsewhere (24). Briefly, 37.6% of K. pneumoniae blood isolates were VIM-1 producers. VIM-1 was mediated by a single integron that had been spread through transferable plasmids to a wide variety of chromosomal types. VPKP isolates were invariably multidrug resistant (resistant to three or more distinct classes of antimicrobial agents); however, MICs of carbapenems varied significantly from the susceptible range to high-level resistance (0.125 to 32 μg/ml) even between isolates of common clonal origin. We report here the clinical characteristics of patients infected with VPKP and the impact of VIM production on the outcome of such patients.
MATERIALS AND METHODS
Study design.
A prospective observational study was conducted in three tertiary-care hospitals located in the Athens metropolitan area; in hospital A, a 500-bed hospital, between February 2004 and March 2006 and in hospitals B and C, 1,000- and 400-bed hospitals, respectively, between February 2005 and March 2006. Consecutive patients with K. pneumoniae BSIs were identified by daily communication with the clinical microbiology laboratory. The medical records of all patients who had one or more blood cultures positive for K. pneumoniae and clinical courses consistent with bacteremia were reviewed upon notification and twice a week until discharge or death. Each patient was included in the study once. Pertinent information regarding demographic characteristics, underlying disease, severity of illness, use of immunosuppressive drugs, prior hospitalizations, prior antibiotic use over the last three months, and the antibiotics used for the episode of bacteremia was abstracted in a predesigned form. The decisions on antimicrobial chemotherapy were made either by the attending physician of the patient or by an infectious diseases specialist according to accepted clinical practices and the local trends of antimicrobial resistance. The endpoint was all-cause mortality at day 14 after the onset of bacteremia. Patients discharged from the hospital before the day 14 were considered survivors. The study was approved by the institutional review board of each hospital; the need for informed consent had been waived.
Microbiology.
Blood cultures were performed by the Wider I automated system (Dade Behring MicroScan, West Sacramento, CA), and species identification was confirmed by the API 20E (bioMerieux, Marcy l'Etoile, France). All blood isolates were collected in our research laboratory for further examination. Susceptibilities to antibiotics were determined by the Etest (AB Biodisk, Solna, Sweden). Other characteristics of K. pneumoniae isolates, including pulsed-field gel electrophoresis (PFGE) types and bla gene carriage, were examined as described previously (24).
Definitions.
BSIs were classified into one of three categories according to the Friedman et al. classification (6): nosocomial (positive cultures obtained from patients already hospitalized for 48 h or longer), healthcare associated (positive cultures obtained at admission or within 48 h or if the patient had received intravenous therapy at home, had attended a hospital or hemodialysis clinic or had received intravenous chemotherapy or was hospitalized in the preceding 3 months, or had resided in a long-term care facility), and community acquired (positive culture obtained at admission or within 48 h of hospitalization, for patients not fulfilling the criteria for healthcare-associated infection). The site of infection was determined to be urinary, intra-abdominal, vascular catheter-related, pulmonary, soft-tissue, primary bloodstream infection or unknown according to Centers for Disease Control and Prevention definitions (7). To obtain comparisons regarding the severity of underlying illnesses, all patients were classified into one of three categories: rapidly fatal, ultimately fatal, and nonfatal, according to the McCabe and Jackson classification (17). Patients on immunosuppressive medication and patients with human immunodeficiency virus infection or neutropenia (<1,000 neutrophils/mm3) were classified as immunocompromised. The severity of bacteremia was assessed by the Pitt bacteremia score as described previously (21). Appropriate empirical antibiotic therapy for an episode of bacteremia was defined as treatment with at least one antibiotic that had in vitro activity against the infecting organism, initiated within 48 h of the initial positive blood culture, and given in adequate dosage. Appropriate definitive treatment was defined as the use for at least 48 h of an antimicrobial agent to which the infecting organism was susceptible in vitro. The classification of the organisms as in vitro susceptible or resistant was based on the Etest results (on several occasions the results were different from those reported by the clinical microbiology laboratories of the participating hospitals) and the current breakpoints for carbapenems as issued by the Clinical and Laboratory Standards Institute (19). For patients with polymicrobial bacteremia the definition of appropriate therapy pertains to therapy that was appropriate for all isolated organisms.
Statistical analysis.
The data were processed and analyzed by using the SPSS statistics software (version 12 for Windows). Initial univariate comparisons were conducted by using the χ2 or the Fisher exact test for categorical variables and the Student t test or the Mann-Whitney U test for continuous variables. Cox regression analysis was performed to assess for factors independently associated with mortality. Variables for which the P value was ≤0.10 in univariate analysis were included in the model. Odds ratios (OR), hazard ratios (HRs), and 95% confidence intervals (CIs) were calculated. Survival curves were prepared with Kaplan-Meier estimation and compared using the log-rank test. All tests were two tailed, and a P < 0.05 was considered significant.
RESULTS
Patients.
A total of 178 patients with K. pneumoniae BSIs were identified; 16 of the BSIs were community acquired, 27 were healthcare associated, and 135 were nosocomial. A total of 162 patients were included in the analysis; 16 patients who had community-acquired BSIs were excluded since they were considered to represent patients with different characteristics (the primary site of infection was the urinary tract in 12, and none of the infections was caused by VPKP). A total of 76 (47.9%) were identified in hospital A, 60 (37%) were identified in hospital B, and 26 (16%) were identified in hospital C. A total of 82 (50.6%) episodes occurred in medical wards, 53 (32.7%) occurred in ICUs, and 27 (16.7%) occurred in surgical wards. One hundred and three (63.6%) of the patients were males, and fifty-nine (36.4%) were females. The mean patient age was 60.3 years (median, 62.5; range, 17 to 97 years). The mean duration of hospitalization before the onset of bacteremia was 25.85 ± 37.87 days. The probable source of bacteremia was the genitourinary tract in 22 cases, the abdomen in 32 cases, the intravascular catheter in 11 cases, the skin or soft tissue in 8 cases, and the lung in 44 cases. In 45 instances no definite portal of entry could be detected. A total of 158 patients had at least one underlying disease; 33 had rapidly fatal disease, 119 had ultimately fatal disease, and 6 had nonfatal disease. The remaining four patients had no underlying disease.
Microorganisms.
Of the 162 isolates, 67 (41.4%) produced metallo-β-lactamase type VIM-1. The carbapenem MICs for the 67 VPKP strains ranged from 0.125 to 32 μg/ml; 14 isolates were resistant to both imipenem and meropenem (MIC > 4 μg/ml), and 53 were susceptible to either imipenem or meropenem (MIC ≤ 4 μg/ml). The MICs of meropenem were one- to twofold lower. The wide diversity in the MICs of carbapenems from the susceptible to fully resistance range has been attributed to loss of porins and duplications of the blaVIM-1 gene in some of VPKP isolates (15). All VPKP isolates were resistant to amoxicillin-clavulanate, ticarcillin-clavulanate, piperacillin, cefoxitin, cefotaxime, and ceftazidime. One VPKP isolate was susceptible to piperacillin-tazobactam, and two isolates were susceptible to cefepime. Forty-seven of the VPKP isolates exhibited either resistance or decreased susceptibility to aztreonam due to SHV-5 type ESBL production, as found in a previous study on this collection of isolates (24). High resistance rates were also observed for tobramycin (100%), gentamicin (47.8%), amikacin (86.6%), cotrimoxazole (100%), tetracycline (85.1%), and ciprofloxacin (86.6%). Thus, the VPKP isolates were resistant to three or more distinct antimicrobial classes. None of the 95 VIM-negative isolates exhibited either resistance or decreased susceptibility to carbapenems (imipenem MICs ranged from 0.064 to 0.5 μg/ml). Nevertheless, 17 (17.9%) of the latter isolates produced SHV-5-type ESBL and displayed resistance to broad-spectrum cephalosporins and aztreonam. The remaining 78 isolates were susceptible to newer β-lactams. Fluoroquinolone resistance was also common in the ESBL-positive group and the respective rate (83.3%) was comparable with that of VIM producers. The VPKP isolates belonged to 12 distinct PFGE types, and several of these types have invaded all three participating hospitals. Interestingly, similar PFGE types were observed among VIM-positive carbapenem-resistant, VIM-positive carbapenem-susceptible, and VIM-negative isolates. Detailed characteristics of the isolates, with regard to PFGE types, bla genes, and presence of integrons, have been described previously (24).
Treatment.
Of 67 patients infected with VIM-positive isolates, 49 (73.1%) received appropriate empirical therapy, and 18 (26.9%) received inappropriate therapy. Of those who received appropriate therapy, 12 received combination therapy with two active drugs (9 received meropenem and 3 received imipenem along with colistin [8 patients] or an active aminoglycoside [4 patients]), and 37 received therapy with one active drug (9 meropenem, 5 imipenem, 15 colistin, and 8 an active aminoglycoside). Among the 95 patients infected with a VIM-negative organism, 84 received appropriate empirical therapy and 11 inappropriate. Of note, the patients infected with an organism for which the MICs of both imipenem and meropenem were >4 μg/ml were more likely (OR, 4.08; 95% CI, 1.29 to 12.85; P = 0.02) to have received inappropriate empirical therapy.
Outcome.
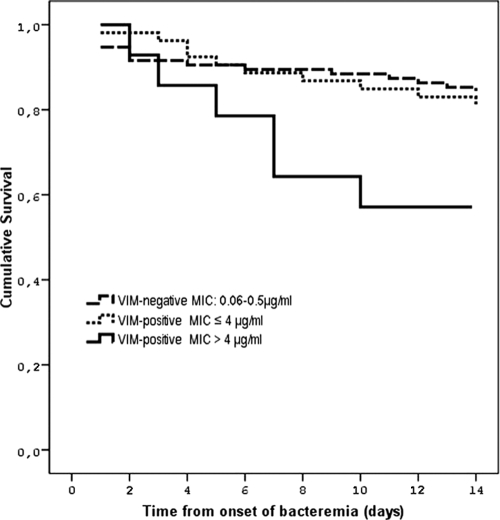
The all-cause 14-day mortality was 19.1%: 15.8% (15 of 95 patients died) for the patients infected with a VIM-negative organism, 18.9% (10 of 53 patients died) for the patients infected with a VIM-positive carbapenem-susceptible organism, and 42.9 (6 of 14 patients died) for the patients infected with a VIM-positive carbapenem-resistant organism. The mortality rates for the patients who received appropriate or inappropriate empirical therapy were 16.5% (22/133) and 31% (9/29), respectively. Mortality rates, according to the empirical treatment regimen, for the patients infected with VIM-positive organisms were as follows: 8.3% (1/12) for those who received combination therapy with two active drugs, 27% (10/37) for those who received therapy with one active drug, 27.8% (5/18) for those who received inappropriate empirical therapy, and 28.6% (4/14) for inappropriate definitive therapy. Table 1 presents the univariate analysis of host-, infection-, and treatment-related factors with their potential effects on mortality. As shown, VIM or ESBL production had no effect on mortality, whereas resistance to carbapenems (MICs of imipenem and meropenem of >4 μg/ml) had a significant impact on 14-day mortality. Also the patients with polymicrobial bacteremia and a Pitt bacteremia score of ≥4 were associated with increased mortality. Patient age, the severity of the underlying disease, the source of the bacteremia, and inappropriate empirical or definitive therapy had a trend for higher mortality. By entering in the Cox regression model all of the host- and infection-related variables for which the P values were ≤0.10 in the univariate analysis, an MIC of carbapenems of >4 μg/ml (HR, 2.83; 95% CI, 1.08 to 7.41; P = 0.035], age (HR, 1.03; 95% CI, 1.01 to 1.06; P = 0.021), and rapidly fatal underlying disease (HR, 2.84; 95% CI, 1.26 to 6.39; P = 0.012) were associated with 14-day mortality (Table 2, model 1). After adjustment for “inappropriate empirical treatment” or “inappropriate definite treatment,” the effect of carbapenem resistance on 14-day mortality was reduced to a level of nonsignificance, while the HRs and the P values of the other variables remained essentially unchanged (Table 2, model 2). Figure 1 shows the survival probability according to VIM production and the susceptibilities of the infecting organisms to carbapenems. The patients infected with an organism for which the MICs of both imipenem and meropenem were >4 μg/ml were at greater risk to die than those infected with a VIM-positive carbapenem-susceptible or VIM-negative organism (log rank = 6.27, P = 0.044).
TABLE 1.
Univariate analysis of factors associated with all-cause 14-day mortality for 162 patients with K. pneumoniae bloodstream infections
| Variable | No. of patients/total (%)
|
OR (95% CI) | P | |
|---|---|---|---|---|
| Survived (n = 131) | Died (n = 31) | |||
| Host-related | ||||
| Mean age (yr) ± SD | 59.01 ± 18.16 | 65.74 ± 16.94 | 1.02 (1.00-1.05) | 0.06 |
| Gender | ||||
| Male | 85/103 (82.5) | 18/103 (17.5) | 0.75 (0.34-1.67) | 0.48 |
| Female | 46/59 (78.0) | 13/59 (22.0) | ||
| Comorbidities | ||||
| ≥2 | 61/79 (77.2) | 18/79 (22.8) | 1.59 (0.72-3.51) | 0.25 |
| <2 | 70/83 (84.3) | 13/83 (15.7) | ||
| Rapidly fatal underlying disease | ||||
| Yes | 23/33 (68.7) | 10/33 (30.3) | 2.23 (0.93-5.38) | 0.07 |
| No | 108/129 (83.7) | 21/129 (16.3) | ||
| Pitt bacteremia score | ||||
| ≥4 | 51/71 (71.8) | 20/71 (28.2) | 2.85 (1.26-6.45) | 0.01 |
| <4 | 80/91 (87.9) | 11/91 (12.1) | ||
| Infection related | ||||
| ESBL production | ||||
| Yes | 50/62 (80.6) | 12/62 (19.4) | 1.02 (0.46-2.29) | 0.95 |
| No | 81/100 (81.0) | 19/100 (19.0) | ||
| VIM-1 production | ||||
| Yes | 51/67 (76.1) | 16/67 (23.9) | 1.67 (0.76-3.68) | 0.20 |
| No | 80/95 (84.2) | 15/95 (15.8) | ||
| MIC of carbapenems | ||||
| >4 μg/mla | 8/14 (57.1) | 6/14 (42.9) | 3.69 (1.18-11.57) | 0.02 |
| ≤4 μg/ml | 123/148 (83.1) | 25/148 (16.9) | ||
| Source of bacteremia | ||||
| Nonurinary | 110/140 (78.6) | 30/140 (21.4) | 5.73 (0.74-44.32) | 0.09 |
| Urinary | 21/22 (95.5) | 1/22 (4.5) | ||
| Polymicrobial bacteremia | ||||
| Yes | 23/34 (67.6) | 11/34 (32.4) | 2.58 (1.09-6.12) | 0.03 |
| No | 108/128 (84.4) | 20/128 (15.6) | ||
| Treatment related | ||||
| Appropriate empirical therapy | ||||
| No | 20/29 (69.0) | 9/29 (31.0) | 2.27 (0.91-5.64) | 0.08 |
| Yes | 111/133 (83.5) | 22/133 (16.5) | ||
| Appropriate definitive therapy | ||||
| No | 17/25 (68) | 8/25 (32) | 2.33 (0.90-6.05) | 0.08 |
| Yes | 114/137 (83.2) | 23/137 (16.8) | ||
The MICs of both imipenem and meropenem were >4 μg/ml.
TABLE 2.
Cox regression analysis of factors associated with all-cause 14-day mortality
| Variable | Model 1
|
Model 2
|
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.03 (1.01-1.06) | 0.021 | 1.03(1.00-1.05) | 0.028 |
| MIC of carbapenems >4 μg/ml | 2.83 (1.08-7.41) | 0.035 | 2.34 (0.83-6.55) | 0.106 |
| Polymicrobial bacteremia | 1.45 (0.65-3.21) | 0.360 | 1.52 (0.69-3.36) | 0.300 |
| Rapidly fatal underlying disease | 2.84 (1.26-6.39) | 0.012 | 2.9 (1.29-6.49) | 0.010 |
| Non-urinary source of bacteremia | 3.33 (0.44-25.05) | 0.242 | 3.46 (0.46-26.00) | 0.23 |
| Pitt bacteremia score ≥4 | 2.1 (0.91-4.81) | 0.08 | 1.96 (0.85-4.52) | 0.114 |
| Inappropriate empiricala therapy | 1.63 (0.68-3.86) | 0.270 | ||
Similar results were obtained when the variable “inappropriate definitive therapy” was included in model 2.
FIG. 1.
Kaplan-Meier curves of survival probability of patients with K. pneumoniae bloodstream infections according to susceptibility of the infecting organism to carbapenems. Patients infected with a VIM-positive organism for which the MICs of both imipenem and meropenem were >4 μg/ml were more likely to die than those infected with a VIM-positive carbapenem-susceptible or VIM-negative organism (log rank = 6.27, P = 0.044).
DISCUSSION
A significant proportion (41.4%) of nosocomial and healthcare-associated K. pneumoniae BSIs in our geographic region is caused by VIM-producing organisms. Overall, the mortality of patients infected with VPKP was not significantly different from that observed in patients infected with non-VPKP. The subgroup of patients, however, infected with VIM-positive carbapenem-resistant organisms had significantly higher mortality compared to patients infected with carbapenem-susceptible organisms. More importantly, carbapenem resistance remained an independent predictor of adverse outcome even after adjusting for a number of host- and infection-related factors.
The effect of carbapenem resistance on mortality was probably mediated by the failure to provide effective antimicrobial therapy. Indeed, by including in the statistical model the variables “inappropriate empirical or definitive treatment” the effect of carbapenem resistance on mortality was reduced to a level of nonsignificance. This hypothesis was further supported by the fact that the patients infected with carbapenem-resistant VPKP had four times increased risk to receive inappropriate empirical treatment which presumably, as has been suggested by several investigators (1, 12), led to worse outcome. Another factor that might have influenced the outcome of patients infected with carbapenem-resistant VPKP could be an enhanced virulence of the resistant organisms. The PFGE analysis of the organisms, however, argues against this hypothesis. As has been reported previously, the VPKP isolates were distributed into a relatively large number of chromosomal types, most of which included both carbapenem-resistant and -susceptible organisms (24). Moreover, no particular clone was associated with increased mortality.
The importance of carbapenem resistance on outcome of patients with K. pneumoniae BSIs had also been indicated in a retrospective study involving a limited number of patients (5). Other investigators have also underscored the clinical importance of carbapenem resistance in infections caused by K. pneumoniae producing KPC carbapenemases (27). Notably, while antimicrobial resistance was mediated by different carbapenemases in the latter report, the case-fatality rate was strikingly similar with that observed in our patients infected with VIM-producing carbapenem-resistant organisms.
While VPKP become increasingly prevalent and cause serious infections resulting in high fatality rates, the optimal treatment of the patients infected with such organisms is currently unknown. In the present study, the lowest mortality (8.3%) was observed in the group of patients who received combination therapy with two active drugs, one of which was a carbapenem and the other either colistin or an active aminoglycoside, whereas therapy with one active drug (a carbapenem, colistin, or an aminoglycoside) resulted in mortality rate similar to that observed in patients who received inappropriate therapy (no active drug). In a recent report, 12 of 17 patients infected with VIM-producing organisms were treated successfully with colistin, either alone or in combination with a carbapenem or an active aminoglycoside (29). The data presented here do not allow us to accurately state whether carbapenems can be used against VPKP; the data do suggest, however, that carbapenems in combination with another active agent (colistin or an aminoglycoside) may provide some therapeutic benefit against VIM-positive carbapenem-susceptible organisms. In this respect, the issue of either reporting such isolates as fully resistant to carbapenems or to consider the respective MICs at face value should remain open.
The present study has several limitations. First, it was an observational study of consecutive patients with VPKP and non-VPKP BSIs without matched controls. Thus, several variables with potential effect on mortality might have been unequally distributed between the patients infected with VIM-positive and with VIM-negative organisms. Although a multivariate analysis was performed to account for bias associated with these variables, several confounding effects might not have been completely controlled for in the statistical model. Second, the patients infected with VPKP for which the MICs of carbapenems were in the susceptible range and treated with a carbapenem were arbitrarily assigned in the “appropriate therapy” group. Although this classification might have influenced the results, our decision was based on the lack of clinical data and on the absence of any official guidelines about what VIM production entails in terms of antimicrobial chemotherapy in patients infected with such organisms. Finally, the study may not have sufficient statistical power to detect an association between VIM production and mortality.
In conclusion, in patients with K. pneumoniae BSIs, carbapenem resistance, advanced age, and severity of underlying disease were independent predictors of adverse outcome, whereas VIM production had no impact on mortality. The higher mortality observed in patients infected with VIM-positive carbapenem-resistant organisms was probably mediated by the failure to provide effective antimicrobial therapy. As VPKP emerges as a life-threatening pathogen, it is critical to develop diagnostic and therapeutic strategies to ensure timely and effective treatment of infections caused by such organisms. More importantly, it is an urgent need to implement enhanced infection control measures to contain their dissemination.
Acknowledgments
This study was partially supported by a grant from the Hellenic Center for Disease Control and Prevention (KEELPNO).
Footnotes
Published ahead of print on 17 February 2009.
REFERENCES
- 1.Anderson, D. J., J. J. Engemann, L. J. Harrell, Y. Carmeli, L. B. Reller, and K. S. Kaye. 2006. Predictors of mortality in patients with bloodstream infection due to ceftazidime-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 50:1715-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush, K. 2001. New beta-lactamases in gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin. Infect. Dis. 32:1085-1089. [DOI] [PubMed] [Google Scholar]
- 3.Conceição, T., A. Brizio, A. Duarte, and R. Barros. 2005. First isolation of blaVIM-2 in Klebsiella oxytoca clinical isolates from Portugal. Antimicrob. Agents Chemother. 49:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornaglia, G., A. Mazzariol, L. Lauretti, G. M. Rossolini, and R. Fontana. 2000. Hospital outbreak of carbapenem-resistant Pseudomonas aeruginosa producing VIM-1, a novel transferable metallo-β-lactamase. Clin. Infect. Dis. 31:1119-1125. [DOI] [PubMed] [Google Scholar]
- 5.Daikos, G. L., A. Karabinis, E. Paramythiotou, V. P. Syriopoulou, C. Kosmidis, A. Avlami, P. Gargalianos, K. Tzanetou, D. Petropoulou, and H. Malamou-Lada. 2007. VIM-1-producing Klebsiella pneumoniae bloodstream infections: analysis of 28 cases. Int. J. Antimicrob. Agents 29:471-473. [DOI] [PubMed] [Google Scholar]
- 6.Friedman, N. D., K. S. Kaye, J. E. Stout, S. A. McGarry, S. L. Trivette, J. P. Briggs, W. Lamm, C. Clark, J. MacFarquhar, A. L. Walton, L. B. Reller, and D. J. Sexton. 2002. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 137:791-797. [DOI] [PubMed] [Google Scholar]
- 7.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control. 16:128-140. [DOI] [PubMed] [Google Scholar]
- 8.Giakkoupi, P., A. Xanthaki, M. Kanelopoulou, A. Vlahaki, V. Miriagou, S. Kontou, E. Papafraggas, H. Malamou-Lada, L. S. Tzouvelekis, N. J. Legakis, and A. C. Vatopoulos. 2003. VIM-1 metallo-β-lactamase-producing Klebsiella pneumoniae strains in Greek hospitals. J. Clin. Microbiol. 41:3893-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirakata, Y., T. Yamaguchi, M. Nakano, K. Izumikawa, M. Mine, S. Aoki, A. Kondoh, J. Matsuda, M. Hirayama, K. Yanagihara, Y. Miyazaki, K. Tomono, Y. Yamada, S. Kamihira, and S. Kohno. 2003. Clinical and bacteriological characteristics of IMP-type metallo-β-lactamase-producing Pseudomonas aeruginosa. Clin. Infect. Dis. 37:26-32. [DOI] [PubMed] [Google Scholar]
- 10.Ito, H., Y. Arakawa, S. Ohsuka, R. Wacharotayankun, N. Kato, and M. Ohta. 1995. Plasmid-mediated dissemination of the metallo-β-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob. Agents Chemother. 39:824-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacoby, G. A., and L. S. Munoz-Price. 2005. The new beta-lactamases. N. Engl. J. Med. 352:380-391. [DOI] [PubMed] [Google Scholar]
- 12.Kollef, M. H. 2000. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin. Infect. Dis. 31(Suppl. 4):S131-S138. [DOI] [PubMed] [Google Scholar]
- 13.Lartigue, M. F., L. Poirel, and P. Nordmann. 2004. First detection of a carbapenem-hydrolyzing metalloenzyme in an Enterobacteriaceae isolate in France. Antimicrob. Agents Chemother. 48:4929-4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laupland, K. B., M. D. Parkins, D. L. Church, D. B. Gregson, T. J. Louie, J. M. Conly, S. Elsayed, and J. D. Pitout. 2005. Population-based epidemiological study of infections caused by carbapenem-resistant Pseudomonas aeruginosa in the Calgary Health Region: importance of metallo-β-lactamase (MBL)-producing strains. J. Infect. Dis. 192:1606-1612. [DOI] [PubMed] [Google Scholar]
- 15.Loli, A., L. S. Tzouvelekis, E. Tzelepi, A. Carattoli, A. C. Vatopoulos, P. T. Tassios, and V. Miriagou. 2006. Sources of diversity of carbapenem resistance levels in Klebsiella pneumoniae carrying blaVIM-1. J. Antimicrob. Chemother. 58:669-672. [DOI] [PubMed] [Google Scholar]
- 16.Luzzaro, F., J. D. Docquier, C. Colinon, A. Endimiani, G. Lombardi, G. Amicosante, G. M. Rossolini, and A. Toniolo. 2004. Emergence in Klebsiella pneumoniae and Enterobacter cloacae clinical isolates of the VIM-4 metallo-β-lactamase encoded by a conjugative plasmid. Antimicrob. Agents Chemother. 48:648-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCabe, W. R., and G. G. Jackson. 1962. Gram-negative bacteremia. I. Etiology and ecology. Arch. Intern. Med. 110:847-855. [Google Scholar]
- 18.Miriagou, V., E. Tzelepi, D. Gianneli, and L. S. Tzouvelekis. 2003. Escherichia coli with a self-transferable, multiresistant plasmid coding for metallo-β-lactamase VIM-1. Antimicrob. Agents Chemother. 47:395-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NCCLS. 2004. Performance standards for antimicrobial susceptibility testing; 14th informational supplement, vol. M100-S14. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 20.Nordmann, P., and L. Poirel. 2002. Emerging carbapenemases in gram-negative aerobes. Clin. Microbiol. Infect. 8:321-331. [DOI] [PubMed] [Google Scholar]
- 21.Paterson, D. L., W. C. Ko, A. Von Gottberg, S. Mohapatra, J. M. Casellas, H. Goossens, L. Mulazimoglu, G. Trenholme, K. P. Klugman, R. A. Bonomo, L. B. Rice, M. M. Wagener, J. G. McCormack, and V. L. Yu. 2004. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum β-lactamase production in nosocomial infections. Ann. Intern. Med. 140:26-32. [DOI] [PubMed] [Google Scholar]
- 22.Peleg, A. Y., C. Franklin, J. M. Bell, and D. W. Spelman. 2005. Dissemination of the metallo-β-lactamase gene blaIMP-4 among gram-negative pathogens in a clinical setting in Australia. Clin. Infect. Dis. 41:1549-1556. [DOI] [PubMed] [Google Scholar]
- 23.Poirel, L., J. N. Pham, L. Cabanne, B. J. Gatus, S. M. Bell, and P. Nordmann. 2004. Carbapenem-hydrolysing metallo-β-lactamases from Klebsiella pneumoniae and Escherichia coli isolated in Australia. Pathology 36:366-367. [DOI] [PubMed] [Google Scholar]
- 24.Psichogiou, M., P. T. Tassios, A. Avlamis, I. Stefanou, C. Kosmidis, E. Platsouka, O. Paniara, A. Xanthaki, M. Toutouza, G. L. Daikos, and L. S. Tzouvelekis. 2008. Ongoing epidemic of blaVIM-1-positive Klebsiella pneumoniae in Athens, Greece: a prospective survey. J. Antimicrob. Chemother. 61:59-63. [DOI] [PubMed] [Google Scholar]
- 25.Queenan, A. M., and K. Bush. 2007. Carbapenemases: the versatile beta-lactamases. Clin. Microbiol. Rev. 20:440-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riccio, M. L., L. Pallecchi, R. Fontana, and G. M. Rossolini. 2001. In70 of plasmid pAX22, a blaVIM-1-containing integron carrying a new aminoglycoside phosphotransferase gene cassette. Antimicrob. Agents Chemother. 45:1249-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwaber, M. J., S. Klarfeld-Lidji, S. Navon-Venezia, D. Schwartz, A. Leavitt, and Y. Carmeli. 2008. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob. Agents Chemother. 52:1028-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scoulica, E. V., I. K. Neonakis, A. I. Gikas, and Y. J. Tselentis. 2004. Spread of blaVIM-1-producing Escherichia coli in a university hospital in Greece: genetic analysis of the integron carrying the blaVIM-1 metallo-β-lactamase gene. Diagn. Microbiol. Infect. Dis. 48:167-172. [DOI] [PubMed] [Google Scholar]
- 29.Souli, M., F. V. Kontopidou, E. Papadomichelakis, I. Galani, A. Armaganidis, and H. Giamarellou. 2008. Clinical experience of serious infections caused by Enterobacteriaceae producing VIM-1 metallo-β-lactamase in a Greek University Hospital. Clin. Infect. Dis. 46:847-854. [DOI] [PubMed] [Google Scholar]
- 30.Tzelepi, E., C. Magana, E. Platsouka, D. Sofianou, O. Paniara, N. L. Legakis, A. C. Vatopoulos, and L. S. Tzouvelekis. 2003. Extended-spectrum β-lactamase types in Klebsiella pneumoniae and Escherichia coli in two Greek hospitals. Int. J. Antimicrob. Agents 21:285-288. [DOI] [PubMed] [Google Scholar]
- 31.Walsh, T. R., M. A. Toleman, L. Poirel, and P. Nordmann. 2005. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:302-306. [DOI] [PMC free article] [PubMed] [Google Scholar]