Abstract
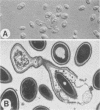
Of 13 clinical isolates of Candida lusitaniae from diverse geographical regions, 7 represented the mating types (6 alpha, 1 a) of the ascomycete Clavispora lusitaniae. Selected nonfertile isolates showed significant DNA relatedness (greater than 90%) to representatives of both mating types. Phenotypic physiological characteristics, such as cellobiose fermentation and rhamnose assimilation, proved insufficient for separation of Clavispora lusitaniae and Clavispora opuntiae.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahearn D. G., McGlohn M. S. In vitro susceptibilities of sucrose-negative Candida tropicalis, Candida lusitaniae, and Candida norvegensis to amphotericin B, 5-fluorocytosine, miconazole, and ketoconazole. J Clin Microbiol. 1984 Mar;19(3):412–416. doi: 10.1128/jcm.19.3.412-416.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinkhorn R. J., Adelstein D., Spagnuolo P. J. Emergence of a new opportunistic pathogen, Candida lusitaniae. J Clin Microbiol. 1989 Feb;27(2):236–240. doi: 10.1128/jcm.27.2.236-240.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield T. L., Smith M. B., Winn R. E., Rinaldi M. G., Guerra C. Mycoses caused by Candida lusitaniae. Rev Infect Dis. 1987 Sep-Oct;9(5):1006–1012. doi: 10.1093/clinids/9.5.1006. [DOI] [PubMed] [Google Scholar]
- Holzschu D. L., Presley H. L., Miranda M., Phaff H. J. Identification of Candida lusitaniae as an opportunistic yeast in humans. J Clin Microbiol. 1979 Aug;10(2):202–205. doi: 10.1128/jcm.10.2.202-205.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Meyer S. A., Phaff H. J. Deoxyribonucleic acid base composition in yeasts. J Bacteriol. 1969 Jan;97(1):52–56. doi: 10.1128/jb.97.1.52-56.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappagianis D., Collins M. S., Hector R., Remington J. Development of resistance to amphotericin B in Candida lusitaniae infecting a human. Antimicrob Agents Chemother. 1979 Aug;16(2):123–126. doi: 10.1128/aac.16.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues de Miranda L. Clavispora, a new yeast genus of the Saccharomycetales. Antonie Van Leeuwenhoek. 1979;45(3):479–483. doi: 10.1007/BF00443285. [DOI] [PubMed] [Google Scholar]
- Schlitzer R. L., Ahearn D. G. Characterization of atypical Candida tropicalis and other uncommon clinical yeast isolates. J Clin Microbiol. 1982 Mar;15(3):511–516. doi: 10.1128/jcm.15.3.511-516.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Mandel M. Quantitative aspects of deoxyribonucleic acid renaturation: base composition, state of chromosome replication, and polynucleotide homologies. J Bacteriol. 1971 May;106(2):608–614. doi: 10.1128/jb.106.2.608-614.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]