Abstract
LB80380, a dipivoxil ester prodrug of LB80331 (metabolite, LB80317), is a novel antiviral agent for chronic hepatitis B (CHB). The pharmacokinetics of LB80331/LB80317 were evaluated in two clinical studies and a study with mice. The clinical studies were dose-escalating pharmacokinetic studies with six healthy subjects per single-dose group and six CHB patients per repeated-dose group. The mouse study was designed to measure the amounts of the phosphorylated portions of LB80331 and LB80317 in the liver. In healthy subjects receiving a single dose of LB80380, the plasma level of LB80331 increased as the dose increased. Although a high-fat diet delayed the time to the maximum concentration in plasma (Tmax) of LB80331, the area under the concentration-time curve from time zero to infinity was similar between the subjects in the fasted group and those in the group who consumed a high-fat diet. In CHB patients, the mean Tmax of LB80331 was 1.0 to 2.0 h postdosing at steady state. The steady-state plasma concentration of LB80331 declined in a monoexponential manner, and the apparent elimination half-life was 2.5 to 3.3 h. The steady-state plasma concentration of LB80317 was maximum at 3 to 8 h postdoing and declined in a monoexponential manner; the apparent elimination half-life was 45 to 62 h at the 30- to 240-mg doses, while LB80317 was measurable in plasma only at higher doses of 120 and 240 mg after the administration of the first dose of LB80380. Forty percent of the amount of LB80331/LB80317 in the mouse liver was detected as the phosphorylated form. In conclusion, LB80380 is rapidly absorbed and converted to LB80331. LB80317 has a long half-life at steady-state, supporting the use of a once-daily dosing regimen. The ingestion of a high-fat diet delays the rate of absorption of LB80380 without affecting the extent of absorption.
Chronic hepatitis B (CHB) is a global endemic disease, and an estimated 400 million people are infected worldwide. The majority of these patients are in Asia. Twenty-five to 40% of these chronically infected people eventually develop hepatocellular carcinoma and cirrhosis-related complications (8). All these facts make the treatment of CHB extremely important.
To date, six drugs are approved for use for the treatment of CHB. They are divided into two main categories: nucleoside/nucleotide analogues (NAs; lamivudine, adefovir dipivoxil, entecavir, telbivudine) and immunomodulators (alpha interferon and pegylated alpha interferon). Two nucleoside analogues will soon be available, namely, clevudine, which is already approved for use in South Korea, and tenofovir disoproxil fumarate, which has been approved for use in some countries. However, there are some intrinsic limitations to all NAs. The prolonged use of lamivudine is associated with a high rate of emergence of lamivudine-resistant hepatitis B virus (HBV) (13). Although adefovir dipivoxil is associated with a lower chance of the emergence of resistance, there is potential renal toxicity with prolonged treatment (3). Entecavir and telbivudine are the more potent NAs (1, 6, 9). Although entecavir is associated with a very low chance of the emergence of resistance (1.2% at 5 years), the chance of entecavir resistance is considerably higher in lamivudine-resistant patients (11). Recently, a warning of the potential for mutant formation in patients coinfected with HBV and human immunodeficiency virus (HIV) has been made (4). Telbivudine is associated with resistance rates of 8.6% and 21.6% at 2 years of treatment for HBV e antigen (HBeAg)-positive and HBeAg-negative CHB patients, respectively (5). It is clear that there is still an unmet need for the treatment of CHB by the development of newer antiviral agents.
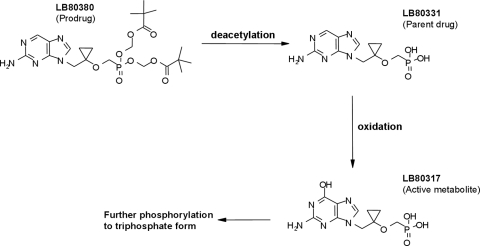
LB80380 is an oral nucleotide prodrug and is chemically similar to adefovir dipivoxil and tenofovir disoproxil fumarate. LB80380 is rapidly converted to the parent drug, LB80331, by the removal of the two pivaloyl groups (deacetylation) in the liver and intestine. LB80331 is further metabolized to LB80317 (by oxidation of the nucleoside base at the 6 position), a nucleotide analogue of GMP (Fig. 1). This is mediated through enzyme oxidases, such as aldehyde oxidase and xanthine oxidase. LB80317 is the active metabolite with the antiviral effect. After phosphorylation to the di- and triphosphate forms, the molecule inhibits viral replication following incorporation into viral DNA. The main route of excretion is via the urinary system (which accounts for approximately 80% of the drug excretion).
FIG. 1.
Chemical structure and conversion of LB80380 to its active metabolite.
It has been shown that LB80380 is effective in suppressing HBV replication in treatment-naïve and lamivudine-resistant CHB patients (7, 12). In those two studies, the dose chosen for clinical development was between 30 mg and 240 mg daily. In an in vitro study, LB80380 was also shown to be effective against HBV strains resistant to lamivudine, adefovir, entecavir, and telbivudine (10). Therefore, the development of this new drug is important, as it will provide complementary coverage against HBV resistant to current antiviral agents which are given to CHB patients on a long-term basis.
We report here the results of three studies examining the pharmacokinetics of LB80380. The first and second studies (studies BVCL001 and BVCL002, respectively) were performed with humans. Study BVCL001 (a phase I, double-blind, placebo-controlled study of the antiviral agent with healthy male subjects) was performed to determine the safety, tolerability, and pharmacokinetics of ascending single oral doses of LB80380 and incorporated a comparison of the pharmacokinetics of the drug in the fed and fasted states. Study BVCL002 (a phase I/II, double-blind, randomized, placebo-controlled study with patients with CHB) was performed to determine the safety and efficacy of 4 weeks of treatment with escalating doses of LB80380. The antiviral properties and safety profile determined in study BVCL002 were reported previously (12). The third study determined the amount of phosphorylation of LB80331 and LB80317 in mouse livers, an experimental model with which studies can be performed without great technical difficulty, although the findings for the mouse liver may not be completely analogous to the human liver.
MATERIALS AND METHODS
Single-dose study with healthy volunteers.
LB80380 was orally administered to healthy male subjects at doses of 10, 30, 60, 120, 240, and 480 mg. Among the subjects in each dose cohort, six subjects received LB80380 and two subjects received placebo. The safety data from each cohort were monitored and were analyzed prior to escalation of the dose. The subjects were fasted overnight, and a witness confirmed that they took the drug. Blood samples were taken from all subjects predosing and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, and 24 h postdosing to measure the plasma LB80331 level after the oral administration of LB80380. After completion of the dose escalation study, the cohort taking the 60-mg dose was assigned to receive an additional oral dose of 60 mg LB80380 after the ingestion of a high-fat meal to determine the effect of food on the absorption of LB80331. The meal provided to the subjects was an FDA-recommended high-fat and high-calorie meal composed of, for example, two fried eggs, two slices of toasted bread spread with butter, two strips of bacon, 4 oz of hash brown potatoes, and 8 oz of whole milk. All subjects provided written informed consent, and the study protocol was approved by the Covance Clinical Research Unit Independent Review Board, United Kingdom.
Single- and multiple-dose studies with CHB patients.
Four consecutive cohorts were assigned to receive four doses of LB80380 (30 mg, 60 mg, 120 mg, and 240 mg daily) in a dose-escalating manner. Seven patients were recruited in each cohort; by computer allocation, six patients were randomized to receive LB80380 at the respective dose and one was randomized to receive placebo. The treatment was given for a total of 4 weeks. Blood samples were taken from all patients at the baseline and during steady-state administration (between weeks 2 and 4) to measure the plasma LB80331 and LB80317 levels. During steady-state administration, the samples were obtained predosing and at 0.5, 1, 2, 3, 4, 6, 8, and 24 h postdosing.
Patients with the following criteria were eligible for study entry: 18 to 65 years of age; HBV surface antigen (HBsAg) positive for at least 6 months; HBeAg positive for at least 1 month; HBV DNA level of ≥1 × 107 copies/ml, as measured by the Cobas Amplicor HBV Monitor test (Roche Diagnostics, Branchburg, NJ); and a serum alanine aminotransferase (ALT) level less than five times the upper limit of normal (ULN).
Patients with any one of the following conditions were excluded: coinfection with hepatitis C or D virus or HIV; pregnancy or breast-feeding; receipt of NAs or any other treatment, including immunomodulatory agents or corticosteroids, for HBV infection within 6 months prior to study entry; receipt of drugs with potential nephrotoxicity or hepatotoxicity within 6 months prior to study entry; evidence of decompensated liver disease; hemoglobin level of <9.0 g/dl; neutrophil count of <1.5 × 109/liter; platelet count of <100 × 109/liter; serum creatinine level of >133 μmol/liter; serum amylase concentration of >165 U/liter (1.5 times the ULN); serum lipase concentration of >304 U/liter (1.5 times the ULN); an alpha-fetoprotein level of >20 ng/ml with evidence of hepatocellular carcinoma by imaging; a history of severe allergic disease; or illicit drug or alcohol abuse.
The demographic data, serum ALT levels, and HBV DNA levels for each cohort are listed in Table 1. All patients were ethnic Asians who were HBsAg and HBeAg positive. There were no significant differences in any of the parameters among the groups. The antiviral efficacies of different doses of LB80380 were reported previously (12).
TABLE 1.
Demographic data for CHB patients in multiple-dose study
| Characteristic | Value for subjects receiving LB80380 doses ofa:
|
|||
|---|---|---|---|---|
| 30 mg | 60 mg | 120 mg | 240 mg | |
| Age (yr) | 34.3 (19.6-54.6) | 31.2 (19.9-44.6) | 29.3 (20.5-42.5) | 23.9 (18.1-29.1) |
| Sex (no. of men:no. of women) | 5:1 | 5:1 | 4:2 | 4:2 |
| Body wt (kg) | 64.1 (10.2) | 61.1 (9.5) | 62.8 (18.2) | 61.2 (8.2) |
| HBV DNA levels (log no. of copies/ml) | 8.6 (7.6-9.7) | 8.7 (6.8-9.4) | 8.7 (7.4-9.0) | 9.1 (7.2-10.1) |
| ALT level (U/ml) | 79.5 (50-100) | 68.7 (22-165) | 34.5 (22-96) | 27 (20-36) |
All continuous variables except body weight are expressed as medians (ranges); body weight data are expressed as means (standard deviations). The data are for six subjects in each dosing group.
All patients provided written informed consent; and the trial was approved by the Institutional Review Board, The University Hong Kong, Queen Mary Hospital, Hong Kong.
Tissue distribution study with mice.
LB80380 dissolved in 100% polyethylene glycol 400 was orally administered to male CD-1 mice at a dose of 30 mg/kg of body weight. Blood and liver tissue samples were collected from three mice per time point at 1, 2, 4, 6, 10, 24, and 36 h postdosing. The plasma was kept frozen after it was separated from the blood, and the liver tissue was snap-frozen by dipping it into liquid nitrogen. All procedures with mice were carried out in accordance with the current NIH guidelines regarding the care and use of laboratory animals and all applicable U.S. laws, regulations, and guidelines.
Liquid chromatography-tandem mass spectrometry analyses of biological samples.
The plasma LB80331 concentration in healthy subjects and CHB patients and the plasma LB80317 concentration in CHB patients only were measured by a validated liquid chromatography-tandem mass spectrometry method. The lower limits of quantitation were 5 and 10 ng/ml for LB80331 and LB80317, respectively, and the intra- and interassay precisions and accuracies were less than 10%.
LB80331, LB80317, and the internal standard (LB80335, a stable isotope of LB80331 and LB80317) were extracted from human plasma by the protein precipitation extraction method.
Mouse plasma and liver extracts were prepared by the protein precipitation method with perchloric acid. After neutralization of an extract with saturated ammonium bicarbonate solution, one aliquot was analyzed to measure the amounts of free LB80331 and LB80317. The other aliquot of the same sample was treated with alkaline phosphatase (calf intestinal phosphatase [CIP]) to catalyze the phosphorolysis of the di- and triphosphates of LB80331 and LB80317 and was subsequently analyzed to measure the total amounts of LB80331 and LB80317, including the di- and triphosphate forms.
Plasma (from both humans and mice) and tissue extracts (from mice) were analyzed with an Agilent model 1100 liquid chromatograph and a CTC HTS PAL autosampler with an MDS/Sciex API4000 triple-quadrupole mass spectrometer. After separation of the analytes from the plasma constituents with a Polaris C18-A analytical column (particle size, 3 μm; 4.6 by 50 mm), quantitation was carried out by monitoring the following mass transitions: m/z 300.1 to 136.1 (LB80331), m/z 316.1 to 152.2 (LB80317), and m/z 343.2 to 179.2 (LB80335). The lower limits of quantitation were 5.0 and 10.0 ng/ml for LB80331 and LB80317, respectively.
Pharmacokinetic analyses.
A noncompartmental model was used to analyze the pharmacokinetics of LB80331 and LB80317 after the administration of single and multiple doses of LB80380. The calculated pharmacokinetic parameters included the maximal concentration in plasma (Cmax); the time required to reach Cmax (Tmax); the area under the plasma-concentration curve (AUC) from time zero to infinity (AUC0-∞) or from time zero to 24 h (AUC0-24) for a single dose and at steady state, respectively; and the apparent half-life at the terminal phase (t1/2λ). If the mean value of the extrapolated AUC was more than 20% of the mean AUC0-24, the value of t1/2λ was marked to indicate that the value was calculated with insufficient sampling time points at the terminal phase. Oral clearance (CL/F) was calculated by dividing the dose (adjusted for the difference in the molecular weights of LB80380 and LB80331) by the AUC. The observed accumulation index was determined by calculation of the ratio of the AUC0-∞ during steady state and that at the baseline. The molar metabolic AUC ratio was calculated by dividing the AUC of LB80317 by that of LB80331 after adjustment for the difference in their molecular weights (by multiplying by 0.94921).
The amounts of phosphorylated LB80331 and LB80317 in mouse liver were obtained by subtraction of the amounts of free LB80331 and LB80317 (before treatment with CIP) from the amounts of total LB80331 and LB80317 (after treatment with CIP).
A nonlinear power model was used to assess dose proportionality (2). The proportionality relationship between each parameter and dose is described as a power function, y = a × doseb, where a is a constant and b is a proportionality constant. The dose proportionality was assessed from the 95% confidence interval for the exponent b, where b equal to 1 indicates the dose proportionality. For practical reasons, the model presented above is log10 linearized as log y = log a + b × log dose and is fitted by using the Proc Reg function in SAS software (SAS Institute Inc., Cary, NC). A dose-proportional relationship is concluded if the 95% confidence interval of the estimated mean slope (b) includes unity.
RESULTS
Single-dose pharmacokinetics in healthy volunteers.
The plasma concentration-time profiles of LB80331 after the administration of a single dose of LB80380 to healthy subjects at 10, 30, 60, 120, 240, and 480 mg are depicted in Fig. 2. The plasma LB80331 concentration increased as the dose increased, and the plasma profiles for the different dose groups were well separated from each other.
FIG. 2.
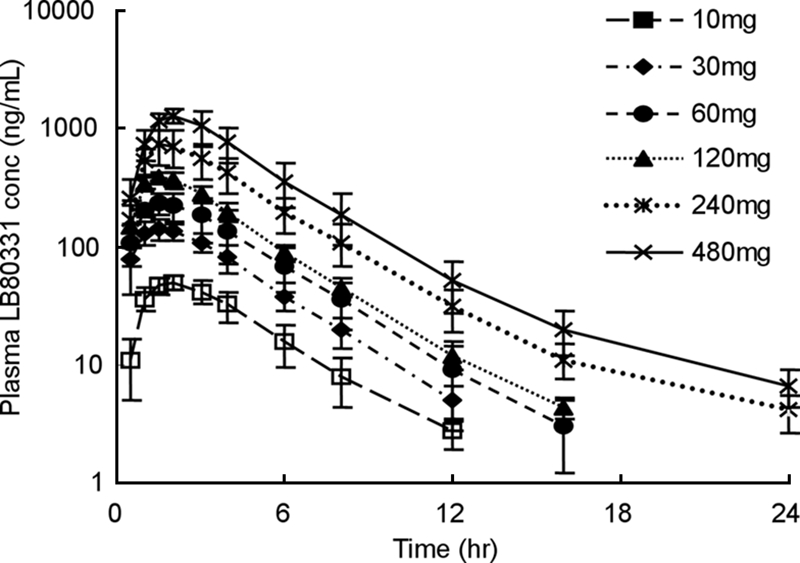
Mean plasma LB80331 concentration versus time following the administration of a single oral dose to healthy subjects during the dose escalation phase. Data are means ± standard deviations.
The pharmacokinetic profiles of LB80331 after the administration of a single 60-mg dose of LB80380 to subjects with and without a high-fat breakfast are depicted in Fig. 3. The mean Cmax for the subjects fasted overnight (286 ± 70 ng/ml) was only slightly higher than that of patients given a high-fat breakfast (246 ± 56 ng/ml). However, the ratio of the mean AUC0-∞ value for the fed group (AUCfed) to the mean AUC0-∞ value for the fasting group (AUCfasting) (metabolic AUC ratio) was close to unity (AUCfed/AUCfasting = 1.10). The median Tmax for subjects fed a high-fat breakfast was delayed compared to the Tmax for subjects fasted overnight (Tmaxs, 1.5 and 4.0 h postdosing, respectively).
FIG. 3.
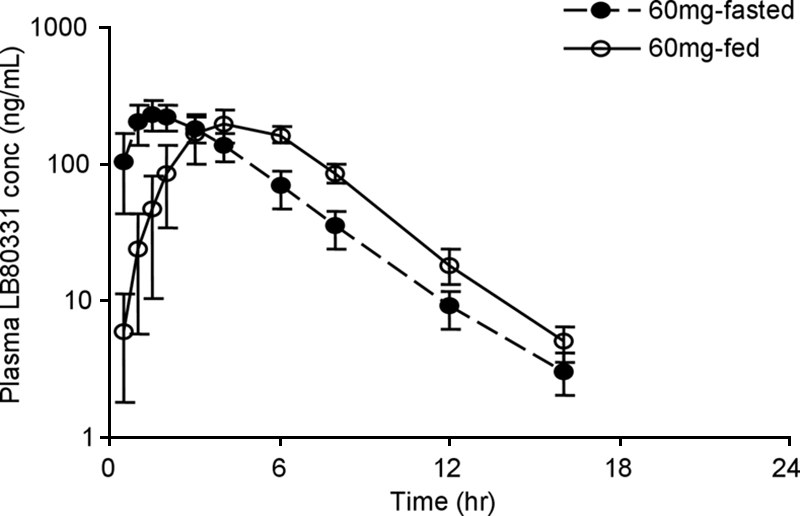
Mean plasma LB80331 concentration versus time following the administration of a single oral dose (60 mg) to healthy subjects after the consumption of a high-fat breakfast and fasting. Data are means ± standard deviations.
Pharmacokinetics in CHB patients after administration of single and multiple doses.
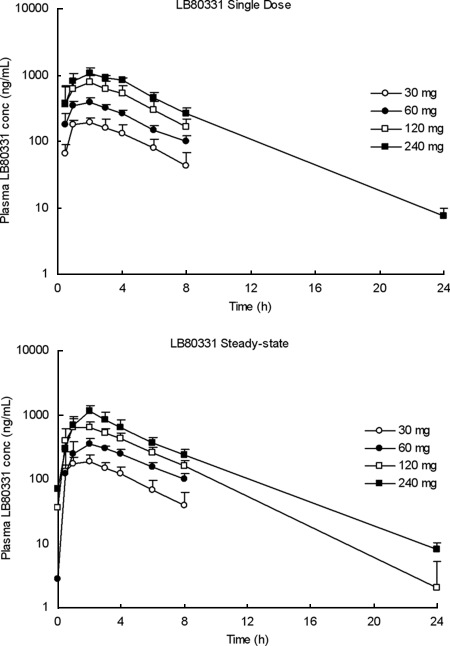
The plasma pharmacokinetic profiles of LB80331 and LB80317 were investigated over 24 h following administration of the initial single dose and at steady state (between weeks 2 and 4). The mean plasma concentration-time profiles of LB80331 in the four dose groups following administration of the initial single dose and at steady state are shown in Fig. 4. The prodrug, LB80380, was rapidly absorbed after oral administration and was converted to its parent drug, LB80331 (LB80380 was not detected in plasma). The median Tmaxs of LB80331 ranged from 1.0 to 2.0 h postdosing. Thereafter, the plasma LB80331 concentration declined in a monoexponential manner, and the mean values of t1/2 ranged from 2.5 to 3.3 h. At 24 h, the plasma concentration was generally below the limit of quantitation (5 ng/ml) except after administration of the highest daily dose of 240 mg.
FIG. 4.
Mean plasma LB80331 concentration profile in CHB patients following the administration of a oral single dose of LB80380 of 30, 60, 120, or 240 mg daily (day 1) and at steady state (weeks 2 to 4). Data are means ± standard deviations.
The intersubject variabilities (coefficients of variation) in Cmax and AUC were generally low and ranged from 9% to 35%. The intersubject variability in CL/F was 14%, when the values obtained following the administration of a single dose and those at steady state were compared. Following 2 to 4 weeks of daily oral administration of LB80380, the values for the apparent steady-state AUC and Cmax were similar to those obtained after the administration of a single dose, thus suggesting no progressive accumulation of LB80331 during the administration of multiple doses; the mean observed accumulation factor (observed accumulation index) was less than 11%.
For the single and multiple doses, the systemic exposure of LB80331 measured in terms of the AUC increased linearly with the dose up to 120 mg, and a slight deviation was shown at doses higher above 240 mg. For an eightfold dose increase from 30 to 240 mg, the mean Cmax and AUC values increased 5.4- and 5.9-fold, respectively, resulting in a dose-proportionality factor of about 0.7. The mean CL/F values for the lower three doses (30 mg, 60 mg, 120 mg) ranged from 244 to 304 ml/min, while those values for the 240-mg dose ranged from 363 to 416 ml/min following the administration of single and multiple doses. The pharmacokinetic parameters of LB80331 following the administration of single and multiple doses of LB80380 are summarized in Table 2.
TABLE 2.
Summary of pharmacokinetic parameters for LB80331 following administration of single and multiple doses of LB80380 in CHB patients
| Dose (mg) | Single dose
|
Multiple doses
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC0-∞ (ng·h/ml) | Cmax (ng/ml) | Tmax (h)a | t1/2 (h) | CL/F (ml/min) | AUC0-∞/AUC0-24 (ng·h/ml) | Cmax (ng/ml) | Tmax (h)a | t1/2 (h) | CL/F (ml/min) | |
| 30 | 1,069 ± 371 | 200 ± 31 | 1.5 (1.0-3.0) | 2.5 ± 0.4 | 288 ± 83 | 1,069 ± 372 | 192 ± 46 | 2.0 (0.5-23.4) | 2.5 ± 0.7 | 289 ± 86 |
| 60 | 2,344 ± 221 | 397 ± 49 | 2.0 (1.0-2.0) | 3.0 ± 0.3 | 244 ± 24 | 2,105 ± 274 | 362 ± 74 | 2.0 (1.0-3.0) | 3.0 ± 0.5 | 273 ± 36 |
| 120 | 4,374 ± 994 | 805 ± 203 | 2.0 (0.5-2.0) | 3.0 ± 1.0 | 273 ± 75 | 3,838 ± 728 | 696 ± 156 | 1.0 (1.0-3.9) | 3.1 ± 0.4 | 304 ± 53 |
| 240 | 6,302 ± 582 | 1,076 ± 218 | 2.0 (1.0-3.9) | 3.0 ± 0.2 | 363 ± 37 | 5,656 ± 1,106 | 1,164 ± 242 | 2.0 (2.0-3.0) | 3.3 ± 0.2 | 416 ± 94 |
Tmax values are shown as medians, and the ranges are given in parentheses.
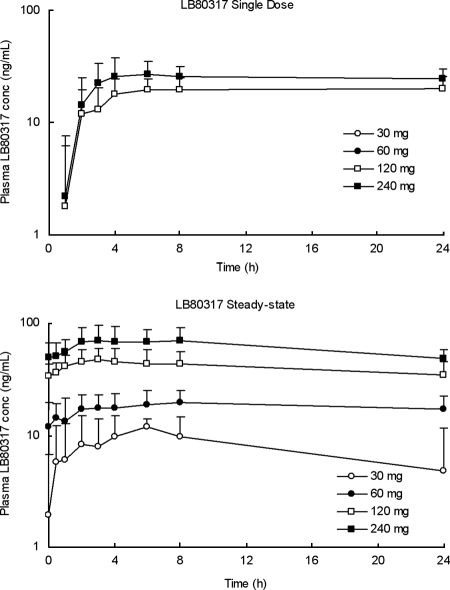
The active metabolite, LB80317, was measurable in plasma samples following the administration of all single doses except the lower doses of 30 and 60 mg. A considerably greater systemic exposure of LB80317 was observed during steady state than after the administration of a single dose (Fig. 5), with the molar metabolic AUC ratios ranging from 119% to 125%. At steady state, the Cmaxs of LB80317 were attained at from 3.0 to 8.0 h postdosing, and thereafter, the plasma concentration declined in a monoexponential manner and the mean apparent t1/2s ranged from 45 to 62 h; these were estimates calculated from the plasma concentration data only up to 24 h postdosing. At steady state, the plasma concentration accumulated two- to threefold from the concentrations achieved following the administration of a single dose. LB80317 had a much longer t1/2 than LB80331, which is indicative of sustained exposure to the active metabolite with a once-a-day dosing regimen. The pharmacokinetic parameters of LB80317 following the administration of multiple doses of LB80380 are summarized in Table 3.
FIG. 5.
Mean plasma LB80317 concentration profile in CHB patients following the administration of a oral single dose of LB80380 of 30, 60, 120, or 240 mg daily (day 1) and at steady state (weeks 2 to 4).
TABLE 3.
Summary of pharmacokinetic parameters for LB80317 following administration of multiple doses of LB80380 in CHB patients
| AUC0-24 (ng·h/ml) | Cmax (ng/ml) | Tmax (h)a | t1/2 (h) | Metabolic AUC ratiob |
|---|---|---|---|---|
| 285 ± 91 | 12 ± 2.2 | 6.0 (4.0-6.0) | 53 ± 37c | 0.20 ± NDd |
| 433 ± 128 | 21 ± 5.4 | 8.0 (3.0-24) | 62 ± NDc | 0.19 ± 0.04 |
| 1,017 ± 339 | 49 ± 14 | 3.0 (2.0-8.0) | 51 ± 20c | 0.25 ± 0.05 |
| 1,449 ± 387 | 75 ± 24 | 4.5 (2.0-8.0) | 45 ± 17c | 0.24 ± 0.04 |
Tmax values are given as the medians, and the ranges are given in parentheses.
Calculated as the ratio of [AUC0-24 for LB80317)/(AUC0-∞/AUC0-24 for LB80331)] × 0.94921.
Calculated on the basis of the plasma concentration data only up to 24 h.
ND, not determined because data were obtained from only one subject.
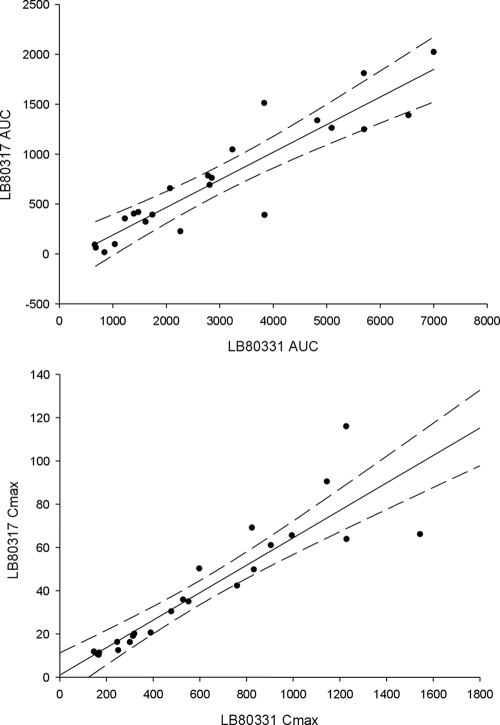
Figure 6 shows the correlation of the pharmacokinetic parameters underlying systemic exposure, AUC and Cmax, between LB80331 and LB80317. There was a strong correlation between the values of the pharmacokinetic parameters for the two compounds, as indicated by correlation coefficients of 0.9181 and 0.8998 for AUC and Cmax, respectively. These findings therefore suggest that the formation of the active metabolite, LB80317, is proportional to the level of systemic exposure to the parent drug, LB80331, during steady state.
FIG. 6.
Correlation of pharmacokinetic parameters (AUC0-24 and Cmax) between LB80331 and LB80317 at steady state.
LB80380 was well tolerated at all doses. No serious adverse events or dose-limiting toxicities were observed during the dosing and postdosing periods. Most of the adverse events reported were mild, self-limiting, and probably unrelated to the drug since they were also observed in the patients receiving placebo. These findings have already been reported previously (12). There were no clinically significant abnormal vital signs or hematology, blood biochemistry, or urinalysis results. Overall, the safety profiles of individuals receiving LB80380 were satisfactory and comparable to those of individuals receiving placebo.
Tissue distribution in mice.
The pharmacokinetic parameters for LB80331 and LB80317 in mouse plasma and liver tissue samples are depicted in Table 4. The total amounts of LB80331 and of LB80317 deposited in the mouse livers as the phosphorylated forms were 39% and 39 to 41%, respectively.
TABLE 4.
Pharmacokinetic parameters for LB80331 and LB80317 in mouse plasma and livera
| Analyte | Tmax (h) | Cmax (μM) | AUC0-36 (μM·h) | AUC0-∞ (μM·h) | t1/2 (h) |
|---|---|---|---|---|---|
| LB80331 (plasma) | 1 | 6 | 17 | 17 | 2.0 |
| LB80331 (liver) | 1 | 44 | 102 | 102 | 2.8 |
| LB80331 di- and triphosphate (liver) | 1 | 17 | 64 | 64 | 3.2 |
| LB80317 (plasma) | 4 | 0.6 | 6 | 6 | 8.6 |
| LB80317 (liver) | 1 | 243 | 3,069 | 3,353 | 9.9 |
| LB80317 di- and triphosphate (liver) | 1 | 107 | 1,883 | 2,152 | 8.3 |
Values round up with one or no decimal space. The units are expressed in μM (micromole per liter), as the extracted proteins were neutralized with the same volume of ammonium bicarbonate for all mice. The drug concentration was then compared on basis of the same volume. The proportion of phosphorylated LB80331 was 39% for both AUC0-36 and AUC0-∞. The proportions of phosphorylated LB80317 were 39% and 41% for AUC0-36 and AUC0-∞, respectively.
DISCUSSION
The clinical pharmacokinetics of LB80331 and LB80317 were investigated in two categories of subjects, namely, healthy volunteers and patients with CHB. In the healthy subjects, the Cmaxs of LB80331 were attained within 2 h at all doses, demonstrating that LB80380 is rapidly absorbed and converted to LB80331 (Fig. 2). After Cmax was reached, the plasma LB80331 concentration declined in a monoexponential manner, and the apparent t1/2 was about 2 h. Among the subjects receiving higher doses (240 and 480 mg), a biexponential pattern of decline in the concentration was observed and the t1/2s of LB80331 seemed to be longer in those dose groups. A long elimination phase after the terminal phase observed in the present study may also exist in the groups receiving lower doses; however, this could not be proven because the plasma concentration was below the limit of quantitation. It is unlikely that the long elimination phase will contribute significantly to the pharmacodynamics of LB80331, since the plasma LB80331 level is too low to show a therapeutic effect at lower doses, such as 30 and 60 mg, on the basis of data from a preclinical study of efficacy. However, it is still possible that a long elimination phase could have an effect on the steady-state pharmacokinetics of LB80331 during repeated dosing. The pharmacokinetics of LB80331 in subjects receiving and not receiving a high-fat diet were compared (Fig. 3). The systemic exposure parameters (AUC0-∞ and Cmax) after the consumption of a high-fat meal were not significantly different from those after the consumption of no food (fasting). While there were no significant differences in t1/2, Cmax, and AUC0-∞ between the two treatments, Tmax was delayed after the consumption of the high-fat meal. Therefore, the ingestion of a high-fat meal delays the oral absorption of LB80380 but does not affect the degree of absorption.
The study evaluated the safety, antiviral activity, and pharmacokinetics of LB80380 in CHB patients during 4 weeks of oral dosing (12). All daily doses of LB80380 studied (30 to 240 mg) were well tolerated, and no clinically relevant adverse events were detected. LB80380 induced a marked suppression of the serum HBV DNA level in HBeAg-positive CHB patients, with the mean viral load reduction being 3.02 to 3.80 log10 copies/ml with daily doses of 30 to 240 mg following 4 weeks of treatment.
For the pharmacokinetics in CHB patients, LB80380 was well absorbed following oral administration and was rapidly converted to its parent drug, LB80331. The Tmax of LB80331 was about 2 h and declined monoexponentially with the apparent t1/2 of about 2 to 3 h (Fig. 4). Following the administration of multiple oral doses, no further accumulation of LB80331 was observed over the study period (Fig. 4). The pharmacokinetics of LB80331 were linear over the dose range of 30 to 120 mg once daily, but a trend toward decreased systemic exposure was observed at the highest dose of 240 mg. The target systemic exposure level of LB80331 was an AUC0-24 of approximately 3,000 ng·h/ml at steady state, and the linear pharmacokinetic range of doses from 30 mg to 120 mg covered the therapeutic target dose of 90 mg. The disproportional decrease in the level of systemic exposure observed with the 240-mg dose is likely related to the solubility and subsequent dose-dependent absorption of LB80380 in the gastrointestinal tract. The expected concentration of LB80380 in the gastrointestinal tract is near its intrinsic aqueous solubility of 1.06 mg/ml when the 240-mg dose is administered orally. The renal clearance of LB80331 is dose independent in healthy volunteers (data not shown), and the in vitro metabolism of LB80331 by hepatic enzymes is not extensive (data not shown), suggesting that the nonlinearity was caused not by renal clearance or hepatic metabolism but by gastrointestinal absorption. LB80331 is further metabolized to its active metabolite, LB80317. The level of formation of the active metabolite, LB80317, is proportional to the level of systemic exposure to the parent drug, LB80331, during steady state (Fig. 6). LB80317 has a much longer t1/2 of about 45 to 62 h (Fig. 5) and accumulated two- to threefold after the administration of multiple doses; this accumulated amount accounts for about 20% of the molar metabolic ratio during steady state. Therefore, the long t1/2 of LB80317 suggests that sustained exposure to active drug can be achieved with once-daily dosing.
The concentrations of LB80331 and LB80317 in the livers of mice instead of the concentrations of the parent drug and its metabolite in human liver were measured. The levels of exposure of the mouse liver to LB80331 and especially to LB80317 were much higher than the systemic exposure level to the two metabolites of LB80380. In addition, a substantial proportion of LB80317 (39 to 41%) was phosphorylated in the mouse livers. This finding suggests that the liver could be exposed to the active form of LB80317 at levels high enough to suppress the replication of HBV, as long as a sufficient level of LB80317 is maintained in plasma. Confirmation of this, however, requires further experiments, since we cannot completely rule out the possibility that the measurable phosphorylated form may be coming from other cells residing in the mouse liver, e.g., red blood cells and peripheral blood mononuclear cells.
In conclusion, LB80380 is rapidly absorbed and converted to the parent drug, LB80331. No further accumulation of LB80331 was observed after steady state was reached. The level of the active metabolite, LB80317, directly correlated with that of LB80331. LB80317 has a t1/2 that is long enough to support a once-daily dosing regimen. The ingestion of a high-fat diet delays the absorption of LB80380 but has no effect on the degree of absorption. In the preliminary study with mice, approximately 40% of LB80317 was phosphorylated in the liver, but this requires further confirmation. These results encourage further studies with animal models to examine whether there is a link between the pharmacokinetics of LB80331/LB80317 and antiviral efficacy.
Acknowledgments
This study was sponsored by LG Life Sciences, Ltd.
Footnotes
Published ahead of print on 17 February 2009.
REFERENCES
- 1.Chang, T. T., R. G. Gish, R. de Man, A. Gadano, J. Sollano, Y. C. Chao, A. S. Lok, K. H. Han, Z. Goodman, J. Zhu, A. Cross, D. DeHertogh, R. Wilber, R. Colonno, D. Apelian, and the BEHoLD AI463022 Study Group. 2006. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 354:1001-1010. [DOI] [PubMed] [Google Scholar]
- 2.Gough, K., M. Hutchison, O. Keene, B. Byrom, S. Ellis, L. Lacey, and J. Mckellar. 1995. Assessment of dose proportionality: report from the statisticians in the pharmaceutical industry/pharmacokinetics UK joint working party. Drug Information J. 29:1039-1048. [Google Scholar]
- 3.Hadziyannis, S. J., N. C. Tassopoulos, E. J. Heathcote, T. T. Chang, G. Kitis, M. Rizzetto, P. Marcellin, S. G. Lim, Z. Goodman, J. Ma, C. L. Brosgart, K. Borroto-Esoda, S. Arterburn, S. L. Chuck, and the Adefovir Dipivoxil 438 Study Group. 2006. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology 131:1743-1751. [DOI] [PubMed] [Google Scholar]
- 4.Jakobsen, M. R., H. Arildsen, H. B. Krarup, M. Tolstrup, L. Østergaard, and A. L. Laursen. 2008. Entecavir therapy induces de novo HIV reverse-transcriptase M184V mutation in an antiretroviral therapy-naive patient. Clin. Infect. Dis. 46:e88-e91. [DOI] [PubMed] [Google Scholar]
- 5.Lai, C. L., E. Gane, C. W. Hsu, S. Thongsawat, Y. Wang, Y. Chen, E. J. Heathcote, J. Rasenack, N. Bzowej, N. Naoumov, S. Zeuzem, A. Di Biscegli, G. C. Chao, A. F. Constance, and N. Brown. 2006. Two-year results from the GLOBE trial in patients with hepatitis B: greater clinical and antiviral efficacy for telbivudine (Ldt) vs. lamivudine. Hepatology 44(4 Suppl. 1):222A. [Google Scholar]
- 6.Lai, C. L., E. Gane, Y. F. Liaw, C. W. Hsu, S. Thongsawat, Y. Wang, Y. Chen, E. J. Heathcote, J. Rasenack, N. Bzowej, N. V. Naoumov, A. M. Di Bisceglie, S. Zeuzem, Y. M. Moon, Z. Goodman, G. Chao, B. F. Constance, N. A. Brown, and the Globe Study Group. 2007. Telbivudine versus lamivudine in patients with chronic hepatitis B. N. Engl. J. Med. 357:2576-2588. [DOI] [PubMed] [Google Scholar]
- 7.Lai, C. L., K. H. Han, S. K. Yoon, S. H. Um, M. F. Yuen, H. S. Kim, H. R. Kim, H. C. Chung, C. R. Kim, P. Hsyu, D. Averett, and J. Kim. 2005. Interim report for a phase II, multi-centre, dose-escalating study of LB80380/ANA380 in hepatitis B patients with lamivudine-resistant YMDD mutant HBV. J. Hepatol 42(Suppl. 2):72A. [Google Scholar]
- 8.Lai, C. L., V. Ratziu, M. F. Yuen, and T. Poynard. 2003. Viral hepatitis B. Lancet 362:2089-2094. [DOI] [PubMed] [Google Scholar]
- 9.Lai, C. L., D. Shouval, A. S. Lok, T. T. Chang, H. Cheinquer, Z. Goodman, D. DeHertogh, R. Wilber, R. C. Zink, A. Cross, R. Colonno, L. Fernandes, and the BEHoLD AI463027 Study Group. 2006. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 354:1011-1020. [DOI] [PubMed] [Google Scholar]
- 10.Min, C. H., C. R. Kim, K. Steffy, D. Averett, S. Locarnini, and T. Shaw. 2007. The active metabolite of LB80380/ANA380, a novel nucleotide analog, exhibits activity in vitro against multiple clinically relevant hepatitis B virus mutants. J. Hepatol. 46(Suppl. 1):S159. [Google Scholar]
- 11.Tenney, D. J., K. A. Pokornowski, R. E. Rose, C. J. Baldick, B. J. Eggers, J. Fang, J. Y. Yang, D. Xu, H. Brett-Smith, and R. J. Colonno. 2008. Entecavir at five years shows long-term maintenance of high genetic barrier to hepatitis B virus resistance. Hepatol. Int. 2:A88-A89. [Google Scholar]
- 12.Yuen, M. F., J. Kim, C. R. Kim, V. Ngai, J. C. H. Yuen, C. Min, H. M. Kang, B. S. Shin, S. D. Yoo, and C. L. Lai. 2006. Dose-finding study of oral LB80380 in HBeAg-positive patients with chronic hepatitis B. Antivir. Ther. 11:977-983. [PubMed] [Google Scholar]
- 13.Yuen, M. F., W. K. Seto, D. H. Chow, K. Tsui, D. K. Wong, V. W. Ngai, B. C. Wong, J. Fung, J. C. Yuen, and C. L. Lai. 2007. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir. Ther. 12:1295-1303. [PubMed] [Google Scholar]