Abstract
Menstrual toxic shock syndrome is a rare but potentially life-threatening illness manifest through the actions of Staphylococcus aureus toxic shock syndrome toxin 1 (TSST-1). Previous studies have shown that tampon additives can influence staphylococcal TSST-1 production. We report here on the TSST-1-suppressing activity of 34 compounds that are commonly used additives in the pharmaceutical, food, and perfume industries. Many of the tested chemicals had a minimal impact on the growth of S. aureus and yet were potent inhibitors of TSST-1 production. The TSST-1-reducing compounds included surfactants with an ether, amide, or amine linkage to their fatty acid moiety (e.g., myreth-3-myristate, Laureth-3, disodium lauroamphodiacetate, disodium lauramido monoethanolamido, sodium lauriminodipropionic acid, and triethanolamine laureth sulfate); aromatic compounds (e.g. phenylethyl and benzyl alcohols); and several isoprenoids and related compounds (e.g., terpineol and menthol). The membrane-targeting and -altering effects of the TSST-1-suppressing compounds led us to assess the activity of molecules that are known to inhibit fatty acid biosynthesis (e.g., cerulenin, triclosan, and hexachlorophene). These compounds also reduced S. aureus TSST-1 production. This study suggests that more additives than previously recognized inhibit the production of TSST-1.
As with other gram-positive organisms, the pathogenic potential of Staphylococcus aureus is tied to its ability to produce extracellular toxins. In one clear case, the production of a staphylococcal superantigen toxin, toxic shock syndrome toxin 1 (TSST-1), is sufficient to impart the clinical manifestations of menstrual toxic shock syndrome (mTSS), a rare but potentially life-threatening illness (22). Although S. aureus TSS is not exclusively a gender-, age-, or S. aureus-specific syndrome, the greatest number of cases have been seen in young menstruating women. In these cases, mTSS was associated with the presence of S. aureus TSST-1 and tampon usage (26).
While TSS has been connected with surgical dressings and nasal packings, the development of methodologies for prophylaxis has focused on catamenial tampons. Building upon the observation that TSST-1 is sufficient to induce mTSS, compounds that either interfere with the growth of S. aureus or, more specifically, with the production or activity of TSST-1 have been identified and introduced into tampons. The desired effect of these methodologies is to prevent mTSS by eliminating the bacteria or by sequestering, detoxifying, or reducing the production of the toxin.
In the early 1980s, different additives were shown to either retard or enhance S. aureus TSST-1 production. For example, myreth-3-myristate (Standamul 1414 E; Cetiol 1414E) retarded the production of TSST-1 (17, 25), while ethylene oxide-propylene oxide block copolymers (Pluronic l-62) increased the growth of S. aureus and toxin production (12). Perhaps the most extensively studied additive is glycerol monolaurate. In a rich medium, glycerol monolaurate has been shown to exhibit a graded concentration-dependent effect on the growth of gram-positive bacteria and the transcription of toxin-encoding genes (8, 17, 19, 21, 25, 33). At high concentrations (150 μg/ml), glycerol monolaurate inhibits the growth of gram-positive organisms and toxin production. At the same concentration of the surfactant with larger bacterial inocula, the compound decreases both bacterial growth and toxin production. At low concentrations (17 μg/ml), glycerol monolaurate only delays toxin production (8, 25). Growth inhibition and the decrease in toxin production seen with the addition of glycerol monolaurate can be destroyed by the action of enzymes with lipase activity (25). Structurally, glycerol monolaurate is a composed of a 12-carbon fatty acid bound to glycerol via an ester bond and can be rapidly hydrolyzed by soluble and membrane-bound staphylococcal lipases to lauric acid and glycerol (20). The transient effect of the nonbactericidal concentrations of this surfactant, measured in terms of hours, may limit its effectiveness as a prophylaxis agent for mTSS.
To overcome the limitations of described measures for mTSS prophylaxis, we sought to identify compounds that can retard or inhibit the production of TSST-1. Based on the studies using myreth-3-myristate and glycerol monolaurate, we examined other surfactants for their ability to inhibit bacterial growth and toxin production. In contrast to the general assumption that the tested compounds lack a biological activity, we report that at low concentrations many of these compounds minimally affect bacterial growth but substantially inhibit TSST-1. The hydrophobicity of these molecules suggests that the biological effect of these molecules is due to interactions with cell membranes. This observation led us to examine the effect of a variety of chemicals capable of interacting with membranes or that are known to affect phospholipid biosynthesis. As with surfactants, certain aromatic and isoprenoid compounds and their analogues, as well as known inhibitors of fatty acid biosynthesis, can be used to repress superantigen toxin expression by S. aureus. With many of these reagents, their structures dictate that their toxin-inhibitory activity will be unaffected by lipase and esterases.
MATERIALS AND METHODS
Bacteria, routine growth, and maintenance.
S. aureus MN8 is an archetype TSST-1-producing strain isolated from a patient with nonmenstrual TSS (a gift from P. M. Schlievert, University of Minnesota Medical School, Minneapolis [23]). In addition, two human vaginal isolates of S. aureus, FRI-1169 and FRI1187 (gifts from the Food Research Institute, Madison, WI), were also used in the present study (34). The Laureth-4-resistant strains were isolated by selecting for bacteria that grew in the presence of 10 mM Laureth-4 after 48 h. These strains maintained their ability to rapidly grow, and they exhibited reduced production of TSST-1 upon culture in the absence of the surfactant. S. aureus isolates were routinely cultivated at 37°C on sheep blood agar plates (Remel, Lenexa, KS) or grown in brain heart infusion broth (Becton Dickinson Microbiology Systems, Franklin Lakes, NJ) with rotary aeration at 250 rpm. Bacteria were stored frozen in brain heart infusion plus 10% glycerol at −70°C.
Test compounds, medium, and preparation of bacteria.
The test compounds and information on their chemical formula, solubility, and source are shown in Table 1. The compounds are grouped as surfactants, aromatics, isoprenoids, and fatty acid synthesis inhibitors. The grouping of compounds is a convenience, with certain compounds assigned to the most appropriate, rather than only, group (e.g., triclosan as a fatty acid biosynthesis inhibitor rather than an aromatic compound). Growth medium (GM) was used to cultivate bacteria in studies on the effect of compounds on growth and TSST-1. The GM consisted of 37 g of brain heart infusion/liter supplemented with 10% (vol/vol) fetal bovine serum, 1.0% (vol/vol) of a 0.02 M solution of MgCl2, and 27 mM l-glutamine in 880 ml of distilled water. GM supplements were obtained from the Sigma-Aldrich Chemical Company (St. Louis, MO). S. aureus was initially cultivated on tryptic soy agar (Difco Laboratories, Cockeysville, MD) at 37°C. After 24 h, three to five colonies were used to inoculate 10 ml of GM in conical polystyrene tubes capped with foam plugs (Jaece Industries, North Tonawanda, NY). The cultures were cultivated at 37°C for 24 h and used as inoculation broth.
TABLE 1.
Compounds
| Compounda | Chemical formula | Formb |
|---|---|---|
| Surfactants | ||
| 1-O-Dodecyl-rac-glycerol | H3C(CH2)10COOCH2CH(OH)CH2OH | L |
| Disodium laureth sulfosucccinate | C22H40Na2O10S | L |
| Myreth-3-myristate* | CH3(CH2)12CO(OCH2CH2)3OCH2(CH2)12CH3 | L |
| Glucopon 220 | C8H17O-(C6H10O5) | L |
| Laureth-3 | CH3(CH2)11(O OCH2CH2)nOH, where n has an avg value of 3 | L |
| Laureth-4 (polyoxyethylene 4 lauryl ether)* | CH3(CH2)11(O OCH2CH2)nOH, where n has an avg value of 4 | L |
| Pluronic L-62† | Ethylene oxide/propylene oxide block copolymer | L |
| Pluronic P-105† | Ethylene oxide/propylene oxide block copolymer | L |
| Disodium lauroamphodiacetate# | C20H36N2Na2O6 | L |
| Sodium lauriminodipropionic acid* | C18H34NNaO4 | L |
| Disodium lauramido MEA sulfosuccinate# | C18H31NNa2O8S | L |
| TEA laureth sulfate§ | C30H65NO8S-2 | L |
| Aromatics | ||
| 2-Phenoxyethanol | C6H5OCH2CH2OH | L |
| Acetaminophen | C8H9NO2 | M |
| Acetyl tyrosine | HOC6H4CH2C(NHCOCH3)HCOOH | M |
| Benzyl alcohol | C6H5CH2OH | L |
| Gallic acid | (OH)3C6H2COOH or C7H6O5 | M |
| Nipagin M (p-hydroxybenzoic acid, methyl ester) | HOC6H5CH2OH | M |
| PABA (p-amino benzoic acid, potassium salt) | NH2C6H4COOH | M |
| Phenylethyl alcohol | C6H5CH2CH2OH | L |
| Salicylic acid (2-hydroxybenzoic acid) | HOOCC6H4OH | M |
| Salicylamide (2-hydroxybenzamide) | HOC6H4CONH2 | M |
| Vanillic acid | C8H8O4 | M |
| Isoprenoids | ||
| β-Ionone | C13H20O | M |
| β-Myrcene | C10H26 | M |
| Geraniol | C10H18O | M |
| Linalool | C10H18O | M |
| Menthol | C10H20O | M |
| p-Menthane-1,8-diol | C10H20O2 | M |
| Terpineol | C10H18O | L |
| Thymol | C10H14O | M |
| Fatty acid biosynthetic inhibitors | ||
| Cerulenin | CH3(CH2)6COCHOCHCOCONH2 | M |
| Hexachlorophene | C13H6Cl6O2 | M |
| Triclosan‡ | C12H7Cl3O2 | M |
| Other | ||
| 4-Hydroxydiphenylmethane | C6H5CH2C6H4OH | M |
Compounds were from the Sigma-AldrichChemical Company unless otherwise noted. *, Henkel; †, BASF; ‡, KIC Chemical;§, Chemron; #, McIntyre Group, Ltd.
L, the compound is in liquid form at room temperature; M, the compound is in solid form at room temperature and was dissolved in methanol prior to use.
Compound test procedure for analysis of growth. (i) Test tube method.
The test compounds were evaluated in 10 ml of GM in 50-ml conical polystyrene tubes inoculated with 5 × 106 CFU/ml (14). The bacteria were grown as described above and enumerated using a standard curve (i.e., the optical density at 600 nm [OD600] versus the viable bacteria). The concentration of bacteria was confirmed by plate counts as described by Koch (9). Water soluble/miscible test compounds were diluted as required in the GM. The highest concentration of water-insoluble or -miscible compounds were dissolved in 200 μl of spectrophotometric grade methanol (Sigma-Aldrich Chemical Company). Each test compound was added to the GM in the amount necessary to obtain the reported final concentration. For water-insoluble or -miscible compounds, tubes of GM with an equivalent amount of methanol, but no test compound, were used as controls. GM tubes with test compounds or controls were inoculated with 0.1 ml of the prepared inoculation broth. Unless otherwise indicated, after 24 h at 37°C with 5% CO2 the OD600 values of the culture fluids and the numbers of viable bacteria were determined. Bacterial growth in the samples is reported as a percentage of CFU/ml for the untreated controls within the same experiment. In preparation for analysis of TSST-1 production, the culture fluid broth was centrifuged, and the supernatant fluids were filter sterilized by using 0.22-μm-pore-size mixed cellulose ester filters (Fisher, Pittsburgh, PA) and then stored at −70°C.
(ii) Flask method.
A second method of growth assessment was used for the evaluation of Laureth-4. Portions (100 ml) of GM were placed in each of nine 500-ml aluminum foil-capped Fleaker flasks (Corning Glass Works, Corning, NY). Laureth-4 (10 mM) was initially added to one flask and to other flasks at 90-min intervals for up to 12 h. All flasks were inoculated with 5 × 106 CFU/ml. The flasks were incubated at 37°C in ambient air on a gyratory shaker. At regular intervals (8, 12, 24, and 48 h), 0.8 ml was removed from each flask, and the OD600 and number of viable bacteria in the culture were determined. At the same time intervals, culture supernatant fluids from each Fleaker were prepared for analysis of TSST-1 as described above.
Quantification of TSST-1.
The concentration of TSST-1 in sterile culture supernatant fluids was determined by a noncompetitive, sandwich enzyme-linked immunosorbent assay. Samples of the culture supernatant fluid and the TSST-1 reference standard were assayed in triplicate. The immunological reagents—purified TSST-1 (catalogue no. TT-606), rabbit polyclonal anti-TSST-1 immunoglobulin G (IgG; LTI-101), rabbit polyclonal anti-TSST-1 IgG conjugated to horseradish peroxidase (catalogue no. LTC-101), and certified anti-TSST-1-free normal rabbit serum (NRS; catalogue no. NRS-10)—were purchased from Toxin Technology (Sarasota, FL). Salts and other reagents were purchased from the Sigma-Aldrich Chemical Company.
A 10-mg/ml solution of anti-TSST-1 IgG was prepared in phosphate-buffered saline (PBS; 16 mM Na2HPO4, 4 mM NaH2PO4, 3 mM KCl, 0.137 M NaCl [pH 7.4]). A 100-μl aliquot of the anti-TSST-1 IgG solution was added into the inner wells of polystyrene microplates (Nunc International, Rochester, NY). Plates were incubated overnight at room temperature. Unbound antitoxin was removed by three washes with PBS-Tween (0.011 M NaH2PO4, 0.9% NaCl [wt/vol], 0.5% Tween 20 [vol/vol]; pH 7.2). Next, the wells were blocked for 1 h at 37°C with 100 μl of a 1% (wt/vol) solution of bovine serum albumin. Unbound bovine serum albumin was removed by three washes with PBS-Tween. TSST-1 was diluted to 10 ng/ml in PBS containing 0.05% (vol/vol) Tween 20 and 1% (vol/vol) NRS and then incubated at 4°C overnight. Bacterial culture supernatants were combined with 1% NRS (vol/vol) and incubated at 4°C overnight. The test samples included TSST-1 reference standards (TSST-1 serially diluted from 10 to 0.3 ng/ml in PBS-Tween) and the NRS-treated bacterial culture supernatant fluids. A 100-μl aliquot of each test sample was added to each well, followed by incubation 2 h at 37°C. Three PBS-Tween washes were used to remove unbound toxin. Rabbit polyclonal anti-TSST-1 IgG conjugated to horseradish peroxidase was diluted according to manufacturer's instructions, and a 100-μl volume was added to each sample well. The plates were covered and incubated 1 h at 37°C. After incubation, the plates were washed five times in PBS-Tween and three times with distilled water. The assay was developed with 100 μl of horseradish peroxidase substrate buffer (5 mg of o-phenylenediamine and 5 μl of 30% hydrogen peroxide in 11 ml of 12 mM anhydrous citric acid and 26 mM dibasic sodium phosphate [pH 5.5]). The plates were incubated for 15 min at 37°C. The reaction was stopped by the addition of 50 μl of a 5% sulfuric acid solution. The intensity of the color reaction, measured as the OD490, was evaluated by using a model EL309 microplate reader (BioTek, Winooski, VT). A standard curve for TSST-1 was constructed for each assay procedure using the results for the toxin standards and linear regression analysis. The procedure was considered acceptable only if the R2 was ≥0.97. TSST-1 concentrations in the test samples were determined from the regression equation. The calculated amount of TSST-1 in each sample was considered acceptable only if the coefficient of variation for each mean OD value of triplicate samples was <20%. TSST-1 in test samples is reported as a percentage of the TSST-1 concentrations in ng/ml in untreated controls within the same experiment.
Evaluation of the interaction of two compounds.
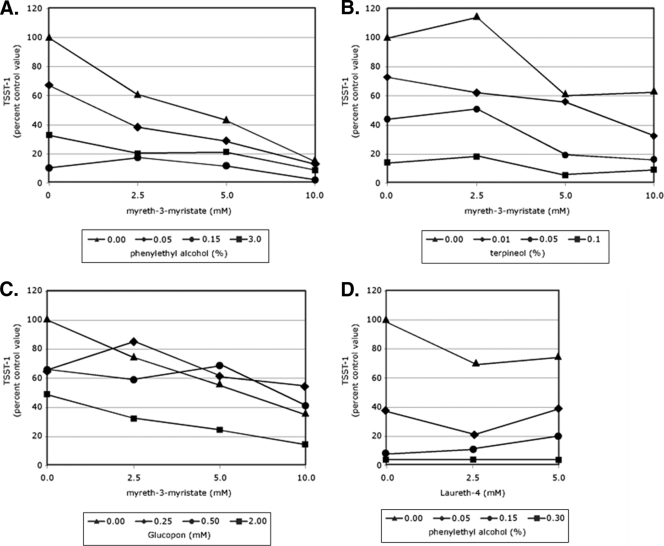
Matrices composed of two increasingly diluted test compounds were used to evaluate the combined effect of the selected chemicals on S. aureus growth and TSST-1 production. For each experiment, tubes contained a medium control or various concentrations of one test compound, augmented with various concentrations of a second test compound. Compounds and their concentrations were as indicated in Fig. 3. Inoculation of the tubes, growth conditions, and assays were preformed as described above.
FIG. 3.
Effect of combinations of test compounds on S. aureus MN8 TSST-1 production. TSST-1 levels are expressed per ml as a percentage of the control (no added compounds) value to combinations of myreth-3-myristrate and phenylethyl alcohol (A), myreth-3-myristrate and terpineol (B), myreth-3-myristrate and Glucopon (C), and Laureth-4 and phenylethyl alcohol (D).
RESULTS
Effect of compounds on S. aureus growth and TSST-1 production.
Myreth-3-myristate and Glucopon had mixed effects on the growth and TSST-1 production by S. aureus strain MN8. For the concentrations examined, these surfactants increased the growth of S. aureus at the higher level test concentrations but were seen to decrease both the amount of total TSST-1 (Table 2) and the amount of TSST-1 per CFU (data not shown) at all of the concentrations tested. Pluronic L-62 and P-105 increased the number of bacteria enumerated after 24 h of cultivation. However, lower rather than higher concentrations of the compounds were observed to increase bacterial growth. The Pluronic compounds were associated with an increased level of TSST-1 compared to control bacterial cultures, in terms of the total amount of TSST-1 (Table 2) and when calculated as TSST-1 per CFU (data not shown).
TABLE 2.
Effectof nonionic surfactants on S. aureusstrain MN8
| Compound | Compound concn (mM) | % Control
|
|
|---|---|---|---|
| Growtha | TSST-1 | ||
| Glucopon | 1.50 | 202.2 | 27.3 |
| 0.75 | 105.5 | 81.2 | |
| Myreth-3-myristate | 10.00 | 143.3 | 22.2 |
| 5.00 | 108.0 | 47.4 | |
| 2.50 | 106.6 | 64.9 | |
| Pluronic L-62 | 10.00 | 123.9 | 215.6 |
| 1.00 | 495.6 | 242.6 | |
| Pluronic P-105 | 10.00 | 101.4 | 162.7 |
| 0.50 | 159.7 | 249.2 | |
Bacterial growth and TSST-1 at 8 h.
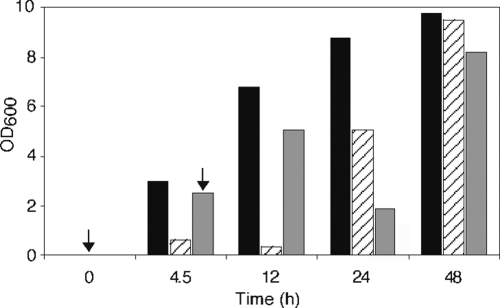
Although all concentrations of the nonionic surfactant Laureth-4 tested reduced TSST-1 to <97% of untreated MN8, profound differences were observed in bacterial growth (Table 3). Laureth-4, when added to a rich medium at concentrations from 2 to 20 mM, inhibited bacterial growth. In time, bacterial growth rebounded to the level seen with unexposed MN8 (Fig. 1). The effect of Laureth-4 was further examined using derivatives of S. aureus MN8 isolated from liquid cultures after the observed rebound in growth. Two derivative strains were stable following storage on tryptic soy agar, and they were able to grow in the presence of Laureth-4 on solid or in liquid medium without the long lag phase seen with MN8 without prior treatment. The reported derivative strains exhibited MN8 levels of growth, although this was not the case for all of the derivatives examined. A difference between the derivative strains was seen in TSST-1 production. TSST-1 production could not be detected in the supernatant fluids from one strain using media with or without Laureth-4 supplementation, while a second strain produced 5% of the wild-type level of TSST-1 when grown in the absence of Laureth-4 and showed a 16-fold reduction of TSST-1 in sample fluids after treatment with 10 mM Laureth-4. We examined the growth and the TSST-1-reducing effect of other surfactants with ether bonds, as well as surfactants with amide, or amine linkages between their head groups and their fatty acid tails. These compounds, at the concentrations tested, had variable effects on growth with the anionic surfactants, with an amine bond exhibiting the greatest repression of growth. All tested surfactants suppressed TSST-1 production (Table 4).
TABLE 3.
Effectof Laureth-4 on S. aureus strains MN8, FRI-1169, and FRI-1187 and their derivativesa
| S. aureus strain and Laureth-4 concn (mM) | OD600 | Time (h) | % Control (TSST-1) |
|---|---|---|---|
| MN8 | |||
| 0 | 1.079 | 10 | 100.0 |
| 2 | 1.035 | 26 | 1.7 |
| 10 | 1.058 | 28 | 2.9 |
| 20 | 1.029 | 26 | 1.5 |
| FRI-1169 | |||
| 0 | 3.970 | 24 | 100.0 |
| 2 | 3.990 | 32 | 0.1 |
| 10 | 4.400 | 32 | 0.0 |
| 20 | 4.180 | 32 | 0.0 |
| FRI-1187 | |||
| 0 | 1.338 | 24 | 100.0 |
| 10 | 0.037 | 24 | ND |
| MN8 derivative 1 | |||
| 0 | 4.400 | 24 | ND |
| 10 | 3.990 | 24 | ND |
| MN8 derivative 2 | |||
| 0 | 3.970 | 24 | 4.9* |
| 10 | 4.180 | 24 | 0.2 |
ND, no TSST-1 detected. *, Value compared to wild-type S. aureus strain MN8.
FIG. 1.
Recovery of growth of S. aureus strain MN8 after treatment with Laureth-4. The OD of MN8 grown either in rich medium (black boxes) or in rich medium supplemented with Laureth-4 (striped and gray boxes) is shown. The arrows indicate the time when Laureth-4 was introduced to a final concentration of 10 mM into the culture medium. During the first 12 h after the addition of Laureth-4, the surfactant either inhibited or reduced growth of MN8. However, by 24 h the treated strains showed renewed growth. Laureth-4 (10 mM) was added to one flask at zero time and to additional flasks at 90-min intervals for up to 12 h of incubation. Only the results for the addition of Laureth-4 at 0 and 4.5 h are shown. Laureth-4 added at other time points resulted in similar growth patterns.
TABLE 4.
Effectof compounds with ether, amide, and amine linkages on growth and TSST-1production for S. aureus strain MN8
| Ether linkage type and surfactant compound | Compound concn (mM) | % Control
|
|
|---|---|---|---|
| Growth | TSST-1 | ||
| Ether linkages | |||
| 1-O-Dodecyl-rac-glycerol | 10.7 | 36.3 | 5.2 |
| Laureth-3 | 9.0 | 7.3 | 1.0 |
| PPG-5 Laureth-5 | 7.4 | 6.1 | 0.4 |
| Disodium laureth sulfosucccinate | 9.8 | 10.3 | 0.6 |
| Amide linkages | |||
| Disodium lauroamphodiacetate | 10.7 | 11.3 | 1.0 |
| Disodium lauramido MEA | 10.7 | 29.4 | 0.4 |
| Amine linkages | |||
| Sodium lauriminodipropionic acid | 2.2 | NDa | 1.0 |
| TEA laureth sulfate | 2.2 | ND | 1.5 |
ND, not determined.
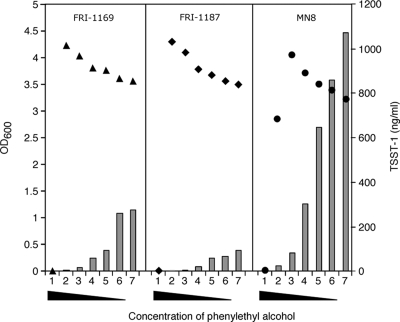
A second class of amphipathic compounds that contain a conjugated ring of unsaturated bonds, the aromatics, was tested for the ability to affect the growth and production of TSST-1 by MN8 (Table 5). The tested aromatic compounds had variable effects on the growth of strain MN8. For example, 0.5% of the methyl ester of p-hydroxybenzoic acid, salicylic acid, or vanillic acid and 0.4% p-aminobenzoic acid completely inhibited bacterial growth. The other aromatics also inhibited growth, but the bacteria grew to measurable levels. TSST-1 production per CFU was universally reduced by the tested aromatic compounds (data not shown). The effect of different concentrations of phenylethyl alcohol on the levels of growth and TSST-1 production for S. aureus strains FRI-1169, FRI-1187, and MN8 after 24 h of cultivation are shown in Fig. 2. The highest concentration of phenylethyl alcohol tested (1%) inhibited the growth of all three strains. At 0.5% phenylethyl alcohol, FRI-1169 and FRI-1187 had accumulated growth that was greater than that seen with the unexposed controls, while MN8 still exhibited phenylethyl alcohol-induced growth inhibition. When this aromatic compound was added to media at concentrations of <0.25%, a dose-dependent augmentation of biomass was observed. The level of TSST-1 produced by each of the strains, while in different absolute amounts, followed an inverse pattern: higher concentrations of phenylethyl alcohol resulted in lower concentrations of TSST-1 in the culture medium.
TABLE 5.
Effectof aromatic compounds on growth and TSST-1 production of S. aureus strain MN8
| Compound | Compound concn (%) | % Control
|
|
|---|---|---|---|
| Growth | TSST-1 | ||
| Acetaminophen | 0.50 | 13.2 | 10.8 |
| 0.10 | 44.2 | 56.3 | |
| Acetyl tyrosine | 0.50 | 19.9 | 47.1 |
| 0.10 | 47.5 | 71.5 | |
| Benzyl alcohol | 0.50 | 8.9 | 13.8 |
| 0.10 | 49.7 | 43.5 | |
| Gallic acid | 0.50 | 2.6 | 8.7 |
| 0.10 | 45.0 | 41.4 | |
| p-Hydroxybenzoic acid | 0.50 | NDa | ND |
| methyl ester | 0.10 | 21.4 | 20.7 |
| PABA | 0.40 | ND | ND |
| 0.20 | 11.8 | 8.6 | |
| Phenoxyethanol | 0.50 | 32.3 | 25.1 |
| 0.10 | 54.3 | 42.3 | |
| Salicylic acid | 0.50 | ND | ND |
| 0.10 | 31.0 | 26.3 | |
| Salicylamide | 0.50 | 14.5 | 11.3 |
| 0.10 | 61.5 | 49.2 | |
| Vanillic acid | 0.50 | ND | ND |
| 0.10 | 24.5 | 38.5 | |
ND, not determined.
FIG. 2.
Effect of different concentrations of phenylethyl alcohol on growth and TSST-1 level of S. aureus strains FRI-1169, FRI-1187, and MN8. The bacteria were cultivated in GM supplemented with 1, 0.5, 0.25, 0.1, 0.05, 0.01% phenylethyl alcohol or with no phenylethyl alcohol (series 1 through 7, respectively). A total of 5 × 106 bacteria were cultivated for 24 h at 35°C, and growth was measured as the OD of the samples (▴, FRI-1169; ⧫, FRI-1187; •, MN8). The amount of TSST-1 in culture supernatant fluids is represented by the bars.
Seven different volatile oils, compounds containing an isoprene unit, were examined for their effect on growth and TSST-1 production using S. aureus MN8 (Table 6). At the concentrations examined, the isoprenoid compounds caused a wide range of growth effects. In general, the concentrations tested were selected to allow observation of the effect of the compound on TSST-1 production. Although the amount of growth ranged from undetectable to 124.7% of the control, TSST-1 production was inhibited at every concentration tested. With the exception of β-myrcene, the compounds all displayed a large reduction in TSST-1 compared to untreated cultures.
TABLE 6.
Effectof isoprenoid compounds on growth and toxin production of S. aureus strain MN8
| Compound | Compound concn (mM) | % Control
|
|
|---|---|---|---|
| Growth | TSST-1 | ||
| β-Ionone | 3.75 | 1.0 | ND |
| 0.75 | 27.1 | 10.0 | |
| β-Myrcene | 75.00 | 76.5 | 75.2 |
| 0.75 | 110.8 | 60.3 | |
| Geraniol | 3.75 | 3.9 | ND |
| 0.75 | 19.3 | 0.7 | |
| Linalool | 3.75 | NDa | ND |
| 0.75 | 121.6 | 12.2 | |
| Menthol | 6.40 | 32.2 | 1.8 |
| 3.20 | 119.5 | 24.4 | |
| p-Menthane-1,8-diol | 37.50 | 118.7 | 23.8 |
| 7.50 | 124.7 | 77.9 | |
| Terpineol | 6.50 | 90.5 | 12.4 |
| 0.65 | 112.7 | 82.3 | |
| Thymol | 3.35 | ND | ND |
| 0.67 | 99.0 | 37.4 | |
ND, not determined.
The final group of compounds are reported to be antimicrobial agents acting on enzymes that are required for fatty acid biosynthesis. Three inhibitors of fatty acid biosynthesis were examined for their effect on bacterial growth and their ability to inhibit TSST-1 production using either MN8 or FRI1187 (Table 7). As with the compounds in the other classes, there were differences in the numbers of bacteria observed after 24 h of growth. For the compounds, the higher concentration level tested resulted in the isolation of fewer viable bacteria compared to samples tested at lower concentrations or untreated controls. The amount of TSST-1 produced was <50% of the control value with two exceptions. The compound 4-hydroxydiphenylmethane, which has a chemical structure similar to that of triclosan, but does not inhibit fatty acid biosynthesis, only modestly reduced amount of TSST-1 produced by strain MN8; similar results were seen with hexachlorophene treatment of FRI-1187.
TABLE 7.
Effectof inhibitors of fatty acid biosynthesis and analogues on growth and TSST-1production by S. aureus
| S. aureus strain and compound | Compound concn (μg/ml) | % Control
|
|
|---|---|---|---|
| Growth | TSST-1 | ||
| MN8 | |||
| 4-Hydroxydiphenylmethane | 2.00 | 37.1 | 77.2 |
| 1.00 | 70.2 | 91.0 | |
| Cerulenin | 120.00 | 50.0 | 10.7 |
| 80.00 | 235.1 | 8.4 | |
| Hexachlorophene | 2.00 | 123.7 | 19.6 |
| 1.00 | 143.81 | 48.1 | |
| Triclosan | 0.05 | 0.0 | NDa |
| 0.01 | 5.6 | ND | |
| FRI-1187 | |||
| Hexachlorophene | 3.00 | 35.2 | 63.5 |
| 1.50 | 75.0 | 74.3 | |
| Triclosan | 0.50 | 35.5 | 3.7 |
| 0.05 | 136.4 | 9.9 | |
ND, not determined.
Evaluation of combinations of test compounds.
Four matrix experiments with mixtures of two compounds at two or three different concentrations were evaluated for their effect on S. aureus growth and TSST-1 concentration. In the first experiment, phenylethyl alcohol with myreth-3-myristrate was shown to reduce the level of TSST-1 per ml compared to using myreth-3-myristrate alone (Fig. 3A). Over the range of concentrations tested, increasing concentrations of phenylethyl alcohol and myreth-3-myristrate (either alone or in combination) reduced the amount of TSST-1 per sample. A second experiment used a range of terpineol concentrations tested in combination with increasing concentrations of myreth-3-myristrate (Fig. 3B). At the two highest concentrations of terpineol tested, there was a reduction in the level of S. aureus TSST-1 per ml at the levels of myreth-3-myristrate tested compared to myreth-3-myristrate alone. In a third experiment, the combination of Glucopon and myreth-3-myristrate were tested (Fig. 3C). With mixtures of these compounds, only the highest level of Glucopon correlated with further decreases in the level of TSST-1 per ml compared to the results obtained with myreth-3-myristrate alone. For example, at 10 mM myreth-3-myristrate, 0.25 mM Glucopon appears to antagonize the toxic-reducing effect of 10 mM myreth-3-myristrate. In a final experiment, Glucopon and phenylethyl alcohol were tested in combination (Fig. 3D). All four concentrations of phenylethyl alcohol, augmented with either 2.5 or 5.0 mM Laureth-4, caused a reduction in the amount of TSST-1 per ml compared to the levels associated with Laureth-4 alone.
DISCUSSION
A tampon-related mTSS epidemic stimulated research aimed at ameliorating the effects of staphylococcal infections. One avenue pursued in these investigations involved rendering S. aureus nonpathogenic through the prophylactic use of compounds that either reduce or eliminate virulence factor expression. We report here that several compounds, grouped in the present study as surfactants, aromatics, isoprenoids, and fatty acid biosynthesis inhibitors, have a minimal impact on bacterial growth and yet suppress S. aureus TSST-1 production. Many of these compounds are hydrophobic and, like myreth-3-myristate and glycerol monolauride, may effectively reduce staphylococcal TSST-1 production when added to tampon cover material (18, 24). In addition, some of the tested compounds may be useful when incorporated into other disposable absorbent articles to reduce the risk of S. aureus-induced TSS.
Of the tested nonionic surfactants, those with ether linkages to fatty acid moieties exhibited the greatest level of toxin-suppressing activity. For example, myreth-3-myristate, a triethylene derivative containing both an ether and an ester bonded 14 carbon fatty acid, and Laureth-4, a polyoxyether of lauryl alcohol, maximally suppressed S. aureus strain MN8 TSST-1 production by 77.8 and 98.5%, respectively. In contrast, a surfactant with glycosidic linkage, Glucopon, when used at a level that allowed growth that was comparable to that of untreated bacteria, reduced toxin production by only 18.8%. At this time, it is not known whether S. aureus is capable of cleaving the glycosidic bond or oxidizing the fatty acid tail to inactivate Glucopon, but a cytoplasmic α-d-glucosidase and a protein similar to alkane 1-monooxygenase and the enzymes for β-oxidation are encoded in the genome of this organism (5, 10). Furthermore, many species of human-associated microorganisms produce α-d-glucosidase, which may further limit the effectiveness of prophylactic or therapeutic effect of Glucopon.
Other investigators have demonstrated that a laurate substituted dodecanoic acid monoester, glycerol monolaurate, retards bacterial virulence factor expression (8, 15, 20, 25, 33). An observed problem with glycerol monolaurate was that the ester bond was degraded by human and staphylococcal esterases and lipases, rendering low concentrations of the compound ineffective (20). The degradation of glycerol monolaurate suggested that surfactants containing single ester linkages are vulnerable to biological degradation and would be of limited prophylactic utility. The TSST-1-reducing ability of myreth-3-myristate suggests that the active portion of the molecule is an ether linked fatty acid alcohol. Laureth-4 represents on such compound. Laureth-4 inhibits the growth of S. aureus over a wide range of concentrations of <50 mM (data not shown); however, given sufficient time, growth recovers to levels associated with untreated bacteria. Without regard to inclusion of Laureth-4 in the culture medium, the recovered bacteria were found to either make very small quantities of TSST-1 or to produce undetectable levels of TSST-1. Other surfactants with ether linkages to fatty acids having the structure R1-O-R2, where R1 is a straight or branched chain alkyl group with from 8 to 18 carbon atom and R2 is an alcohol, a polyalkoxylated sulfate salt, or a polyalkoxylated sulfosuccinate salt, are presumably not degraded, and these compounds display an ability to suppress TSST-1 production. These findings also appear applicable to low levels (>10.7 mM) of surfactants that contain amine linkages such as sodium lauriminodipropionic acid and triethanolamine (TEA) laureth sulfate. Surfactants with amide linkages to fatty acids such as disodium lauroamphodiacetate and disodium lauramido monoethanolamido (MEA) sulfosuccinate appear susceptible to amidases, much as esters are susceptible to lipases and esterases.
In studies of S. aureus, phenylethyl alcohol has been shown to inhibit the production alpha-toxin and other exoproteins that contain a membrane translocation signal sequence (11). In Escherichia coli, the mechanism by phenylethyl alcohol has been associated with induced, yet reversible, alterations of the cell membrane (27). Low concentrations of phenylethyl alcohol has been reported to affect the rate of synthesis of individual phospholipids and, as a consequence, it alters the composition and function of the cell membrane (13). Further study showed that minimal concentrations of phenylethyl alcohol also impairs the synthesis of saturated fatty acids at the level of the acetyltransferase (13). Unlike the toxin inhibition observed in S. aureus, where phenylethyl ethanol has been shown to interfere with protein translocation, in E. coli this compound has been reported to affect de novo protein synthesis (29).
Phenylethyl alcohol is a member of a large class of substituted aromatic compounds commonly used in the pharmaceutical, food, and cosmetic industries. Similar compounds, such as the aromatic ether alcohol, phenoxyethanol, have been used as preservatives, as perfume fixatives, and in insect repellents. Many compounds of this class, including esters of p-hydroxybenzoic acid, salicylic acid, and benzyl alcohol, are known to have antimicrobial effects. Given the effect of phenylethyl alcohol on the inhibition of protein expression, we examined this compound and a series of related compounds for their effect on S. aureus TSST-1 production and bacterial growth. We demonstrated an inhibitory effect of phenylethyl alcohol on TSST-1 production and showed that this effect was not strain dependent using three independently isolated strains of S. aureus. In addition, other aromatic compounds were shown to reduce TSST-1 production. In terms of bacterial growth in the presence of phenylethyl alcohol, FRI-1169 and FRI1187 exhibited a dose-dependent increase in bacteria as measured by the OD, whereas MN8 only followed this pattern at phenylethyl alcohol concentrations of <0.5%. The other tested aromatics had a minimal effect on the growth of S. aureus.
Alteration of membranes, membrane function, and the suppression of the TSST-1 production by phenylethyl alcohol led to testing of isoprenoid (terpenoid) compounds. Isoprenoid compounds are the building blocks of membrane components, many of which play a role in membrane permeability and fluidity (4). Isoprenoid compounds include important secondary metabolites such as carotenoids, sterols, polyphenyl alcohols. Isoprenoid compounds are commonly used in hygienic products, antioxidant medications, perfumes, precursors for organic syntheses, and in flavor enhancers. We examined a range of compounds including: a monoisoprenoid, monoisoprenoid alcohols, monoisopremoid phenols, and menthol, commercially synthesized as a derivative of myrcene. The selected compounds, at the concentrations tested, exhibited a range in their ability to suppress growth and TSST-1 production.
Cerulenin, an antibiotic compound produced by Cephalosporium caerulens, was selected for testing because previous studies have shown that it interferes with fatty acid metabolism and inhibits the synthesis of secreted proteins (6, 7). Cerulenin is a potent inhibitor of the subtypes of β-keto-acyl-ACP synthase, the gene products of fabB and fabF (16). The mechanism of inhibition involves cerulenin directly binding to these enzymes. This binding blocks the initial condensation step of fatty acid biosynthesis, preventing the formation of the chain-elongating molecule acetoacetyl-ACP. The effect of sub-growth-inhibitory levels of cerulenin has been shown to inhibit the production of alpha-toxin and enterotoxin B (2, 3, 16). Recently, these findings have been extended to show that cerulenin profoundly inhibits the transcription of secreted proteins but has only a minimal effect on the elaboration of cytoplasmic proteins (1). Here we show that cerulenin is inhibitory to growth at the higher of the two test concentrations and stimulatory to growth at the lower test concentration. The concentrations tested were in line with previously reported MICs for S. aureus treated with cerulenin but were at least 16-fold higher than those reported to inhibit secreted toxin production (1).
In addition to cerulenin, we tested the fatty acid synthesis inhibitor triclosan. Triclosan specifically targets and inhibits bacterial β-ketoacyl-ACP reductase, the gene product of fabI (7, 28). This enzyme is required for the formation of D-3-hydroxyacyl-ACP, an early intermediate in the synthesis of fatty acids. Two strains of S. aureus, MN8 and FRI-1187, were tested for growth and TSST-1 production in the presence of triclosan because naturally occurring resistant isolates, strains that overexpress fabI, have been reported (28). At the lowest concentration of triclosan tested, the growth of strain MN8 was almost totally inhibited. However, FRI-1187 was intrinsically resistant or more readily adapted to the growth-inhibitory action of triclosan than was strain MN8, and we could show a TSST-1-inhibitory effect of triclosan.
Finally, we tested two compounds that are structurally related to triclosan, hexachlorophene and 4-hydroxydiphenylmethane. Like triclosan, hexachlorophene is polychlorinated phenolic compound with an ether linkage joining the ring moieties. 4-Hydroxydiphenylmethane differs from triclosan and hexachlorophene in that it is nonhalogenated and has a carbon bridge between its ring structures. Hexachlorophene is reported to disrupt bacterial membranes and mechanistically, like triclosan, functions by inhibiting β-ketoacyl-ACP reductase (7). In contrast, 4-hydroxydiphenylmethane does not inhibit this enzyme. As expected, subinhibitory concentrations of hexachlorophene suppressed TSST-1 production in both strains MN8 and FRI-1187, whereas 4-hydroxydiphenylmethane lacks this activity.
Selected compounds can inhibit S. aureus TSST-1 production and that of other secreted toxins (see references 30, 31, and 32) at concentrations that allow for wild-type growth. This novel activity typically eludes detection in screens for antimicrobial agents. Although the intended use of these compounds is in medical or personal hygiene products, their value may extend to use as supplements in solutions or lotions where antivirulence properties would be beneficial. Although the exact mechanism of TSST-1 suppression by these compounds remains to be elucidated, all of the chemicals appear to affect membrane fluidity and, subsequently, the expression of toxins. The matrix experiments, wherein the TSST-1-reducing effect of different levels of two compounds were tested in combination, illustrated that the tested compounds had complementary activities. Further studies aimed at understanding the mechanism of action of the selected compounds will delineate their potential for a role in prophylaxis or treatment of staphylococcal infections and infections due to other microorganisms.
Acknowledgments
This work was supported by the Kimberly-Clark Corporation.
Footnotes
Published ahead of print on 17 February 2009.
REFERENCES
- 1.Adhikari, R. P., and R. P. Novick. 2005. Subinhibitory cerulenin inhibits staphylococcal exoprotein production by blocking transcription rather than by blocking secretion. Microbiology 151:3059-3069. [DOI] [PubMed] [Google Scholar]
- 2.Altenbern, R. A. 1977. Enterotoxin B production by Staphylococcus aureus under controlled fatty acid nutrition induced by cerulenin. Can. J. Microbiol. 23:1145-1150. [DOI] [PubMed] [Google Scholar]
- 3.Altenbern, R. A. 1977. Extreme sensitivity of staphylococcal enterotoxin B and C production to inhibition by cerulenin. Antimicrob. Agents Chemother. 11:906-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloch, K. E. 1983. Sterol structure and membrane function. CRC Crit. Rev. Biochem. 14:47-92. [DOI] [PubMed] [Google Scholar]
- 5.Gill, S. R., D. E. Fouts, G. L. Archer, E. F. Mongodin, R. T. Deboy, J. Ravel, I. T. Paulsen, J. F. Kolonay, L. Brinkac, M. Beanan, R. J. Dodson, S. C. Daugherty, R. Madupu, S. V. Angiuoli, A. S. Durkin, D. H. Haft, J. Vamathevan, H. Khouri, T. Utterback, C. Lee, G. Dimitrov, L. Jiang, H. Qin, J. Weidman, K. Tran, K. Kang, I. R. Hance, K. E. Nelson, and C. M. Fraser. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 187:2426-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg, I., J. R. Walker, and K. Bloch. 1973. Inhibition of lipid synthesis in Escherichia coli cells by antibiotic cerulenin. Antimicrob. Agents Chemother. 3:549-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heath, R. J., J. Li, G. E. Roland, and C. O. Rock. 2000. Inhibition of the Staphylococcus aureus NADPH-dependent enoyl-acyl carrier protein reductase by triclosan and hexachlorophene. J. Biol. Chem. 18:4654-4659. [DOI] [PubMed] [Google Scholar]
- 8.Holland, K. T., D. Taylor, and A. M. Farrell. 1994. The effect of glycerol monolaurate on growth of, and production of toxic shock syndrome toxin-1 and lipase by, Staphylococcus aureus. Antimicrob. Agents Chemother. 33:41-55. [DOI] [PubMed] [Google Scholar]
- 9.Koch, A. L. 1994. Growth measurement, p. 248-277. In P. Gerhardt, R. G. E. Murray, R. N. Costilow, E. W. Nester, W. A. Wood, N. R. Krieg, and G. B. Phillips (ed.), Manual of methods for general bacteriology. American Society for Microbiology, Washington, DC.
- 10.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Hosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshino, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 11.Lee, K. Y., and T. H. Birbeck. 1985. Effect of phenethyl alcohol on Staphylococcus aureus alpha-lysin production. Infect. Immun. 47:112-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melish, M., S. Fukunaga, and S. Murata. 1998. Effect of glycerol monolaurate (GML) and pluronic L92 (PL92) on illness and TSST-1 production in a subcutaneous depot model for TSS, p. 144. In J. Arbuthmott and B. Furman (ed.), European Conference on Toxic Shock Syndrome, vol. 229. Royal Society of Medicine Services, London, United Kingdom. [Google Scholar]
- 13.Nunn, W. D. 1975. The inhibition of phospholipids synthesis in Escherichia coli by phenethyl alcohol. Biochim. Biophys. Acta 380:403-413. [DOI] [PubMed] [Google Scholar]
- 14.Onderdonk, A. B., G. R. Zamarchi, M. L. Rodriguez, M. L. Hirsch, A. Munoz, and E. H. Kass. 1987. Qualitative assessment of vaginal microflora during use of tampons of various compositions. Appl. Environ. Microbiol. 53:2779-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson, M. L., and P. M. Schlievert. 2006. Glycerol monolaurate inhibits the effects of gram-positive select agents on eukaryotic cells. Biochemistry 45:2387-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price, A. C., K. H. Choi, R. J. Heath, Z. Li, S. W. White, and C. O. Rock. 2001. Inhibition of β-ketoacyl-aacyl carrier protein synthase by thiolactomycin and cerulenin: structure and mechanism. J. Biol. Chem. 276:6551-6559. [DOI] [PubMed] [Google Scholar]
- 17.Projan, S. J., S. Brown-Skrobot, P. M. Schlievert, F. Vandenesch, and R. P. Novick. 1994. Glycerol monolaurate inhibits the production of β-lactamase, toxic shock syndrome toxin-1, and other staphylococcal exoproteins by interfering with signal transduction. J. Bacteriol. 176:4204-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins, R. N., B. J. Kelly, G. L. Hehl, and M. S. Bergdoll. 1989. Effect of tampon wraps on production of toxic shock syndrome toxin-1. Rev. Infect. Dis. 11:S197-S202. [DOI] [PubMed] [Google Scholar]
- 19.Ruzin, A., and R. P. Novick. 1998. Glycerol monolaurate inhibits induction of vancomycin resistance in Enterococcus faecalis. J. Bacteriol. 180:182-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruzin, A., and R. P. Novick. 2000. Equivalence of lauric acid and glycerol monolaurate as inhibitors of signal transduction in Staphylococcus aureus. J. Bacteriol. 182:2668-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saleh, F. A., and J. H. Freer. 1982. Inhibition of secretion of staphylococcal alpha toxin by cerulenin. J. Med. Microbiol. 18:205-216. [DOI] [PubMed] [Google Scholar]
- 22.Schlievert, P. M. 2005. Staphylococcal toxic shock syndrome: still a problem. Med. J. Aust. 182:651-652. [DOI] [PubMed] [Google Scholar]
- 23.Schlievert, P. M., and D. A. Blomster. 1983. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J. Infect. Dis. 147:236-242. [DOI] [PubMed] [Google Scholar]
- 24.Schlievert, P. M., D. A. Blomster, and J. A. Kelly. 1984. Toxic shock syndrome Staphylococcus aureus: effect of tampons on toxic shock syndrome toxin 1 production. Obstet. Gynecol. 64:666-671. [PubMed] [Google Scholar]
- 25.Schlievert, P. M., J. R. Deringer, M. H. Kim, S. J. Projan, and R. P. Novick. 1992. Effect of glycerol monolaurate on bacterial growth and toxin production. Antimicrob. Agents Chemother. 36:626-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shands, K. N., G. P. Schmid, B. B. Dan, D. Blum, R. J. Guidotti, N. T. Hargrett, R. L. Anderson, D. L. Hill, C. V. Broome, J. D. Band, and D. W. Fraser. 1980. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N. Engl. J. Med. 303:1436-1442. [DOI] [PubMed] [Google Scholar]
- 27.Silver, S., and L. Wendt. 1967. Mechanism of action of phenethyl alcohol: breakdown of the cellular permeability barrier. J. Bacteriol. 93:560-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slater-Radosti, C., G. Van Aller, R. Greenwood, R. Nicholas, P. M. Keller, W. E. DeWolf, Jr., F. Fan, D. J. Payne, and D. D. Jaworski. 2001. Biochemical and genetic characterization of the action of triclosan on Staphylococcus aureus. Antimicrob. Chemother. 48:1-6. [DOI] [PubMed] [Google Scholar]
- 29.Stim-Herndon, K. P. 1995. Effect of the local anesthetics phenethyl alcohol and procaine on hns mutants of the acid-induced biodegradative arginine (adi) and lysine (cad) decarboxylases of Escherichia coli. Curr. Microbiol. 130:281-285. [DOI] [PubMed] [Google Scholar]
- 30.Syverson, R. E. June 1995. Inhibition of exoprotein in absorbent article. U.S. patent 5,612,045.
- 31.Syverson, R. E. June 1995. Inhibition of exoprotein using amide compositions in absorbent article. U.S. patent 5,685,872.
- 32.Syverson, R. E. June 1995. Inhibition of exoprotein using amine compositions in absorbent article and method thereof. U.S. patent 5,618,554.
- 33.Vetter, S. M., and P. M. Schlievert. 2005. Glycerol monolaurate inhibits virulence factor production in Bacillus anthracis. Antimicrob. Agents Chemother. 49:1302-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong, A. C. L. 1987. Factors affecting growth of Staphylococcus aureus and production of toxic shock syndrome-1. Ph.D. thesis. University of Wisconsin, Madison.