Abstract
Polymorphisms in the Plasmodium falciparum crt (Pfcrt), Pfmdr1, and Pfmrp genes were not significantly associated with quinine (QN) 50% inhibitory concentrations (IC50s) in 23 strains of Plasmodium falciparum. An increased number of DNNND repeats in Pfnhe-1 microsatellite ms4760 was associated with an increased IC50 of QN (P = 0.0007). Strains with only one DNNND repeat were more susceptible to QN (mean IC50 of 154 nM). Strains with two DNNND repeats had intermediate susceptibility to QN (mean IC50 of 548 nM). Strains with three DNNND repeats had reduced susceptibility to QN (mean IC50 of 764 nM). Increased numbers of NHNDNHNNDDD repeats were associated with a decreased IC50 of QN (P = 0.0020). Strains with profile 7 for Pfnhe-1 ms4760 (ms4760-7) were significantly associated with reduced QN susceptibility (mean IC50 of 764 nM). The determination of DNNND and NHNDNHNNDDD repeats in Pfnhe-1 ms4760 could be a good marker of QN resistance and provide an attractive surveillance method to monitor temporal trends in P. falciparum susceptibility to QN. The validity of the markers should be further supported by analyzing more isolates.
Malaria is the most important parasitic disease in the world, affecting 300 to 500 million people and killing 3 million people every year. Quinine (QN) has been used as a malaria treatment for more than 350 years in Africa, with little emergence and spread of resistance. QN remains the first-line antimalarial drug for the treatment of complicated malaria in Europe and Africa. However, despite QN′s efficacy against chloroquine-resistant strains, the emergence of QN resistance (QNR) has been documented. The first cases of QN clinical failure were observed in Brazil and Asia in the 1960s (4, 12). In the 1980s, clinical failures became more frequent in Southeast Asia, South America, and Africa (13, 15, 19, 22, 33). However, QNR is not yet a significant problem. QN remains the first-line drug for severe malaria and remains widely used at present as a second-line therapy for uncomplicated malaria in Africa and other areas. Artemisinin-based combination therapies were proposed as a first-line treatment for uncomplicated malaria 6 years ago. Since 2001, more than 56 countries have officially adopted artemisinin-based combination therapies for the treatment of Plasmodium falciparum malaria. However, individual P. falciparum isolates that are resistant to artemisinin in vitro in Cambodia have been described (14, 21). It is not clear whether these strains are associated with clinical failures. One strategy that health officials can pursue to reduce the prevalence of malaria is to combine QN with other antimalarial drugs such as tetracycline (8, 18) or clindamycin (16).
Although some reports of treatment failure of QN exist, it is difficult to fully document QNR because of its short elimination half-life, the requirement to administer the drug three times a day for at least 5 days, drug intolerance often leading to poor compliance, and the lack of reliable data on the correlation between QN 50% inhibitory concentrations (IC50s) and clinical failure. Maximizing the efficacy and longevity of QN as a tool for malaria control will depend critically on pursuing intensive research into identifying in vitro markers as well as implementing in vitro and in vivo surveillance programs such as those championed by the World Antimalarial Resistance Network (30, 31). In this context, there is a need to identify molecular markers that predict QNR and that can provide an active surveillance method to monitor temporal trends in parasite susceptibility (23).
QNR appears to share common characteristics with chloroquine resistance. QNR is associated with mutations in both the P. falciparum multidrug resistance gene mdr1 (Pfmdr1) (20, 26) and the chloroquine resistance transporter gene Pfcrt (6, 7, 20). Nevertheless, the mechanism of QNR is still unclear. In addition to Pfmdr1 and Pfcrt, other genetic polymorphisms such as variations in microsatellite length on the sodium/hydrogen exchanger gene Pfnhe-1 (11) and mutations on the multidrug resistance protein gene Pfmrp might contribute to QNR (20). The evidence for the involvement of Pfnhe-1 or Pfmrp in QNR is limited. Only one previous study investigated the association of QN IC50 and polymorphisms in the Pfnhe-1 gene in P. falciparum isolates (11).
The objective of the present study was to investigate genetic polymorphisms in Pfcrt, Pfmrp, Pfmdr1, and Pfnhe-1 that could be associated with QNR in order to identify molecular markers of QNR that could be used for surveillance of resistance.
MATERIALS AND METHODS
Plasmodium falciparum cultures.
In total, 23 parasite strains (familiar laboratory strains or strains obtained from isolates after growth in culture for an extended period of time) from a wide panel of countries were maintained in culture in RPMI 1640 medium (Invitrogen, Paisley, United Kingdom), supplemented with 10% human serum (Abcys S.A., Paris, France), and buffered with 25 mM HEPES and 25 mM NaHCO3. Parasites were grown in type A+ human blood under controlled atmospheric conditions that consisted of 10% O2, 5% CO2, and 85% N2 at 37°C with a humidity of 95%. Clonality was verified using PCR genotyping of polymorphic genetic markers, msp1, msp2, and microsatellite loci. All strains were synchronized twice with sorbitol before use (17).
Drugs.
QN was purchased from Sigma (St. Louis, MO). QN was first dissolved in methanol and then diluted in water to obtain final concentrations between 0.125 and 400 nM for strains with QN IC50s of <500 nM and between 5.3 and 3,387 nM for strains with QN IC50s of >500 nM.
In vitro assay.
For in vitro isotopic microtests, 25 μl/well of QN and 200 μl/well of the suspension of synchronous parasitized red blood cells (>95% of rings; final parasitemia, 0.5%; final hematocrit, 1.5%) were distributed in 96-well plates. Parasite growth was assessed by adding 1 μCi of tritiated hypoxanthine with a specific activity of 14.1 Ci/mmol (Perkin-Elmer, Courtaboeuf, France) to each well at time zero. The plates were then incubated for 48 h under controlled atmospheric conditions. Immediately after incubation, plates were frozen and then thawed to lyse the erythrocytes. The content of each well was collected on standard filter microplates (Unifilter GF/B; Perkin-Elmer) and washed using a cell harvester (Filter-Mate cell harvester; Perkin-Elmer). Filter microplates were dried, and 25 μl of scintillation cocktail (Microscint O; Perkin-Elmer) was placed into each well. Radioactivity incorporated into nucleotides by the parasites was measured with a scintillation counter (Top Count; Perkin-Elmer).
The IC50 was assessed by the drug concentration resulting in 50% of the incorporation of tritiated hypoxanthine by the parasite in the drug-free control wells. The IC50 was determined by nonlinear regression analysis of log-based dose-response curves (Riasmart; Packard).
Nucleic acid extraction.
Total genomic DNA of each strain was isolated using the EZNA blood DNA kit (Omega Bio-Tek, GA). The RNA of each strain was purified using the QIAamp blood minikit (Qiagen, Germany).
Pfcrt single-nucleotide polymorphisms (SNPs).
A 1,250-nucleotide-length fragment of the Pfcrt gene was amplified by reverse transcription-PCR using primers F1-sense (5′-TAA TTT CTT ACA TAT AAC AAA ATG AAA TTC-3′) and F1-antisense (5′-TTA TTG TGT AAT AAT TGA ATC GAC-3′) and sequenced using primers F2-sense (5′-TAG GTG GAG GTT CTT GTC TTG GTA-3′) and F2-antisense (5′-TCG ACG TTG GTT AAT TCT CCT TC-3′) as previously described (9). Amplifications were performed according to the instructions provided in the Access reverse transcription-PCR system kit (Promega, Madison, WI). Sequencing was conducted using ABI Prism Big Dye Terminator v1.1 (Applied Biosystems, CA) cycle sequencing ready reaction kits according to the manufacturer's instructions.
Pfmdr1 SNPs.
Pfmdr1 was amplified by PCR using primer pairs 5′-TTA CAT TTT ATT TGA TTT TGT GTT G-3′ and 5′-CAT CTT TTC TAG TAT CAT AAT GAA-3′ to amplify codons 86 and 184 and 5′-ACG GGT TTA GTA AAT AAT ATT GTT-3′ and 5′-ATG GGT TCT TGA CTA ACT ATT G-3′ to amplify codons 1034, 1042, and 1246. Amplifications were performed using the Titanium PCR kit (Clontech, Ozyme, France) according to the manufacturer's instructions. The amplified fragments were sequenced as described above.
Pfmrp SNPs.
PCR amplification followed by sequencing was used to detect SNPs in Pfmrp at positions 191 and 437. The primers used for amplification and sequencing were pfmrp-501F (5′-TTT CAA AGT ATT CAG TGG GT-3′) and pfmrp-1409R (5′-GGC ATA ATA ATT GAT GTA AA-3′).
Pfnhe-1 microsatellite profiles.
A sequence containing the ms4760 microsatellite (11) was amplified using primers pfnhe-3802F (5′-TTATTAAATGAATATAAAGA-3′) and pfnhe-4322R (5′-TTTTTTATCATTACTAAAGA-3′). The amplified fragments were sequenced as described above.
Statistical analysis.
Because of the lack of reliable data on the correlation between QN IC50 and clinical failure and the arbitrary nature of cutoff values chosen for in vitro resistance (300 nM, 500 nM, or 800 nM in different studies) (5, 24, 25), the association between markers and QN response was assessed using the IC50 as a continuous variable rather than as a categorical variable (i.e., in vitro QNR). Differences in IC50s for QN were tested using the Mann-Whitney U test or the Kruskal-Wallis test. The results of these tests were compared according to the alleles at each locus. The differences in IC50s for QN were then tested 18 times (i.e., one per locus). The probability of getting a significant result with 18 tests at the level of significance of an α value of 0.05 was 1 − 0.9518 (where 1 is probability of not getting a significant result with 18 tests). According to the Bonferroni correction, it was concluded that a difference was significant when at least 1 of the 18 comparisons yielded a significance level below 0.05/18 = 0.0028 (1).
RESULTS
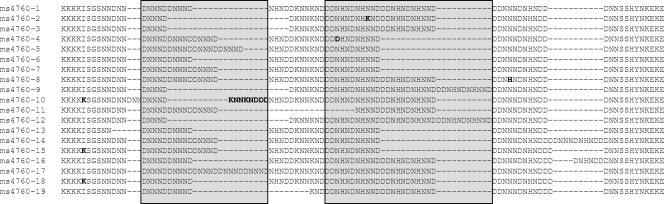
The following mutations were identified in at least one strain: C72S, M74I, N75E, K76T, A220S, Q271(E/V), N326(S/D), I356(T/L), and I371R for Pfcrt; H191Y and S437A for Pfmrp; and N86Y, Y184F, S1034C, N1042D, and D1246Y for Pfmdr1 (Table 1). ms4760 microsatellite profiles of Pfnhe-1 were incremented from 1 to 19 (Fig. 1). Eight different profiles were described. The number of DNNND and NHNDNHNNDDD repeats on ms4760 ranged from 1 to 3 (Fig. 1 and Table 1).
TABLE 1.
Pfcrt, Pfmdr1, Pfmrp, and Pfnhe-1 polymorphisms in 23 Plasmodium falciparum strainsa
| Strain | Origin | QN IC50 (nM) | Amino acid encoded by Pfcrt codon:
|
Amino acid encoded by Pfmdr1 codon:
|
Amino acid encoded by Pfmrp codon:
|
Pfnhe-1 microsatellite ms4760b
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 74 | 75 | 76 | 220 | 271 | 326 | 356 | 371 | 86 | 184 | 1034 | 1042 | 1246 | 191 | 437 | No. of DNNND repeats | No. of DDNHNDNHNN repeats | Mutation | Profile | |||
| IMT 9881 | Niger | 57 | M | N | K | A | Q | N | I | R | N | Y | S | N | D | H | S | 3 | 2 | N | 9 |
| IMT 10354 | Comoros | 58 | I | E | T | S | Q | N | I | I | Y | F | S | N | Y | H | S | 1 | 3 | N | 12 |
| D6 | Sierra Leone | 62 | M | N | K | A | Q | N | I | R | N | Y | S | N | D | H | S | 1 | 2 | N | 3 |
| IMT 16332 | Congo | 83 | I | E | T | S | E | N | T | I | Y | Y | S | N | D | H | S | 1 | 2 | N | 3 |
| 3D7 | Africa | 128 | M | N | K | A | Q | N | I | R | N | Y | S | N | D | H | S | 1 | 2 | K | 2 |
| IMT 8425 | Senegal | 148 | M | N | K | A | Q | N | I | R | N | Y | S | N | D | H | S | 1 | 2 | K | 2 |
| IMT 16116 | Congo | 204 | I | E | T | S | E | N | I | I | Y | F | S | N | D | H | S | 2 | 2 | N | 18 |
| IMT 10500 | Comoros | 210 | M | N | K | A | E | N | I | R | N | Y | S | N | D | H | S | 1 | 2 | K | 2 |
| IMT 10336 | Comoros | 271 | M | N | K | A | E | N | I | R | N | Y | S | N | D | H | S | 1 | 2 | N | 3 |
| FCR3 | The Gambia | 334 | I | E | T | S | E | S | I | I | Y | Y | S | N | D | Y | A | 2 | 1 | N | 6 |
| IMT 31 | Senegal | 371 | M | N | K | A | Q | N | I | R | N | Y | S | N | D | H | S | 1 | 2 | N | 3 |
| IMT Vol | Djibouti | 421 | I | E | T | S | E | S | I | I | Y | Y | S | N | D | Y | A | 2 | 1 | N | 6 |
| IMT 6311 | Senegal | 453 | I | E | T | S | E | N | T | I | Y | F | S | N | D | H | S | 3 | 1 | N | 7 |
| IMT Bres | Brazil | 500 | I | E | T | S | E | S | I | I | Y | Y | S | N | D | Y | A | 2 | 1 | N | 6 |
| IMT L1 | Niger | 575 | I | E | T | S | E | N | I | I | Y | Y | S | N | D | Y | A | 2 | 1 | N | 6 |
| FCM29 | Cameroon | 580 | I | E | T | S | E | S | I | I | Y | Y | S | N | D | Y | A | 3 | 1 | N | 7 |
| IMT K2 | Cambodia | 599 | I | E | T | S | V | S | T | I | N | F | C | D | D | Y | A | 3 | 1 | N | 7 |
| IMT A4 | Thailand | 605 | I | E | T | S | E | S | I | I | N | F | C | D | Y | H | S | 3 | 1 | N | 7 |
| PA | Uganda | 625 | I | E | T | S | E | S | I | I | Y | Y | S | N | D | Y | A | 2 | 1 | N | 6 |
| W2 | Indochina | 686 | I | E | T | S | E | S | T | I | Y | Y | S | N | D | Y | A | 2 | 2 | N | 1 |
| IMT K4 | Cambodia | 1,034 | I | E | T | S | E | S | T | I | N | Y | C | D | D | Y | A | 3 | 1 | N | 7 |
| IMT 9996 | Comoros | 1,039 | M | N | K | A | Q | N | I | R | N | Y | S | N | D | H | S | 2 | 1 | N | 6 |
| IMT K14 | Cambodia | 1,310 | I | E | T | S | E | S | T | I | N | F | C | D | Y | Y | A | 3 | 1 | N | 7 |
Boldface indicates point mutations.
Polymorphism types are detailed in Fig. 1.
FIG. 1.
Sequences of Pfnhe-1 microsatellite ms4760 detected among the 23 P. falciparum strains studied.
The QN IC50 was not significantly associated with polymorphisms in the Pfcrt gene (P = 0.0454 to 0.0687, depending on position), the Pfmdr1 gene (P = 0.0119 to 1.0000), or the Pfmrp gene (P = 0.0036) after Bonferroni correction (Table 2). Only one locus of Pfcrt (N326R) was found to be significantly associated with QN IC50.
TABLE 2.
Association of in vitro QN response (IC50) and polymorphisms on the Pfnhe-1, Pfcrt, Pfmdr1, and Pfmrp genes of 23 strains of Plasmodium falciparuma
| Genotype | P value | Significanceb |
|---|---|---|
| Pfnhe-1 ms4760 profiles | 0.0021 | S |
| Pfnhe-1, no. of DNNND repeats | 0.0007 | S |
| Pfnhe-1, no. of NHNDNHNNDDD repeats | 0.0020 | S |
| Mutation in codon 74 of Pfcrt gene | 0.0454 | NS |
| Mutation in codon 75 of Pfcrt gene | 0.0454 | NS |
| Mutation in codon 76 of Pfcrt gene | 0.0454 | NS |
| Mutation in codon 220 of Pfcrt gene | 0.0454 | NS |
| Mutation in codon 271 of Pfcrt gene | 0.0688 | NS |
| Mutation in codon 326 of Pfcrt gene | 0.0019 | S |
| Mutation in codon 356 of Pfcrt gene | 0.0687 | NS |
| Mutation in codon 371 of Pfcrt gene | 0.0454 | NS |
| Mutation in codon 86 of Pfmdr1 gene | 1.0000 | NS |
| Mutation in codon 184 of Pfmdr1 gene | 0.5754 | NS |
| Mutation in codon 1034 of Pfmdr1 gene | 0.0119 | NS |
| Mutation in codon 1042 of Pfmdr1 gene | 0.0119 | NS |
| Mutation in codon 1246 of Pfmdr1 gene | 0.5228 | NS |
| Mutation in codon 191 of Pfmrp gene | 0.0036 | NS |
| Mutation in codon 437 of Pfmrp gene | 0.0036 | NS |
Determined by Mann-Whitney U test or Kruskal-Wallis test. The variable was the QN IC50. The significance cutoff was 0.0028 (0.05/18, 18 tests, Bonferroni correction).
S, significant; NS, nonsignificant.
Moreover, there were significant associations between the QN IC50 and Pfnhe-1 ms4760 profiles (P = 0.0021) and the number of DNNND (P = 0.0007) or NHNDNHNNDDD repeats (P = 0.0020) (Table 2).
A total of eight Pfnhe-1 ms4760 profiles were described for the 23 strains tested (Fig. 1 and Table 1). Only the most numerous profiles were included in the statistical analysis. Thus, a significant association between QN response and profiles was observed for seven strains with profile 2 (ms4760-2) or profile 3 (ms4760-3), six strains with profile 6 (ms4760-6), and six strains with profile 7 (ms4760-7) (P = 0.0021). These were the four most frequently found profiles, present in 83% of the P. falciparum parasites evaluated in this study. The mean QN IC50 (and standard deviation) for ms4760-2 or ms4760-3 were 178 ± 114 nM (first quartile, 62 nM; third quartile, 271 nM), meaning that these strains are susceptible to QN (inferior to the arbitrary cutoff of 500 nM). The mean IC50 for ms4760-6 was 582 ± 247 nM (first quartile, 421 nM; third quartile, 625 nM), suggesting that these strains had intermediate susceptibility to QN. The mean IC50 for ms4760-7 was 764 ± 332 nM (first quartile, 580 nM; third quartile, 1,034 nM), meaning that these strains had reduced susceptibility to QN.
The number of DNNND repeats ranged from 1 to 3 and was significantly associated with QN response (P = 0.0007). Strains with one DNNND repeat were more susceptible to QN (n = 9; mean IC50 ± standard deviation of 154 ± 110 nM; first quartile, 62 nM; third quartile, 210 nM) than strains with two DNNND repeats, which showed intermediate susceptibility to QN (n = 8; mean IC50 of 548 ± 253 nM; first quartile, 378 nM; third quartile, 656 nM). Strains with three DNNND repeats tended to have reduced susceptibility to QN (n = 6; mean IC50 of 764 ± 332 nM; first quartile, 580 nM; third quartile, 1,034 nM). A greater number of DNNND repeats was significantly associated with increased IC50 of QN (P = 0.0007).
The number of NHNDNHNNDDD repeats ranged from 1 to 3 and was significantly associated with QN response (P = 0.0020). Only one strain had three repeats (IC50 = 58 nM). Having one NHNDNHNNDDD repeat was associated with intermediate susceptibility to QN (n = 12; mean IC50 of 673 ± 295 nM; first quartile, 477 nM; third quartile, 830 nM). Having two NHNDNHNNDDD repeats was associated with QN susceptibility (n = 10; mean IC50 of 222 ± 190 nM; first quartile, 83 nM; third quartile, 271 nM). A greater number of NHNDNHNNDDD repeats was significantly associated with decreased IC50 of QN (P = 0.0020).
DISCUSSION
Although some reports of treatment failure of QN exist, it is difficult to fully document QNR because of its short elimination half-life, drug intolerance often leading to poor compliance, and lack of reliable data on the correlation between QN IC50 and clinical failure. In this context, molecular markers that predict QNR would be a major advance for the monitoring of drug-resistant malaria.
The evidence for the involvement of PfNHE in resistance to QN is limited and still debated. Studies to identify markers of QNR in P. falciparum parasites have been very limited (3, 11, 34). Only one of these studies tried to identify molecular markers according to QN IC50 values (11). In the present study, Pfnhe-1 polymorphism was significantly associated with QN response. The number of DNNND and NHNDNHNNDDD repeats was significantly associated with the response to QN. Strains with one DNNND repeat were susceptible to QN (QN IC50 of <260 nM), whereas strains with two or three DNNND repeats had intermediate (300 nM < QN IC50 < 700 nM) or reduced (QN IC50 > 600 nM) susceptibility to QN. A greater number of DNNND repeats was associated with an increased IC50 of QN. The three strains with IC50 values of >800 nM all had at least two DNNND repeats and a single NHNDNHNNDDD repeat. The distribution of the QN IC50 in three groups according to the number of DNNND repeats (IC50 < 300 nM, 300 nM < IC50 < 700 nM, and IC50 > 600 to 800 nM) is close to the arbitrary cutoff values defined for in vitro resistance and used in different studies: 300 nM (5), 500 nM (24), or 800 nM (25). The relationship between QN response and the number of DNNND repeats was previously observed (11). When the parasite isolates were grouped by the number of DNNND repeats (one versus two or more repeats), one repeat was more often found in the relatively QN-susceptible strains.
The number of NHNDNHNNDDD repeats was significantly associated with the response to QN. A greater number of NHNDNHNNDDD repeats was associated with a decreased IC50 of QN. Strains with one NHNDNHNNDDD repeat had intermediate susceptibility to QN (400 nM < QN IC50 < 800 nM). Having two NHNDNHNNDDD repeats was associated with QN susceptibility (QN IC50 < 300 nM).
In addition, some microsatellite profiles (ms4760) were significantly associated with QN response. A total of eight different ms4760 genotypes of Pfnhe-1 were observed among these 23 P. falciparum strains (19 profiles already described). Only the most numerous profiles were included in the statistical analysis. Having profile 7 for Pfnhe-1 ms4760 (ms4760-7) was significantly associated with reduced QN susceptibility (QN IC50 > 600 nM). Profile ms4760-6 was associated with strains with intermediate susceptibility to QN (400 nM < QN IC50 < 800 nM), and ms4760-2 or ms4760-3 were associated with strains that were susceptible to QN (QN IC50 < 300 nM). Only one strain, the reduced-susceptibility QN W2 clone from Indochina, showed an ms4760-1 profile. This profile was found in relatively QN-resistant strains Dd2 and 7G8 from Southeast Asia and South America (11) but also appeared frequently in other parasites with low QN responsivenesses from Southeast Asia, South America, and Africa (11). The frequency of ms4760 genotypes varied among regions. Profiles ms4760-2, ms4760-4, and ms4760-8 (not detected in the present study) were not detected among Indian isolates (34).
The numbers of DNNND and NHNDNHNNDDD repeats seem to be better molecular markers for reduced QN susceptibility than ms4760 profiles because the number of repeats varied less (from 1 to 5) than ms4760 genotypes (19 different genotypes) (34). However, this is a preliminary report. Twenty-three strains may not be sufficient to make definite conclusions. The validity of the markers should be further supported by analyzing more isolates.
The fact that PfNHE might be involved in QNR is supported by a physiological study. A recent study proposed a model for PfNHE activity in which the QN response could be altered by pH modifications into the parasite cytoplasm or digestive vacuole (3). These pH perturbations could depend on H+ transporters such as PfNHE, an Na+/H+ exchanger. DNNND repeat polymorphisms might alter the regulation of PfNHE activity (3).
In the present study, polymorphisms in the Pfcrt, Pfmdr1, or Pfmrp gene were not significantly associated with variations in the QN IC50. Some studies have reported contradictory results showing that polymorphisms of Pfcrt (11), Pfmrp (20), and other ABC transporter-encoded genes (2) were associated with QN response. The role of the protein encoded by Pfmdr1, Pgh1, in QNR is still unclear. Amplification of the Pfmdr1 gene was previously associated with QNR (32), but polymorphisms in Pfmdr1 had no effect on QN accumulation in the food vacuole (29). Nevertheless, the same authors showed that wild-type Pgh1 transports QN, whereas polymorphic Pgh1 variants do not transport QN (28). A recent study has shown that Pgh1 can transport dye into the parasite food vacuole and that this transport activity is inhibited in the presence of QN (27). A similar finding has been reported for human MDR, where QN also inhibited MDR transport function (35). It seems that a direct QN-PfCRT interaction was associated with altered QN susceptibility (6, 24). Nevertheless, the significance levels of the associations between QN IC50 and polymorphism in Pfcrt (codons 74, 75, 76, 220, 326, and 371), Pfmdr1 (codons 1034 and 1042), and Pfmrp (codons 191 and 437) were above the Bonferroni-corrected P value threshold (0.0028) in the present study but below 0.05. This drastic correction may explain the differences in the association between polymorphisms and QNR. The QNR phenotype is complex and appears to be affected by multiple genes located at different loci with either additive or pairwise effects on resistance (10, 11).
In conclusion, Pfnhe-1 polymorphism was significantly associated with QNresponse. The identification of Pfnhe-1 ms4760 profiles and the determination of DNNND and NHNDNHNNDDD repeats in ms4760 could be good markers of QNR for monitoring of drug resistance. However, this is a preliminary report. Twenty-three strains may not be sufficient to make definite conclusions. The validity of the makers should be further supported by analyzing more isolates. This paper is the first installment in what promises to be a continuing line of study. Epidemiological studies with large numbers of parasite samples with reduced susceptibility to QN are required to confirm the possible use of the Pfnhe-1 gene as a QNR marker. A multisite study with more than 250 isolates is in progress.
Acknowledgments
This work was supported by grant number 03CO006-05 from the Délégation Générale pour l'Armement (French Ministry of Defense).
We have no conflict of interest to declare.
Footnotes
Published ahead of print on 9 March 2009.
REFERENCES
- 1.Abdi, A. 2007. Bonferroni and Sidak corrections for multiple comparisons, p. 1-9. In N. J. Salkind (ed.), Encyclopedia of measurement and statistics. Sage, Thousand Oaks, CA.
- 2.Anderson, T. J., S. Nair, H. Qin, S. Singlam, A. Brockman, L. Paiphun, and F. Nosten. 2005. Are transporter genes other than the chloroquine resistance locus (pfcrt) and multidrug resistance gene (pfmdr) associated with antimalarial drug resistance? Antimicrob. Agents Chemother. 49:2180-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett, T. N., J. Patel, M. T. Ferdig, and P. D. Roepe. 2007. Plasmodium falciparum Na(+)/H(+) exchanger activity and quinine resistance. Mol. Biochem. Parasitol. 153:48-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorkman, A., and P. A. Phillips-Howard. 1990. The epidemiology of drug-resistant malaria. Trans. R. Soc. Trop. Med. Hyg. 84:177-180. [DOI] [PubMed] [Google Scholar]
- 5.Brasseur, P., J. Kouamouo, R. Moyou-Somo, and P. Druilhe. 1992. Multi-drug resistant falciparum malaria in Cameroon in 1987-1988. I. Stable figures of prevalence of chloroquine- and quinine-resistant isolates in the original foci. Am. J. Trop. Med. Hyg. 46:1-7. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, R. A., M. T. Ferdig, X. Z. Su, L. M. Ursos, J. Mu, T. Nomura, H. Fujioka, D. A. Fidock, P. D. Roepe, and T. E. Wellems. 2002. Alternative mutations at position 76 of the vacuolar transmembrane protein PfCRT are associated with chloroquine resistance and unique stereospecific quinine and quinidine responses in Plasmodium falciparum. Mol. Pharmacol. 61:35-42. [DOI] [PubMed] [Google Scholar]
- 7.Cooper, R. A., K. D. Lane, B. Deng, J. Mu, J. J. Patel, T. E. Wellems, X. Su, and M. T. Ferdig. 2007. Mutations in transmembrane domains 1, 4 and 9 of the Plasmodium falciparum chloroquine resistance transporter alter susceptibility to chloroquine, quinine and quinidine. Mol. Microbiol. 63:270-282. [DOI] [PubMed] [Google Scholar]
- 8.Duarte, E. C., C. J. Fontes, T. W. Gyorkos, and M. Abrahamowicz. 1996. Randomized controlled trial of artesunate plus tetracycline versus standard treatment (quinine plus tetracycline) for uncomplicated Plasmodium falciparum malaria in Brazil. Am. J. Trop. Med. Hyg. 54:197-202. [DOI] [PubMed] [Google Scholar]
- 9.Durrand, V., A. Berry, R. Sem, P. Glaziou, J. Beaudou, and T. Fandeur. 2004. Variations in the sequence and expression of the Plasmodium falciparum chloroquine resistance transporter (Pfcrt) and their relationship to chloroquine resistance in vitro. Mol. Biochem. Parasitol. 136:273-285. [DOI] [PubMed] [Google Scholar]
- 10.Ekland, E. H., and D. A. Fidock. 2007. Advances in understanding the genetic basis of antimalarial drug. Curr. Opin. Microbiol. 10:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferdig, M. T., R. A. Cooper, J. Mu, B. Deng, D. A. Joy, X. Z. Su, and T. E. Wellems. 2004. Dissecting the loci of low-level quinine resistance in malaria parasites. Mol. Microbiol. 52:985-997. [DOI] [PubMed] [Google Scholar]
- 12.Giboda, M., and M. B. Denis. 1988. Response of Kampuchean strains of Plasmodium falciparum to antimalarials: in-vivo assessment of quinine and quinine plus tetracycline; multiple drug resistance in vitro. J. Trop. Med. Hyg. 91:205-211. [PubMed] [Google Scholar]
- 13.Harinasuta, T., D. Bunnag, and R. Lasserre. 1990. Quinine resistant falciparum malaria treated with mefloquine. Southeast Asian J. Trop. Med. Public Health 21:552-557. [PubMed] [Google Scholar]
- 14.Jambou, R., E. Legrand, M. Niang, N. Khim, P. Lim, M. T. Ekala, C. Bouchier, P. Esterre, T. Fandeur, and O. Mercereau-Puijalon. 2005. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet 366:1960-1963. [DOI] [PubMed] [Google Scholar]
- 15.Jelinek, T., P. Schelbert, T. Löscher, and D. Eichenlaub. 1995. Quinine resistant falciparum acquired in east Africa. Trop. Med. Parasitol. 46:38-40. [PubMed] [Google Scholar]
- 16.Kremsner, P. G. 1990. Clindamycin in malaria treatment. J. Antimicrob. Chemother. 25:9-14. [DOI] [PubMed] [Google Scholar]
- 17.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 18.Looareesuwan, S., P. Wilairatana, S. Vanijanonta, D. Kyle, and K. Webster. 1992. Efficacy of quinine-tetracycline for acute uncomplicated falciparum malaria in Thailand. Lancet 339:369. [DOI] [PubMed] [Google Scholar]
- 19.Molinier, S., P. Imbert, D. Verrot, M. Morillon, D. Parzy, and J. E. Touze. 1994. Plasmodium falciparum malaria: type R1 quinine resistance in East Africa. Presse Med. 23:1494. [PubMed] [Google Scholar]
- 20.Mu, J., M. T. Ferdig, X. Feng, D. A. Joy, J. Duan, T. Furuya, G. Subramanian, L. Aravind, R. A. Cooper, J. C. Wootton, M. Xiong, and X. Z. Su. 2003. Multiple transporters associated with malaria parasite responses to chloroquine and quinine. Mol. Microbiol. 49:977-989. [DOI] [PubMed] [Google Scholar]
- 21.Noedl, H., Y. Se, K. Schaecher, B. L. Smith, D. Socheat, and M. M. Fukuda. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359:2619-2620. [DOI] [PubMed] [Google Scholar]
- 22.Palmieri, F., N. Petrosillo, M. G. Paglia, A. Conte, D. Goletti, L. P. Pucillo, M. Menegon, A. Sannella, C. Severini, and G. Majori. 2004. Genetic confirmation of quinine-resistant Plasmodium falciparum malaria followed by postmalaria neurological syndrome in a traveler from Mozambique. J. Clin. Microbiol. 42:5424-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plowe, C. V., C. Rooper, J. W. Barnwell, C. T. Happi, H. H. Joshi, W. Mbacham, S. R. Meshnick, K. Mugittu, I. Naidoo, R. N. Price, R. W. Shafer, C. H. Sibley, C. J. Sutherland, P. A. Zimmerman, and P. J. Rosenthal. 2007. World Antimalarial Resistance Network (WARN) III: molecular markers for drug resistant malaria. Malar. J. 6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pradines, B., C. Rogier, T. Fusai, A. Tall, F. F. Trape, and J. C. Doury. 1996. Sensibilité in vitro de 85 isolats de Plasmodium falciparum dans la region de Fatick, Sénégal. Med. Trop. 56:141-146. [PubMed] [Google Scholar]
- 25.Ralaimazava, P., R. Durand, N. Godineau, Z. Jezic, B. Pradines, O. Bouchaud, and J. Le Bras. 2002. Profile and evolution of the chemosusceptibility of falciparum malaria imported into France in 2000. Euro Surveill. 7:113-118. [DOI] [PubMed] [Google Scholar]
- 26.Reed, M. B., K. J. Saliba, S. R. Caruana, K. Kirk, and A. F. Cowman. 2000. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403:906-909. [DOI] [PubMed] [Google Scholar]
- 27.Rohrbach, P., C. P. Sanchez, K. Hayton, O. Friedrich, J. Patel, A. B. Sidhu, M. T. Ferdig, D. A. Fidock, and M. Lanzer. 2006. Genetic linkage of pfmdr1 with food vacuolar solute import in Plasmodium falciparum. EMBO J. 25:3000-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez, P. C., A. Rotmann, W. D. Stein, and M. Lanzer. 2008. Polymorphisms within PfMDR1 alter the substrate specificity for antimalarial drugs in Plasmodium falciparum. Mol. Microbiol. 70:786-798. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez, P. C., W. D. Stein, and M. Lanzer. 2008. Dissecting the components of quinine accumulation in Plasmodium falciparum. Mol. Microbiol. 67:1081-1093. [DOI] [PubMed] [Google Scholar]
- 30.Sibley, C. H., K. I. Barnes, and C. V. Plowe. 2007. The rationale and plan for creating a World Antimalarial Resistance Network (WARN). Malar. J. 6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sibley, C. H., K. I. Barnes, W. M. Watkins, and C. V. Plowe. 2008. A network to monitor antimalarial drug resistance: a plan for moving forward. Trends Parasitol. 24:43-48. [DOI] [PubMed] [Google Scholar]
- 32.Sidhu, A. B., A. C. Uhlemann, S. G. Valderramos, J. C. Valderramos, S. Krishna, and D. A. Fidock. 2006. Decreasing pfmdr1 copy number in Plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J. Infect. Dis. 194:528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tish, K. N., and P. I. Pillans. 1997. Recrudescence of Plasmodium falciparum malaria contracted in Lombok, Indonesia after quinine/doxycycline and mefloquine: case report. N. Z. Med. J. 110:255-256. [PubMed] [Google Scholar]
- 34.Vinayak, S., M. T. Alam, M. Upadhyay, M. K. Das, V. Dev, N. Singh, A. P. Dash, and Y. D. Sharma. 2007. Extensive genetic diversity in the Plasmodium falciparum Na+/H+ exchanger 1 transporter protein implicated in quinine resistance. Antimicrob. Agents Chemother. 51:4508-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weaver, J. L., G. Szabo, P. S. Pine, M. M. Gottesman, S. Goldenberg, and A. Aszalos. 1993. The effect of ion channel blockers, immunosuppressive agents, and other drugs on the activity of the multi-drug transporter. Int. J. Cancer 54:456-461. [DOI] [PubMed] [Google Scholar]