Abstract
An amantadine-resistant influenza A/Duck/MN/1525/81 (H5N1) virus was developed from the low-pathogenic North American wild-type (amantadine-sensitive) virus for studying treatment of infections in cell culture and in mice. Double combinations of amantadine, oseltamivir (or the cell culture-active form, oseltamivir carboxylate), and ribavirin were used. Amantadine-oseltamivir carboxylate and amantadine-ribavirin combinations showed synergistic interactions over a range of doses against wild-type virus in Madin-Darby canine kidney (MDCK) cell culture, but oseltamivir carboxylate-ribavirin combinations did not. Primarily additive interactions were seen with oseltamivir carboxylate-ribavirin combinations against amantadine-resistant virus. The presence of amantadine in drug combinations against the resistant virus did not improve activity. The wild-type and amantadine-resistant viruses were lethal to mice by intranasal instillation. The resistant virus infection could not be treated with amantadine up to 100 mg/kg body weight/day, whereas the wild-type virus infection was treatable with oral doses of 10 (weakly effective) to 100 mg/kg/day administered twice a day for 5 days starting 4 h prior to virus exposure. Drug combination studies showed that treatment of the amantadine-resistant virus infection with amantadine-oseltamivir or amantadine-ribavirin combinations was not significantly better than using oseltamivir or ribavirin alone. In contrast, the oseltamivir-ribavirin (25- and 75-mg/kg/day combination) treatments produced significant reductions in mortality. The wild-type virus infection was markedly reduced in severity by all three combinations (amantadine, 10 mg/kg/day combined with the other compounds at 20 or 40 mg/kg/day) compared to monotherapy with the three compounds. Results indicate a lack of benefit of amantadine in combinations against amantadine-resistant virus, but positive benefits in combinations against amantadine-sensitive virus.
The effective treatment of influenza virus infections remains a public health priority. In the 2007-2008 influenza season there was a rise in the number of infected individuals, due to two of the vaccine virus strains being suboptimally matched with viruses circulating in nature (2). The afflicted individuals would have benefited from antiviral drug treatment. The threat of emerging highly pathogenic avian influenza A (H5N1) viruses for which no vaccines exist is also a concern (29). Recent data indicate the widespread viral resistance to the antiviral drug amantadine (6, 11) and the growing frequency of resistance to the other widely used antiviral drug oseltamivir (5, 19). Certain clades of highly pathogenic H5N1 viruses are resistant to amantadine, whereas other clades are not (3, 15).
Highly pathogenic H5N1 virus infections of humans have a high human mortality rate, exceeding 60% (18). Such severe infections are difficult to treat with oseltamivir (4). Thus, there is a need for more potent therapy, as well as for treatment that may decrease the frequency of the emergence of drug-resistant viruses (14). Combination chemotherapy with the right medications may be the answer to both problems. Investigators over the years have studied various compounds in combination in vitro (10, 13, 14, 21, 26) and in mouse models (8, 16, 17, 20, 22, 26, 28) against the H1N1, H3N2, H5N1, and H9N2 strains of influenza viruses. These include the testing of M2 channel blockers amantadine and rimantadine; the neuraminidase inhibitors oseltamivir carboxylate, peramivir, and zanamivir; and the nucleoside analog ribavirin (an inhibitor of influenza virus RNA polymerase [7]). Since the M2 channel blockers, neuraminidase inhibitors, and ribavirin all have separate modes of antiviral action, various combinations of these inhibitors have been more beneficial than monotherapy in treating infections in cell culture and in mice. Due to the widespread occurrence of viruses that are resistant to amantadine, a recent study has focused on the treatment of amantadine-resistant influenza virus infections (17).
We have developed a mouse model using a low-pathogenic North American strain, influenza A/Duck/MN/1525/81 (H5N1) virus for antiviral drug testing. The virus causes a severe lethal respiratory infection in mice that is treatable by antiviral therapy (25). The experimental influenza A/Duck mouse infection model described in the present set of experiments is not optimal, as it does not fully reflect the type of pathogenesis of the highly pathogenic avian influenza H5N1 viruses. The low-pathogenic A/Duck virus does not contain the multibasic amino acid R-X-R/K-R motif in the hemagglutinin protein, whereas the highly pathogenic avian viruses do (8). Having this motif allows for the highly pathogenic viruses to be proteolytically activated by ubiquitous subtilisin-like cellular proteases, favoring systemic spread in vivo beyond the respiratory tract, causing multiorgan failure. Indeed, Ilyushina et al. demonstrated spread of highly pathogenic H5N1 virus strains to other organs besides the lungs with a mouse model (16, 17). A high virus titer (approximately 104 cell culture 50% infective doses [CCID50] per mouse) is required to induce lethality with the wild-type A/Duck virus, compared to 1 to 4 PFU of A/Vietnam or A/Turkey viruses (16, 17). Thus, the highly pathogenic viruses are more virulent in mice. The A/Duck virus is sensitive to neuraminidase inhibitors (27) and to the RNA polymerase inhibitors ribavirin and T-705 (25, 27). It is also sensitive to inhibition by amantadine in vitro, as reported herein.
For these studies we also developed an amantadine-resistant A/Duck virus that is lethal to mice. Treatment of infections caused by this virus was compared with the treatment of wild-type virus infections, using the drugs amantadine, oseltamivir (or the cell culture-active form oseltamivir carboxylate), and ribavirin. The results with mice correlate with recent reports by Ilyushina et al. (16, 17) using highly pathogenic H5N1 amantadine-sensitive and amantadine-resistant viruses. In those studies, amantadine-oseltamivir and oseltamivir-ribavirin combinations were evaluated, but not amantadine-ribavirin combinations. An advantage to using the A/Duck virus mouse model is that studies can be conducted in a low-containment laboratory.
To our knowledge, the present investigation represents the first report of the use of amantadine and ribavirin in combination in vitro against an amantadine-resistant H5N1 virus and of oseltamivir carboxylate and ribavirin in combination against either sensitive or resistant H5N1 viruses in vitro. This is also the first report of treatment of an H5N1 virus infection in mice with the combination of amantadine plus ribavirin.
MATERIALS AND METHODS
Antiviral compounds.
Amantadine was purchased from Sigma (St. Louis, MO). Jack Nguyen of Adamas Pharmaceuticals (Emeryville, CA) provided oseltamivir carboxylate, the active form of oseltamivir. Oseltamivir was purchased from a local pharmacy. Ribavirin was obtained from ICN Pharmaceuticals (Costa Mesa, CA). The compounds were dissolved in cell culture medium for antiviral testing or in water for oral gavage delivery to mice. Full chemical names of these compounds have been reported (16, 17). Since oseltamivir was used from pharmaceutical capsules that also contained ingredients besides the drug (as filler material), the contents of entire capsules (minus the shell) were added to water to make appropriate doses of mg/kg of body weight/day.
Viruses.
The low-pathogenic North American influenza A/Duck/MN/1525/81 (H5N1) virus was provided by Robert Webster (St. Jude Children's Research Hospital, Memphis, TN). It was passaged three times in mice to enhance its virulence in mice. The amantadine-resistant virus was developed by two passages of the virus in cell culture in the presence of 100 μM amantadine. Several clones were picked and assayed for resistance to amantadine. One clone was chosen and amplified twice in cell culture for further characterization. It was confirmed to be resistant to amantadine in cell culture studies and was later titrated in mice for lethality. The clone required no additional passages in mice to be lethal. The amantadine-resistant virus has an A30T mutation in the M2 protein (determined by TGen North, Flagstaff, AZ), consistent with a drug resistance genotype (1).
Cell culture assays.
The assays were performed with 96-well microplates infected with approximately 50 CCID50 per 0.1 ml of virus, by quantifying virus yield by the end-point dilution method (24, 27). Virus yields were determined from samples collected 72 h after infection, when untreated control microwells exhibited 100% cytopathic effect. To quantify the amount of virus produced in the presence of the inhibitors, the microplates were frozen on the day of collection and the samples were later titrated in new 96-well plates of MDCK cells by 10-fold serial dilution. Medium for replicating the viruses was Eagle's minimal essential medium, 0.18% sodium bicarbonate, 10 units/ml trypsin, 1 μg/ml EDTA, and 50 μg/ml gentamicin. Drug-drug interactions for three replicate assays were analyzed by the three-dimensional model of Prichard and Shipman (23), using the MacSynergy II software program at 95% confidence limits. Descriptions of additive, synergistic, and antagonistic interactions using this computer model have been described in detail for influenza studies (16).
Animal experiment design.
Specific pathogen-free BALB/c mice weighing approximately 17 to 19 g were purchased from Charles River Laboratories (Wilmington, MA). They were maintained on standard rodent chow and given water ad libitum with water bottles. The animals were quarantined 48 h prior to the onset of studies. Mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and then by intranasal infection with a 90-μl suspension of influenza virus. The infecting virus titers were approximately 104 CCID50 of wild-type virus or 105 CCID50 of amantadine-resistant virus per mouse. Groups of mice were treated orally (by gavage) with amantadine, oseltamivir, ribavirin, or placebo. The compounds were given twice a day (at 12-hour intervals) for 5 days starting 4 h before virus exposure. The placebo was administered in parallel. Drug combinations were given as treatment with one compound followed by treatment with the second compound. Ten drug-treated infected mice and 20 placebo-treated controls were observed daily for death through 21 days. Mice were weighed collectively in each group every other day during the infection. Normal controls (10 uninfected, untreated animals) were weighed in parallel with the infected groups.
Lung virus titers were determined 72 h after infection in one experiment using 5 mice per group. Harvested lungs from sacrificed mice were weighed and then frozen at −80°C until processed. Thawed lungs were homogenized in 1 ml of cell culture medium (minimal essential medium) and then were refrozen. Later, thawed samples were centrifuged at 2,000 × gravity for 10 min and titrated by end-point dilution in 96-well microplates as described above in “Cell culture assays.” Virus titers were converted to CCID50/g of lung tissue.
Ethical treatment of laboratory animals.
This study was conducted in accordance with the approval of the Institutional Animal Care and Use Committee of Utah State University. The work was performed in the university's AAALAC-accredited Laboratory Animal Research Center. The research was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Statistical analysis of animal experimental data.
Increases in total survivors were evaluated by Fisher's exact test. Differences in the mean days of death and lung virus titers were evaluated by the Mann-Whitney U test. All analyses were two tailed and calculated using Instat (GraphPad Software, San Diego, CA). Statistical comparisons were made between treated and placebo groups and between monotherapy and drug combination groups. Synergistic interactions were analyzed by the three dimensional method of Prichard and Shipman using the MacSynergy II software program (23), as was more fully described above for the cell culture assays.
RESULTS
Cell culture drug combination experiments using amantadine-sensitive virus.
Virus yield reduction data for the wild-type virus infections are presented in Table 1. In these experiments, amantadine alone was 100% effective in reducing virus titers at 10 μM, as was oseltamivir carboxylate at 1.0 μM and ribavirin at 100 μM.
TABLE 1.
Effect of combinations of amantadine, oseltamivir carboxylate, and ribavirin on an influenza A/Duck/MN/1525/81 (H5N1) amantadine-sensitive virus infection in MDCK cells
| Drug | Concn (μM) | Virus titers with:b
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amantadine (μM)a
|
Ribavirin (μM)c
|
||||||||||||
| 10 | 3.2 | 1.0 | 0.32 | 0.1 | 0 | 100 | 32 | 10 | 3.2 | 1.0 | 0 | ||
| Oseltamivir carboxylate | 1.0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 0.32 | 0 ± 0 | 0 ± 0 | 0.4 ± 0.8 | 0.3 ± 0.6 | 1.9 ± 2.3 | 3.5 ± 2.5 | 0 ± 0 | 0.3 ± 0.6 | 2.5 ± 2.3 | 2.7 ± 3.4 | 2.3 ± 2.8 | 2.7 ± 3.4 | |
| 0.1 | 0 ± 0 | 0 ± 0 | 2.9 ± 2.7 | 3.2 ± 3.2 | 3.7 ± 3.3 | 5.1 ± 0.7 | 0 ± 0 | 1.1 ± 1.9 | 3.1 ± 2.9 | 3.2 ± 3.3 | 2.7 ± 3.1 | 3.0 ± 3.0 | |
| 0.032 | 0 ± 0 | 0 ± 0 | 3.6 ± 2.6 | 3.5 ± 2.2 | 4.0 ± 2.7 | 5.2 ± 0.6 | 0 ± 0 | 0.9 ± 1.0 | 4.9 ± 0.8 | 5.0 ± 0.7 | 5.3 ± 0.9 | 5.3 ± 0.9 | |
| 0.01 | 0 ± 0 | 0 ± 0 | 4.1 ± 2.1 | 4.5 ± 1.1 | 4.7 ± 2.0 | 5.7 ± 0.7 | 0 ± 0 | 1.7 ± 1.9 | 5.0 ± 1.2 | 5.2 ± 1.3 | 5.4 ± 1.0 | 5.5 ± 0.7 | |
| 0 | 0 ± 0 | 0 ± 0 | 5.3 ± 0.9 | 5.9 ± 0.4 | 5.9 ± 0.5 | 5.6 ± 0.6 | 0 ± 0 | 1.5 ± 1.4 | 5.0 ± 0.6 | 5.7 ± 0.9 | 5.5 ± 0.2 | 5.5 ± 0.2 | |
| Amantadine | 10 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | ||||||
| 3.2 | 0 ± 0 | 0 ± 0 | 0.4 ± 0.8 | 0.5 ± 0.9 | 0.5 ± 0.9 | 4.3 ± 2.1 | |||||||
| 1.0 | 0 ± 0 | 0.3 ± 0.6 | 1.0 ± 1.7 | 3.5 ± 1.3 | 2.6 ± 2.7 | 5.8 ± 0.7 | |||||||
| 0.32 | 0 ± 0 | 0 ± 0 | 5.2 ± 0.6 | 4.9 ± 1.0 | 5.4 ± 0.2 | 5.9 ± 0.5 | |||||||
| 0.1 | 0 ± 0 | 1.5 ± 2.6 | 5.8 ± 0.8 | 5.7 ± 0.4 | 5.7 ± 0.9 | 5.6 ± 0.7 | |||||||
| 0 | 0 ± 0 | 2.4 ± 0.3 | 5.6 ± 0.3 | 5.4 ± 0.6 | 6.1 ± 0.5 | 5.7 ± 0.4 | |||||||
The volume of synergy, as calculated by the method of Prichard and Shipman (23), for oseltamivir carboxylate and amantadine was 22.11.
Values are means ± standard deviations (n = 3 independent assays) of virus titers (log10 CCID50/0.1 ml). Boldface values indicate regions of synergy.
The volume of synergy was 1.21 for oseltamivir carboxylate and ribavirin and 22.74 for amantadine and ribavirin.
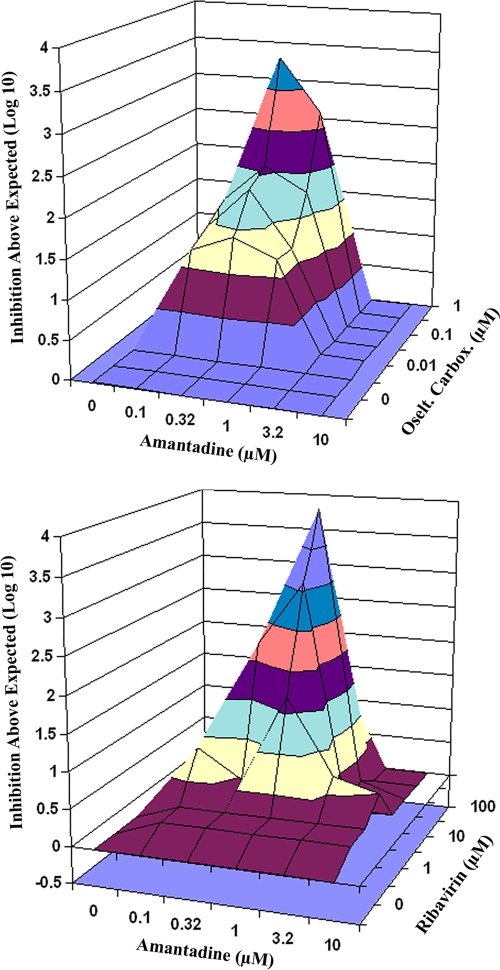
Table 1 shows the effects of the combination of oseltamivir carboxylate plus amantadine on viral titers. Virus yield reductions greater than expected (i.e., synergistic interactions) occurred in the region of amantadine at 0.1 to 1.0 μM combined with oseltamivir carboxylate at 0.01 to 0.32 μM. A three-dimensional plot of the data was generated by MacSynergy software and is presented in Fig. 1 (top). In this figure, the region of synergy is shown above the plane. The volume of synergy calculated for the data at a 95% confidence limit was 22.11, which was highly significant.
FIG. 1.
Three-dimensional synergy plots of interactions of amantadine and oseltamivir carboxylate (Oselt. Carbox.) (top) and amantadine and ribavirin (bottom) on influenza A/Duck/MN/1525/81 (H5N1) virus yields from MDCK cells. The data used are those presented in Table 1. Plots were made at the 95% confidence level.
Table 1 also shows interactions of oseltamivir carboxylate plus ribavirin. There were no regions of synergistic interaction. The interactions were additive, with no antagonism present (no synergy plot is shown). The volume of synergy calculated for these data was 1.21, which was not significant.
Table 1 also shows interactions of amantadine plus ribavirin. Synergistic interactions occurred in the region of amantadine at 1.0 to 3.2 μM combined with ribavirin at 1.0 to 32 μM and when amantadine at 0.32 μM was combined with ribavirin at 32 μM. The synergy plot for these data is shown in Fig. 1 (bottom). The volume of synergy for these data was 22.74, which was highly significant.
A summary of findings for this series of experiments indicated synergistic interactions with combinations of amantadine plus oseltamivir and amantadine plus ribavirin. The degree of synergistic interaction between amantadine and oseltamivir carboxylate was essentially the same as that exhibited between amantadine and ribavirin, based upon the volume of synergy values. Interactions between oseltamivir carboxylate and ribavirin were additive.
Cell culture drug combination experiments using amantadine-resistant virus.
Effects of compounds used alone and in combination against amantadine-resistant virus infections are shown in Table 2. Monotherapy with amantadine was ineffective against the infection at concentrations as high as 100 μM. Oseltamivir carboxylate was nearly 100% effective in reducing virus titer at 10 μM, and ribavirin was completely effective at 100 μM.
TABLE 2.
Effect of combinations of amantadine, oseltamivir carboxylate, and ribavirin on an influenza A/Duck/MN/1525/81 (H5N1) amantadine-resistant virus infection in MDCK cells
| Drug | Concn (μM) | Virus titers with:a
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amantadine (μM)
|
Ribavirin (μM)
|
||||||||||||
| 100 | 32 | 10 | 3.2 | 1 | 0 | 100 | 32 | 10 | 3.2 | 1.0 | 0 | ||
| Oseltamivir carboxylate | 10 | 0 ± 0 | 0.7 ± 1.2 | 0.6 ± 1.0 | 0.7 ± 1.2 | 0.6 ± 1.0 | 0.7 ± 1.2 | 0 ± 0 | 0 ± 0 | 0.6 ± 1.0 | 0.5 ± 0.9 | 0.4 ± 0.8 | 0.7 ± 1.2 |
| 3.2 | 1.6 ± 1.5 | 1.8 ± 0.7 | 1.5 ± 1.4 | 1.8 ± 1.7 | 1.4 ± 1.4 | 1.6 ± 1.5 | 0 ± 0 | 0 ± 0 | 1.3 ± 1.2 | 1.7 ± 1.5 | 1.7 ± 1.5 | 1.5 ± 1.5 | |
| 1.0 | 4.1 ± 0.5 | 4.5 ± 0.5 | 4.3 ± 0.8 | 4.6 ± 0.7 | 3.9 ± 0.8 | 4.2 ± 0.5 | 0 ± 0 | 0.2 ± 0.4 | 3.7 ± 0.3 | 4.3 ± 0.0 | 4.4 ± 0.1 | 4.6 ± 0.5 | |
| 0.32 | 5.2 ± 0.8 | 5.4 ± 0.2 | 5.3 ± 0.4 | 5.4 ± 0.6 | 5.2 ± 0.6 | 4.7 ± 0.9 | 0 ± 0 | 1.2 ± 1.0 | 4.6 ± 0.9 | 4.9 ± 0.9 | 5.2 ± 0.8 | 5.1 ± 0.5 | |
| 0.1 | 5.3 ± 0.8 | 5.5 ± 0.0 | 5.7 ± 0.4 | 5.7 ± 0.3 | 5.6 ± 0.1 | 5.0 ± 0.9 | 0 ± 0 | 2.1 ± 1.9 | 4.9 ± 0.7 | 5.0 ± 0.9 | 5.4 ± 0.6 | 5.5 ± 0.3 | |
| 0 | 5.6 ± 0.3 | 5.8 ± 0.2 | 5.7 ± 0.3 | 5.6 ± 0.4 | 5.6 ± 0.4 | 5.5 ± 0.5 | 0 ± 0 | 2.5 ± 2.2 | 5.4 ± 0.5 | 5.2 ± 0.5 | 5.5 ± 0.2 | 5.6 ± 0.4 | |
| Amantadine | 100 | 0 ± 0 | 1.6 ± 1.7 | 4.5 ± 0.5 | 5.1 ± 0.5 | 5.5 ± 0.9 | 5.5 ± 0.2 | ||||||
| 32 | 0 ± 0 | 2.0 ± 1.8 | 4.9 ± 0.3 | 5.5 ± 0.2 | 5.5 ± 0.2 | 5.9 ± 0.1 | |||||||
| 10 | 0 ± 0 | 2.3 ± 2.0 | 4.9 ± 0.4 | 5.5 ± 0.4 | 5.5 ± 0.2 | 5.8 ± 0.1 | |||||||
| 3.2 | 0 ± 0 | 2.1 ± 1.9 | 4.7 ± 0.0 | 5.3 ± 0.5 | 5.3 ± 0.5 | 5.6 ± 0.4 | |||||||
| 1 | 0 ± 0 | 2.1 ± 1.9 | 4.7 ± 0.3 | 5.2 ± 0.5 | 5.4 ± 0.2 | 5.6 ± 0.4 | |||||||
| 0 | 0 ± 0 | 2.6 ± 2.3 | 4.8 ± 0.3 | 5.4 ± 0.1 | 5.5 ± 0.1 | 5.6 ± 0.1 | |||||||
The values are means ± standard deviations (n = 3 independent assays) of virus titers (log10 CCID50/0.1 ml). The volume of synergy value, as calculated by the method of Prichard and Shipman (23), was 1.81 for oseltamivir carboxylate and amantadine, 3.95 for oseltamivir carboxylate and ribavirin, and 5.74 for amantadine and ribavirin. Boldface values indicate regions of synergy.
Table 2 shows the effects of the combination of oseltamivir carboxylate plus amantadine. There were no regions of synergistic interaction. The data were a mixture of slight additivity and slight antagonism, resulting in a volume of synergy of 1.81 at a 95% confidence limit, which was not significant.
Table 2 also shows interactions of oseltamivir carboxylate plus ribavirin. A very small region of synergistic interaction occurred when ribavirin at 32 μM was combined with oseltamivir carboxylate at 0.32, 1.0, and 3.2 μM. The volume of synergy was 3.95. The overall effect was additive.
Table 2 also shows interactions of amantadine plus ribavirin. Weak synergistic interaction occurred with amantadine at 100 μM plus ribavirin at 32 μM. The volume of synergy was 5.74, with the overall effect being additive.
A summary of findings for this series of experiments indicated weakly synergistic interactions with combinations of oseltamivir plus ribavirin and amantadine plus ribavirin. These interactions occurred only at the 32 μM ribavirin concentration and the higher concentrations of the other inhibitors. Interactions between oseltamivir carboxylate and amantadine were indifferent.
Dose-responsive effects of amantadine in infected mice.
The effects of amantadine treatment on influenza A/Duck/MN/1525/81 (H5N1) virus infections have not previously been reported. Therefore, a dose-response study was conducted with the drug for the treatment of wild-type and amantadine-resistant virus infections in mice (Table 3). In each infection the infectious dose killed 95% of the placebo-treated animals. Against the wild-type virus infection, amantadine protected all mice from death at 30- and 100-mg/kg/day doses, and the 10-mg/kg/day dose was partially protective, with 40% survival observed. The 3-mg/kg/day dose of amantadine was not active. The amantadine-resistant virus infection was refractive to treatment with amantadine, consistent with the results from the cell culture drug experiments. The 30- and 100-mg/kg/day doses of amantadine resulted in no survival. There were two survivors in the 10-mg/kg/day group, the results of which were not statistically significant. This study served as a basis for evaluation of amantadine in combination with oseltamivir and ribavirin.
TABLE 3.
Dose-responsive effects of treatment with amantadine on influenza A/Duck/MN/1525/81 (H5N1) virus infections in mice induced by wild-type (amantadine-sensitive) and amantadine-resistant virus strains
| Infecting virus type | Amantadine dose (mg/kg/day)a | No. of survivors/total no.c | MDD ± SDb |
|---|---|---|---|
| Wild type | 100 | 10/10*** | |
| 30 | 10/10*** | ||
| 10 | 4/10* | 8.3 ± 2.0 | |
| 3 | 0/10 | 7.1 ± 0.7 | |
| Placebo | 1/20 | 6.7 ± 0.6 | |
| Amantadine resistant | 100 | 0/10 | 6.8 ± 1.1 |
| 30 | 0/10 | 6.8 ± 1.0 | |
| 10 | 2/10 | 6.6 ± 1.6 | |
| 3 | 0/10 | 6.8 ± 1.0 | |
| Placebo | 1/20 | 6.8 ± 1.3 |
Oral treatments were given twice a day for 5 days starting 4 h prior to virus exposure.
MDD, mean day of death of mice that died prior to day 21.
*, P < 0.05, compared to placebo; ***, P < 0.001, compared to placebo.
Treatment of wild-type virus infection in mice.
The experiment reported in Table 3 established that 10 mg/kg/day of amantadine was weakly effective in the treatment of the wild-type influenza A/Duck virus infection. This dose was chosen to be combined with oseltamivir and ribavirin, and oseltamivir was combined with ribavirin to treat the infection (Table 4). Amantadine was not significantly active at 10 mg/kg/day in preventing death from the infection, although 30% survival was noted. Oseltamivir was not protective at 20 and 40 mg/kg/day, nor was ribavirin at 20 mg/kg/day. Ribavirin at 40 mg/kg/day was 40% protective, which represented a level of statistical significance. Oseltamivir and ribavirin, each at 20 and 40 mg/kg/day, combined with amantadine markedly improved survival over oseltamivir, ribavirin, or amantadine alone. The mean time to death was extended by monotherapy with the three drugs, except with the 40-mg/kg/day dose of ribavirin.
TABLE 4.
Treatment of an influenza A/Duck/MN/1525/81 (H5N1) wild-type (amantadine-sensitive) virus infection in mice with amantadine, oseltamivir, and ribavirin used alone or in combinationa
| Compound 1 (mg/kg/day) | Compound 2 (mg/kg/day) | No. of survivors/total no.c,d | MDD ± SDb |
|---|---|---|---|
| Amantadine (10) | 3/10 | 10.1 ± 3.2** | |
| Oseltamivir (40) | 1/10 | 8.9 ± 0.6*** | |
| Oseltamivir (20) | 3/10 | 10.0 ± 2.4*** | |
| Ribavirin (40) | 4/10* | 8.0 ± 1.3 | |
| Ribavirin (20) | 2/10 | 8.8 ± 1.3** | |
| Amantadine (10) | Oseltamivir (40) | 10/10***,φφ,ϕ | |
| Amantadine (10) | Oseltamivir (20) | 9/10***,φ | 11.0 |
| Amantadine (10) | Ribavirin (40) | 10/10***,φ | |
| Amantadine (10) | Ribavirin (20) | 9/10***,φφ | 8.0 |
| Oseltamivir (40) | Ribavirin (40) | 9/9***,φ,ϕ | |
| Oseltamivir (40) | Ribavirin (20) | 10/10***,φφ,ϕϕ | |
| Oseltamivir (20) | Ribavirin (40) | 10/10***,φ | |
| Oseltamivir (20) | Ribavirin (20) | 9/10***,φ | 10.0 |
| Placebo | 1/20 | 7.2 ± 1.3 |
Oral treatments were given twice a day for 5 days starting 4 h prior to virus exposure. *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared to placebo.
MDD, mean day of death of mice that died prior to day 21.
φ, P < 0.05; φφ, P < 0.01, compared to either compound used alone.
ϕ, P < 0.05; ϕϕ, P < 0.01, compared to the sum of survivors from the respective groups treated with monotherapy, indicative of synergy.
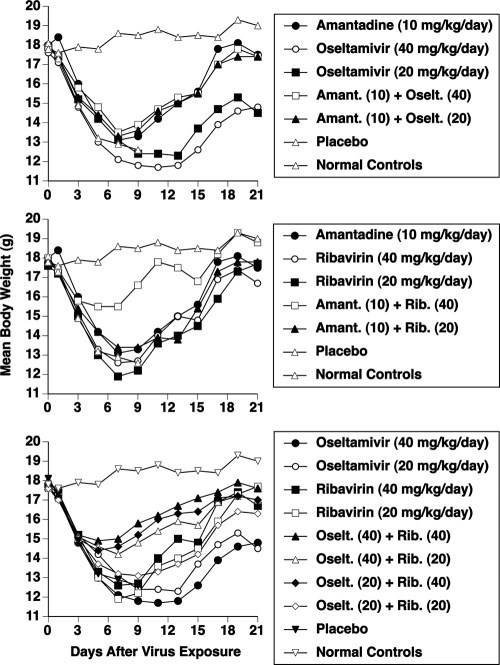
Weight loss and recovery during wild-type virus infection are depicted in Fig. 2. The top panel shows the effects of amantadine ± oseltamivir on body weights. Treatment with amantadine was more beneficial than oseltamivir in preventing excessive weight loss. The combination gave results similar to that of amantadine alone. It was curious that the combination did not provide an improvement in body weight, since survival with the combination was much better than that with monotherapy. The middle panel shows the effects of amantadine ± ribavirin on body weights. The combination of amantadine plus ribavirin at 40 mg/kg/day caused a marked improvement in body weight, relative to all other treatments. The bottom panel shows the effects of oseltamivir ± ribavirin on body weights. The three top-performing treatments were combinations of oseltamivir plus ribavirin, in which ribavirin was at 40 mg/kg/day combined with either dose of oseltamivir and in which oseltamivir was at 40 mg/kg/day combined with ribavirin at 20 mg/kg/day.
FIG. 2.
Effects of treatment with amantadine, oseltamivir, and ribavirin, used alone or in combination, on mean body weights during an influenza A/Duck/MN/1525/81 (H5N1) amantadine-sensitive virus infection in mice. Oral treatments were given twice a day for 5 days starting 4 h before virus exposure. Data represent mean values using 10 drug-treated mice and 20 placebos per group. By day 11 the monotherapy groups had 1 to 4 survivors and the combination treatment groups had 9 or 10 survivors (per Table 4).
The effects of treatment of an amantadine-sensitive virus infection on 72-h lung virus titer are reported in Fig. 3. Twice-daily oral treatments starting 4 h prior to infection were administered for 3 days prior to harvesting lungs from sacrificed animals. Figure 3A shows amantadine-plus-oseltamivir combinations. Virus titers were reduced relative to placebo, but only statistically significant reductions were seen with amantadine at 10 mg/kg/day or amantadine at 10 mg/kg/day combined with oseltamivir at 20 mg/kg/day. Figure 3B depicts treatments with amantadine plus ribavirin. Here, the only treatment that was not significantly active was ribavirin at 20 mg/kg/day. The effects of oseltamivir at 40 mg/kg/day combined with ribavirin are reported in Fig. 3C. Oseltamivir alone or ribavirin at 20 mg/kg/day alone was not significantly active, whereas ribavirin at 40 mg/kg/day and the two combinations were significantly active. Figure 3D shows the activities of oseltamivir at 20 mg/kg/day combined with ribavirin. Monotherapy with oseltamivir or ribavirin at 20 mg/kg/day was not significantly effective. Monotherapy with ribavirin at 40 mg/kg/day and combination treatments were active.
FIG. 3.
Effects of treatment with amantadine, oseltamivir, and ribavirin, used alone or in combination, on lung virus titers determined 72 h after influenza A/Duck/MN/1525/81 (H5N1) amantadine-sensitive virus infection of mice. Oral treatments were given twice a day starting 4 h before virus exposure, with the last treatment administered 4 h prior to sacrifice of the mice. Data are mean values ± standard deviations using lungs from five mice per group. A, amantadine plus oseltamivir; B, amantadine plus ribavirin; C and D, oseltamivir plus ribavirin. Values are shown as mg/kg/day.
Overall, the effects of treatment on lung virus titers by any of the treatments were weak or moderate, with a maximum 10-fold inhibition observed. The best effects were seen with certain combinations, although the trends were not uniformly consistent. For example, lower virus titers were found in lungs treated with 10 mg/kg/day amantadine plus 20 mg/kg/day oseltamivir than in lungs treated with 10 mg/kg/day amantadine plus 40 mg/kg/day oseltamivir. Similarly, more virus was seen in lungs treated with 40 mg/kg/day oseltamivir plus 40 mg/kg/day ribavirin than with 40 mg/kg/day oseltamivir plus 20 mg/kg/day ribavirin. Apparently a single time point of analysis does not give a definitive picture of the benefits from combination chemotherapy. Because of these results, we did not perform the same experiment with amantadine-resistant virus. Instead, only mortality data are presented (below).
Taken together, these results indicate that the combinations of amantadine plus oseltamivir, amantadine plus ribavirin, and oseltamivir plus ribavirin were more beneficial than monotherapy in preventing death and preventing weight loss during the infection. All three combinations were judged to be equally effective in terms of survival. However, results of body weight measurements indicated that when ribavirin was a component of the combination, the infected mice lost less weight during the acute phase of the infection prior to the recovery phase. Effects on virus titers showed a trend toward improvement with drug combinations over monotherapy.
Treatment of amantadine-resistant virus infections in mice.
A high (100-mg/kg/day) dose of amantadine was used in combination with oseltamivir or ribavirin to treat infections in mice caused by an amantadine-resistant influenza A/Duck virus (Table 5). Amantadine alone was not active in preventing death from the infection, similar to the results reported in Table 3. Oseltamivir was moderately protective at 25 and 75 mg/kg/day, as was ribavirin at 25 mg/kg/day. Ribavirin at 100 mg/kg/day was 100% protective. Oseltamivir at 75 mg/kg/day combined with amantadine did not improve survival over oseltamivir alone. More survivors (100%) were noted with oseltamivir (25 mg/kg/day) plus amantadine than with oseltamivir alone (50% survival). The amantadine-plus-ribavirin (25-mg/kg/day) combination was slightly more effective than ribavirin alone, but not significantly. Doses of 100 mg/kg/day ribavirin were 100% protective either alone or combined with amantadine or oseltamivir. The other combinations of oseltamivir plus ribavirin (25 mg/kg/day) resulted in 100% survival, which was an improvement over either compound used alone. Three drug combinations caused increases in the numbers of survivors that are suggestive of synergy: amantadine plus oseltamivir at 40 mg/kg/day, oseltamivir at 40 mg/kg/day plus ribavirin at 40 mg/kg/day, and oseltamivir at 40 mg/kg/day plus ribavirin at 20 mg/kg/day. The other combinations that improved numbers of survivors are considered to be additive. Increases in the mean time to death were seen in the 25-mg/kg/day ribavirin groups (alone or combined with amantadine).
TABLE 5.
Treatment of an influenza A/Duck/MN/1525/81 (H5N1) amantadine-resistant virus infection in mice with amantadine, oseltamivir, and ribavirin used alone or in combinationa
| Compound 1 (mg/kg/day) | Compound 2 (mg/kg/day)a | No. of survivors/total no.c,d | MDD ± SDb |
|---|---|---|---|
| Amantadine (100) | 0/10 | 6.9 ± 1.1 | |
| Oseltamivir (75) | 7/10*** | 6.0 ± 0.0 | |
| Oseltamivir (25) | 5/10** | 7.2 ± 1.6 | |
| Ribavirin (75) | 10/10*** | ||
| Ribavirin (25) | 5/10** | 10.2 ± 3.0** | |
| Amantadine (100) | Oseltamivir (75) | 7/10*** | 7.3 ± 0.6 |
| Amantadine (100) | Oseltamivir (25) | 10/10***,φ,ϕ | |
| Amantadine (100) | Ribavirin (75) | 10/10*** | |
| Amantadine (100) | Ribavirin (25) | 7/10*** | 9.7 ± 1.5** |
| Oseltamivir (75) | Ribavirin (25) | 10/10*** | |
| Oseltamivir (25) | Ribavirin (75) | 10/10*** | |
| Oseltamivir (25) | Ribavirin (25) | 10/10***,φ | |
| Placebo | 1/20 | 6.4 ± 1.6 |
Oral treatments were given twice a day for 5 days starting 4 h prior to virus exposure. **, P < 0.01; ***, P < 0.001, compared to placebo.
MDD, mean day of death of mice that died prior to day 21.
φ, P < 0.05, compared to either compound used alone.
ϕ, P < 0.05, compared to the sum of survivors from the respective groups treated with monotherapy, indicative of synergy.
There was an indication from the data reported in Table 5 that the combination of amantadine at 100 mg/kg/day plus oseltamivir at 25 mg/kg/day was significantly more beneficial than either compound used alone. By comparing the sum of survivors in the monotherapy groups to those in the drug combination group, the results are suggestive of synergy. To confirm whether these results are reproducible, a second (repeated) experiment was conducted (Table 6). As was seen previously, amantadine was not active at 100 mg/kg/day in preventing death from the infection. Oseltamivir was moderately or highly protective at 25 and 75 mg/kg/day. Oseltamivir at these doses combined with amantadine did not significantly improve survival over oseltamivir alone, although there was an additional surviving mouse in each combination group. Body weights were slightly improved during combination treatment over monotherapy with oseltamivir or amantadine (data not shown).
TABLE 6.
Treatment of an influenza A/Duck/MN/1525/81 (H5N1) amantadine-resistant virus infection in mice with amantadine and oseltamivir used alone or in combination (second experiment)a
| Compound 1 (mg/kg/day) | Compound 2 (mg/kg/day) | No. of survivors/ total no.c | MDD ± SDb |
|---|---|---|---|
| Amantadine (100) | 0/10 | 6.9 ± 1.1 | |
| Oseltamivir (75) | 5/10** | 7.6 ± 2.3 | |
| Oseltamivir (25) | 8/10*** | 6.5 ± 0.7 | |
| Amantadine (100) | Oseltamivir (75) | 6/10*** | 7.5 ± 1.3 |
| Amantadine (100) | Oseltamivir (25) | 9/10*** | 6.0 |
| Placebo | 0/20 | 7.0 ± 1.1 |
Oral treatments were given twice a day for 5 days starting 4 h prior to virus exposure.
MDD, Mean day of death of mice that died prior to day 21.
**, P < 0.01; ***, P < 0.001, compared to placebo.
Both studies (Tables 5 and 6) provide an indication of slight improvement in survival rendered by high-dose amantadine combined with oseltamivir or ribavirin. Because monotherapy with either oseltamivir or ribavirin was very effective, the improvement seen with the combinations could reach only a level of additivity. Lower, less-effective doses of these inhibitors would need to be tested to determine synergistic interactions.
DISCUSSION
In these studies we determined that combinations of amantadine plus oseltamivir or ribavirin produced synergistic interactions in MDCK cell culture against wild-type influenza A/Duck (H5N1) virus. These positive effects were abolished against the amantadine-resistant virus, since the antiviral activity was no better than that with oseltamivir or ribavirin alone. Published in vitro studies have documented additive or synergistic benefits of combination treatment of influenza A virus infections with amantadine plus oseltamivir (14), amantadine plus ribavirin (13), rimantadine plus ribavirin (13, 21), and rimantadine plus neuraminidase inhibitors (zanamivir, oseltamivir carboxylate, and peramivir [10]). Enhanced efficacy was also seen with combinations of ribavirin plus zanamivir (21) or peramivir (26). In each case, the drug combinations were tested against viruses that were sensitive to both drugs.
Although synergistic interactions occurred between amantadine and oseltamivir or ribavirin in these cell culture studies against wild-type virus infections (Fig. 1), the combination of oseltamivir carboxylate and ribavirin at any concentration of either drug did not result in synergistic inhibition of A/Duck virus replication but was additive, with each drug having the same effect in the combination as it had when used alone. More research will be required to determine if this interaction is virus specific, meaning that other strains of influenza virus might be inhibited by this combination in a synergistic manner. Generally, compounds with different modes of action are predicted to act synergistically against viral infections.
Although synergistic interactions were not seen with the combination of oseltamivir carboxylate and ribavirin in vitro, great improvement in survival was seen with the combinations with mice infected with amantadine-sensitive virus. Thus, there appears to be an obvious discrepancy between effects seen in vitro versus those in vivo. The discrepancy may be related to the assay method used, which for the in vivo studies was survival benefit. With influenza infections in mice there seems to be a fine line between death and survival. Mice can appear equally ill from infection (as determined by body weight), yet one animal recovers and another expires. Slight improvement in the degree of virus inhibition may be enough to change the outcome from death to survival. This was not clearly demonstrated by the results reported in Fig. 3. But there was a trend of greater virus titer inhibition with drug combinations. And the mice clearly benefited from the combination treatment, as indicated by improved survival and body weights (Fig. 2). A high virus titer (approximately 104 CCID50 per mouse) is required to induce lethality with the A/Duck virus, compared to 1 to 4 PFU of A/Vietnam or A/Turkey viruses (16, 17). Thus, the highly pathogenic viruses are more lethal in BALB/c mice. This makes the challenge of reducing virus titer more difficult in the A/Duck infection (Fig. 3), since the infection is initiated with a larger virus inoculum. This may partly explain why virus titers were not greatly inhibited in the A/Duck virus model, as opposed to the higher degrees of inhibition reported for the highly pathogenic viruses (16, 17).
The amantadine-resistant A/Duck virus mouse model was established in our laboratory to study the effects of combination treatment using amantadine and other inhibitors. When we first began developing this model, treatments of infected mice with amantadine unexpectedly resulted in survival. Our suspicion at the time was that the virus pool might have contained some wild-type virus. Cloning out the virus into a number of isolates and assaying individual clones for amantadine sensitivity in cell culture confirmed this. The amantadine-resistant clone selected for the present studies exhibited an amantadine-resistant phenotype both in vitro and in mice. Ilyushina et al. (17) tested the effects of double combinations of amantadine and oseltamivir against amantadine-sensitive and -resistant influenza A/Vietnam/1203/04 (H5N1) in mice. Their results demonstrated that the double combination of amantadine and oseltamivir provided improved benefit over either drug alone against the amantadine-sensitive virus, whereas the double combination was no better than oseltamivir alone against the amantadine-resistant virus. Our data may have suggested a slight benefit of using amantadine against the resistant virus, but the dose used was high and may have had some inhibitory effect. These results are in contrast to the work of Masihi et al. (22), who reported that amantadine provided a significant benefit in combination with oseltamivir against influenza A/PR/8/34 (H1N1) amantadine-resistant virus infection. Their virus may need to be verified for purity to determine whether amantadine-sensitive virus particles may also be present.
It is known that amantadine has two modes of action, being active at both low and high concentrations by different mechanisms. Its virus-specific mode of action is to inhibit influenza A virus uncoating at low concentrations via the M2 proton channel, and it is an inhibitor of membrane fusion activity of the virus hemagglutinin at high concentrations (12). If whatever slight benefit resulting from combination treatment is attributable to the action of amantadine rather than the result of biological variation, then its effect may relate to the action of the drug due to its membrane activity.
Recently, Ilyushina and colleagues published work with oseltamivir-ribavirin combinations against A/Vietnam/1203/04 (H5N1) and the A/Turkey/15/06 (H5N1) virus infections in mice (16). They found that the interaction was largely additive and that higher drug levels were required to inhibit the A/Turkey virus than the A/Vietnam virus. Thus, these viruses and the A/Duck viral strain do not all respond the same way to treatment. We anticipate that differences in treatment will be seen among other H5N1 viral strains. To date, other investigators have not published animal studies comparing amantadine and ribavirin in combination against H5N1 viruses. Thus, our present in vivo work represents novel findings with these two inhibitors against an H5N1 virus.
It was clear that the treatment of the wild-type virus infection (Table 4) demonstrated an advantage of combination treatment over monotherapy, interpreted to be additive or synergistic. Often it is difficult to establish that drug combinations produce synergistic responses in animal studies. This is partly due to the group sizes that are used and the variability in response rates at particular doses. For example, against the amantadine-resistant virus infection, oseltamivir at 75 mg/kg/day provided 70% protection in the first experiment (Table 5) and 50% protection in the second study (Table 6). Oseltamivir at 25 mg/kg/day gave 50% and 80% protection in the first and second experiments, respectively. Comparing the results of these two studies, oseltamivir at 75 mg/kg/day would be considered more active than 25 mg/kg/day in one test but less effective in another. By looking at the results together, one would conclude that the responses to treatment were similar for these doses. The combination of amantadine (100 mg/kg/day) plus oseltamivir (25 mg/kg/day) gave an indication of synergy in the first study but not in the second. In the first experiment, the higher dose combination of amantadine (100 mg/kg/day) plus oseltamivir (75 mg/kg/day) should have given a similar result to that of the lower-dose combination, but in reality produced no more survivors than oseltamivir alone.
In the influenza mouse model, single agents alone prevent death, but there is substantial morbidity manifested by weight loss. In this study, loss of weight could not be prevented during infection by treatment with the three compounds tested alone. However, when two compounds were combined, much less weight loss occurred (Fig. 2). The most effective treatments for reducing weight loss were ribavirin (40 mg/kg/day) combined with either oseltamivir (40 mg/kg/day) or amantadine (10 mg/kg/day) against the wild-type virus infection. We could have used higher doses of compounds in this model to produce a better effect but that would have made it more difficult to assess drug interactions.
A number of animal studies have been published illustrating the value of drug combinations for the treatment of influenza virus infections (9, 16, 17, 20, 22, 26, 28). The present experiments demonstrated that many of the drug interactions were additive or synergistic, particularly in the treatment of the wild-type virus infection. This translated into considerable improvement in the number of survivors (Table 5). Because amantadine (and the related drug rimantadine), oseltamivir, and ribavirin are all approved drugs (albeit ribavirin is not approved for the treatment of influenza virus infections), it is prudent to consider the use of these agents in combination to treat severe influenza virus infections for which monotherapy with oseltamivir has not been particularly effective (4), such as those caused by highly pathogenic H5N1 viruses. Severe cases of infection caused by seasonal influenza viruses could also benefit from such treatments.
Acknowledgments
This work was supported by contracts NO1-AI-15435, NO1-AI-30048, and NO1-AI-30063 from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
The contents of this article do not necessarily reflect the position or policy of the government, and no official endorsement should be inferred.
The investigators adhered to the “Guide for the Care and Use of Laboratory Animals,” prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council, and used facilities fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Footnotes
Published ahead of print on 9 March 2009.
REFERENCES
- 1.Abed, Y., N. Goyette, and G. Boivin. 2005. Generation and characterization of recombinant influenza A (H1N1) viruses harboring amantadine resistance mutations. Antimicrob. Agents Chemother. 49:556-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2008. Interim within-season estimate of the effectiveness of trivalent inactivated influenza vaccine - Marshfield, Wisconsin, 2007-08 influenza season. MMWR Morb. Mortal. Wkly. Rep. 57:393-398. [PubMed] [Google Scholar]
- 3.Cheung, C. L., J. M. Rayner, G. J. Smith, P. Wang, T. S. Naipospos, J. Zhang, K. Y. Yuen, R. G. Webster, J. S. Peiris, Y. Guan, and H. Chen. 2006. Distribution of amantadine-resistant H5N1 avian influenza variants in Asia. J. Infect. Dis. 193:1626-1629. [DOI] [PubMed] [Google Scholar]
- 4.Crusat, M., and M. D. de Jong. 2007. Neuraminidase inhibitors and their role in avian and pandemic influenza. Antivir. Ther. 12:593-602. [PubMed] [Google Scholar]
- 5.de Jong, M. D., T. T. Tran, H. K. Truong, M. H. Vo, G. J. Smith, V. C. Nguyen, V. C. Bach, T. Q. Phan, Q. H. Do, Y. Guan, J. S. Peiris, T. H. Tran, and J. Farrar. 2005. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N. Engl. J. Med. 353:2667-2672. [DOI] [PubMed] [Google Scholar]
- 6.Deyde, V. M., X. Xu, R. A. Bright, M. Shaw, C. B. Smith, Y. Zhang, Y. Shu, L. V. Gubareva. N. J. Cox, and A. I. Klimov. 2007. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J. Infect. Dis. 196:249-257. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson, B., E. Helgstrand, N. G. Johansson, A. Larsson, A. Misiorny, J. O. Norén, L. Philipson, K. Stenberg, G. Stening, S. Stridh, and B. Oberg. 1977. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob. Agents Chemother. 11:946-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fereidouni, S. R., T. C. Harder, and E. Starick. 2008. Rapid pathotyping of recent H5N1 highly pathogenic avian influenza viruses and of H5 viruses with low pathogenicity by RT-PCR and restriction enzyme cleavage pattern (RECP). J. Virol. Methods 154:14-19. [DOI] [PubMed] [Google Scholar]
- 9.Galabov, A. S., L. Simeonova, and G. Gegova. 2006. Rimantadine and oseltamivir demonstrate synergistic combination effect in an experimental infection with type 1 (H3N2) influenza virus in mice. Antivir. Chem. Chemother. 17:251-258. [DOI] [PubMed] [Google Scholar]
- 10.Govorkova, E. A., H. B. Fang, M. Tan, and R. G. Webster. 2004. Neuraminidase inhibitor-rimantadine combinations exert additive and synergistic anti-influenza virus effects in MDCK cells. Antimicrob. Agents Chemother. 48:4855-4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hata, M., M. Tsuzuki, Y. Goto, N. Kumagai, M. Harada, M. Hashimoto, S. Tanaka, K. Sakae, T. Kimura, H. Minagawa, and Y. Miyazaki. 2007. High frequency of amantadine-resistant influenza A (H3N2) viruses in the 2005-2006 season and rapid detection of amantadine-resistant influenza A (H3N2) viruses by MAMA-PCR. Jpn. J. Infect. Dis. 60:202-204. [PubMed] [Google Scholar]
- 12.Hay, A. J., A. J. Wolstenholme, J. J. Skehel, and M. H. Smith. 1985. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 4:3021-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayden, F. G., R. G. Douglas, Jr., and R. Simons. 1980. Enhancement of activity against influenza viruses by combinations of antiviral agents. Antimicrob. Agents Chemother. 18:536-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilyushina, N. A., N. V. Bovin, R. G. Webster, and E. A. Govorkova. 2006. Combination chemotherapy, a potential strategy for reducing the emergence of drug-resistant influenza A variants. Antivir. Res. 70:121-131. [DOI] [PubMed] [Google Scholar]
- 15.Ilyushina, N. A., E. A. Govorkova, and R. G. Webster. 2005. Detection of amantadine-resistant variants among avian influenza viruses isolated in North America and Asia. Virology 341:102-106. [DOI] [PubMed] [Google Scholar]
- 16.Ilyushina, N. A., A. Hay, N. Yilmaz, A. C. M. Boon, R. G. Webster, and E. A. Govorkova. 2008. Oseltamivir-ribavirin combination therapy for highly pathogenic H5N1 influenza virus infection in mice. Antimicrob. Agents Chemother. 52:3889-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilyushina, N. A., E. Hoffmann, R. Salomon, R. G. Webster, and E. A. Govorkova. 2007. Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antiviral Ther. 12:363-370. [PubMed] [Google Scholar]
- 18.Komar, N., and B. Olsen. 2008. Avian influenza virus (H5N1) mortality surveillance. Emerg. Infect. Dis. 14:1176-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le, Q. M., M. Kiso, K. Someya, Y. T. Sakai, T. H. Nguyen, K. H. Nguyen, N. D. Pham, H. H. Nguyen, S. Yamada, Y. Muramoto, T. Horimoto, A. Takada, H. Goto, T. Suzuki, Y. Suzuki, and Y. Kawaoka. 2005. Avian flu: isolation of drug-resistant H5N1 virus. Nature 437:1108. [DOI] [PubMed] [Google Scholar]
- 20.Leneva, I. A., N. Roberts, E. A. Govorkova, O. G. Goloubeva, and R. G. Webster. 2000. The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza virus. Antivir. Res. 48:101-115. [DOI] [PubMed] [Google Scholar]
- 21.Madren, L. K., C. Shipman, and F. G. Hayden. 1995. In vitro inhibitory effects of combinations of anti-influenza agents. Antivir. Chem. Chemother. 6:109-113. [Google Scholar]
- 22.Masihi, K. N., B. Schweiger, T. Finsterbusch, and H. Hengel. 2007. Low dose oral combination chemoprophylaxis with oseltamivir and amantadine for influenza A virus infections in mice. J. Chemother. 19:295-303. [DOI] [PubMed] [Google Scholar]
- 23.Prichard, M. N., and C. Shipman, Jr. 1990. A three dimensional model to analyze drug-drug interactions. Antivir. Res. 14:181-206. [DOI] [PubMed] [Google Scholar]
- 24.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 25.Sidwell, R. W., D. L. Barnard, C. W. Day, D. F. Smee, K. W. Bailey, M.-H. Wong, J. D. Morrey, and Y. Furuta. 2007. Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob. Agents Chemother. 51:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smee, D. F., K. W. Bailey, A. C. Morrison, and R. W. Sidwell. 2002. Combination treatment of influenza A virus infections in cell culture and in mice with the cyclopentane neuraminidase inhibitor RWJ-270201 and ribavirin. Chemotherapy 48:88-93. [DOI] [PubMed] [Google Scholar]
- 27.Smee, D. F., J. H. Huffman, A. C. Morrison, D. L. Barnard, and R. W. Sidwell. 2001. Cyclopentane neuraminidase inhibitors with potent in vitro anti-influenza virus activity. Antimicrob. Agents Chemother. 45:743-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smee, D. F., M.-H. Wong, K. W. Bailey, and R. W. Sidwell. 2006. Activities of oseltamivir and ribavirin used alone and in combination against infections in mice with recent isolates of influenza A (H1N1) and B viruses. Antivir. Chem. Chemother. 17:185-192. [DOI] [PubMed] [Google Scholar]
- 29.Webster, R. G., and E. A. Govorkova. 2006. H5N1 influenza - continuing evolution and spread. N. Engl. J. Med. 355:2174-2177. [DOI] [PubMed] [Google Scholar]