Abstract
RWJ-416457 is an investigational pyrrolopyrazolyl-substituted oxazolidinone with activity against antibiotic-susceptible and -resistant gram-positive pathogens. Efficacies of RWJ-416457, linezolid, and vancomycin against methicillin-susceptible Staphylococcus aureus (MSSA) and community-associated methicillin-resistant S. aureus (CA-MRSA) in murine skin and systemic infections were compared, as were efficacies against Streptococcus pneumoniae in a lower respiratory infection. In staphylococcal systemic infections, RWJ-416457 was equipotent with to twofold more potent than linezolid, with 50% effective dose values ranging from 1.5 to 5 mg/kg of body weight/day. RWJ-416457 was two- to fourfold less potent than vancomycin against MSSA but up to fourfold more potent than vancomycin against CA-MRSA. In MSSA and CA-MRSA skin infections, RWJ-416457 demonstrated an efficacy similar to that of linezolid, reducing CFU/g skin approximately 1.0 log10 at all doses tested; vancomycin yielded greater reductions than the oxazolidinones, with decreases in CFU/g skin of 3 log10 (MSSA) and 2 log10 (CA-MRSA). In the pneumococcal model, RWJ-416457 was two- to fourfold more potent than linezolid. The free-drug area under the concentration-time curves at 24 h (fAUC24) were similar for RWJ-416457 and linezolid. The half-life of RWJ-416457 was up to threefold longer than that of linezolid for all routes of administration. The fAUC24/MIC ratio, the pharmacodynamic parameter considered predictive of oxazolidinone efficacy, was approximately twofold greater for RWJ-416457 than for linezolid. Since the fAUC values were similar for both compounds, the higher fAUC/MIC ratios of RWJ-416457 appear to result from its greater in vitro potency. These results demonstrate that RWJ-416457 is a promising new oxazolidinone with efficacy in S. aureus or S. pneumoniae mouse infection models.
The increasing incidence of antibiotic resistance among pathogenic bacteria threatens the utility of existing antibiotics, resulting in the need for new antibiotics with efficacy against susceptible and resistant isolates (21). Staphylococcus aureus, once largely susceptible to antibiotics, is now often resistant to multiple classes, including β-lactams, macrolides, aminoglycosides, and fluoroquinolones (5, 32). Methicillin-resistant S. aureus (MRSA), defined by resistance to oxacillin, contains the mecA gene, providing resistance to all available β-lactams (3, 6, 19). Historically, these multiresistant MRSA isolates have been found predominantly in hospitals and health care facilities, whereas community-associated strains remained largely methicillin susceptible. In a recent development, methicillin resistance has been found increasingly in community-associated S. aureus, resulting from the acquisition of an abbreviated version of the mec element type IV (4, 25, 26). These community-associated MRSA (CA-MRSA) strains, first described in 1981 and responsible for recent outbreaks in diverse settings and groups, such as schools, prisons, athletic teams, the homeless, drug users, and the military (7, 15, 17, 23, 27, 31), are usually involved in skin disease but have also been linked to lethal pneumonia and sepsis (18, 25, 31).
Although many CA-MRSA isolates are also resistant to erythromycin and, by some reports, fluoroquinolones, to date CA-MRSA strains remain susceptible to a variety of other antibiotic classes (10, 19, 31). Notably, with increased exposure of CA-MRSA to antibiotics, increasing resistance to tetracyclines and clindamycin has been reported (10, 20, 31).
To address the need for new oral agents effective in the treatment of CA-MRSA as well as health care-associated MRSA, the novel oxazolidinone RWJ-416457 was identified. This compound has a spectrum of activity similar to that of linezolid, the sole regulatory agency-approved representative of the oxazolidinone class, against antibiotic-susceptible and -resistant gram-positive pathogens, including MRSA, vancomycin-resistant enterococci, and penicillin-resistant pneumococci (11, 29). RWJ-416457 MICs are two- to fourfold lower than those of linezolid against relevant gram-positive clinical pathogens, including health care-associated MRSA and CA-MRSA strains (13, 22). Thus, RWJ-416457, in phase 1 development for the treatment of respiratory tract and skin and skin structure infections, may represent a viable option for patients, including those unable to tolerate β-lactams or macrolides.
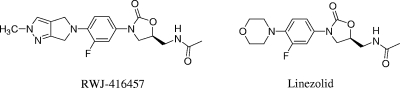
We report herein on the in vivo activities of RWJ-416457, linezolid, and vancomycin (Fig. 1) in S. aureus and Streptococcus pneumoniae murine models of infection and on selected pharmacokinetic and pharmacodynamic parameters.
FIG. 1.
Structures of RWJ-416457 and linezolid.
MATERIALS AND METHODS
Bacterial strains.
Methicillin-susceptible S. aureus (MSSA) ATCC 13709 and penicillin-susceptible S. pneumoniae (PSSP) ATCC 6301 were obtained from the American Type Culture Collection. OC8525, a CA-MRSA strain, was a gift from B. Kreiswirth (Public Health Research Institute, Newark, NJ).
Compound preparation.
RWJ-416457 was synthesized at Johnson & Johnson Pharmaceutical Research and Development. Vancomycin was purchased from MP Biomedicals, Inc. (Irvine, CA), and linezolid was obtained from Pfizer (Groton, CT). RWJ-416457 was prepared as a suspension in 0.5% Methocel for oral (p.o.) dosing. For subcutaneous (s.c.)- and intravenous (i.v.)-dosage formulations, RWJ-416457 was prepared as a solution in 20% aqueous hydroxypropyl-β-cyclodextrin. Linezolid and vancomycin were prepared in water for all administration routes.
MIC.
MICs were determined by the broth microdilution method according to CLSI guidelines (8). The MIC was defined as the lowest concentration of antibiotic that prevented visible growth after an incubation period of 16 to 20 h.
Microcarrier dextran bead preparation.
Stock suspensions (2%, wt/vol) of Cytodex 1 microcarrier dextran beads, 131 to 220 μm (Sigma-Aldrich Chemical Company, St. Louis, MO), were prepared in 0.85% phosphate-buffered saline and sterilized by autoclaving.
Preparation of inoculum.
For systemic S. aureus infections, ATCC 13709 or OC8525 was grown in brain heart infusion broth at 37°C and 200 rpm for 18 h. Following centrifugation, the cell pellet was washed twice by resuspension in 0.85% NaCl. After the second wash, the pellet was resuspended to 1/3 the original volume in 0.85% NaCl, followed by a second (1:100) dilution into 7% mucin (hog gastrin mucin; Sigma-Aldrich Chemical Company, St. Louis, MO)-0.85% NaCl to yield the final inoculum. Serial 100-fold dilutions in saline (0.85% saline; Remel, Lenexa, KS) were plated using spiral plate methodology (Autoplate 4000; Spiral Biotech, Inc., Norwood, MA) on Trypticase soy agar (TSA) plates. The plates were incubated for 18 h at 37°C and analyzed (Q-Count; Spiral Biotech, Inc., Norwood, MA) for CFU.
For skin infection studies, an overnight culture was centrifuged (10 min, 3,450 × g), and the cells were washed with 1/2 volume of saline. The cells were recentrifuged, the pellet was suspended in brain heart infusion medium, and the 2% Cytodex 1 bead suspension was added until a final bead concentration of 0.1% was attained.
For lower respiratory tract infections, S. pneumoniae was grown overnight at 35°C with an atmosphere of 5% CO2 on TSA containing 5% sheep blood. The inoculum was suspended in TSB containing 5% heat-inactivated, filter-sterilized goat serum (Rockland Immunochemicals, Inc., Gilbertsville, PA) and incubated until an optical density at 600 nm of 0.2 to 0.4 was reached. The culture was centrifuged and the cell pellet resuspended to 10% of the original culture volume in TSB with 5% goat serum. The CFU of the inoculum was determined on TSA containing 5% sheep blood, as described above.
In vivo models.
All animal studies were approved by the Johnson & Johnson Institutional Animal Care and Usage Committee.
Staphylococcal systemic lethal infection.
Six-week-old female Swiss-Webster mice (Taconic, Germantown, NY) were inoculated intraperitoneally with 4 × 105 CFU of ATCC 13709 or 1 × 107 CFU of OC8525. For p.o. or s.c. administration, mice were dosed at 1 and 3 h postinfection with either RWJ-416457 or linezolid. For i.v. administration, animals were given a single dose 1 h postinfection. Vancomycin was dosed s.c. and i.v. as described above for RWJ-416457 and linezolid. Infected mice not treated with drug served as controls. Mortality was monitored for 3 days postinfection. Historical studies in our laboratory showed that no additional deaths were observed after 3 days. At least three independent studies were conducted, with five to eight mice per dose group in each study. The 50% effective dose (ED50), i.e., the dose allowing survival of 50% of the infected animals, was calculated using the logistic function of the SAS statistical package.
Staphylococcal skin infection.
Female SKH1 mice (Charles River Laboratories, Kingston, NY) were anesthetized with 3% isoflurane (Abbott, Chicago, IL) maintained with oxygen at 3 liters/min. On the left flank, the animals were given a 0.2-ml s.c. injection of the Cytodex bead inoculum containing 2.0 × 106 CFU MSSA. On the right flank, the animals were given 0.2 ml of Cytodex bead suspension containing 2.0 × 106 CFU MRSA. Mice received RWJ-416457 or linezolid p.o. or vancomycin s.c. 1, 3, 25, and 27 h postinfection. Animals were euthanized 48 h after infection for determination of CFU/g skin. The skin from the infected areas was then disinfected with chlorhexidine diacetate (Nolvasan; Fort Dodge Animal Health, Fort Dodge, IA), excised, weighed, and homogenized. In control studies, chlorhexidine diacetate had no significant effect on CFU recovery. Serial 100-fold dilutions in saline were plated as described above, and the CFU were determined. Each experiment was performed twice, and the results were combined for analysis.
Pneumococcal lower respiratory infection.
Four-week-old female CF-1 mice (Charles River Laboratories) were briefly anesthetized with 3% isoflurane and then infected intranasally with approximately 2 × 107 CFU of S. pneumoniae. Twenty-four hours postinfection, mice were given a single p.o., s.c., or i.v. dose of RWJ-416457 or linezolid. Infected mice not treated with drug served as controls. Mortality was monitored for 48 h postinfection. At least two independent studies, with five to eight mice per dose group, were conducted. The ED50 was calculated as described above.
Single-dose pharmacokinetics.
Four-week-old female CF-1 mice (Charles River Laboratories) were dosed with RWJ-416457 or linezolid i.v. (2 mg/kg of body weight), p.o. (10 mg/kg), or s.c. (10 mg/kg). At time points ranging from 0.13 to 8 h after dosing (three mice per time point), mice were euthanized and blood was collected by cardiac puncture. Plasma was obtained from whole blood by centrifugation. Plasma samples were mixed with 2 volumes of acetonitrile and centrifuged, and the supernatant was analyzed by a validated liquid chromatography-mass spectrometry method (internal reports). Protein binding of RWJ-416457, determined at 5 μg/ml in mouse plasma, was 46% (measured by equilibrium dialysis) (unpublished data). The protein binding value of 32% for linezolid in mice was obtained from the literature (16).
RESULTS
In vitro susceptibilities.
The in vitro activities of RWJ-416457, linezolid, and vancomycin against the S. aureus and S. pneumoniae strains used in the infection models are shown in Table 1. RWJ-416457 was twofold more potent than linezolid against the tested isolates. Vancomycin MICs against the MSSA and MRSA strains were similar to those of RWJ-416457.
TABLE 1.
In vitro and in vivo activities of RWJ-416457, linezolid, and vancomycin against gram-positive pathogens in mouse lethal infection models
| Model and strain (type) | Compound | MIC (μg/ml) | ED50, mg/kg/day (95% fiducial limit)
|
||
|---|---|---|---|---|---|
| p.o. | s.c. | i.v. | |||
| S. aureus systemic infection models | |||||
| ATCC 13709 (MSSA) | RWJ-416457 | 1 | 3.4 (2.7-4.5) | 4.7 (3.2-6.8) | 4.0 (3.0-5.2) |
| Linezolid | 2 | 6.4 (5.2-7.8) | 7.0 (5.4-9.1) | 6.3 (4.2-9.8) | |
| Vancomycin | 1 | NAa | 1.7 (0.9-2.9) | 0.9 (0.6-1.4) | |
| OC8525 (CA-MRSA) | RWJ-416457 | 2 | 2.1 (1.3-3.0) | 3.5 (2.5-4.5) | 1.5 (0.6-2.3) |
| Linezolid | 4 | 5.1 (2.7-7.8) | 3.7 (1.7-5.7) | 8.5 (6.5-11) | |
| Vancomycin | 1 | NA | 12 (10-14) | 5.6 (3.7-11) | |
| S. pneumoniae lung infection model | |||||
| ATCC 6301 (PSSP) | RWJ-416457 | 1 | 3.1 (2.0-4.9) | 9.3 (5.6-31) | 18 (13-26) |
| Linezolid | 2 | 14 (9.5-28) | 20 (13-26) | >40 | |
NA, not applicable.
Staphylococcal systemic lethal infection.
In the mouse systemic infection model with the S. aureus strains ATCC 13709 (MSSA) and OC8525 (CA-MRSA), the effects of RWJ-416457 were similar to and up to 5.7-fold more potent than the effects of linezolid (Table 1) when dosed p.o. or parenterally, with ED50 values ranging from 1.5 to 4.7 mg/kg/day for RWJ-416457 and 3.7 to 8.5 mg/kg/day for linezolid. Although ED50 values for RWJ-416457 were generally lower than those for linezolid, the 95% fiducial limits frequently overlapped. Vancomycin demonstrated lower ED50 values than the oxazolidinones against the MSSA strain but was generally less potent in the CA-MRSA model.
Staphylococcal skin infection studies.
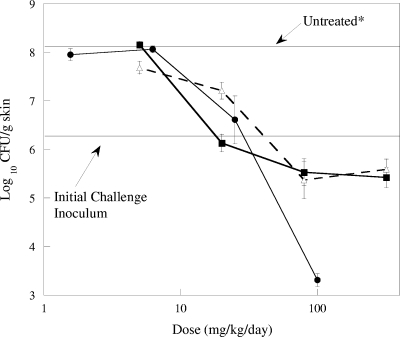
The efficacies of RWJ-416457, linezolid, and vancomycin against S. aureus Smith ATCC 13709 (MSSA) are summarized in Fig. 2. In untreated control animals, the MSSA CFU increased 1.9 log10 above the infecting inoculum level to 8.2 log10 CFU/g skin in 48 h. In contrast, during this same time period, treatment of S. aureus Smith with RWJ-416457 at doses of ≥80 mg/kg/day resulted in approximately a 0.9-log10 decrease in CFU/g skin relative to the level for the initial inoculum. At a dose of 5 mg/kg/day of RWJ-416457, no effect on growth of the MSSA strain was observed, with CFU values similar to those for untreated control animals. At a dose of 20 mg/kg/day, RWJ-416457 exhibited static activity, with CFU remaining the same as the level for the initial inoculum. Similar results were observed with linezolid (doses from 5 mg/kg/day to 320 mg/kg/day) and vancomycin (doses from 1.6 mg/kg/day to 25 mg/kg/day). At 100 mg/kg/day, vancomycin was more potent than either oxazolidinone, decreasing CFU/g skin by 3 log10.
FIG. 2.
Effects of RWJ-416457 (▪), vancomycin (•), and linezolid (▵) on S. aureus Smith ATCC 13709 in a murine skin infection model. Each symbol represents the mean ± standard error for 10 mice. *, bacterial counts in untreated animals at 48 h.
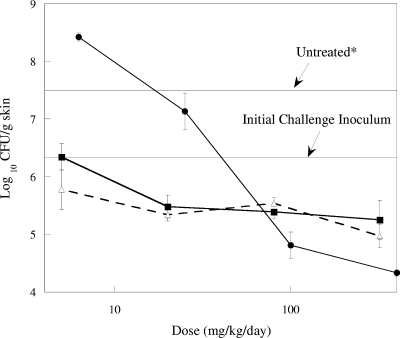
The efficacies of RWJ-416457, linezolid, and vancomycin against the CA-MRSA strain are summarized in Fig. 3. In control animals, the bacteria grew 1.6 log10 CFU from the level for the initial inoculum over the 48-h treatment period. In animals treated with RWJ-416457, CFU in the skin remained unchanged relative to the level for the initial inoculum (dose of 5 mg/kg/day) or decreased approximately 1 log10 CFU/g skin tissue (doses of 20 to 320 mg/kg/day). Similar results were obtained for linezolid, with decreases of 0.5 to 1.3 log10 CFU/g skin over the same dose range. Vancomycin treatments at 6.2 and 25 mg/kg/day had a minimal effect on MRSA skin titers, which remained above those for the initial inoculum. The highest doses of vancomycin (100 and 400 mg/kg/day) resulted in decreases that were 1.5 and 2 log10 CFU/g skin tissue, respectively, the largest decreases observed for any of the MRSA treatment groups.
FIG. 3.
Effects of RWJ-416457 (▪), vancomycin (•), and linezolid (▵) on MRSA OC8525 in a murine skin infection model. Each symbol represents the mean ± standard error for 10 mice. *, bacterial counts in untreated animals at 48 h.
Pneumococcal lower respiratory infection.
In the lethal lower respiratory infection model with S. pneumoniae ATCC 6301, RWJ-416457 was at least 2- to 4.5-fold more potent than linezolid (Table 1) across the different routes of administration, with ED50 values ranging from 3.1 to 18 mg/kg/day for RWJ-416457 and 14 to ≥40 mg/kg/day for linezolid. In this model, for both oxazolidinones, potency appeared to decrease when the compounds were dosed parenterally, with the highest ED50 values observed with i.v. dosing.
Single-dose pharmacokinetics.
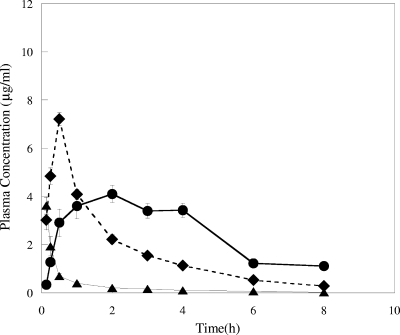
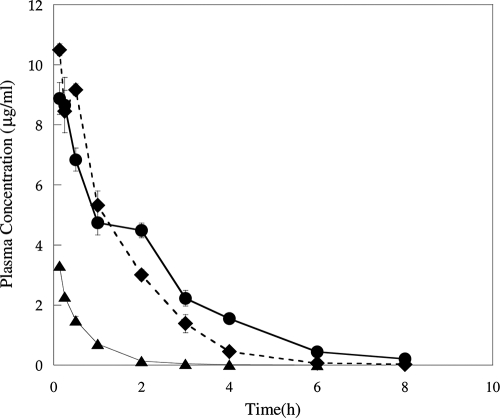
The plasma pharmacokinetic parameters for RWJ-416457 and linezolid are shown in Fig. 4 and 5 and in Table 2. Both total and free-drug values for the area under the concentration-time curve at 24 h (AUC24 and fAUC24, respectively) and the maximum concentration of drug in serum (Cmax and fCmax, respectively) were determined (Table 2). The fAUC24 values of RWJ-416457 ranged from 8 to 13 h·μg/ml (with the i.v. value normalized to a dose of 10 mg/kg/day, assuming a linear response) and were similar to those of linezolid for all three dosing routes. fCmaxs for RWJ-416457 were comparable to those for linezolid following i.v. dosing but were 1.7-fold and 3.2-fold lower than those for linezolid when dosed s.c. and p.o., respectively. RWJ-416457 time to maximum concentration of drug in serum values (s.c. and p.o. dosing) were 0.5 and 2 h, at least 4- and 16-fold longer, respectively, than those for linezolid. The half-life (t1/2) of RWJ-416457, 1.5 to 2 h when dosed parenterally and 3.4 h when dosed p.o., was 2.4- to 3-fold longer than that of linezolid. In agreement with the longer t1/2 of RWJ-416457, clearance (CL) values for RWJ-416457 when dosed i.v. were slightly lower than those for linezolid (Table 2). When CL was adjusted for bioavailability, values for both compounds (p.o. or s.c.) were similar.
FIG. 4.
Plasma pharmacokinetics of RWJ-416457 in normal CF-1 mice following a single 2-mg/kg i.v. (▴), 10-mg/kg p.o. (•), or 10-mg/kg s.c. (⧫) dose.
FIG. 5.
Plasma pharmacokinetics of linezolid in normal CF-1 mice following a single 2-mg/kg i.v. (▴), 10-mg/kg p.o. (•), or 10-mg/kg s.c. (⧫) dose.
TABLE 2.
Plasma pharmacokinetic parameters for RWJ-416457 and linezolid in CF-1 mice
| Compound | Administration route | Dose (mg/kg) | AUC24 (h·μg/ml) | fAUC24a (h·μg/ml) | Cmax (μg/ml) | fCmax (μg/ml) | Tmaxb (h) | t1/2 (h) | CL (ml/h/kg) | CL/Fc (ml/h/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| RWJ-416457 | i.v.d | 2 | 3.2 | 1.7 | 4.4 | 2.4 | NAe | 1.5 | 630 | |
| Linezolid | i.v.d | 2 | 2.1 | 1.4 | 3.3 | 2.3 | NA | 0.5 | 940 | |
| RWJ-416457 | s.c. | 10 | 15 | 8.3 | 7.5 | 4.1 | 0.5 | 2.0 | 650 | |
| Linezolid | s.c. | 10 | 16 | 11 | 11 | 7.1 | ≤0.13 | 0.8 | 640 | |
| RWJ-416457 | p.o. | 10 | 23 | 13 | 3.5 | 1.9 | 2.0 | 3.4 | 430 | |
| Linezolid | p.o. | 10 | 19 | 13 | 8.9 | 6.0 | ≤0.13 | 1.4 | 520 |
The free-drug plasma exposure following a single 10-mg/kg dose (p.o. and s.c.) or a single 2-mg/kg dose (i.v.) was determined from the total drug plasma levels corrected for protein binding.
Tmax, time to maximum concentration of drug in serum.
CL/F, CL adjusted for bioavailability.
Normalization of i.v. values to a 10-mg/kg dose (assuming linear response) yielded RWJ-416457 and linezolid AUC24 values of 16 and 11 h·μg/ml, respectively, and fAUC24 values of 8.5 and 7 h·μg/ml, respectively.
NA, not applicable.
Pharmacodynamic analyses.
The AUC24/MIC ratios were calculated for the data in this study (Table 3) to determine whether the relative magnitudes for RWJ-416457 and linezolid were consistent with the results observed in these efficacy studies. When i.v. data were normalized to a 10-mg/kg dose and compared with data for the other routes of administration, the fAUC24/MIC ratios for RWJ-416457 ranged from 4.1 to 13, whereas those for linezolid were 1.8 to 6.6. The greater fAUC/MICs for RWJ-416457 than for linezolid are similar to the relative differences in the ED50 values of RWJ-416457 and linezolid described above for the animal infection models.
TABLE 3.
Plasma pharmacodynamic fAUC/MIC parameters for RWJ-416457 and linezolid
| Strain (type) | Compound | MIC (μg/ml) |
fAUC24/MICa
|
||
|---|---|---|---|---|---|
| p.o. | s.c. | i.v.b | |||
| S. aureus ATCC 13709 | RWJ-416457 | 1 | 13 | 8.3 | 8.6 |
| (MSSA) | Linezolid | 2 | 6.6 | 5.3 | 3.6 |
| S. aureus OC8525 | RWJ-416457 | 2 | 6.3 | 4.1 | 4.3 |
| (CA-MRSA) | Linezolid | 4 | 3.3 | 2.6 | 1.8 |
| S. pneumoniae ATCC | RWJ-416457 | 1 | 13 | 8.3 | 8.6 |
| 6301 (PSSP) | Linezolid | 2 | 6.6 | 5.3 | 3.6 |
The free-drug plasma exposure following a single 10-mg/kg dose (p.o. and s.c.) or a single 2-mg/kg dose (i.v.) was determined from the total drug plasma levels corrected for protein binding.
The i.v. fAUC24/MIC ratios were determined from fAUC24 values projected for a 10-mg/kg i.v. dose.
DISCUSSION
The oxazolidinone class of antibiotics has been developed to address the need for new agents to treat antibiotic-susceptible and -resistant gram-positive pathogens, such as MRSA and PRSP, in health care and community settings. RWJ-416457 has previously been described as an investigational oxazolidinone with more potent in vitro activity against selected gram-positive pathogens than linezolid (13, 22). In this study, we report on the pharmacokinetics, pharmacodynamics, and efficacy of RWJ-416457 in experimental mouse models.
In these murine models, ED50 values for RWJ-416457 were generally lower than those for linezolid across all models and dosing routes. These in vivo results are consistent with the twofold- to fourfold-lower MICs of RWJ-416457 for the strains used in this study. However, because 95% fiducial limits frequently overlapped, it is possible that some ED50 values were comparable.
While the trends between in vitro and in vivo potencies were maintained in comparisons of RWJ-416457 and linezolid, inconsistencies were observed when comparing the in vitro and in vivo potencies between the MSSA and CA-MRSA models. Although MICs of RWJ-416457 and linezolid were twofold higher with the CA-MRSA strain than with the MSSA strain, ED50 values were generally equivalent or lower in the CA-MRSA model. This may reflect differences in virulence of the MSSA and CA-MRSA strains. Similarly, whereas the vancomycin MICs were equivalent for the MSSA and CA-MRSA strains, the in vivo efficacy of vancomycin in the CA-MRSA systemic model was reduced relative to that in the MSSA systemic model. Notably, the in vivo inoculum of CA-MRSA was 1.5 log10 CFU higher than that used in the MSSA model; these data are consistent with the reported phenomenon that an increased vancomycin inoculum can selectively decrease the in vivo efficacy relative to in vitro potency (9). The ED50 values reported for linezolid in this study are similar to those described by other investigators using systemic murine infection models, where ED50 values for S. aureus ranged from 2.9 to 7.0 mg/kg (14, 30). The improved in vivo potency of RWJ-416457 may be important clinically for the treatment of staphylococcal and enterococcal infections where MICs of linezolid approach nonsusceptibility breakpoints.
RWJ-416457 showed an oral efficacy similar to that of linezolid in the reduction of MSSA and MRSA CFU in skin infections in mice; however, at doses of ≥100 mg/kg/day, vancomycin appeared to be more effective than the oxazolidinones in this model. The oxazolidinones are bacteriostatic, whereas vancomycin can be bactericidal against some staphylococcal infections, consistent with these results. In earlier studies (12) evaluating MSSA or MRSA skin lesion size, RWJ-416457 yielded lesion volumes that were 14 to 56% or 23 to 37% smaller than those of linezolid, respectively, suggesting a potential advantage to RWJ-416457. Based on these data, there appear to be several measures of outcome that can be used to evaluate skin lesions.
In a pharmacokinetic/pharmacodynamic mouse thigh infection model, the AUC24/MIC was reported to be the major parameter predictive of the in vivo efficacy of linezolid, while the time above the MIC, a factor contributing to efficacy, correlated with outcome less consistently (2). The utility of AUC/MIC as a predictor of efficacy for linezolid has been confirmed clinically (24, 28). The calculated fAUC24/MICs of RWJ-416457 in our studies ranged from 1.6- to 2.4-fold greater than those of linezolid (Table 3) for the MSSA, MRSA, and S. pneumoniae strains used, similar to the trends of 1.1-fold- to 5.7-fold-greater in vivo potency for RWJ-416457. These data are consistent with the utility of AUC/MIC as a predictive index for the efficacy of RWJ-416457 in these mouse infection models. The AUC values of RWJ-416457 and linezolid were similar across the different routes of administration (i.v., s.c., and p.o.) at comparable doses (Table 2). The larger fAUC/MIC ratios for RWJ-416457 than for linezolid thus appear to result from the contribution of the lower RWJ-416457 MICs, suggesting that the increased systemic efficacy of RWJ-416457 stems from its greater in vitro potency. Although the pharmacodynamic indices were normalized to plasma free-drug levels, the slightly higher mouse protein binding of RWJ-416457 (46%) than of linezolid (32%) may also have contributed to the relative potencies of the two compounds observed in the animal infection models.
A mouse pharmacokinetic/pharmacodynamic study by Andes et al. (2) reported s.c. static-dose AUC24/MIC ratios of linezolid from 22 to 97 for S. pneumoniae strains and from 38 to 167 for S. aureus strains. Normalization of these ratios in the Andes et al. study to a 10-mg/kg dose, with 68% free drug (32% mouse protein binding), yielded ratios for the S. pneumoniae strains ranging from 6.3 to 12.6 and ratios for the S. aureus strains ranging from 2.4 to 9.5; these values are similar to the S. pneumoniae and S. aureus fAUC24/MICs of 5.3 and 2.6, respectively (s.c. dosing), reported for linezolid in the current study (Table 3). A single linezolid dose of 600 mg in humans (equivalent to 8.6 mg/kg) is similar to the 10-mg/kg dose administered to mice in this study. In previously published reports, linezolid single-dose AUC values (i.v.) for humans were approximately five- to sevenfold higher than the single-dose AUC values (i.v.) reported in this study for mice, and, consistently, CL in humans was approximately five- to eightfold slower than CL in mice (1, 24). With a linezolid MIC of 4 μg/ml, the calculated AUC/MIC ratio would be approximately five- to sevenfold higher in humans than in mice.
In our studies, the RWJ-416457 and linezolid p.o. AUCs were approximately 1.5-fold higher than the respective dose-adjusted i.v. AUC values (Table 2), suggesting that oral bioavailability of the oxazolidinones exceeded 100%. In general, increased exposure with p.o. or s.c. dosing did not correlate with increased efficacy. This lack of correlation may be explained by differences in metabolism or CL of the oxazolidinones with the lower i.v. dose or by rate-limiting absorption following p.o. or s.c. dosing. Nonetheless, when RWJ-416457 and linezolid were compared, trends in pharmacokinetic and pharmacodynamic parameters were consistent with efficacy values observed in the systemic infection models.
In addition to the improved in vitro potency of RWJ-416457 relative to that of linezolid, RWJ-416457 MICs were similar to or lower than those of vancomycin against vancomycin-susceptible and -resistant gram-positive pathogens (13), suggesting that the potency, as well as the spectrum of activity, of RWJ-416457 is appropriate for the treatment of antibiotic-susceptible and -resistant gram-positive pathogens. The murine infection studies described herein demonstrate that RWJ-416457 has in vivo activity that is consistent with its in vitro potency, relative to these two important clinical agents. RWJ-416457, like linezolid, was effective in the treatment of staphylococcal murine skin infections, further supporting the clinical evaluation of RWJ-416457 for the treatment of skin and skin structure infections.
Acknowledgments
We thank Steven Stryker for technical assistance in conducting in vivo studies.
Footnotes
Published ahead of print on 9 March 2009.
REFERENCES
- 1.Adembri, C., S. Fallani, M. I. Cassetta, S. Arrigucci, A. Ottaviano, P. Pecile, T. Mazzei, R. De Gaudio, and A. Novelli. 2008. Linezolid pharmacokinetic/pharmacodynamic profile in critically ill septic patients: intermittent versus continuous infusion. Int. J. Antimicrob. Agents 31:122-129. [DOI] [PubMed] [Google Scholar]
- 2.Andes, D., M. L. VanOgrop, J. Peng, and W. A. Craig. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob. Agents Chemother. 46:3484-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appelbaum, P. C. 2007. Microbiology of antibiotic resistance in Staphylococcus aureus. Clin. Infect. Dis. 45:S165-S170. [DOI] [PubMed] [Google Scholar]
- 4.Avdic, E., and S. E. Cosgrove. 2008. Management and control strategies for community-associated methicillin-resistant Staphylococcus aureus. Expert Opin. Pharmacother. 9:1463-1479. [DOI] [PubMed] [Google Scholar]
- 5.Barrett, J. F. 2005. MRSA—what is it, and how do we deal with the problem? Expert Opin. Ther. Targets 9:253-265. [DOI] [PubMed] [Google Scholar]
- 6.Berger-Bachi, B., and S. Rohrer. 2002. Factors influencing methicillin resistance in staphylococci. Arch. Microbiol. 178:165-171. [DOI] [PubMed] [Google Scholar]
- 7.Boubaker, K., P. Diebold, D. S. Blanc, F. Vandenesch, G. Praz, G. Dupuis, and N. Troillet. 2004. Panton-Valentine leukocidin and staphylococcal skin infections in schoolchildren. Emerg. Infect. Dis. 10:121-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CLSI. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. Clinical and Laboratory Standards Institute M7-A7. CLSI, Wayne, PA.
- 9.Craig, W. A., D. Lee, S. Kethireddy, and D. R. Andes. 2008. Comparison of in vitro and in vivo activity of vancomycin (V) against MRSA at 105 and 107 inocula, abstr. A-986, p. 23. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother./Infect. Dis. Soc. Am. 46th Annu. Meet. American Society for Microbiology, Washington, DC.
- 10.Davis, S. L., M. B. Perri, S. M. Donabedian, C. Manierski, A. Singh, D. Vager, N. Z. Haque, K. Speirs, R. R. Muder, B. Robinson-Dunn, M. K. Hayden, and M. J. Zervos. 2007. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J. Clin. Microbiol. 45:1705-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dresser, L. D., and M. J. Rybak. 1998. The pharmacologic and bacteriologic properties of oxazolidinones, a new class of synthetic antimicrobials. Pharmacotherapy 18:456-462. [PubMed] [Google Scholar]
- 12.Fernandez, J., J. J. Hilliard, W. Zhang, J. L. Melton, D. Abbanat, and K. Bush. 2007. In vivo efficacy of RWJ-416457 in a staphylococcal murine skin infection model, abstr. 431, p. 138. 45th Annu. Meet. Infect. Dis. Soc. Am.
- 13.Foleno, B. D., D. Abbanat, R. M. Goldschmidt, R. K. Flamm, S. D. Paget, G. C. Webb, E. Wira, M. J. Macielag, and K. Bush. 2007. In vitro antibacterial activity of the pyrrolopyrazolyl-substituted oxazolidinone RWJ-416457. Antimicrob. Agents Chemother. 51:361-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford, C. W., G. E. Zurenko, and M. R. Barbachyn. 2001. The discovery of linezolid, the first oxazolidinone antibacterial agent. Curr. Drug Targets Infect. Disord. 1:181-199. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert, M., J. MacDonald, D. Gregson, J. Siushansian, K. Zhang, S. Elsayed, K. Laupland, T. Louie, K. Hope, M. Mulvey, J. Gillespie, D. Nielsen, V. Wheeler, M. Louie, A. Honish, G. Keays, and J. Conly. 2006. Outbreak in Alberta of community-acquired (USA300) methicillin-resistant Staphylococcus aureus in people with a history of drug use, homelessness or incarceration. Can. Med. Assoc. J. 175:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill, C. J., G. K. Abruzzo, A. M. Flattery, A. S. Misura, K. Bartizal, and E. J. Hickey. 2007. In vivo efficacy of a novel oxazolidinone compound in two mouse models of infection. Antimicrob. Agents Chemother. 51:3434-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hota, B., C. Ellenbogen, M. K. Hayden, A. Aroutcheva, T. W. Rice, and R. A. Weinstein. 2007. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections at a public hospital: do public housing and incarceration amplify transmission? Arch. Intern. Med. 167:1026-1033. [DOI] [PubMed] [Google Scholar]
- 18.Klevens, R. M., M. A. Morrison, J. Nadle, S. Petit, K. Gershman, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, E. R. Zell, G. E. Fosheim, L. McDougal, R. B. Carey, and S. K. Fridkin. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763-1771. [DOI] [PubMed] [Google Scholar]
- 19.LaPlante, K. L., M. J. Rybak, M. Amjad, and G. W. Kaatz. 2007. Antimicrobial susceptibility and staphylococcal chromosomal cassette mec type in community- and hospital-associated methicillin-resistant Staphylococcus aureus. Pharmacotherapy 27:3-10. [DOI] [PubMed] [Google Scholar]
- 20.Liu, C., C. J. Graber, M. Karr, B. A. Diep, L. Basuino, B. S. Schwartz, M. C. Enright, S. J. O'Hanlon, J. C. Thomas, F. Perdreau-Remington, S. Gordon, H. Gunthorpe, R. Jacobs, P. Jensen, G. Leoung, J. S. Rumack, and H. F. Chambers. 2008. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004-2005. Clin. Infect. Dis. 46:1637-1646. [DOI] [PubMed] [Google Scholar]
- 21.Livermore, D. M. 2007. Introduction: the challenge of multiresistance. Int. J. Antimicrob. Agents 29:S1-S7. [DOI] [PubMed] [Google Scholar]
- 22.Livermore, D. M., M. Warner, S. Mushtaq, S. North, and N. Woodford. 2007. In vitro activity of the oxazolidinone RWJ-416457 against linezolid-resistant and -susceptible staphylococci and enterococci. Antimicrob. Agents Chemother. 51:1112-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu, D., and P. Holtom. 2005. Community-acquired methicillin-resistant Staphylococcus aureus, a new player in sports medicine. Curr. Sports Med. Rep. 4:265-270. [DOI] [PubMed] [Google Scholar]
- 24.MacGowan, A. P. 2003. Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with gram-positive infections. J. Antimicrob. Chemother. 51:ii17-ii25. [DOI] [PubMed] [Google Scholar]
- 25.Maltezou, H. C., and H. Giarmarellou. 2006. Community-acquired methicillin-resistant Staphylococcus aureus infections. Int. J. Antimicrob. Agents 27:87-96. [DOI] [PubMed] [Google Scholar]
- 26.Okuma, K., K. Iwakawa, J. D. Turnidge, W. B. Grubb, J. M. Bell, F. G. O'Brien, G. W. Coombs, J. W. Pearman, F. C. Tenover, M. Kapi, C. Tiensasitorn, T. Ito, and K. Hiramatsu. 2002. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J. Clin. Microbiol. 40:4289-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagac, B. B., R. W. Reiland, D. T. Bolesh, and D. L. Swanson. 2006. Skin lesions in barracks: consider community-acquired methicillin-resistant Staphylococcus aureus infection instead of spider bites. Mil. Med. 171:830-832. [DOI] [PubMed] [Google Scholar]
- 28.Rayner, C. R., A. Forrest, A. K. Meagher, M. C. Birmingham, and J. J. Schentag. 2003. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin. Pharmacokinet. 42:1411-1423. [DOI] [PubMed] [Google Scholar]
- 29.Riedl, B., and R. Endermann. 1999. Recent developments with oxazolidinone antibiotics. Expert Opin. Ther. Patents 9:625-633. [Google Scholar]
- 30.Sreenivas, K., P. V. S. Amarnath, A. Mallik, H. Sarnaik, N. Selvar Kumar, M. Takhi, S. Trehan, M. Sitaram Kumar, J. Iqbal, R. Rajagopalan, and R. Chakrabarti. 2007. In vitro and in vivo antibacterial evaluation of DRF 8417, a new oxazolidinone. J. Antimicrob. Chemother. 60:159-161. [DOI] [PubMed] [Google Scholar]
- 31.Stryjewski, M. E., and H. F. Chambers. 2008. Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 46(Suppl. 5):S368-S377. [DOI] [PubMed] [Google Scholar]
- 32.Styers, D., D. J. Sheehan, P. Hogan, and D. F. Sahm. 2006. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann. Clin. Microbiol. Antimicrob. 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]