Abstract
At present, voriconazole (VOR) is the drug of first choice for treating invasive pulmonary aspergillosis (IPA). However, particularly in advanced stages of disease and in the severely immunocompromised host, the mortality remains substantial. The combination of VOR with an echinocandin may improve the therapeutic outcome. We investigate here whether combining VOR and anidulafungin (ANI) in advanced IPA in transiently neutropenic rats results in a higher therapeutic efficacy. Since VOR is metabolized more rapidly in rodents than in humans, dosage adjustment for VOR is necessary to obtain an area under the plasma concentration-time curve (AUC) in rodents that is equivalent to that of humans. In this study, the pharmacokinetics of VOR and ANI in rats were elucidated, and dosage schedules were applied that produced AUCs similar to those of humans. The developed dose schedules were well tolerated by the rats, without effects on renal and hepatic functions. VOR showed excellent efficacy in early IPA (100% rat survival). In advanced IPA, VOR was less efficacious (50% rat survival), whereas a significant decrease in galactomannan concentrations in lungs and sera was found in surviving rats. ANI administered in advanced IPA resulted in 22% rat survival, and the serum concentrations of fungal galactomannan were slightly but not significantly decreased. The addition of ANI to VOR did not result in significantly increased therapeutic efficacy in advanced IPA, resulting in 67% rat survival and a significant decrease in galactomannan concentration in serum. In conclusion, VOR monotherapy is therapeutically effective in the treatment of advanced-stage IPA and superior to the use of ANI. Combining both agents does not significantly improve the therapeutic outcome.
Invasive pulmonary aspergillosis (IPA) continues to be a major problem in immunocompromised patients (18). Since voriconazole (VOR) was shown to be superior to amphotericin B (AMB) in a large randomized trial, it became the drug of choice for the treatment of IPA (22, 44). More recently, another class of antifungal agents, the echinocandins, has been used to salvage patients with refractory IPA (44). Echinocandins target 1,3-β-d-glucan synthesis of most pathogenic fungi. Caspofungin (CAS) was the first drug of this class, and more recently anidulafungin (ANI) has become available. However, despite these new treatment options for IPA, the mortality remains substantial, with mortality rates of 25 to 35% 12 weeks after diagnosis (21, 22). Combination therapy might reduce mortality rates further. From clinical observational studies, it appeared that in 57 to 68% of the favorable results were obtained in patients when combination therapy with VOR and CAS was applied (27, 37). In neither of these studies, however, was the combination therapy compared to VOR monotherapy in a randomized fashion.
In this respect, preclinical research in animal models of IPA is important for investigating the potency of antifungal agents in combination. In our laboratory, we developed a model of unilateral IPA in rats that closely mimics the human disease in patients with transient neutropenia (8, 9, 41). This model is characterized by a transiently neutropenic state, fungal inoculation through the respiratory route, fungal broncho- and angioinvasion, and extrapulmonary dissemination. Since VOR and the echinocandins appear to be active agents and have a different mode of action, combination therapy might further improve the therapeutic outcome.
The triazole VOR is metabolized by the hepatic P450 isoenzymes CYP3A4, CYP2C9, and CYP2C19 and converted into a N-oxide, which has no antifungal activity (35). In rodents (except guinea pigs), metabolism occurs faster than in humans, resulting in a more rapid clearance and a shorter half-life of the drug (12, 34). Therefore, in rodent models, the dosage schedule of VOR should be adjusted to compensate for rapid metabolism in order to enable clinically relevant studies in rodents. The development of human equivalent dosage schedules in the rat is further complicated by the presence of saturable pharmacokinetics and enzyme induction, which are both not observed in the clinical situation. Human pharmacokinetic equivalent VOR dosage schedules in rats have not been described. Like VOR, some of the echinocandins are metabolized in the liver. While CAS undergoes N acetylation and nonenzymatic hydrolysis, ANI is metabolized by hydrolysis of a cyclic peptide (28). For this chemical degradation the CYP450 system is not involved and, as a result, a compensation for a rapid drug metabolism in rats is probably not needed (39).
For the triazoles, the area under the concentration-time curve (AUC)/MIC ratio is the pharmacokinetic/pharmacodynamic (PK/PD) index predicting therapeutic efficacy (4, 6, 7). For the echinocandins, both the AUC/MIC and the Cmax/MIC have been indicated to predict efficacy (2, 3, 5, 25, 43). The Cmax/MIC ratio was more indicative early in therapy and associated with serum kinetics (5, 25), whereas the AUC/MIC ratio was more indicative later in therapy and in tissue kinetics (25). Therefore, dosage schedules for VOR and ANI in animal models should be developed that produce AUCs comparable to those in humans.
In the present study, we developed human equivalent dosage schedules for VOR and ANI in rats. The therapeutic efficacy of both agents, administered alone or in combination, was investigated in our model of IPA in transiently neutropenic rats in relation to the stage of infection.
MATERIALS AND METHODS
Aspergillus fumigatus strain.
A clinical isolate of A. fumigatus was used in all experiments. This isolate was obtained from a hemato/oncological patient with IPA. To maintain its virulence, the strain was passed regularly through neutropenic rats and maintained on Sabouraud maltose agar slants. In our in vitro susceptibility test, the ATCC 204305 A. fumigatus strain as a control strain was used as well.
In vitro antifungal susceptibility assay.
The in vitro antifungal susceptibility test for VOR and ANI was adapted from a quantitative viability-based susceptibility assay as described elsewhere (40). In short, the MIC was determined independently in triplicate with the broth microdilution method described by the Clinical and Laboratory Standards Institute (CLSI) (15). In this microwell tray a checkerboard titration of the antifungal agents VOR and ANI was performed, with final drug concentrations ranging from 0.06 to 4 μg/ml for VOR and from 0.015 to 128 μg/ml for ANI. For ANI, next to the MIC, the minimal effective concentration (MEC) was also determined. The MEC was considered to be the concentration in the first well that contained altered microscopic morphology. After endpoint reading according to the CLSI criteria, the substrate 2,3-bis(2-methoxy-4-nitro-5-[(sulfenylamino)carbonyl]-2H-tetrazolium-hydroxide (XTT)/menadione was added for quantitative determination of the inhibition. The inhibitory concentration endpoint for each antifungal combination was defined as the first concentration at which spectrophotometrically 80% or more reduction of mitochondrial dehydrogenase activity was measured.
Evaluation of the activity of combinations of antifungal agents was done by using fractional inhibitory concentration indexes, which were calculated by the method published by den Hollander et al. (17).
Animal model.
The rat model of aerogenic IPA in transiently neutropenic rats was used, as described previously (8, 9, 41, 42). In short, in neutropenic female RP strain albino rats left-sided invasive IPA was induced by instillation of an A. fumigatus conidial suspension, followed by inhalation. The infection, if untreated, resulted in 100% mortality of rats within 5 to 10 days.
The experimental protocols adhered to the rules specified in the Dutch Animal Experimentation Act of 1977 and the published Guidelines on the Protection of Experimental Animals by the Council of the European Community of 1986. The present protocols were approved by the Institutional Animal Care and Use Committee of the Erasmus Medical Center, Rotterdam, The Netherlands.
Pharmacokinetics of VOR in uninfected rats.
In order to establish a VOR dosing schedule in rats mimicking VOR plasma levels in humans, the pharmacokinetics of VOR were investigated in several groups composed of nine uninfected neutropenic rats in each group. Pharmacokinetic parameters were derived and used to adjust the dose in the subsequent group of rats in order to obtain a human pharmacokinetic equivalent dosage schedule. For confirmation, the pharmacokinetics of this VOR schedule was assessed in four groups of nine rats each. The first and second groups received VOR doses at 2.6 and 5 mg/kg every 12 h (q12h) intraperitoneally (i.p.), respectively, for 10 days. The third group received increasing doses of 6, 8, 10, and 11 mg/kg q12h i.p. on 4 consecutive days, followed by doses of 12 mg/kg q12h i.p. on the remaining 6 days. The fourth group received increasing dosages of 7.5, 10, 12.5, and 15 mg/kg q12h i.p. on the first 4 consecutive days, followed by doses of 17.5 mg/kg q12h i.p. on the remaining 6 days. The dosing schedule applied in the fourth group produced VOR plasma levels similar to those observed in humans. In the pharmacokinetic studies, serial blood samples were withdrawn from the retro-orbital plexus from animals under isoflurane anesthesia on days 0, 5, and 9 at 0, 2, and 8 h; at 0.5, 4, and 10 h; or at 1, 6, and 12 h postdosing. Nine samples were available from each rat. Plasma concentrations of VOR were determined by using a standard large plate agar diffusion method with diagnostic sensitivity test agar (Oxoid, Basingstoke, United Kingdom) and a clinical Candida albicans strain as test organism. Samples of 200 μl were assayed. Twofold increasing standard concentrations of 0.125 to 4 μg/ml were used.
Pharmacokinetic models were fitted to the VOR plasma concentration from all rats simultaneously using NONMEM software (for nonlinear mixed effects modeling, version 1.1, double precision, first-order estimation) (11). The nonlinear pharmacokinetics of VOR were described by a model, which includes both autoinduction and saturation of the metabolizing enzyme (23). The pharmacokinetic model is represented by equations 1 to 5.
The change of amount of VOR in the peritoneum (Aip) over time (t) is described by:
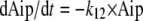 |
(1) |
where k12 is a first-order rate constant describing the transport from the peritoneum to the central compartment. The change of amount of VOR in the central compartment (Ac) over time is described by:
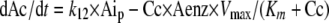 |
(2) |
where Cc is the VOR concentration in the central compartment Cc = Ac/V (V is the volume of distribution), Aenz is the (relative) amount of metabolizing enzyme, Vmax is the maximum elimination rate, and Km is the Michaelis-Menten constant (the concentration at half Vmax).
The change of Aenz over time in the enzyme compartment is dependent on Cc as follows:
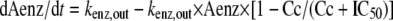 |
(3) |
where kenz,out is a first-order rate constant for enzyme degradation and IC50 is the VOR plasma concentration at 50% of the maximum inhibition of enzyme degradation. At t = 0 and Aenz = 1, the enzyme formation rate (kenz,in) is equal to the degradation rate (kenz,out).
In the NONMEM model between-rat variability in V, Vmax, and kenz was estimated by using an exponential model:
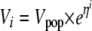 |
(4) |
where Vi is the clearance of the ith rat, Vpop is the typical value in the population, and ηi is a between-rat random variable with mean of zero and a variance of ω2.
For a nonlinear mixed-effects model, the residual variance corresponds to the difference between the observed concentration (Cobs) and the predicted concentration (Cpred). The latter is predicted on basis of individual parameters (e.g., Vmaxi, Vi, etc.). The residual variance was modeled with an additive (ɛ1) and a proportional part (ɛ2) as follows:
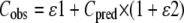 |
(5) |
where ɛ1 and ɛ2 are independent random variables with zero means and variances of σ12 and σ22, respectively.
Model adequacy was evaluated by using various residual plots (“goodness-of-fit” plots), values of random-effects variances, and precision of the parameter estimates.
Pharmacokinetics of ANI in uninfected rats.
A human pharmacokinetic equivalent dosage schedule for ANI was obtained in a manner similar to that used for VOR. The pharmacokinetics of ANI was studied in three groups of rats. The first and second group (both composed of nine rats) received subcutaneously (s.c.) an ANI loading dose of 20 mg/kg q24h, followed by doses of 10 mg/kg q24h (group 1) and 5 mg/kg q24h (group 2), respectively, for 2 days. In the third group, 24 rats received s.c. an ANI loading dose of 20 mg/kg q24h, followed by doses of 5 mg/kg q24h for 9 days. The dosing schedule in group 3 produced ANI plasma levels similar to those observed in humans. In the pharmacokinetic studies blood samples were withdrawn on days 0, 1, 2, 6, and 9 at 6, 12, and 24 h postdosing. Nine samples were available from each rat. The plasma concentrations for ANI were determined by using a standard large plate agar diffusion method as described for VOR.
The pharmacokinetics of ANI were assessed by NONMEM modeling as described above. A one-compartment pharmacokinetic model was used with first-order absorption and elimination. The following parameters were estimated: k12, V, and clearance (CL).
Antifungal treatment of infected rats.
Treatment with AMB (Fungizone; Bristol-Myers Squibb B.V., Woerden, The Netherlands) was started at 24 h after fungal inoculation. AMB was diluted in 5% dextrose and was administered intravenously via the lateral tail vein at 1 mg/kg/day once daily for 10 days (the maximum tolerated dose for rats). Treatment with VOR (Vfend; Pfizer B.V., Capelle a/d IJsel, The Netherlands) was started 24, 48, or 72 h after fungal inoculation. VOR was administered i.p. at 12-h intervals for 10 days. The treatment regime of VOR included two daily doses 7.5, 10, 12.5, and 15 mg/kg on days 0, 1, 2, and 3 and two daily doses of 17.5 mg/kg on day 4 and beyond, as stated in Table 1. Treatment with ANI (Eraxis; Pfizer) was started 72 h after fungal inoculation. ANI was reconstituted in solvent. The solvent consisted of 20% (vol/vol) ethanol. The final drug concentrations administered to the rats contained no more than 2.5% (vol/vol) ethanol. ANI was administered s.c. once daily for either 3 days or 10 days. The treatment regimen included an uploading ANI dose 20 mg/kg/day on day 1, followed by a maintaining dosage of 5 mg/kg/day for the following 9 days.
TABLE 1.
Human pharmacokinetic equivalent dosage schedule of VOR in ratsa
| Time (h) after first injection | VOR dose (mg/kg, q12h) | AUC12 (μg·h/ml) | % of the human target AUC12 |
|---|---|---|---|
| 0-12 | 7.5 | 37.2 | 124 |
| 12-24 | 7.5 | 38.4 | 128 |
| 24-36 | 10.0 | 36.6 | 122 |
| 36-48 | 10.0 | 27.6 | 92 |
| 48-60 | 12.5 | 30.0 | 100 |
| 60-72 | 12.5 | 26.4 | 88 |
| 72-84 | 15.0 | 31.5 | 105 |
| 84-96 | 15.0 | 29.4 | 98 |
| 96-108 | 17.5 | 31.8 | 106 |
| 108-120 | 17.5 | 30.9 | 103 |
| 120-132 | 17.5 | 30.6 | 102 |
| 132-144 | 17.5 | 30.3 | 101 |
| 144-156 | 17.5 | 33.0 | 113 |
| 156-168 | 17.5 | 33.3 | 111 |
| 168-180 | 17.5 | 33.0 | 110 |
| 180-192 | 17.5 | 33.0 | 110 |
| 192-204 | 17.5 | 33.0 | 110 |
| 204-216 | 17.5 | 33.0 | 110 |
| 216-228 | 17.5 | 33.0 | 110 |
| 228-240 | 17.5 | 32.7 | 109 |
| 240-252 | 0 | 32.7 | 109 |
Doses were administered at 12-h intervals (q12h) as indicated. The AUC12 for VOR was calculated on the basis of the population pharmacokinetic parameters given in Table 2. The percentage of the human target was obtained by dividing the AUC12 by the human target value (29.5 μg·h/ml) × 100.
Toxic side effects of antifungal agents.
To determine potential toxic side effects of VOR or ANI, the hepatic and renal functions were monitored in uninfected neutropenic animals. This was done by sampling blood on the last day of therapy for both VOR and ANI at 24 h after the last dosage. This was for VOR after 10 days of treatment and for ANI after 3 days or after 10 days of treatment. Also, at 11 days after the termination of treatment a sample was taken. In the serum samples obtained creatinine (CREAT) levels and blood-urea-nitrogen (BUN) levels were determined to assess the renal functions, while serum alanine aminotransferase (ALAT) and aspartate aminotransferase (ASAT) levels were measured to assess the hepatic functions. The same parameters were determined for a healthy control group, consisting of 35 rats, to calculate the normal values for this rat strain. Mild toxicity was defined when the level for either one of these parameters was more than three times the upper limit of normal (three times the 95th percentile boundary of the healthy control group), and severe toxicity was defined as levels higher than five times the upper limit of normal.
Parameters for therapeutic efficacy of antifungal treatment.
The survival rate of rats was monitored daily for 23 days after fungal inoculation. In addition, the concentration of circulating A. fumigatus galactomannan (an antigenic constituent of the fungal cell wall) in both serum and infected left lung homogenate was detected to quantify the fungal burden. The galactomannan concentrations were determined with a commercially available sandwich enzyme-linked immunosorbent assay (Platelia Aspergillus; Bio-Rad, Marnes-la-Coquette, France) as described previously (42).
Statistical analysis.
Kaplan-Meier survival curves were generated, and the differences in rat survival rate were assessed with a log rank test. Differences in quantitative parameters of fungal infection were assessed by using the nonparametric Mann-Whitney test (GraphPad Instat). P values are reported without correction for multiple testing.
RESULTS
Establishment of the human pharmacokinetic equivalent VOR dosage schedule in the rat.
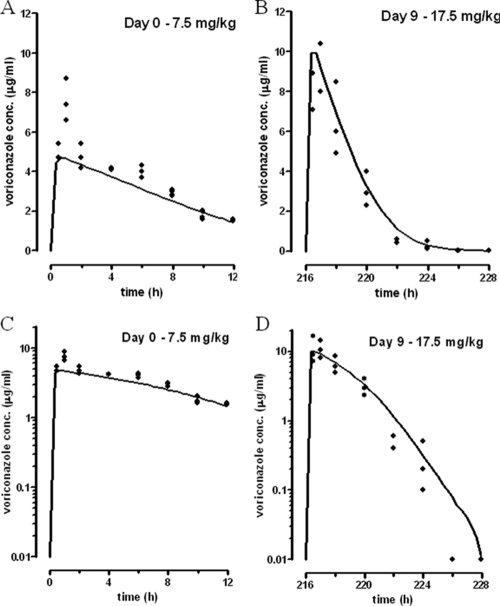
For triazoles such as VOR, the AUC/MIC ratio is considered the PK/PD index determining therapeutic efficacy (1). The human steady-state AUC for 12 h (AUC12) is 29.5 μg·h/ml (33); for the uninfected neutropenic rats in the present study the doses were iteratively adjusted to obtain drug exposures similar to those in humans. To do this, higher VOR doses than those used in humans were administered to the rats in order to compensate for the rapid metabolism of VOR in the rat. From the pharmacokinetic model developed, it appeared that doses of VOR of 7.5, 10, 12.5, and 15 mg/kg q12h on the first four consecutive days and of 17.5 mg/kg q12h on the remaining 6 days provided AUC12 values comparable to those of humans. Table 1 summarizes for each 12-h interval the AUC values calculated on the basis of the derived population pharmacokinetic parameters in rats and the percentages of the human target AUC. The mean AUC12 in rats was 32.3 μg·h/ml, which represents 109% of the human target AUC12. From these data, the estimated population pharmacokinetic parameters were derived and summarized in Table 2. Figure 1 shows the measured VOR plasma concentration-versus-time profile on the first and last days of this schedule, as predicted on basis of the pharmacokinetic parameter values summarized in Table 2. In Fig. 1 the logarithmic plot clearly demonstrates Michaelis-Menten pharmacokinetics since the elimination half-life is shortened with decreasing concentrations on both days. Compared to the first day, the amount of enzyme is increased sixfold on the last day, producing a comparable increase in the Vmax. The latter is responsible for the faster elimination at day 9 after the administration of VOR. In order to compensate for this higher Vmax, VOR doses were increased during the 10 day-treatment period. Escalating doses produced increasing maximal VOR concentrations, while the AUC remained constant (Table 1).
TABLE 2.
Population pharmacokinetic parameters of VOR in ratsa
| Parameter | Mean | Between-rat variability (%) |
|---|---|---|
| k12 (h−1) | 7.8 | |
| V (ml) | 310 | 15 |
| Km (μg/ml) | 5.6 | |
| Vmax (μg/h) | 180 | 103 |
| kenz (h−1) | 0.076 | 223 |
| IC50 (μg/ml) | 0.045 | |
| Residual variability | ||
| Additive error (μg/ml) | 0.02 | |
| Proportional error (%) | 28 |
Standard errors of the estimates were <42%.
FIG. 1.
Measured VOR plasma concentrations (dots) on day 0 (A and C) and day 9 (B and D) following doses of 7.5 and 17.5 mg/kg, respectively. The solid lines represent the concentration profile as predicted on basis of the population pharmacokinetic parameters presented in Table 2. In the logarithmic plots (C and D) the convex shape of the curves clearly demonstrates saturable metabolism. The elimination slope increases with diminishing concentrations. In comparison with day 0 the rate of elimination (Vmax) is increased on day 9 due to enzyme induction. At comparable concentrations the elimination slope is steeper on day 9. In order to compensate for the increased elimination rate, a larger dose was given on day 9 (17.5 mg/kg versus 7.5 mg/kg), which produces higher maximal concentrations but comparable exposure. See linear plots (A and B) with respective values of the AUC12 of 37.2 and 33.0 μg·h/ml.
The tolerability of this VOR dosage schedule in rats was monitored by evaluation of the renal and hepatic functions as measured in terms of serum CREAT, BUN, ALAT, and ASAT. The VOR dosage schedule was well tolerated and did not result in elevated levels of either parameter. For none of the parameters was mild toxicity (three times the upper limit of normal boundary) or severe toxicity (five times the upper limit of normal boundary) observed.
Therapeutic efficacy of VOR compared to AMB in relation to the stage of IPA.
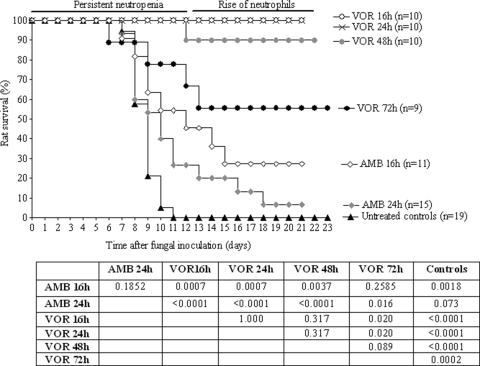
In our transiently neutropenic rat model of IPA, the therapeutic efficacy of VOR, at the human pharmacokinetic equivalent dosage schedule, was investigated in reference to AMB at 1 mg/kg/day, the maximum tolerated dose in this model. As shown in Fig. 2, untreated rats all died between day 5 and day 10 after fungal inoculation. Treatment with VOR started 16 h after fungal inoculation, when hyphal outgrowth was established, resulted in 100% survival of rats. In contrast, treatment with AMB resulted in only 27% rat survival at day 21 after fungal inoculation.
FIG. 2.
Therapeutic efficacy of AMB and VOR in transiently neutropenic rats with IPA. AMB was administered intravenously once daily at 1 mg/kg/day. VOR was administered i.p. at 12-h intervals according to the human pharmacokinetic equivalent dosage schedule. Treatment was started either 16, 24, 48, or 72 h after fungal inoculation and continued for 10 days. P values were determined with the log rank test by comparing the different dosing schedules.
Delay of start of treatment from 16 to 24 h after fungal inoculation resulted in a slight, although not statistically significant (P = 0.1852), decrease in the therapeutic efficacy of AMB (Fig. 2). Rat survival at day 21 decreased from 27 to 7%. In contrast, delay in start of treatment with VOR to 24 h did not reduce the efficacy of VOR since 100% survival of rats was again observed. A further delay in VOR treatment to 48 h resulted in only a minor, but not significant, reduction in therapeutic efficacy (P = 0.3173), with 90% rat survival. When treatment with VOR was started as late as 72 h after fungal inoculation, efficacy was significantly reduced (P = 0.0123) compared to starting the treatment at 16 h. However, in this advanced stage of severe IPA the efficacy of VOR still reached a significantly increased rat survival rate of 56% compared to untreated controls (P = 0.0002).
In vitro antifungal activity of VOR and ANI alone or in combination.
The MICs for VOR and MECs for ANI were determined for both the A. fumigatus clinical isolate used in our animal model and the ATCC A. fumigatus reference strain. MICs found for VOR were 0.25 and 0.125 μg/ml, respectively; the MECs for ANI were 0.03 μg/ml for both isolates, while the MICs for ANI were >128 μg/ml for both isolates. The activity of the combination of both antifungals was determined in a checkerboard antifungal susceptibility assay. The MICs for VOR were not influenced by the addition of ANI; the MECs for ANI were not influenced by the addition of VOR. Indifference was observed with a fractional inhibitory concentration index of 0.8 for both A. fumigatus isolates when exposed to the combination of VOR and ANI.
Establishment of the human pharmacokinetic equivalent ANI dosage schedule in the rat.
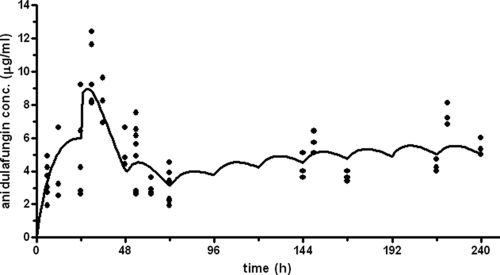
As for the triazoles, for the echinocandins the AUC/MIC ratio is considered the PK/PD index determining therapeutic efficacy (25). The human steady-state AUC24 is 120.3 μg·h/ml. To achieve human target AUC for ANI in the rat various dosage schedules of ANI were applied to groups of uninfected neutropenic rats. A one-compartment pharmacokinetic model with first-order absorption and elimination adequately described the plasma concentration-time profiles of ANI in all groups. The population pharmacokinetic parameters for ANI are given in Table 3. The pharmacokinetics of ANI were time dependent. Compared to day 0, ANI clearance was 41 and 61% higher on day 1 and day 2, respectively, whereas the volumes of distribution were 74 and 48% lower. The values on days 6 and 9 after the administration of ANI were comparable to that on day 0. An ANI loading dose of 20 mg/kg followed by 5 mg/kg q24h for 10 days provided an ANI human equivalent pharmacokinetic profile (Table 4). Figure 3 shows the measured ANI plasma concentrations for this schedule and the profile calculated on basis of the population the pharmacokinetic parameters in rats. The AUC values on days 0, 1, 2, 6, and 9, as predicted by the model, were 77.2, 197.0, 125.1, 114.5, and 150.2 μg·h/ml, respectively (Table 4). The mean AUC24 in rats was 129.8 μg·h/ml, which correlates to 108% of the target AUC24. For this ANI dosage schedule and for the ANI solvent, the renal and hepatic functions were measured to determine whether any toxic side effects occurred. Serum CREAT, BUN, and ALAT levels were all lower than the upper limit of normal (data not shown). However, only shortly after administering the loading ANI dose of 20 mg/kg/day, ASAT levels (median, 136.0 U/liter; range, 72 to 1,005 U/liter) were higher than the upper limit of normal (118.5 U/liter) but lower than the three times upper-limit boundary (355.5 U/liter). This elevation of ASAT levels appeared to be caused by the ANI solvent, which resulted in elevated ASAT levels as well (median, 184.0 U/liter; range, 62 to 648 U/liter).
TABLE 3.
Population pharmacokinetic parameters of ANI in ratsa
| Parameter | Mean | Between-rat variability (%) |
|---|---|---|
| k12 (h−1) | 0.089 | |
| V, day 0 and days 3 to 9 (ml) | 455 | 28 |
| V, day 1 (ml) | 118 | |
| V, day 2 (ml) | 236 | |
| CL, day 0 and days 3 to 9 (ml/h) | 7.6 | 8 |
| CL, day 1 (ml/h) | 10.7 | |
| CL, day 2 (ml/h) | 12.2 | |
| Residual variability | ||
| Additive error (μg/ml) | 0.30 | |
| Proportional error (%) | 20 |
Standard errors of the estimates were <36%.
TABLE 4.
Human pharmacokinetic equivalent dosage schedule of ANI in ratsa
| Time (h) after first injection | ANI dose (mg/kg, q24h) | AUC24 (μg·h/ml) | % of the human target AUC24 (%) |
|---|---|---|---|
| 0-24 | 20 | 77.2 | 64 |
| 24-48 | 5 | 197.0 | 164 |
| 48-72 | 5 | 125.1 | 104 |
| 72-96 | 5 | 114.5 | 95 |
| 96-120 | 5 | ND | ND |
| 120-144 | 5 | ND | ND |
| 144-168 | 5 | 114.5 | 95 |
| 168-192 | 5 | ND | ND |
| 192-216 | 5 | ND | ND |
| 216-240 | 5 | 150.2 | 125 |
Doses were administered at 24-h intervals as indicated. The AUC24 for ANI was calculated on the basis of the population pharmacokinetic parameters given in Table 3. The percentage of the human target was obtained by dividing the AUC24 by the human target value (120.3 μg·h/ml) × 100. ND, not done.
FIG. 3.
Measured ANI plasma concentrations (dots) following a loading dose of 20 mg/kg and once-daily doses of 5 mg/kg. The solid line represents the concentration profile as predicted by the developed pharmacokinetic model (Table 3). Clearance on days 1 and 2 was increased compared to day 0 and day 9.
Therapeutic efficacy of VOR in combination with ANI in rats with an advanced stage of IPA.
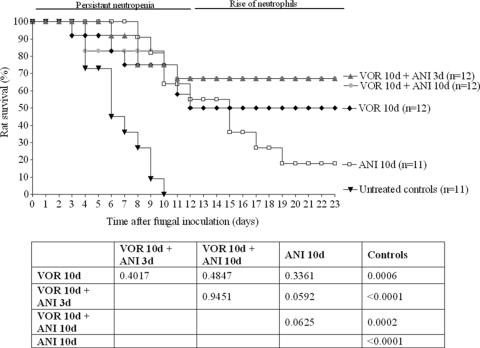
To enhance therapeutic efficacy of antifungal treatment in advanced stage of IPA, we investigated whether the addition of ANI to the VOR dosage schedule would improve the outcome of treatment. ANI was added to VOR in two different schedules: either during the first 3 days of treatment or during the entire 10 days VOR treatment period. The efficacy of the 10-day treatment with ANI alone was also determined. As shown in Fig. 4, untreated control rats all died between day 4 and day 10 after fungal inoculation. Treatment with VOR alone resulted in 56% rat survival at day 23, while treatment with ANI alone resulted in only 18% rat survival. Although in terms of rat survival percentages evaluated at day 23 VOR was superior to ANI, the survival at the end of the entire treatment period was comparable for both agents (P = 0.3361). Combination of VOR and ANI in this advanced stage of IPA resulted in increased rat survival rates compared to either monotherapy, although the differences were not significant. The duration of the combination therapy was not important, since similar rat survival was obtained after addition of ANI for only the first 3 days or the entire 10-day treatment period (P = 0.9451).
FIG. 4.
Therapeutic efficacy of VOR and ANI in transiently neutropenic rats with IPA. VOR was administered i.p. at 12-h intervals according to the human pharmacokinetic equivalent dosage schedule for 10 days. ANI was administered s.c. once daily according to the human pharmacokinetic equivalent dosage schedule for either 3 or 10 days. Treatment was started 72 h after fungal inoculation. P values were determined with the log rank test by comparing the different dosing schedules.
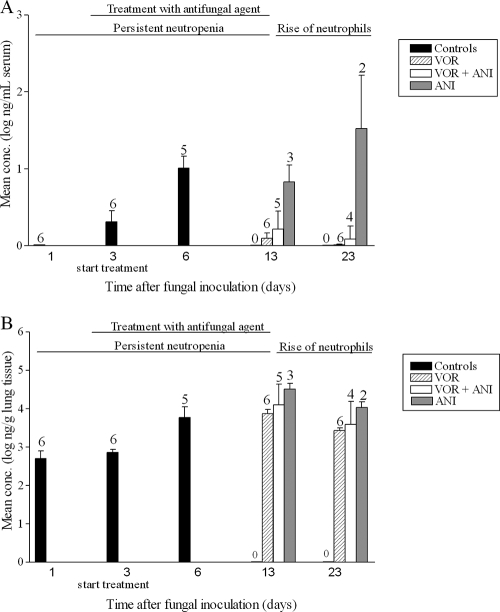
In addition to the animal survival rate as an overall parameter for treatment outcome, we also evaluated the fungal burden in the rat in terms of fungal galactomannan levels in infected left lungs and serum. As is shown in Fig. 5, the fungal burden in the lung in terms of galactomannan was high with all treatment schedules. Only at day 13 were fungal galactomannan concentrations in the lung significantly lower after VOR treatment compared to ANI monotherapy (P = 0.0238). Significant differences were not found at day 13 with combination therapy compared to either VOR monotherapy (P = 0.3290) or ANI monotherapy (P = 0.5714). In contrast, in serum a decrease in galactomannan levels was observed. At day 13, the galactomannan concentrations in serum were significantly higher after ANI monotherapy compared to either VOR monotherapy (P = 0.0238) or combination therapy (P = 0.0357). No difference was observed between the VOR monotherapy and the combination therapy (P = 0.3290). At day 23, for both lung and serum statistical analysis could not be performed due to the low number of surviving rats in the ANI monotherapy group.
FIG. 5.
Concentration of galactomannan in serum (A) or in the infected left lung (B) of surviving transiently neutropenic rats with IPA treated with VOR, ANI, or a combination of both. Treatment was started 72 h after fungal inoculation. The numbers above the bars are the numbers of surviving rats out of groups of six rats at each time interval. The error bars represent the standard deviations. On days 1, 3, and 6 galactomannan concentrations were determined for the untreated controls. On days 13 and 23 galactomannan concentrations were determined for the treated animals.
DISCUSSION
Since monotherapy for IPA is still associated with frequent therapeutic failure, interest has emerged in combination therapy for the treatment of IPA. Particularly combination regimens targeting both the Aspergillus cell membrane synthesis (azoles) and the cell wall synthesis (echinocandins) are under investigation. In the present study the therapeutic efficacy of VOR combined with ANI was compared to both monotherapies in transiently neutropenic rats with advanced stage of IPA. To increase the clinical relevance of animal studies the dosage regimens of VOR and ANI in rats were optimized to achieve a human pharmacokinetic equivalent dosage in the rat. For both agents the AUC/MIC was determined to be the PK/PD index predicting therapeutic efficacy. It should be stated that this was concluded in studies performed mainly in C. albicans-infected animals (2-4, 6, 7, 25, 43) and does not necessarily means that the AUC/MIC ratio is also the determining PK/PD parameter in A. fumigatus infections (2).
When establishing AUCs in rats, one should be aware of the degradation of VOR by hepatic cytochrome P450 enzymes. Due to amino acid differences in these enzymes between rodents and humans, VOR is metabolized more rapidly in rodents (45). In order to compensate for this process, other investigators used guinea pigs or mice on a grapefruit diet to increase serum levels of VOR (13, 20, 36, 38). In the present study a different approach was elaborated to achieve a human pharmacokinetic equivalent dosage of VOR in rats.
A pharmacokinetic model was iteratively developed which could be used to simulate VOR exposure for different schedules. Compared to the doses used in humans, higher doses were needed in rats due to the presence of saturable and inducible metabolism of VOR in rats, which is not observed in humans. By applying a dosage increment schedule in the rats human treatment was simulated, and VOR exposure was comparable to humans during the entire treatment period. The estimated population values for Km (5.6 μg/ml) and Vmax (180 μg/h ≈ 0.58 μg/ml/h) were comparable to values reported by Roffey et al. in humans (Km = 2.4 μg/ml, Vmax = 1.2 μg/ml/h) (34). ANI is not metabolized by the cytochrome P450 enzymes, and human pharmacokinetic equivalent dosages were mimicked by administering a dose of 20 mg/kg and a maintenance dose of 5 mg/kg. In this way, the human target AUC for ANI during the entire treatment period was achieved. Since coadministration of VOR and ANI has no influence on the pharmacokinetic parameters of either agent, a further dosage adjustment was not needed (19).
Administration of the human pharmacokinetic equivalent VOR and ANI dosage schedules in rats did not induce toxic side effects in rats in terms of significant changes in renal and hepatic functions. Only with ANI was a slight increase in ASAT levels observed. This might be due to the ethanol present in the solvent.
With respect to therapeutic activity, VOR as monotherapy in the early stage of IPA was completely effective in terms of rat survival and highly superior compared to AMB administered at the maximum tolerated dose. This high efficacy was comparable to that found in other IPA animal models, which reported animal survival rates of 80 to 100% for VOR and 20% for AMB (13, 29). In the present study, the efficacy of VOR was only slightly reduced with the delay of the start of treatment. When VOR treatment was started at an advanced stage of IPA, the therapeutic activity, although substantially reduced, was still highly significant. In an attempt to increase the therapeutic efficacy of VOR at that stage ANI was added to the treatment schedule.
The rationale for the combination therapy was found in the in vitro studies on antifungal activity. A. fumigatus was very susceptible to both VOR and ANI, with low MICs for VOR and low MECs for ANI. In vitro combining VOR with ANI resulted in indifference in activity (30). Indifference was also reported when VOR was combined with the echinocandin CAS (16). In vitro synergy between VOR and ANI was only demonstrated in studies providing alternative interpretations of MIC endpoints being prominent growth reduction endpoints (32).
In the present in vivo study we demonstrated that combining VOR and ANI in the treatment of IPA did not significantly improve rat survival compared to either VOR or ANI monotherapy. Our results obtained for the combination of VOR with ANI were comparable to three other animal studies comparing the therapeutic effect of VOR in combination with an echinocandin. In two studies of invasive aspergillosis in guinea pigs VOR was combined with CAS (24, 26), whereas in one study of IPA in guinea pigs VOR was combined with micafungin (14). Although a significantly lower fungal burden with combination therapy was observed in one of these studies (24), in all studies significant improvement in terms of animal survival was never obtained compared to VOR monotherapy. To the best of our knowledge, only one study with ravuconazole, another azole with activity against aspergillus, in combination with micafungin showed a significantly improved survival in comparison with azole monotherapy (31).
In the present study, VOR and ANI both administered as monotherapy were similarly effective in partially preventing the mortality of rats with advanced stages of IPA. To assess the therapeutic efficacy, not only was animal survival monitored but also the reduction of the fungal load was determined. To this aim, the reduction in concentration of galactomannan was used as parameter, and not the reduction in CFU counts, since CFU count as a parameter is not reliable for filamentous fungi. Earlier studies in the same model of IPA in our lab in which the parameters A. fumigatus galactomannan and A. fumigatus DNA were compared showed that quantitative galactomannan detection is superior to DNA detection as a parameter for monitoring IPA (10). It was also demonstrated that a significant increase in animal survival (as an overall parameter for therapeutic success) correlated with a significant decrease in fungal burden in terms of the amount of A. fumigatus galactomannan in the infected lung and in serum (probably reflecting active fungal multiplication in the infected lung) (10, 41, 42). In the present study we found that the galactomannan levels representing the fungal load in the infected left lung of surviving rats were high, with a small, but significant, decrease in the VOR-treated animals at the end of the treatment period. Serum galactomannan levels in VOR-treated animals were undetectable and were very low in animals treated with the combination of VOR and ANI. In contrast, rats treated with ANI alone had relatively high serum galactomannan levels.
Apparently, compared to VOR, ANI was not able to significantly reduce the viability of the fungal mass in the infected lung in advanced IPA, suggesting that VOR is a more potent drug in this respect.
In conclusion, VOR is a highly potent drug in the treatment of IPA even at an advanced stage of infection. The superior therapeutic activity of VOR compared to AMB and the echinocandin ANI is probably related to a significant reduction in viable fungal load in the infected lung tissue, which is only seen after treatment with VOR. The potency of this drug in the advanced stage of IPA is not enhanced by the addition of ANI to the treatment schedule.
Acknowledgments
This study was financially supported in part by Pfizer, Inc.
Footnotes
Published ahead of print on 23 February 2009.
REFERENCES
- 1.Andes, D. 2004. Clinical utility of antifungal pharmacokinetics and pharmacodynamics. Curr. Opin. Infect. Dis. 17:533-540. [DOI] [PubMed] [Google Scholar]
- 2.Andes, D. 2003. Pharmacokinetics and pharmacodynamics in the development of antifungal compounds. Curr. Opin. Investig. Drugs. 4:991-998. [PubMed] [Google Scholar]
- 3.Andes, D., D. J. Diekema, M. A. Pfaller, R. A. Prince, K. Marchillo, J. Ashbeck, and J. Hou. 2008. In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 52:539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andes, D., K. Marchillo, R. Conklin, G. Krishna, F. Ezzet, A. Cacciapuoti, and D. Loebenberg. 2004. Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob. Agents Chemother. 48:137-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andes, D., K. Marchillo, J. Lowther, A. Bryskier, T. Stamstad, and R. Conklin. 2003. In vivo pharmacodynamics of HMR 3270, a glucan synthase inhibitor, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:1187-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andes, D., K. Marchillo, T. Stamstad, and R. Conklin. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:3165-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andes, D., and M. van Ogtrop. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob. Agents Chemother. 43:2116-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker, M. J., S. De Marie, M. H. Fens, J. J. Haitsma, H. A. Verbrugh, B. Lachmann, and I. A. Bakker-Woudenberg. 2006. Pathophysiology of unilateral pulmonary aspergillosis in an experimental rat model. Med. Mycol. 44:133-139. [DOI] [PubMed] [Google Scholar]
- 9.Becker, M. J., S. de Marie, M. H. Fens, H. A. Verbrugh, and I. A. Bakker-Woudenberg. 2003. Effect of amphotericin B treatment on kinetics of cytokines and parameters of fungal load in neutropenic rats with invasive pulmonary aspergillosis. J. Antimicrob. Chemother. 52:428-434. [DOI] [PubMed] [Google Scholar]
- 10.Becker, M. J., S. de Marie, D. Willemse, H. A. Verbrugh, and I. A. Bakker-Woudenberg. 2000. Quantitative galactomannan detection is superior to PCR in diagnosing and monitoring invasive pulmonary aspergillosis in an experimental rat model. J. Clin. Microbiol. 38:1434-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boeckman, A. J., L. W. Sheiner, and S. L. Beal. 1994. NONMEM users guide. V. Introductory guide. University of California, San Francisco, CA.
- 12.Cao, X., S. T. Gibbs, L. Fang, H. A. Miller, C. P. Landowski, H. C. Shin, H. Lennernas, Y. Zhong, G. L. Amidon, L. X. Yu, and D. Sun. 2006. Why is it challenging to predict intestinal drug absorption and oral bioavailability in human using rat model. Pharm. Res. 23:1675-1686. [DOI] [PubMed] [Google Scholar]
- 13.Chandrasekar, P. H., J. Cutright, and E. Manavathu. 2000. Efficacy of voriconazole against invasive pulmonary aspergillosis in a guinea-pig model. J. Antimicrob. Chemother. 45:673-676. [DOI] [PubMed] [Google Scholar]
- 14.Chandrasekar, P. H., J. L. Cutright, and E. K. Manavathu. 2004. Efficacy of voriconazole plus amphotericin B or micafungin in a guinea-pig model of invasive pulmonary aspergillosis. Clin. Microbiol. Infect. 10:925-928. [DOI] [PubMed] [Google Scholar]
- 15.CLSI. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. NCCLS document M38-A22. CLSI, Wayne, PA.
- 16.Dannaoui, E., O. Lortholary, and F. Dromer. 2004. In vitro evaluation of double and triple combinations of antifungal drugs against Aspergillus fumigatus and Aspergillus terreus. Antimicrob. Agents Chemother. 48:970-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.den Hollander, J. G., J. W. Mouton, and H. A. Verbrugh. 1998. Use of pharmacodynamic parameters to predict efficacy of combination therapy by using fractional inhibitory concentration kinetics. Antimicrob. Agents Chemother. 42:744-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23:608-615. [DOI] [PubMed] [Google Scholar]
- 19.Dowell, J. A., J. Schranz, A. Baruch, and G. Foster. 2005. Safety and pharmacokinetics of coadministered voriconazole and anidulafungin. J. Clin. Pharmacol. 45:1373-1382. [DOI] [PubMed] [Google Scholar]
- 20.Graybill, J. R., L. K. Najvar, G. M. Gonzalez, S. Hernandez, and R. Bocanegra. 2003. Improving the mouse model for studying the efficacy of voriconazole. J. Antimicrob. Chemother. 51:1373-1376. [DOI] [PubMed] [Google Scholar]
- 21.Greene, R. E., J. Mauskopf, C. S. Roberts, T. Zyczynski, and H. T. Schlamm. 2007. Comparative cost-effectiveness of voriconazole and amphotericin B in treatment of invasive pulmonary aspergillosis. Am. J. Health Syst. Pharm. 64:2561-2568. [DOI] [PubMed] [Google Scholar]
- 22.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 23.Kerbusch, T., R. A. Mathot, H. J. Keizer, G. P. Kaijser, J. H. Schellens, and J. H. Beijnen. 2001. Influence of dose and infusion duration on pharmacokinetics of ifosfamide and metabolites. Drug Metab. Dispos. 29:967-975. [PubMed] [Google Scholar]
- 24.Kirkpatrick, W. R., S. Perea, B. J. Coco, and T. F. Patterson. 2002. Efficacy of caspofungin alone and in combination with voriconazole in a Guinea pig model of invasive aspergillosis. Antimicrob. Agents Chemother. 46:2564-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louie, A., M. Deziel, W. Liu, M. F. Drusano, T. Gumbo, and G. L. Drusano. 2005. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob. Agents Chemother. 49:5058-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacCallum, D. M., J. A. Whyte, and F. C. Odds. 2005. Efficacy of caspofungin and voriconazole combinations in experimental aspergillosis. Antimicrob. Agents Chemother. 49:3697-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maertens, J., A. Glasmacher, R. Herbrecht, A. Thiebaut, C. Cordonnier, B. H. Segal, J. Killar, A. Taylor, N. Kartsonis, T. F. Patterson, M. Aoun, D. Caillot, and C. Sable. 2006. Multicenter, noncomparative study of caspofungin in combination with other antifungals as salvage therapy in adults with invasive aspergillosis. Cancer 107:2888-2897. [DOI] [PubMed] [Google Scholar]
- 28.Morrison, V. A. 2006. Echinocandin antifungals: review and update. Expert Rev. Anti-Infect. Ther. 4:325-342. [DOI] [PubMed] [Google Scholar]
- 29.Murphy, M., E. M. Bernard, T. Ishimaru, and D. Armstrong. 1997. Activity of voriconazole (UK-109,496) against clinical isolates of Aspergillus species and its effectiveness in an experimental model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 41:696-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perkhofer, S., D. Jost, M. P. Dierich, and C. Lass-Florl. 2008. Susceptibility testing of anidulafungin and voriconazole alone and in combination against conidia and hyphae of Aspergillus spp. under hypoxic conditions. Antimicrob. Agents Chemother. 52:1873-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petraitis, V., R. Petraitiene, A. A. Sarafandi, A. M. Kelaher, C. A. Lyman, H. E. Casler, T. Sein, A. H. Groll, J. Bacher, N. A. Avila, and T. J. Walsh. 2003. Combination therapy in treatment of experimental pulmonary aspergillosis: synergistic interaction between an antifungal triazole and an echinocandin. J. Infect. Dis. 187:1834-1843. [DOI] [PubMed] [Google Scholar]
- 32.Philip, A., Z. Odabasi, J. Rodriguez, V. L. Paetznick, E. Chen, J. H. Rex, and L. Ostrosky-Zeichner. 2005. In vitro synergy testing of anidulafungin with itraconazole, voriconazole, and amphotericin B against Aspergillus spp. and Fusarium spp. Antimicrob. Agents Chemother. 49:3572-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purkins, L., N. Wood, P. Ghahramani, K. Greenhalgh, M. J. Allen, and D. Kleinermans. 2002. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob. Agents Chemother. 46:2546-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roffey, S. J., S. Cole, P. Comby, D. Gibson, S. G. Jezequel, A. N. Nedderman, D. A. Smith, D. K. Walker, and N. Wood. 2003. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab. Dispos. 31:731-741. [DOI] [PubMed] [Google Scholar]
- 35.Scott, L. J., and D. Simpson. 2007. Voriconazole: a review of its use in the management of invasive fungal infections. Drugs 67:269-298. [DOI] [PubMed] [Google Scholar]
- 36.Serena, C., F. J. Pastor, M. Marine, M. M. Rodriguez, and J. Guarro. 2007. Efficacy of voriconazole in a murine model of cryptococcal central nervous system infection. J. Antimicrob. Chemother. 60:162-165. [DOI] [PubMed] [Google Scholar]
- 37.Singh, N., A. P. Limaye, G. Forrest, N. Safdar, P. Munoz, K. Pursell, S. Houston, F. Rosso, J. G. Montoya, P. Patton, R. Del Busto, J. M. Aguado, R. A. Fisher, G. B. Klintmalm, R. Miller, M. M. Wagener, R. E. Lewis, D. P. Kontoyiannis, and S. Husain. 2006. Combination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational study. Transplantation 81:320-326. [DOI] [PubMed] [Google Scholar]
- 38.Sugar, A. M., and X. P. Liu. 2001. Efficacy of voriconazole in treatment of murine pulmonary blastomycosis. Antimicrob. Agents Chemother. 45:601-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theuretzbacher, U. 2004. Pharmacokinetics/pharmacodynamics of echinocandins. Eur. J. Clin. Microbiol. Infect. Dis. 23:805-812. [DOI] [PubMed] [Google Scholar]
- 40.van de Sande, W. W. J., M. Tavakol, W. van Vianen, and I. A. J. M. Bakker-Woudenberg. The antifungal effect of amphotericin B, itraconazole, voriconazole and caspofungin on conidia versus hyphae of Aspergillus fumigatus. Med. Mycol., in press.
- 41.van de Sande, W. W. J., W. van Vianen, M. T. ten Kate, J. Vissers, J. Laurijsens, M. Tavakol, B. Rijders, R. A. Mathot, and I. A. Bakker-Woudenberg. 2008. Caspofungin prolongs survival of transiently neutropenic rats with advanced-stage invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 52:1345-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Vianen, W., S. de Marie, M. T. ten Kate, R. A. Mathot, and I. A. Bakker-Woudenberg. 2006. Caspofungin: antifungal activity in vitro, pharmacokinetics, and effects on fungal load and animal survival in neutropenic rats with invasive pulmonary aspergillosis. J. Antimicrob. Chemother. 57:732-740. [DOI] [PubMed] [Google Scholar]
- 43.Wiederhold, N. P., D. P. Kontoyiannis, J. Chi, R. A. Prince, V. H. Tam, and R. E. Lewis. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 190:1464-1471. [DOI] [PubMed] [Google Scholar]
- 44.Zmeili, O. S., and A. O. Soubani. 2007. Pulmonary aspergillosis: a clinical update. Q. J. Med. 100:317-334. [DOI] [PubMed] [Google Scholar]
- 45.Zuber, R., E. Anzenbacherova, and P. Anzenbacher. 2002. Cytochromes P450 and experimental models of drug metabolism. J. Cell Mol. Med. 6:189-198. [DOI] [PMC free article] [PubMed] [Google Scholar]