Abstract
The emergence of Mycobacterium tuberculosis resistant to first-line antibiotics has renewed interest in second-line antitubercular agents. Here, we aimed to extend our understanding of the mechanisms underlying para-aminosalicylic acid (PAS) resistance by analysis of six genes of the folate metabolic pathway and biosynthesis of thymine nucleotides (thyA, dfrA, folC, folP1, folP2, and thyX) and three N-acetyltransferase genes [nhoA, aac(1), and aac(2)] among PAS-resistant clinical isolates and spontaneous mutants. Mutations in thyA were identified in only 37% of the clinical isolates and spontaneous mutants. Overall, 24 distinct mutations were identified in the thyA gene and 3 in the dfrA coding region. Based on structural bioinformatics techniques, the altered ThyA proteins were predicted to generate an unfolded or dysfunctional polypeptide. The MIC was determined by Bactec/Alert and dilution assay. Sixty-three percent of the PAS-resistant isolates had no mutations in the nine genes considered in this study, revealing that PAS resistance in M. tuberculosis involves mechanisms or targets other than those pertaining to the biosynthesis of thymine nucleotides. The alternative mechanism(s) or pathway(s) associated with PAS resistance appears to be PAS concentration dependent, in marked contrast to thyA-mutated PAS-resistant isolates.
The discovery of the antitubercular activity of para-aminosalicylic acid (PAS) by Lehmann in 1943 (15) was followed by two successful clinical trials conducted in 1944 and 1949 (16, 31). These breakthroughs, combined with the almost simultaneous discovery and introduction of streptomycin (STR), brought much hope in the fight against tuberculosis (TB) (22). The initial success was soon thwarted by the emergence of PAS and STR resistance. This was overcome by coadministering PAS and STR, resulting in the advent of combination therapy (19). In 1951, isoniazid was added to anti-TB regimens until the mid-1960s. Although including PAS combination therapy proved efficacious, side effects attributed to PAS were documented as early as 1951 (6, 25). In addition to PAS-associated gastrointestinal toxicity, elevated and repetitive dosing complicated therapeutic regimens. PAS therapy was discontinued after the introduction of rifampin (rifampicin) and pyrazinamide. PAS was reintroduced in the United States in 1992, following several outbreaks of multidrug-resistant (MDR) isolates (4). Since then, the need for new antibiotics for the treatment of MDR TB has led to the development of novel formulations of PAS, which have proven to be less toxic (5). Today, PAS is used primarily as a second-line drug to treat MDR TB (34).
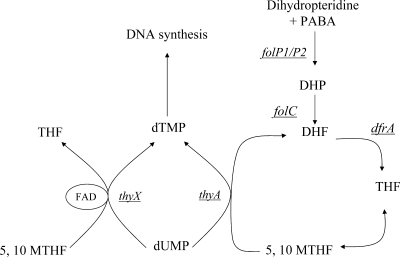
PAS has structural similarities to sulfonamides. Sulfonamides are structural analogues of para-aminobenzoic acid, the substrate of dihydropteroate synthase (encoded by folP1/folP2), and hence function as competitive inhibitors. FolP1 and its putative homologue FolP2 catalyze the condensation of para-aminobenzoic acid and 6-hydroxymethyl-7,8-dihydropterein pyrophosphate to 7,8-dihydropteroate, which is converted to dihydrofolate and reduced to generate the cofactor tetrahydrofolate (THF) by the enzyme dihydrofolate reductase (encoded by dfrA) (Fig. 1). Unlike the actions of some sulfonamides or analogues in other pathogens, the PAS-inhibitory activity of folP1 has proven to be unexpectedly poor in vitro (24). More recently, Rengarajan and colleagues (26), using transposon mutagenesis, have shown that PAS resistance is associated with mutations of thymidylate synthase A, encoded by the thyA gene and required for thymine biosynthesis in the folate pathway. This result implies that PAS functions as a folate antagonist, a suggestion supported by the identification of mutations within the thyA coding region in PAS-resistant (PASr) clinical isolates (26).
FIG. 1.
The folate pathway and plausible targets for PAS inhibition. The six genes analyzed in this study are underlined.
ThyA catalyzes the reductive methylation of dUMP to yield dTMP, required for de novo dTTP synthesis (12). ThyA requires the 5,10-methylene THF cofactor both as a reductant and as a carbon donor in the methylation reaction. The presence of the thyX gene, encoding a functional homologue of thymidylate synthase but with the clear distinction that it utilizes flavin adenine dinucleotide as a cofactor instead of THF, in the Mycobacterium tuberculosis genome is noteworthy (8). Although ThyX utilizes flavin adenine dinucleotide as a cofactor, it still requires 5,10-methylene THF as the methyl donor. It is hypothesized that the bacteriostatic activity of PAS results from perturbation of the folate pathway, although the underlying mechanism has yet to be elucidated (26).
Here, we set out to investigate the mutations associated with PAS resistance in a collection of well-characterized M. tuberculosis clinical isolates and PASr spontaneous mutants. Five genes, thyA, dfrA, folC, folP1, and folP2, encoding enzymes of the folate pathway; thyX, encoding an alternative thymine-biosynthetic enzyme; and three N-acetyltransferase genes possibly associated with the modification of PAS were analyzed. To better understand PAS resistance, the identified mutations were correlated with MICs and the protein three-dimensional (3D) structure. The structural stability was modeled for all mutants. To our surprise, only 37% of the PASr clinical isolates or spontaneous mutants encoded mutations in enzymes of the folate pathway, indicating that other mechanisms associated with PAS resistance have yet to be elucidated.
MATERIALS AND METHODS
M. tuberculosis laboratory reference strains.
The laboratory PASr mutant and PAS-susceptible (PASs) H37Rv strains were used as positive and negative controls, respectively (Table 1) (3, 29, 30). M. tuberculosis complex strains used as reference strains included Mycobacterium bovis (TMC 401-Ravenel-PASr, TMC 410-NADL-PASr, and TMC 407-Branch-PASs), M. bovis-BCG-Pasteur (ATCC 35734-PASs), Mycobacterium africanum PASs (TMC 5122), and Mycobacterium microti PASs (ATCC 19422).
TABLE 1.
Molecular characteristics and drug susceptibility profiles of isolates studied
| FPa | TNb | ORIc | No. of IS6110d | PGGe | Clusterf | Spoligo (octal)g | Resistanceh
|
|
|---|---|---|---|---|---|---|---|---|
| First line | Second line | |||||||
| Unclustered clinical isolates | ||||||||
| A7 | 6196 | US | 7 | 2 | III | 777777777720771 | INH, RIF, EMB, PZA, STR | ETH, KAN, PAS |
| CC3 | 4469 | US | 12 | 2 | VI | ′776177607760771 | INH, RIF, EMB, PZA, STR | ETH, PAS |
| CI12 | 18117 | KS | 15 | 1 | II | ′000000000003771 | INH, EMB | PAS |
| CI1 | 4862 | US | 10 | 1 | II | 000000000000771 | INH, RIF, EMB, STR | CIP, PAS |
| H | 14905 | CH | 2 | 2 | IV | 777776777760601 | INH, RIF, PZA | RBT, RMC, PAS |
| H | 13528 | IN | 2 | 2 | IV | 777776777760601 | INH, RIF, EMB, PZA | PAS |
| H | 16213 | US | 2 | 2 | IV | 777776777760601 | INH | PAS |
| BA27 | 14204 | HA | 13 | 2 | VI | ′777777607760771 | INH | PAS |
| BA19 | 14303 | US | 12 | 2 | VI | 677777607760771 | INH | PAS |
| BA19 | 14550 | US | 12 | 2 | VI | 677777607760771 | INH | PAS |
| BA34 | 15180 | DR | 11 | 2 | VI | 677777607760771 | INH, STR | PAS |
| OO1 | 15758 | BG | 10 | 1 | I | 776177607763771 | INH, STR | PAS |
| OO1 | 16054 | CH | 12 | 1 | I | 777777777763771 | INH, RIF, EMB, STR | RMC, PAS |
| OO1 | 18048 | CH | 9 | 1 | II | ′000000000003771 | INH, RIF, PZA, STR | RBT, RMC, PAS |
| BE | 18460 | MI | 1 | 1 | I | 757777777413731 | INH, RIF, EMB, STR | ETH, CYC, CIP, KAN, CAP, RBT, RMC, PAS |
| BE | 17182 | IN | 1 | 1 | I | 777777777413731 | INH | ETH, PAS |
| BE1 | 9560 | IN | 1 | 1 | I | 477777777413771 | Susceptible | PAS |
| BE3 | 13310 | IN | 1 | 2 | IV | 777737777760771 | INH, EMB, STR | PAS |
| BF53 | 16931 | US | 9 | 3 | VII | ′777777777760771 | INH, STR | PAS |
| W | 2550 | US | 18 | 1 | II | ′000000000003771 | INH, RIF, EMB, PZA, STR | ETH, OFL, KAN, CYC, PAS |
| W | 14003 | RQ | 18 | 1 | II | ′000000000003771 | INH, RIF, EMB, STR | ETH, RBT, RMC, PAS |
| W269 | 15016 | IN | 14 | 1 | II | ′000000000003771 | INH, RIF, EMB, PZA, STR | ETH, KAN, RBT, RMC, PAS |
| W283 | 14178 | IN | 14 | 1 | II | ′000000000003771 | INH, RIF, EMB, PZA, STR | KAN, CAP, RBT, PAS |
| W563 | 18052 | CH | 1 | II | ′000000000003771 | STR | PAS | |
| HR102 | 19546 | RP | 10 | 1 | I | 677777477413771 | INH, EMB, STR | PAS |
| HD15 | 18985 | CH | 11 | 1 | II | ′000000000003771 | INH, RIF, EMB, PZA, STR | CYC, CIP, OFL, KAN, AMO, CAP, RBT, RMC, PAS |
| Clustered clinical isolates | ||||||||
| P | 693 | US | 10 | 2 | VI | ′777777607760771 | INH, RIF, EMB, PZA | ETH, PAS |
| P | 758 | US | 10 | 2 | VI | ′777777607760771 | INH, RIF, STR | ETH, KAN, CAP, CYC, PAS |
| P | 768 | US | 10 | 2 | VI | ′777777607760771 | INH, RIF, EMB, PZA, STR | ETH, PAS |
| P | 1618 | US | 10 | 2 | VI | ′777777607760771 | INH, RIF, EMB, STR | ETH, CIP, CAP, PAS |
| P | 2557 | US | 10 | 2 | VI | ′777777607760771 | INH, RIF, EMB, STR | CAP, PAS |
| P | 3158 | US | 10 | 2 | VI | ′777777607760771 | INH, RIF, EMB, PZA, STR | ETH, CIP, CAP, KAN, CYC, PAS |
| P | 3814 | US | 10 | 2 | VI | ′777777607760771 | INH, RIF | CIP, PASs |
| P | 5902 | US | 10 | 2 | VI | ′777777607760771 | INH, RIF, PZA | PAS |
| P | 8327 | US | 10 | 2 | VI | ′777777607760771 | INH, RIF, EMB, PZA, STR | PAS |
| P | 10454 | US | 10 | 2 | VI | ′777777607760771 | NA | PAS |
| P | 16442 | RQ | 10 | 2 | VI | ′777777607760771 | INH, RIF, PZA, STR | RMC, PAS |
| P6 | 13249 | US | 10 | 2 | VI | ′777777607760771 | INH, RIF, EMB, PZA, STR | PAS |
| P6 | 16796 | US | 10 | 2 | VI | ′777777607760771 | INH, PZA | PAS |
| P23 | 16906 | US | 11 | 2 | VI | ′777777607760771 | INH, RIF, EMB, PZA, STR | ETH, RMC, PAS |
| P | 18137 | US | 10 | 2 | VI | ′777777607760771 | INH, PZA, STR | PAS |
| P1 | 18900 | US | 11 | 2 | VI | ′777777607760771 | INH, RIF, EMB, PZA | RBT, RMC, PAS |
| AB | 1283 | US | 11 | 2 | VI | 676600000000371 | INH, RIF, STR | PASs |
| AB | 6202 | US | 11 | 2 | VI | 676600000000371 | INH, RIF, EMB, PZA | PAS |
| AB | 1202 | US | 11 | 2 | VI | 676600000000371 | INH, RIF | PAS |
| AB8 | 7958 | US | 11 | 2 | VI | 676600000000371 | INH, RIF, EMB, STR | PAS |
| AU | 2450 | US | 10 | 2 | III | 773777774020771 | INH, RIF, EMB | PAS |
| AU | 4718 | US | 10 | 2 | III | 773777774020771 | RIF | CYC, PASs |
| AU | 4526 | US | 10 | 2 | III | 773777774020771 | INH, RIF | PASs |
| AU | 1868 | US | 10 | 2 | III | 773777774020771 | INH, RIF, EMB | PASs |
| AU4 | 4619 | US | 11 | 2 | III | 773777774020771 | Susceptible | PASs |
| Reference susceptible clinical strains | ||||||||
| 006 | 17994 | US | 0 | ND | ND | 777700000000000 | Susceptible | PASs |
| AF | 9139 | US | 12 | 3 | VIII | ′777777777760771 | Susceptible | PASs |
| AI36 | 12556 | RU | 11 | 2 | VI | ′777760007760771 | Susceptible | PASs |
| BE | 6921 | US | 1 | 1 | I | ND | Susceptible | PASs |
| CDC1551 | 5170 | US | 4 | 2 | V | ′700076757760771 | Susceptible | PASs |
| J | 1694 | US | 12 | 3 | VIII | ′777777607560771 | Susceptible | PASs |
| W4 | 1147 | US | 18 | 1 | II | ′000000000003771 | Susceptible | PASs |
| Laboratory reference strains | ||||||||
| H37Ra1 | 25177i | LAB | 16 | 3 | VIII | ′777777477760771 | Susceptible | PASs |
| H37Rv2 | 25618i | LAB | 14 | 3 | VIII | ′777777477760771 | Susceptible | PASs |
| H37Rv2 | 27294i | LAB | 14 | 3 | VIII | ′777777477760771 | Susceptible | PASs |
| H37Rv2 | 35821i | LAB | 14 | 3 | VIII | ′777777477760771 | Susceptible | PAS |
| H37Rv5 | 35824i | LAB | 13 | 3 | VIII | ′777777477760771 | STR | PAS |
| H37Rv6 | 35825i | LAB | 14 | 3 | VIII | ′777777477760771 | INH, STR | PAS |
| BCG-Branch | 407j | LAB | 1 | 1 | I | ND | Susceptible | PASs |
| BCG-Pasteur | 35734i | LAB | 1 | 1 | I | ND | Susceptible | PASs |
| M. bovis-NADL | 410j | LAB | 1 | 1 | I | ND | Susceptible | PASs |
| M .bovis-Rav | 401j | LAB | 1 | 1 | I | ND | Susceptible | PASs |
| M. africanum | 5122j | LAB | ND | 1 | I | ND | Susceptible | PASs |
| M . microti | 19422i | LAB | ND | 1 | I | ND | Susceptible | PASs |
FP, fingerprint name, based on IS6110 typing and PHRI nomenclature.
TN, tracking number, a PHRI unique identifier for each isolate.
ORI, country of origin (BG, Bangladesh; CH, China; DR, Dominican Republic; HA, Haiti; IN, India; KS, South Korea; MI, Malawi; RP, Philippines; RQ, Puerto Rico; RU, Russia; US, United States).
No. of IS6110, number of IS6110 insertions in the isolate genome.
PGG, principal genetic group (groups 1 to 3), based on polymorphisms in katG and gyrA.
Cluster, genetic cluster (clusters I to VIII), based on 101 SNPs.
Spoligo (octal), spoligotype, octal depiction.
Resistance, drug susceptibility profile. INH, isoniazid; EMB, ethambutol; ETH, ethionamide; PZA, pyrazinamide; CYC, cycloserin; CIP, ciprofloxacin; OFL, ofloxacin; KAN, kanamycin; AMI, amikacin; CAP, capreomycin; RMC, rifamycin; RBT, rifabutin; ND, data not available.
ATCC.
Trudeau Mycobacterial Collection.
M. tuberculosis clinical isolates.
Twenty-six PASr clinically unrelated isolates were selected from the M. tuberculosis collection (n > 25,000) maintained at the Tuberculosis Center, Public Health Research Institute, Newark, NJ (PHRI), for PASr phenotypic and genotypic analyses (Table 1). These PASr clinical isolates were selected for their genetic diversity and as representatives of the nine major M. tuberculosis phylogenetic clusters as determined by Gutacker et al. (10). In addition to the 26 diverse clinical isolates, representative samples from three strain clusters (related isolates belonging to the M. tuberculosis genotypes P, AB, and AU) associated with three unrelated MDR outbreaks in New York City (2, 21) were also analyzed. Representative P strains comprised 15 MDR-PASr and 1 MDR-PASs isolates. Three PASr and one PASs AB isolates and five AU clustered strains were analyzed. The AU isolates were MDR and polyresistant, but only one isolate had a PASr phenotype (2).
All of the isolates were typed by multiple genetic techniques in order to establish their genetic diversity (unrelated clinical isolates) or relatedness (clustered clinical isolates). The nomenclature used for the classification of the strains was based first on IS6110 restriction fragment length polymorphism patterns, followed by other genotyping techniques as described elsewhere (Table 1) (18).
Selection of spontaneous PASr mutants.
Spontaneous mutants resistant to PAS were selected on 7H11 plates containing 16 μg/ml of PAS (Sigma). To avoid strain bias, spontaneous mutants of eight well-characterized clinical strains (W4, J, CDC1551, OO6, BE, AF AU, and AI) were used. The protocol for selection of spontaneous mutants was adapted from Luria and Delbruck (17) and Morlock et al. (20). Briefly, for each of the eight strains, a sample was cultured under standard conditions in Sauton medium for 3 weeks, the bacterial density was adjusted to an optical density at 600 nm of 1.2 (approximately 7.2 × 107 CFU/ml), and bacteria were inoculated into 220 individual tubes (5 ml Sauton broth each; no antibiotic) for 32 days and plated in toto on 7H11 plates containing 16 μg/ml PAS. Colonies were picked and subcultured in the presence of various concentrations of PAS for determination of the MIC, followed by DNA extraction and stocking. Only a single colony per plate was picked for this study.
Sequencing drug target regions.
The loci including the folP1 (Rv3608c), folP2 (Rv1207), thyX (Rv2754c), dfrA (Rv2763c), and thyA (Rv2764c) genes and the corresponding 100 nucleotides (nt) upstream were sequenced in both directions for all isolates. The putative bifunctional M. tuberculosis dihydrofolate synthetase-folylpolyglutamate synthetase gene, known as folC (Rv2447c), was sequenced in 12 PASr isolates that did not include any other mutations in genes of the folate biosynthetic pathway. Three additional genes were further sequenced in five of these PASr isolates, including the arylamine N-acetyltransferase gene, nhoA (Rv3566c); the aminoglycoside 2′-N-acetyltransferase gene, aac(1) (Rv0262); and the aminoglycoside N-acetyltransferase (GCN5-related N-acetyltransferase) gene, aac(2) (Rv1347c). A complete list of the sequencing primers can be found in Table S1 in the supplemental material. Amplicon sequencing was outsourced.
MIC.
The MICs of all the clinical strains and spontaneous mutants were determined by the agar dilution method and BacT/Alert 3D system (bioMérieux, France). Briefly, samples (107 CFU/ml; diluted 1:100; 100 μl plated) were plated simultaneously on 7H11 plates containing 0, 16, 32, 64, and 128 μg/ml of PAS, and the colony formation was tabulated. Likewise, MICs were determined using the BacT/Alert 3D system as recommended by the supplier. Finally, bacillary growth was monitored spectrophotometrically (optical density at 600 nm) every 72 h for 32 consecutive days in triplicate. Growth curves were determined for four to six strains per group, including clinical isolates (n = 6), spontaneous mutants with an early stop codon in the thyA gene (n = 2) or with other mutations within the thyA gene (n = 6), and PASr isolates including wild-type genes in the folate and pyrimidine biosynthesis pathway (n = 6). Growth curves were done on 7H9 broth in the presence of 0, 16, 32, 64, and 128 μg/ml of PAS.
Structural analysis and homology modeling.
A 3D model of the M. tuberculosis thymidylate synthase homodimer was built with the automated comparative-modeling program Modeler v8.2 (27) using the very high-resolution X-ray structure of Escherichia coli thymidylate synthase as a homologous protein template (Protein Data Bank entry 2G8O; X-ray resolution, 1.3 Å). The stereochemical quality of the model was evaluated with the procheck-nmr program (14). The Naccess program (11) was used to identify buried and solvent accessible residues. Residues interacting with the substrate or cofactor were defined using the Ligplot program (33) based on the X-ray structure of E. coli thymidylate synthase, which was cocrystallized with the substrate dUMP and the cofactor 10-propargyl-5,8-dideazafolate, an analogue of THF (23). Thermodynamic stability changes resulting from a single-site mutation were predicted using the PoPMuSiC web server (13). This program computes the free-energy difference (ΔG) for a given protein between its folded and unfolded states and evaluates the changes (ΔΔG) in this unfolding free-energy difference upon mutations. A positive ΔΔG indicates that the mutation is predicted to thermodynamically decrease the protein stability. Conversely, a negative ΔΔG predicts a mutant protein more stable than the wild type. The magnitude of the predicted ΔΔG is also important for estimating the reliability of predictions, as the errors of the PoPMuSiC program are evaluated to be on the order of ±0.3 to 0.4 kcal/mol for mutations of solvent-accessible residues and to be on the order of ±1.2 to 1.9 kcal/mol for mutations of buried residues (7). Finally, at each mutated position, the conservation of wild-type residues and the occurrence of mutant residues were evaluated on an alignment of 279 thymidylate synthase sequences. These sequences were retrieved by a BLAST query (1) and were aligned using the ClustalX program (32).
RESULTS
Polymorphism in the enzymes of the folate metabolic pathway and thymine biosynthesis in clinical isolates and spontaneous mutants.
The thyA, thyX, dfrA, folP1, and folP2 genes and corresponding upstream regions (∼100 nt upstream) were sequenced for 12 reference strains (Table 2), 3 groups of clustered strains (Table 2), and 55 spontaneous mutants (Table 3). In total, 118 samples were sequenced (Table 1). Drug susceptibility profiles were also determined for each isolate. To generate spontaneous mutants, all parental strains were drug susceptible except for a mono-rifampin-resistant AU4718 strain (2).
TABLE 2.
Mutations associated with PAS resistance in reference strains and clustered and nonclustered clinical isolates (n = 70)a
| Fingerprint (tracking no.) | PAS resistanceb | No. of isolates | thyA mutation [amino acid/(SNP)] | dfrA | folP2 |
|---|---|---|---|---|---|
| Reference strains | |||||
| Ra1; Rv2 (25618); Rv2 (27294) | S | 3 | WTb | WT | WT |
| Rv2 (35821); Rv5; Rv6 | R | 3 | G91E (GGG→GAG) | WT | WT |
| M. microti, M. africanum | S | 2 | WT | WT | −19A→G |
| BCG-Branch, BCG-Pasteur | S | 2 | WT | WT | −19A→G |
| M. bovis-NADL, M. bovis-Ravenel | R | 2 | V263I (GTA-→ATA) | WT | −19A→G |
| Clustered clinical isolates | |||||
| P strain 3814 | S | 1 | WT | WT | WT |
| P (18137), P23 (16906) and P6 (13249) | R | 3 | T202A (ACC→GCC) | WT | WT |
| All other P strains from Table 1 | R | 10 | T202A (ACC→GCC)/ 264STOP→R (TGA→CGA) | WT | WT |
| P strain 3158 | R | 1 | T202A (ACC→GCC)/ 264STOP→R (TGA→CGA) | 54V→A/110C→R | WT |
| P strain 693 | R | 1 | T202A (ACC→GCC)/ 264STOP→R (TGA→CGA) | 66S→C | WT |
| AB strain 1283 | S | 1 | WT | WT | WT |
| AB strains 6202, 1202 and AB8 (7958) | R | 3 | T202A (ACC→GCC) | WT | WT |
| AU strains 4526 and 1868; AU4 strains 4718 and 4619 | S | 4 | WT | WT | VNTR-Del |
| AU strain 2450 | R | 1 | WT | WT | VNTR-Del |
| Nonclustered clinical isolates | |||||
| CDC1551 (5170), J (1694), 006 (17994), BE (6921), AF (9139), AI36 (12556), W4 (1147) | S | 7 | WT | WT | WTc |
| 001 (15758) | R | 1 | V261G (GTC→GGC) | WT | WT |
| W283 (14178) | R | 1 | L183V (TTG→GTG) | WT | WT |
| BA19 (14303); BA19 (14550); BA27 (14204); BA34 (15180); CC3 (4469) | R | 5 | T202A (ACC→GCC) | WT | WT |
| A7 (6169); BE (17182); BE (18460); BE1 (9560); BE3 (13310) | R | 5 | WT | WT | −19A→G |
| BF53 (16931); 001 (16054); 001 (18048); CI1 (4862); CI12 (18117); H (13528); H (14905); H (16213); HD15 (18985); HR102 (19546); W (14003); W563 (18052); W (2550); W269 (15016) | R | 14 | WT | WT | WT |
The DNA of all isolates includes wild-type (WT) thyX, folP, folC, nhoA, aac(1), and aac(2) genes and flanking region.
PAS-resistant (R) and -susceptible (S) phenotypes.
Strains 006 (17994) and BE (6921), −19A→G.
TABLE 3.
Mutations in the thyA gene associated with PAS resistance in spontaneous mutants (n = 55)a
| Fingerprint (tracking no.) | PAS resistanced | No. of isolates | thyA mutation [codon (amino acid)] | folP2 mutation |
|---|---|---|---|---|
| (A)b | R | 24 | WT | WT |
| (B)c | R | 10 | WT | −19A→G |
| BE_149 | R | 1 | TGG→TAG (W83STOP) | −19A→G |
| AF_62/AF_52 | R | 2 | CAG→TAG (Q111STOP) | WT |
| BE_147 | R | 1 | TTG→TAG (L118STOP) | −19A→G |
| BE_43 | R | 1 | TAC→TAA (Y153STOP) | −19A→G |
| BE_148 | R | 1 | TAC→TAA (Y164STOP) | −19A→G |
| OO6_160 | R | 1 | TAC→TAG (Y251STOP) | WT |
| W4_22 | R | 1 | INS 2 ntds; Frameshift 11 | WT |
| OO6_15 | R | 1 | DEL 5 ntds; Frameshift 72 | −19A→G |
| W4_3 | R | 1 | DEL 2 ntds; Frameshift 217 | WT |
| OO6_79 | R | 1 | GGT→CGT (G15R) | WT |
| OO6_18 | R | 1 | GGG→AGG (G91R) | −19A→G |
| OO6_10 | R | 1 | CGC→CTC (R127L) | −19A→G |
| AI_64 | R | 1 | CTG→CCG (L143P) | WT |
| J_152 | R | 1 | TGT→CGT (C146R) | WT |
| BE_144 | R | 1 | CTG→CCG (L172P) | −19A→G |
| AI_163 | R | 1 | GCG→CCG (A182P) | WT |
| OO6_17 | R | 1 | CAG→CGG (Q191R) | −19A→G |
| CDC-10 | R | 1 | ACC→GCC (T202A) | WT |
| AU_121 | R | 1 | GCT→CCT (A259P) | WT |
| CDC_78 | R | 1 | GTA→GGA (V263G) | WT |
Isolates are ordered by mutation codon number from the N to C terminus. All are PASr spontaneous mutants with wild-type (WT) thyX, dfrA, folP1, folC, nhoA, aac(1), and aac(2) genes and flanking regions.
PASr spontaneous mutants with wild-type thyA gene.
PASr spontaneous mutants as in (B) with an additional SNP upstream of folP2 not associated with PAS resistance.
R, resistant.
Mutations in the dfrA, folP1/folP2, and thyX genes are not associated with PAS resistance.
Three mutations in the dfrA gene (66S→C, 54V→A, and 110C→R) were identified in two clinical isolates that in addition already bore a mutation in the thyA gene (Table 2). Specifically, strain P-3158 encoded SNP 66S→C, while P-693 included two mutations in the dfrA gene (54V→A and 110C→R). No PASr isolate with a polymorphism only within the dfrA gene was found; consequently, it is not known whether the three dfrA mutations alone contribute to a PASr phenotype. No mutations were found within the thyX gene or flanking regions in either the clinical isolates or the spontaneous mutants. This gene is also highly conserved (100%) among other M. tuberculosis complex strains (GenBank database; data not shown). Likewise, the folP1 gene and flanking sequences were conserved throughout, while some polymorphisms were noted in the folP2 gene and its upstream region. FolP2 has been listed as a nonessential enzyme by transposon mutagenesis (28). A single-nucleotide substitution was found upstream from the starting codon of folP2 (−19A→G) in both PASs and PASr isolates. This single-nucleotide polymorphism (SNP) is also present in PASr/s M. bovis and M. bovis BCG. All isolates characterized by this SNP grouped in genetic cluster I (9) and were correlated with a phylogenetic lineage, rather than PAS resistance. The N terminus of the folP2 gene comprises three and a half imperfect 27-nt-long tandem repeats, except for PASr/s AU strains. All AU strains have lost most of the second repeat while retaining the coding region in frame. The observation that both PASr/s AU isolates carry this alteration indicates that this mutation is not associated with PASr, but rather, is a molecular characteristic of AU and related strains. Thus, no mutations within the genes encoding enzymes in the folate and thymine biosynthetic pathway, other than thyA, were correlated with a PASr phenotype. Additionally, no SNPs were identified in folC (Rv2447c) and three N-acetyltransferase genes, including nhoA (Rv3566c), aac(1) (Rv0262), and aac(2) (Rv1347c).
Thirty-seven percent of PASr strains have a mutation within the thymidylate synthase A (thyA) gene.
Sequence analysis of the clinical isolates and spontaneous mutants revealed 24 different mutations in the thyA gene, including 4 and 20 distinct polymorphisms in clinical isolates and spontaneous mutants, respectively (Tables 2 and 3). It is noteworthy that most polymorphisms found in the clinical isolates and the spontaneous mutants were distinct. Only the most common mutation identified in clinical isolates (202ACC→GCC; 202T→A) was also identified in a single spontaneous mutant. This SNP was characteristic of all PASr clinical isolates belonging to two unrelated strain clusters (genotypes P and AB), as well as an additional four strains accounting for six different genotypes altogether (Table 2), but was not found in corresponding related PASs isolates. The mutation 91GGG→GAG (91G→E) was identified in all three laboratory PASr H37Rv isolates, but not in the PASs H37Rv/a. The three PASr H37Rv isolates were generated by Steenken and Wolinsky in the 1950s (30). Interestingly, a different SNP was identified at the same position (91GGG→AGG; 91G→R) in a single spontaneous mutant. Other SNPs, deletions, or insertions leading to either stop codons or frameshifts were noted among the spontaneous mutants (Table 3). Other polymorphisms in the thyA gene were distributed throughout the gene. Although no “hot spot” drug resistance-determining region within the thyA gene was identified, protein structural predictions indicate that all the mutations recorded reside within essential functional or structural sites, as discussed below (Table 4). An unexpectedly high number of PASr isolates were found to have wild-type genotypes for the enzymes of the folate and thymidine biosynthetic pathways among clinical isolates and the spontaneous mutants.
TABLE 4.
Features of PASr thyA mutants
| Mutant | ΔΔGa | MICb | Consc | Structural features |
|---|---|---|---|---|
| Truncated protein mutants | ||||
| 83W→Stop | >128 | Truncated polypeptide | ||
| 111Q→Stop | >128 | Truncated polypeptide | ||
| 118L→Stop | >128 | Truncated polypeptide | ||
| 153Y→Stop | >128 | Truncated polypeptide | ||
| 164Y→Stop | >128 | Truncated polypeptide | ||
| 251Y→Stop | >128 | Truncated polypeptide | ||
| 264Stop→R | >128 | Truncated polypeptide | ||
| Mutations affecting catalytic site | ||||
| 127R→L | +0.18 | >128 | 99.2 (0) | In interaction with substrate dUMP |
| 143L→P | +2.45 | >128 | 98.9 (0) | In interaction with THF cofactor |
| 146C→R | +1.56 | >128 | 100 (0) | Catalytic residue |
| 172L→P | +1.28 | >128 | 99.2 (0) | In interaction with THF cofactor |
| 182A→P | +3.94 | >128 | 77.6 (0) | Completely buried into the hydrophobic core of the enzyme |
| 259A→P | +1.56 | <64 | 55.1 (0) | Close to the binding site of cofactor folate derivative |
| 261V→G | +0.52 | >128 | 43.0 (0) | In interaction with THF cofactor |
| 263V→I | +0.45 | ND | 80.5 (10) | Close to binding site of THF cofactor |
| Structurally destabilized mutants | ||||
| 15G→R | +1.20 | >8 <32 | 99.5 (0) | Makes 1-residue-long link between two secondary structures |
| 91G→E | +1.68 | ND | 99.2 (0) | Buried and in positive backbone j torsion angle |
| 91G→R | +1.89 | ND | 99.2 (0) | Buried and in positive backbone j torsion angle |
| 183L→V | +2.13 | <64 | 80.7 (0) | Completely buried into the hydrophobic core of the enzyme |
| 191Q→R | +1.39 | <64 | 20.6 (0) | Buried residue in C-terminal region of an α-helix |
| 202T→A | +3.24 | <128 | 70.2 (1) | In dimeric interface opposite to the equivalent residue |
ΔΔG (in kcal/mol) is the predicted change in folding free energy upon single-site mutation computed on an M. tuberculosis homodimer 3D model with the PoPMuSiC program (13). The computed values were averaged on both chains.
In μg/ml. ND, not determined.
Cons is the conservation percentage of a given residue as determined from the multiple-amino-acid alignment of 279 different ThyA sequences. Mutant residues are in parentheses.
PASr M. tuberculosis P strains have dual mutations in thyA.
Fifteen isolates known to be PASr and one PASs isolate belonging to a single cluster known as the P strain family were analyzed. These isolates belong to a larger cluster of over 120 isolates associated with MDR outbreaks in New York City and neighboring states (21). One isolate sharing the same molecular genotype and resistance phenotype (rifampin and isoniazid resistance) as the other P strains was found to be PASs and hence was used as a control strain. The 15 PASr P strains had a characteristic mutation in codon 202 (202T→A) of thyA, and 12 of them had a second mutation in the thyA gene consisting of a single-nucleotide substitution at the termination codon (264TGA→CGA; 264stop→R) (Table 2). A hairpin structure 3 nt downstream from the stop codon could function as a terminator of the mRNA, allowing dfrA transcript initiation. It is unlikely that thyA-dfrA forms an operon. A putative ribosome binding site is present 15 nt upstream from the dfrA gene. Alternatively, an unlikely run-through transcript would result in an extended thyA mRNA overlapping out of frame by 251 nt with the downstream coding region of the dfrA gene. These data suggest that the P strain acquired the 202ACC→GCC mutation prior to the mutation in the termination codon. Moreover the identification of mutation 202 alone in two closely related P variants (P6 and P23) indicates that these isolates diverged after the acquisition of mutation 202 but prior to the acquisition of the SNP on the termination codon. Two of the double-ThyA-mutant P strains developed secondary mutations on the flanking dfrA gene (66S→C and a double mutation, 54V→A and 110C→R, on a second strain), as described above.
Predicting the structural implications of the thyA mutations identified.
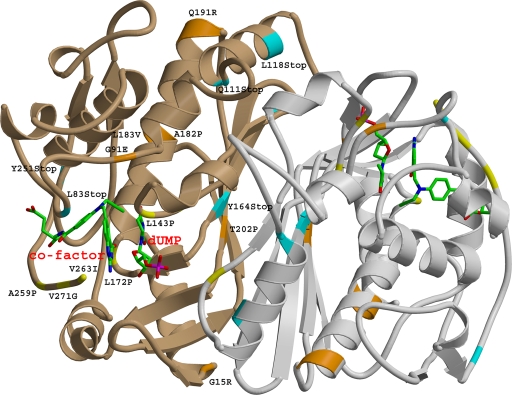
To understand the impacts of the identified mutations on protein function, a 3D homodimer structure of M. tuberculosis thymidylate synthase was modeled based on the X-ray structure of the E. coli thymidylate synthase as a homologous template. M. tuberculosis and E. coli ThyA protein sequences share strong identity (67%) and similarity (83%). The model showed an equivalent resolution of 1.7 Å according to hydrogen bond energy criteria, with no residue found in the disallowed regions of the Ramachandran plot. Analysis of the mutations positioned within the structure model revealed three groups of mutants (Table 4 and Fig. 2).
FIG. 2.
Ribbon view of a homodimer model of the M. tuberculosis thymidylate synthase enzyme. The mutated positions are labeled and depicted in one monomer and colored coded in the other monomer. Blue, stop codon mutations; orange, mutations affecting protein stability; yellow, mutations of residues involved in substrate or cofactor binding. The dUMP substrate and folate cofactor are also depicted and labeled in red.
The first group (Fig. 2) consists of mutations that modify the length of the protein. Six stop mutations (83W→stop, 111Q→stop, 118L→stop, 153Y→stop, 164Y→stop, and 251Y→stop) and three insertion/deletion alterations, including 5- and 2-nt deletions and a 2-nt insertion in codons 11, 72, and 217, respectively, introduce early frameshift. Such modifications are expected to render the enzyme nonfunctional due to the loss of its ternary structure, which harbors many residues of active sites or of cofactor binding sites located within the C-terminal part of the enzyme. A 10th mutation potentially extending the size of the protein by 76 amino acids through the obliteration of the stop codon was also identified in the P clinical cluster. Existing structural data on other bacterial and eukaryotic ThyA enzymes have shown that the C-terminal amino acid has a functional role in catalysis (Fig. 2).
The second group of mutants contains mutations of amino acids involved in the active catalytic site or within the cofactor or substrate binding site (Fig. 2). For example, mutation 127R→L affects the residue involved in dUMP substrate binding, while 172L→P, 143L→P, 261V→G, and 263V→I interact with the folate derivative. 146C→R forms part of the catalytic site.
The third group encompasses mutations that could destabilize the ternary structure of the ThyA protein (Fig. 2), rendering the enzyme dysfunctional or less active (26). Mutants with 15G→R, 91G→E, and 91G→R mutations change glycine residues otherwise conserved among all known thymidylate synthase structures to satisfy conformational restraints. Residues 82Ala and 183Leu are completely embedded in the hydrophobic clusters of the protein core. 202Thr is an amino acid located at the monomer-monomer interface facing the 202Thr of its counterpart. Therefore, all mutants were predicted to encode destabilizing mutations, according to the positive changes of free folding energy computed by the PoPMuSiC program (Table 4).
Elevated MICs in PASr spontaneous mutants and clinical isolates.
The MICs of selected clinical isolates and spontaneous mutants were determined by the dilution assay and Bactec/Alert culture (Table 4). In a departure from the conventional application of Bactec/Alert for diagnostic purposes, the growth of PASr samples was followed over a period of 2 weeks in the presence of increasing concentrations of PAS. Samples including mutations within the thyA gene proved to be highly resistant to PAS. This observation was further confirmed by plating the samples at various concentrations of PAS (up to 128 μg/ml).
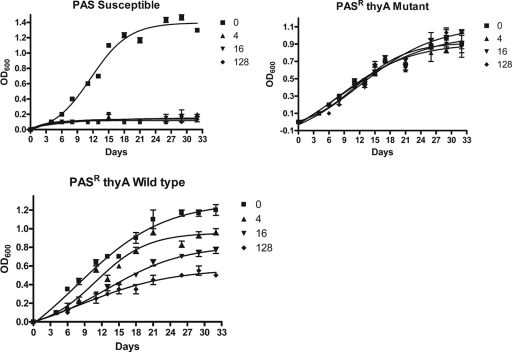
In addition, the growth curves of representative PASr samples were followed in triplicate over a period of 32 days (Fig. 3). The growth curve profiles of strains with a mutated ThyA protein, which were found to be equally resistant to increasing concentrations of PAS up to the 128 μg/ml tested, were noteworthy, displaying overlap with the corresponding untreated strains. In contrast, the growth of PASr isolates encoding wild-type proteins of the folate pathway was found to be dose dependent. For these isolates, growth was proportionally inhibited as the dose of PAS was increased, clearly indicating the presence of a different mechanism of PAS resistance.
FIG. 3.
Representative growth curves of PASs and PASr isolates in the presence of various concentrations of PAS (in μg/ml). PASr isolates with a mutated thyA gene (truncated or with an altered catalytic site) were equally resistant to increasing concentrations of PAS, while PASr isolates with a wild-type genotype were dose dependent. For this isolate, growth was inhibited at increasing concentrations of PAS. The error bars indicate standard deviations of three independent experiments. OD600, optical density at 600 nm.
DISCUSSION
Recently, the thyA gene of the folate pathway was shown to be associated with PAS resistance in M. tuberculosis (26). In the present study, we analyzed the thyA sequences in 118 strains, including references strains, clinically diverse and clustered PASr strains, and PASr spontaneous mutants, and found that only 37% of the samples included a thyA mutation implicated in PAS resistance. The limited association between the PASr phenotype and mutations in the thyA gene led us to examine the nucleotide sequences of five other enzymes in the folate pathway and thymine biosynthesis, as well as three N-acetyltransferase genes. To our surprise no mutations were identified in these eight genes.
Twenty-four distinct mutations were identified in the thyA gene, including 4 SNPs found uniquely in clinical isolates and 20 observed in the spontaneous mutants. A single polymorphism (202ACC→GCC), previously reported, was seen in clinical isolates and one spontaneous mutant (26). The divergence in mutation type observed in clinical isolates and spontaneous mutants could result from experimental bias due to PASr selection at elevated concentrations of PAS or to the limited number of PASr isolates investigated. Alternatively, ThyA may not be an essential enzyme in vitro while assuming a more significant function in vivo. This could be due to a higher demand in vivo for thymine, lower availability of extracellular thymine, or even a functional difference in the complementary ThyX, which may require complementation for an active ThyA. The observation that nine of the spontaneous mutants encoded truncated ThyA proteins supports this notion; however, further work is needed to confirm this possibility. Other mutations in ThyA among the spontaneous mutants were found to be equally destabilizing. They include alterations affecting the substrate or cofactor binding site or the catalytic site or resulting in major structural changes as determined by analysis of predicted 3D-mutated ThyA.
The most prevalent mutation, 202ACC→GCC, was found in spontaneous mutants and molecularly/epidemiologically unrelated clinical isolates (n = 4), as well as in strain clusters belonging to P and AB genotypes. Sequence analysis of the P strain cluster isolates revealed that all resistant samples had the characteristic 202ACC→GCC mutation and that 12 of them additionally included a stop codon, suggesting sequential acquisition. The deletion of the stop codon alone was not shown to be associated with PASr, as no isolates with the obliterated 264stop codon alone were identified. Structural predictions of the ThyA protein and literature review suggest that the carboxyl-terminal amino acid folds back into the catalytic groove of the enzyme and therefore may be functionally relevant.
Overall, protein structure modeling predicts that all 24 different mutations identified in the thyA gene map to essential amino acids affecting either the structure, the functional site (substrate, cofactor binding site, or catalytic site), or the dimer interface of the ThyA homodimer. Six mutations were found in key positions of spatial structure, radically altering the conformation of the enzyme. Seven of the mutations involved essential amino acids in close proximity to the catalytic site, including a PASr spontaneous mutant with a single point mutation in amino acids of the catalytic site (261V→G and 263V→I) and mutant 127R→L, encoding an amino acid substitution at the substrate dUMP binding site.
The observation that most mutants reported here affect highly conserved positions otherwise rarely found in the protein family of thymidylate synthase is noteworthy. Our results indicate that the observed mutations affect residues under strong functional selection that are uncommon in nature (Table 4). This detrimental alteration to ThyA is permissible in M. tuberculosis due to the presence of the complementary functional homologue ThyX. ThyX per se does not seem to be susceptible to PAS. This is further exemplified in the work of Rengarajan and colleagues, who have shown that the resistant phenotype of transposon-generated PASr mutants could be reversed through complementation/overexpression of the wild-type thyA gene (26).
Mutations in the thyA gene were associated with elevated levels of PAS resistance, as determined by dilution assays, Bactec/Alert susceptibility testing, and growth curve experiments. Interestingly, spontaneous mutants encoding a ThyA modification responded equally to increasing concentrations of PAS, while PASr mutants encoding wild-type ThyA proved to be dose dependent. This observation indicates that the yet unidentified alternative mechanism(s) or target(s) associated with PASr is concentration dependent.
The observation that PAS is active only in the presence of a functional ThyA enzyme suggests that, like other antimycobacterials (isoniazid, ethionamide, and pyrizinamide), PAS is a prodrug whose activation somehow requires a viable ThyA, as was also previously proposed (26).
Supplementary Material
Acknowledgments
V.M. is the recipient of a doctoral scholarship from the FNRS (Belgian Fund for Scientific Research). R.W. is a Research Associate at the FNRS. A.S. is supported by the Novartis Institute for Tropical Diseases, NITD. This work was supported in part by Les Amis de L'Institut Pasteur de Bruxelles. We thank bioMérieux Belgium for sponsoring the MIC determinations by Bactec/Alert and for technical support.
We are grateful to J. Rengarajan and E. Rubin, Harvard School of Public Health, Boston, MA, for insightful suggestions during the course of the work.
Footnotes
Published ahead of print on 23 February 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Bifani, P., B. Mathema, N. E. Kurepina, E. Shashkina, J. Quatannens, A. S. Blanchis, S. Moghazeh, J. Driscoll, B. Gicquel, R. Frothingham, and B. N. Kreiswirth. 2008. The evolution of drug-resistance in Mycobacterium tuberculosis: from a mono-rifampin resistant cluster into increasingly multidrug resistant variants in an HIV sero-positive population. J. Infect. Dis. 198:90-94. [DOI] [PubMed] [Google Scholar]
- 3.Bifani, P., S. Moghazeh, B. Shopsin, J. Driscoll, A. Ravikovitch, and B. N. Kreiswirth. 2000. Molecular characterization of Mycobacterium tuberculosis H37Rv/Ra variants: distinguishing the mycobacterial laboratory strain. J. Clin. Microbiol. 38:3200-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC. 1992. Update: availability of streptomycin and para-aminosalicylic acid—Unites States. MMWR Morb. Mortal. Wkly. Rep. 41:482. [Google Scholar]
- 5.De Logu, A., V. Onnis, B. Saddi, C. Congiu, M. L. Schivo, and M. T. Cocco. 2002. Activity of a new class of isonicotinoylhydrazones used alone and in combination with isoniazid, rifampicin, ethambutol, para-aminosalicylic acid and clofazimine against Mycobacterium tuberculosis. J. Antimicrob. Chemother. 49:275-282. [DOI] [PubMed] [Google Scholar]
- 6.Fisher, B. M., G. Roberts, and H. C. Hinshaw. 1951. The subcutaneous administration of the sodium salt of para-aminosalicylic acid in the treatment of tuberculosis. Am. Rev. Tuberc. 64:557-563. [DOI] [PubMed] [Google Scholar]
- 7.Gilis, D., and M. Rooman. 2000. PoPMuSiC, an algorithm for predicting protein mutant stability changes: application to prion proteins. Protein Eng. 13:849-856. [DOI] [PubMed] [Google Scholar]
- 8.Graziani, S., J. Bernauer, S. Skouloubris, M. Graille, C. Z. Zhou, C. Marchand, P. Decottignies, H. van Tilbeurgh, H. Myllykallio, and U. Liebl. 2006. Catalytic mechanism and structure of viral flavin-dependent thymidylate synthase ThyX. J. Biol. Chem. 281:24048-24057. [DOI] [PubMed] [Google Scholar]
- 9.Gutacker, M. M., B. Mathema, H. Soini, E. Shashkina, B. N. Kreiswirth, E. A. Graviss, and J. M. Musser. 2006. Single-nucleotide polymorphism-based population genetic analysis of Mycobacterium tuberculosis strains from 4 geographic sites. J. Infect. Dis. 193:121-128. [DOI] [PubMed] [Google Scholar]
- 10.Gutacker, M. M., J. C. Smoot, C. A. Migliaccio, S. M. Ricklefs, S. Hua, D. V. Cousins, E. A. Graviss, E. Shashkina, B. N. Kreiswirth, and J. M. Musser. 2002. Genome-wide analysis of synonymous single nucleotide polymorphisms in Mycobacterium tuberculosis complex organisms: resolution of genetic relationships among closely related microbial strains. Genetics 162:1533-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubbard, S. J., and J. M. Thornton. 1993. NACCESS Computer Program. University College, London, United Kingdom.
- 12.Kunz, B. A., and S. E. Kohalmi. 1991. Modulation of mutagenesis by deoxyribonucleotide levels. Annu. Rev. Genet. 25:339-359. [DOI] [PubMed] [Google Scholar]
- 13.Kwasigroch, J. M., D. Gilis, Y. Dehouck, and M. Rooman. 2002. PoPMuSiC, rationally designing point mutations in protein structures. Bioinformatics 18:1701-1702. [DOI] [PubMed] [Google Scholar]
- 14.Laskowski, R. A., J. A. Rullmannn, M. W. MacArthur, R. Kaptein, and J. M. Thornton. 1996. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 8:477-486. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann, J. 1946. Para-aminosalicylic acid in the treatment of tuberculosis. Lancet 247:15-16. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann, J. 1949. The treatment of tuberculosis in Sweden with para-aminosalicylic acid; a review. Dis. Chest 16:684-703. [DOI] [PubMed] [Google Scholar]
- 17.Luria, S. E., and M. Delbruck. 1943. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28:491-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathema, B., N. E. Kurepina, P. J. Bifani, and B. N. Kreiswirth. 2006. Molecular epidemiology of tuberculosis: current insights. Clin. Microbiol. Rev. 19:658-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medical Research Council. 1949. Treatment of pulmonary tuberculosis with para-aminosalicylic acid and streptomycin. Br. Med. J. 2:1521. [PMC free article] [PubMed] [Google Scholar]
- 20.Morlock, G. P., J. T. Crawford, W. R. Butler, S. E. Brim, D. Sikes, G. H. Mazurek, C. L. Woodley, and R. C. Cooksey. 2000. Phenotypic characterization of pncA mutants of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 44:2291-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munsiff, S. S., T. Bassoff, B. Nivin, J. Li, A. Sharma, P. Bifani, B. Mathema, J. Driscoll, and B. N. Kreiswirth. 2002. Molecular epidemiology of multidrug-resistant tuberculosis, New York City, 1995-1997. Emerg. Infect. Dis. 8:1230-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray, J. F. 2004. A century of tuberculosis. Am. J. Respir. Crit. Care Med. 169:1181-1186. [DOI] [PubMed] [Google Scholar]
- 23.Newby, Z., T. T. Lee, R. J. Morse, Y. Liu, L. Liu, P. Venkatraman, D. V. Santi, J. S. Finer-Moore, and R. M. Stroud. 2006. The role of protein dynamics in thymidylate synthase catalysis: variants of conserved 2′-deoxyuridine 5′-monophosphate (dUMP)-binding Tyr-261. Biochemistry 45:7415-7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nopponpunth, V., W. Sirawaraporn, P. J. Greene, and D. V. Santi. 1999. Cloning and expression of Mycobacterium tuberculosis and Mycobacterium leprae dihydropteroate synthase in Escherichia coli. J. Bacteriol. 181:6814-6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pugh, D. L., G. S. Edwords, R. G. McLaren, and E. R. Jones. 1952. Toxic psychiatric manifestations in the treatment of tuberculosis with sodium para-aminosalicylate. Tubercle 33:369-376. [DOI] [PubMed] [Google Scholar]
- 26.Rengarajan, J., C. M. Sassetti, V. Naroditskaya, A. Sloutsky, B. R. Bloom, and E. J. Rubin. 2004. The folate pathway is a target for resistance to the drug para-aminosalicylic acid (PAS) in mycobacteria. Mol. Microbiol. 53:275-282. [DOI] [PubMed] [Google Scholar]
- 27.Sali, A., and T. L. Blundell. 1993. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234:779-815. [DOI] [PubMed] [Google Scholar]
- 28.Sassetti, C. M., D. H. Boyd, and E. J. Rubin. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48:77-84. [DOI] [PubMed] [Google Scholar]
- 29.Steenken, W., Jr., and E. Wolinsky. 1956. Cycloserine: antituberculous activity in vitro and in the experimental animal. Am. Rev. Tuberc. 73:539-546. [DOI] [PubMed] [Google Scholar]
- 30.Steenken, W., Jr., and E. Wolinsky. 1950. Effects of antimicrobial agents on the tubercle bacillus and on experimental tuberculosis. Am. J. Med. 9:633-653. [DOI] [PubMed] [Google Scholar]
- 31.Therapeutic Trials Committee of the Swedish National Association against Tuberculosis. 1950. Para-aminosalicylic acid treatment in pulmonary tuberculosis. Am. Rev. Tuberc. 61:597-612. [PubMed] [Google Scholar]
- 32.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallace, A. C., R. A. Laskowski, and J. M. Thornton. 1995. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 8:127-134. [DOI] [PubMed] [Google Scholar]
- 34.WHO. 2000. Guidelines for establishing DOTS-Plus pilot projects for the management of multidrug-resistant tuberculosis (MDR-TB). WHO/CDS/TB/2000.279. WHO, Geneva, Switzerland.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.