Abstract
Phosphorodiamidate morpholino oligomers (PMOs) are uncharged nucleic acid-like molecules designed to inactivate the expression of specific genes via the antisense-based steric hindrance of mRNA translation. PMOs have been successful at knocking out viral gene expression and replication in the case of acute viral infections in animal models and have been well tolerated in human clinical trials. We propose that antisense PMOs represent a promising class of therapeutic agents that may be useful for combating filoviral infections. We have previously shown that mice treated with a PMO whose sequence is complementary to a region spanning the start codon of VP24 mRNA were protected against lethal Ebola virus challenge. In the present study, we report on the abilities of two additional VP24-specific PMOs to reduce the cell-free translation of a VP24 reporter, to inhibit the in vitro replication of Ebola virus, and to protect mice against lethal challenge when the PMOs are delivered prior to infection. Additionally, structure-activity relationship evaluations were conducted to assess the enhancement of antiviral efficacy associated with PMO chemical modifications that included conjugation with peptides of various lengths and compositions, positioning of conjugated peptides to either the 5′ or the 3′ terminus, and the conferring of charge modifications by the addition of piperazine moieties. Conjugation with arginine-rich peptides greatly enhanced the antiviral efficacy of VP24-specific PMOs in infected cells and mice during lethal Ebola virus challenge.
Members of the Filoviridae family of viruses, Ebola virus (EBOV) and Marburg virus (MARV), represent severe threats to human health not only from infections to populations in regions of endemicity but also from the possible use of weaponized versions by bioterrorists. Case fatality rates up to 90% have been reported (6), and outbreaks have occurred as recently as November 2007, during which a possible new species emerged in the Bundibugyo District of Uganda (38). Although no vaccines or antiviral therapies are licensed for use for the prevention or treatment of filovirus infections, approaches to therapeutic development efforts include the administration of type I interferons, therapeutic vaccines, immune globulins, and ribavirin and other nucleoside analogues (8, 10). The development of effective therapies has been hampered by the biosafety level 4 containment requirements for filoviruses, limitations in the understanding of filoviral pathogenesis, and differences between animal models. Several antiviral treatments have been shown to offer protection in mouse models of infection but have only partially protected nonhuman primates following challenge with EBOV Zaire (10, 17, 34).
The filovirus genome consists of seven genes encoded by a 19-kb negative-sense RNA molecule. These genes encode an RNA-dependent RNA polymerase (L protein), nucleoprotein, glycoprotein, and four smaller products (VP24, VP30, VP35, and VP40). VP24, a minor matrix protein embedded in the lipid bilayer, is a 24-kDa product capable of forming homotetramers (13) and has the capability to direct nucleocapsid formation (27). VP24 interacts with the nuclear localization receptor for tyrosine-phosphorylated STAT1, which may provide a mechanistic explanation for its role as an antagonist of the type I interferon signaling pathway (29). Additionally, recent investigations have shown that VP24 associates with the ribonucleoprotein complex, inhibiting viral RNA replication and transcription (36).
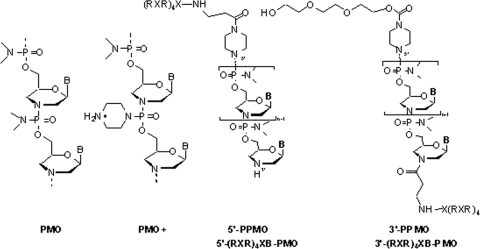
Synthetic antisense agents interfere with the translation of products by sterically blocking mRNA or by triggering RNase H-mediated cleavage of the RNA-DNA duplex, resulting in the inhibition of gene expression (20). Phosphorodiamidate morpholino oligomers (PMOs) are uncharged antisense agents that are composed of moieties with a morpholino base (versus RNA, whose moieties have a ribose base) linked through methylene phosphorodiamidate (Fig. 1). PMOs inhibit gene expression and alter pre-mRNA splicing by binding with the transcript, thus sterically blocking translational processes. PMOs are attractive as antiviral agents due to their favorable base stacking, high degree of duplex stability (40), high degree of solubility, and lack of hybridization complexities (15, 16, 20, 32). PMOs and peptide-conjugated PMOs have been used to inhibit the replication of vesiviruses (31), flaviviruses (6, 18), and the severe acute respiratory syndrome-associated coronavirus (25). Conjugation of arginine-rich cell-penetrating peptides with PMOs (PPMOs) has shown promise toward facilitating cell entry and have increased efficacy compared to the efficacies of neutrally charged PMO molecules (1, 20, 21, 28, 30, 42).
FIG. 1.
Structures of a PMO, PMO+, and PPMO conjugated to either the 5′ or the 3′ PMO terminus. The PPMOs are shown as (RXR)4XB conjugates and are representative of PPMOs conjugated to other peptides used in the experiments described herein.
PMOs complementary to viral products have been shown to be efficacious in in vivo models of EBOV infection. Intraperitoneal (i.p.) administration of a VP24-specific PMO provided nearly complete protection in mice following a lethal EBOV challenge (35). Enterlein et al. (7) have demonstrated that conjugation of a VP35-specific PMO with an arginine-rich cell-penetrating peptide improved its efficacy, completely protecting mice following lethal EBOV challenge. The success of PPMO led us to develop a new PMO chemistry, designated PMO+, in which the positively charged piperazine groups were introduced to selected positions on the PMO linkage (Fig. 1).
In the present studies, PMOs were designed to target the EBOV VP24 transcript at one of three positions either 5′ to, overlapping, or immediately downstream of the translational start codon. We screened these PMOs, which were conjugated with various peptides rich in basic amino acids, for their abilities to affect the translation of a VP24 reporter construct, inhibit in vitro infection, and protect mice against lethal EBOV challenge. In addition, we carried out a structure-activity relationship study to determine the effects of peptide length, type of amino acid, and conjugation position of the peptide on the efficacies of the resulting PPMOs targeting EBOV in both in vitro and in vivo assays. Several PMO+ compounds that contained two to four positive charges on the backbone were also evaluated and compared with unmodified PMOs and PPMOs. Lastly, EBOV-inhibiting PMOs that may be suitable for potential development efforts were identified.
MATERIALS AND METHODS
PMOs.
PMOs whose sequences had identity with sequences upstream, overlapping, or immediately downstream of the AUG translational start site of the EBOV VP24 mRNA were designed. The names of these sequences and the sequences are as follows: VP24 5′ terminus (VP24-5′Term; 5′-TTCAACCTTGAAACCTTGCG-3′), VP24-AUG (5′-GCCATGGTTTTTTCTCAGG-3′), and VP24-AUG+4 (5′-TGTATCGTCCCGTAGCTTTACG-3′) (Fig. 2). A PMO whose sequence had identity with the MARV VP24 mRNA sequence (VP24-AUG [5′-CGTTGATAATTCTGCCATG-3′]) was also designed. The MARV VP24 mRNA-specific PMO and an unrelated, scrambled PMO (5′-AGTCTCGACTTGCTACCTCA-3′) were used as controls in these experiments. The PMOs were synthesized by AVI BioPharma, Inc. (Corvallis, OR), as described previously (32, 35). The synthesis of cell-penetrating peptides, the PPMO compounds, and the piperazine-modified PMO+ compounds is described elsewhere (37, 39).
FIG. 2.
Targeting the EBOV VP24 gene. (A) Schematic diagram of the negative-sense EBOV genome showing relative locations for sequences homologous to VP24-5′Term, VP24-AUG, and VP24-AUG+4. (B) Partial sequence for EBOV VP24 mRNA showing regions complementary to the PMO sequences. The VP24 start codon is indicated.
In vitro translation assay.
A rabbit reticulocyte lysate in vitro translation assay was performed as described previously (7, 26, 35). Briefly, oligonucleotides complementary to the EBOV VP24 (positions −84 to +43; bases 10261 to 10390) were subcloned upstream of and in frame with the protein-coding sequence for firefly luciferase, without the initiator Met codon ATG, into plasmid pCiNeo (Promega, Madison, WI). Subsequently, the linearized plasmid was transcribed into RNA in vitro by using a T7 polymerase-based Megascript kit (Ambion, Austin, TX), according to the manufacturer's protocols. Briefly, nuclease-treated rabbit reticulocyte lysate, complete amino acid mixture, 1 nM of RNA, and either water (for the control reactions) or PMO were combined. All treatments were conducted in triplicate in 96-well plates. The reaction mixtures were incubated at 37°C for 1 h. The luciferase signal was generated by adding the luciferase assay reagent (Promega) to the wells. The relative light units produced by each reaction were quantified with a plate reader, normalized to the mean of the reactions for the water control included on each plate, and expressed as the percent inhibition of luciferase translation. To determine the effective concentration that provided 50% inhibition (EC50), the luminescence values from the replicate treatments were averaged and used to graph the percent inhibition associated with PMO doses. The EC50s for each PMO were calculated by using Graph Pad (San Diego, CA) Prism graphing software to determine the concentration of PMO required to reduce the luminescent signal generated from the reactions for the water control on the same plate by 50%.
In vitro inhibition of viral replication by PMOs, as assessed by infection with ZEBOV-GFP.
Vero E6 cells were grown to confluence in a 96-well plate. Two hours before infection, the medium was removed from the cells and PMOs were added in 100 μl of serum-free Eagle minimum essential medium to achieve a PMO concentration of 20 μM. After 2 h, the cells were infected at a multiplicity of infection (MOI) of 1 by adding 100 μl of Eagle minimum essential medium containing 10% fetal calf serum and green fluorescent protein (GFP)-expressing EBOV strain Zaire (ZEBOV) (ZEBOV-GFP) (33). After 48 h, the cells were fixed with 10% neutral-buffered formalin and the cell nuclei were labeled with Hoechst dye. The percentage of GFP-expressing cells was measured with a Discovery-1 automated microscope (Molecular Devices Corp., Sunnyvale, CA) by measuring nine individual spots per well, with an average of 2,000 cells per spot being counted (19). In each experiment, duplicate or triplicate wells were examined for each PMO treatment condition. Percent inhibition values were calculated as follows: 100 × [1 − (average GFP fluorescence from specific PMO-treated cells)/(average fluorescence from scrambled PMO-treated cells)]. The experiments were performed at least three times.
Animals.
Male or female C57BL/6 mice (National Cancer Institute, Frederick Cancer Research and Development Center, Frederick, MD) aged 6 to 10 weeks at the start of each experiment were housed in microisolator cages and were provided autoclaved water and chow ad libitum. To assess the efficacies of the indicated PMOs, the mice were treated twice, once at 24 h and again at 4 h, prior to EBOV challenge with 50, 5, or 1 μg of PMO. The mice were challenged i.p. with 1,000 PFU of mouse-adapted EBOV (3). All EBOV-infected animals were handled under maximum containment in a biosafety level 4 laboratory at the United States Army Medical Research Institute of Infectious Diseases. The research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals (23). The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
RESULTS
In vitro efficacy of VP24 PMO formulated with various delivery chemistries.
A plasmid containing 127 nucleotides encompassing the PMO target area from EBOV VP24 was fused to firefly luciferase and used to generate RNA. Inhibition of the VP24 RNA target was assessed on the basis of the level of luciferase expression (relative light units) by using reaction mixtures containing different concentrations of PMO. At a concentration of 300 nM, addition of unmodified VP24-5′Term to the cell-free translation assay substantially reduced the level of luciferase expression relative to the level achieved with the scrambled PMO control treatment (Fig. 3). Of the VP24-5′Term PMOs evaluated, treatment with (RXR)4XB-conjugated PMO suppressed the level of translation, relative to the levels achieved with both scrambled PMO and unconjugated VP24-5′Term PMO treatments, to the greatest degree. While modest suppression was observed at the lowest concentration of the (RXR)4XB-conjugated PMO (1 nM), the level of luciferase expression decreased in a dose-dependent fashion and was nearly completely inhibited at 300 nM. The three piperazine-modified VP24-5′Term PMO+ compounds suppressed translation of the VP24 reporter to approximately equivalent degrees; however, treatment with these PMO+ compounds failed to substantially inhibit translation relative to the level of inhibition achieved with the unmodified PMO control.
FIG. 3.
Sequence-specific inhibition of EBOV VP24 gene targets in a cell-free translation assay. A plasmid containing ∼150 nucleotides encompassing the VP24-5′Term PMO target area from EBOV VP24 was fused to firefly luciferase and was used to generate RNA. Inhibition of the VP24 RNA target was assessed on the basis of the level of luciferase expression (relative light units) by using in vitro translation reaction mixtures containing different concentrations of the PMO. The PMOs used in the assay included scrambled PMO, unmodified VP24-5′Term, (RXR)4XB-conjugated VP24-5′Term, or VP24-5′Term containing piperazine modifications to the PMO backbone at the indicated positions relative to the 5′ end. All treatments were conducted in triplicate in 96-well plates. The luciferase signal produced by each reaction mixture was quantified with a plate reader, normalized to the mean of the reaction mixtures for the water control included on each plate, and expressed as percent inhibition of luciferase translation. The data are shown as means of three replicates per data point, with error bars representing standard deviations.
Inhibition of viral amplification by PMOs and PPMOs in cultured cells.
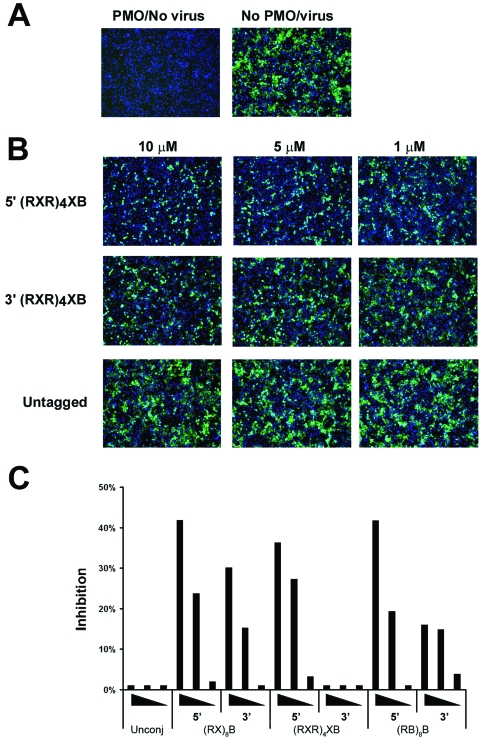
Vero E6 cells were pretreated for 2 h with 0 to 10 μM EBOV VP24-5′Term PMO before infection (MOI, 1) with ZEBOV-GFP (33). After 48 h, the cells were fixed in 10% formalin and examined by fluorescence microscopy. GFP was well expressed in cells infected with ZEBOV-GFP, whereas uninfected cells exhibited only negligible background levels of fluorescence (Fig. 4A). Treatment of cells with concentrations up to 10 μM unmodified VP24-5′Term PMO failed to appreciably diminish the level of ZEBOV-GFP replication compared to the level of replication by untreated infected cells (Fig. 4B, bottom panels). Although cells treated with (RXR)4XB conjugated to the 5′ end of VP24-5′Term exhibited a reduced level of fluorescence (Fig. 4B, top panels) relative to the level of fluorescence produced by infected cells treated with unmodified VP24-5′Term, this PMO conjugated with (RXR)4XB at the 3′ end inhibited ZEBOV-GFP replication to a lesser degree (Fig. 4B, middle panels). Conjugation with other peptides to either the 5′ or the 3′ end of VP24-5′Term consistently demonstrated that positioning of the peptides to the 5′ end of the PMOs provided viral inhibition greater than that achieved when the peptides were added to the 3′ end (Fig. 4C). Treatment of the cells with 10 μM VP24-5′Term conjugated at the 5′ end with either (RX)8B, (RXR)4XB, or (RB)8B provided >35% inhibition of ZEBOV-GFP relative to the level of inhibition achieved with infected cells treated with unconjugated VP24-5′Term. The PMOs exhibited no overt indications of cellular toxicity at the concentrations tested, as determined by visual examination of infected cells throughout the assay. Additionally, the nuclear staining patterns suggested that the cell monolayers remained viable throughout the assay.
FIG. 4.
Inhibition of ZEBOV-GFP replication by PMOs in vitro. Vero E6 cells were pretreated for 2 h with 0 to 10 μM of either unconjugated or peptide-conjugated VP24-5′Term PMO before infection (MOI, 1) with ZEBOV-GFP (33). After 48 h, the cells were fixed in 10% formalin and examined by fluorescence microscopy. Representative photos of cells treated with VP24 PMOs with various delivery chemistries are shown. The width of the field represented is 880 μm. (A) Vero E6 cells were treated with PMO and mock infected or infected with ZEBOV-GFP without PMO treatment. (B) Vero E6 cells were treated with 1, 5, or 10 μM of (RXR)4XB-conjugated (at either the 5′ or the 3′ PMO terminus) VP24-5′Term or unconjugated VP24-5′Term and infected with ZEBOV-GFP. (C) GFP fluorescence was measured by the use of Discovery-1 automated microscopy (Molecular Devices Corp.) by measuring nine individual spots per well. The percent inhibition achieved by the treatments was calculated by dividing the average GFP fluorescence from treatment wells by the average GFP fluorescence from the control wells that contained medium only. The experiments were performed at least three times; the results of a single and representative experiment are shown. Peptides to which VP24-5′Term was conjugated are indicated on the x axis along with the PMO terminus (5′ or 3′) to which each was conjugated. PMOs were tested at concentrations of 10, 5, and 1 μM, with the displayed wedges indicating the relative concentration.
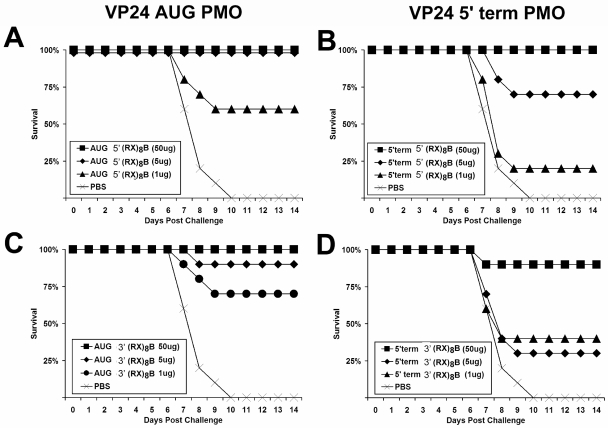
Effects of peptide length and conjugation position on VP24 PPMOs against lethal EBOV infection of mice.
To evaluate the in vivo efficacies of the PPMOs in the context of EBOV challenge, the VP24-AUG and VP24-5′Term PMOs were delivered i.p. to mice (n = 20 to 30) at 24 h and 4 h prior to challenge with 1,000 PFU of mouse-adapted EBOV. The mice were pretreated twice with the PMOs to provide an interval during which the PMOs could biodistribute, bind to target cells, and enter the cytoplasm if the molecules were capable of doing so. This dosing regimen maximized the likelihood that the antiviral potentials of the PMOs would be actualized during the infection.
Delivered at 5-μg and 50-μg doses, (RX)8B conjugated to the 5′ end of VP24-AUG completely protected the mice against lethal challenge, and 1-μg doses provided 60% protection (Fig. 5A). The mice were also well protected following the administration of 50-μg and 5-μg doses of VP24-AUG conjugated with (RX)8B at the 3′ end, with the treatments providing 100% and 90% protection, respectively (Fig. 5C). Additionally, 70% of mice were protected following the delivery of 1-μg doses of this PMO. Complete protection was also observed in mice treated with 50-μg doses of (RX)8B-conjugated VP24-5′Term, and 70% were protected at the 5-μg dose level (Fig. 5B). A high degree of protection was observed in mice treated with 50-μg doses of VP24-5′Term conjugated with (RX)8B at the 3′ end, while the 5- and 1-μg dosing regimens protected 40% and 30% of the treated mice, respectively (Fig. 5D).
FIG. 5.
Dose-dependent protective efficacy of arginine-rich, peptide-tagged VP24 PMOs against lethal EBOV infection of mice. Groups of mice (n = 20 to 30) were pretreated twice at 4 h and 24 h prior to EBOV infection with either 500-μg, 5-μg, or 1-μg doses of VP24-5′Term or VP24-AUG PMOs delivered i.p. PMOs were tagged with arginine-rich peptides on either the 5′ or the 3′ end, as indicated in the keys associated with the individual graphs. All animals in these studies were challenged i.p. with 1,000 PFU of mouse-adapted EBOV and were monitored for illness and survival for at least 28 days. PBS, phosphate-buffered saline.
To evaluate whether altering the number of arginine-aminohexanoic acid amino acid residues in the PMO would influence the in vivo efficacy of VP24-5′Term, we constructed PMOs conjugated to peptides (RX)nB, where n was equal to two, four, six, or eight RX residues and the peptides were positioned at either the 5′ or the 3′ end of the PMO. These PMOs were administered i.p. at 24 h and 4 h prior to lethal challenge with EBOV. The number of RX residues was generally positively correlated with the percent survival (Fig. 6). The greatest degree of protection (95%) was seen for mice treated with the 50-μg/dose of VP24-5′Term conjugated to eight RX repeats on the 5′ end, while the same PMO with six, four, and two RX repeats conferred 75%, 25%, and 15% protection, respectively. The unconjugated PMO did not provide any protection at this dose. A similar positive correlation between protection and the number of conjugated RX repeats was observed when residues were positioned on the 3′ end of VP24-5′Term. These data strongly suggest that the conjugation of VP24-5′Term with arginine-rich peptides enhances its in vivo antisense efficacy.
FIG. 6.
Various protective efficacies associated with multiple arginine peptide-tagged VP24 PMOs against lethal EBOV challenge in mice. Groups of mice (n = 20 to 30) were pretreated with 500-μg doses (delivered i.p.) of the VP24-5′Term PMO at 4 h and 24 h prior to i.p. EBOV challenge. PMOs were conjugated to peptides (RX)nB, where n is equal to two, four, six, or eight arginine-6-aminohexanoic acid repeats, and the peptides were positioned at either the 5′ or the 3′ end of the PMO. Mice were challenged with 1,000 PFU of mouse-adapted EBOV and monitored for illness and survival for at least 28 days.
Effects of amino acid type, composition, and PMO backbone modification on the antiviral activities of VP24-5′Term PMO and PMO+.
To evaluate whether the antiviral properties of VP24-5′Term could be enhanced through chemical modifications, this PMO was conjugated at either the 5′ or the 3′ terminus with various peptides bearing arginine (R), ornithine (O), methylated ornithine [O(Me)2)], or histidine (H) or was chemically modified at multiple positions along the PMO backbone with the positively charged piperazine groups. We evaluated the abilities of these altered versions of VP24-5′Term to affect the cell-free translation of a VP24-GFP reporter, inhibit the in vitro replication of ZEBOV-GFP, and confer protection to mice during EBOV infection. In Table 1, we present a summary of the results in which the activity of VP24-5′Term can be directly compared with the activities of the modified versions of this PMO in the three assays.
TABLE 1.
VP24-5′Term PMOs and activities against EBOV
| Chemical modificationa | Location of chemical modificationb | EC50 (nM) for inhibition of in vitro translation by RRL assayc | In vitro inhibition of EBOV growth (% GFP expression)d | Protective efficacy in C57BL/6 mice (%)e |
|---|---|---|---|---|
| None | 364 | 22 | 0 | |
| (RX)2B | 5′ | 37 | 1 | 15 |
| 3′ | 39 | 0 | 25 | |
| (RX)4B | 5′ | 18 | 0 | 25 |
| 3′ | 17 | 0 | 50 | |
| (RX)6B | 5′ | 4 | 0 | 75 |
| 3′ | 5 | 0 | 30 | |
| (RX)8B | 5′ | 8 | 41 | 93 |
| 3′ | 11 | 23 | 70 | |
| (RB)8B | 5′ | 4 | 26 | 30 |
| 3′ | 8 | 33 | 60 | |
| (RXR)4XB | 5′ | 2 | 38 | 80 |
| 3′ | 85 | 3 | 40 | |
| H1 | 5′ | 240 | 4 | 0 |
| 3′ | 200 | 0 | 20 | |
| (RXR)4C | 5′ | 4 | 27 | 40 |
| 3′ | 7 | 23 | 20 | |
| [O(Me)2X]4B | 5′ | 14 | 1 | 0 |
| 3′ | 51 | 2 | 0 | |
| (OX)4B | 5′ | 7 | 0 | 0 |
| 3′ | 9 | 0 | 0 | |
| (OX)8B | 5′ | 5 | 0 | 40 |
| 3′ | 5 | 0 | 80 | |
| RRXRXB | 5′ | 16 | 23 | 25 |
| 3′ | NDf | ND | ND | |
| (RB)7RXB | 5′ | 3 | 37 | 80 |
| 3′ | 3 | 19 | 70 | |
| Piperazine linkage within PMO backbone | 1, 2 | 35 | 15 | 10 |
| 16, 17 | 25 | 15 | 30 | |
| 8, 9 | 24 | 21 | 3 | |
| 1, 2, 7, 8, 15, 16 | 12 | ND | 50 |
The chemistry designations represent the peptide conjugated to the VP24-5′Term PMO. R, arginine; X, 6-aminohexanoic acid; H, histidine; B, beta-alanine; O, ornithine; C, cyteine; Me, methyl; H1 peptide sequence, HLFHAIAHFIHHGWHGLHHC.
The peptides were covalently conjugated to either the 5′ or the 3′ end. Piperazine was conjugated to the internal PMO backbone at the indicated nucleotide position relative to the 5′ end.
In vitro translational activity was assessed in rabbit reticulocyte lysates (RRL) by using a linearized reporter plasmid (pCiNeo) containing 127 nucleotides encompassing the PMO target area from EBOV VP24 subcloned upstream and in frame with the gene for firefly luciferase. RNA was generated from plasmids by using a T7 polymerase-based Megascript kit. Translation reactions were conducted, in triplicate, by combining rabbit reticulocyte lysates, amino acids, PMO or water (as a control), and 1 nM RNA. The relative light units produced by reaction mixtures containing different concentrations of VP24-5′Term were determined, normalized to the results for reaction mixtures for the water control, averaged, and expressed as the percent inhibition of the luciferase signal obtained from water controls on the same plate. The EC50 of the reporter signal was determined by calculating the concentration of PMO required to reduce the signal generated from the control reaction mixtures with water by 50%.
Vero E6 monolayers were pretreated for 2 h with 20 μM VP24-5′Term prior to infection (MOI, 1) with ZEBOV-GFP, producing a final PMO concentration of 10 μM. After 48 h, the cells were fixed and the GFP-expressing cells were quantified in duplicate or triplicate wells at nine individual positions per well. Percent inhibition values were calculated as follows: 100 × [1 − (average GFP fluorescence from specific PMO-treated cells)/(average fluorescence from scrambled PMO-treated cells)]. Any negative inhibition values were changed to 0%.
VP24-5′Term PMOs (50 μg/dose) were administered i.p. to male or female C57BL/6 mice (n = 20 to 30) at 24 and 4 h prior to i.p. challenge with 1,000 PFU of mouse-adapted EBOV. Survival was monitored for at least 28 days.
ND, not determined.
Compared with unconjugated PMO, conjugation with arginine-rich peptides generally enhanced the antiviral effect of VP24-5′Term in ZEBOV-GFP-infected cells and conferred a greater level of protection in treated mice challenged with EBOV (Table 1). Of the peptides tested, conjugation with (RX)8B led to the greatest enhancement of antiviral properties during the in vivo evaluations, conferring a survival rate of 93% in mice challenged with a lethal dose of EBOV. The PMO terminus with which the arginine-based peptide was conjugated did not consistently affect in vivo protection in these evaluations. Treatment with VP24-5′Term conjugated with peptides containing H residues or less than eight O or O(Me)2 residues provided no or very low levels of protection against lethal challenge; however, treatment with (OX)8B-conjugated VP24-5′Term protected 80% of the infected mice.
In contrast to in vivo evaluations, conjugation of VP24-5′Term PMO with peptides containing less than eight arginine residues failed to enhance the antiviral activity relative to that achieved with untagged PMO in ZEBOV-GFP inhibition assays (Table 1). However, VP24-5′Term conjugated with (RX)8B, (RXR)4XB, or (RB)7RXB inhibited the replication of ZEBOV-GFP in Vero E6 cells to a greater degree than unconjugated PMO did. Neither the addition of positively charged piperazine moieties to VP24-5′Term PMO nor conjugation of the PMO with peptides containing ornithine or histidine residues inhibited ZEBOV-GFP replication to a greater degree than unmodified PMO did.
Conjugation of VP24-5′Term with each of the peptides tested enhanced the ability of this PMO to inhibit the in vitro translation of the VP24 reporter construct (Table 1). The EC50 for unconjugated VP24-5′Term was 364 nM, while the EC50s for conjugated versions ranged from 2 nM [for (RXR)4XB-conjugated PMO] to 240 nM (for H1-conjugated PMO). Conjugation of VP24-5′Term with many of the peptides—specifically, (RX)4B, (RX)6B, (RX)8B, (RB)8B, (RXR)4XB, (RXR)4C, [O(Me)2X]4B, (OX)4B, (OX)8B, RRXRXB, and (RB)7RXB—decreased the EC50s to <20 nM. Conjugation with H1 only moderately enhanced the inhibition of VP24 reporter translation compared to the level of inhibition achieved with unconjugated PMO. Piperazine modifications to the VP24-5′Term PMO backbone enhanced the ability of the PMO to inhibit in vitro translation, with all of the piperazine-modified PMOs exhibiting EC50s >10-fold lower than the EC50 of the unmodified PMO. These results clearly demonstrate that the antiviral potency of VP24-5′Term can be enhanced by chemically modifying the PMO, either by conjugating it with peptides or by introducing positively charged piperazine moieties to the PMO scaffold.
Enhanced efficacy of chemically modified EBOV VP24-AUG and VP24-AUG+4.
We previously demonstrated that the i.p. administration of two 500-μg doses of VP24-AUG nearly completely protected mice against lethal EBOV challenge, although 50-μg doses provided only 25% protection (35). To evaluate whether the antiviral activity of this PMO could be enhanced, arginine-based peptides were conjugated to either the 5′ or the 3′ end or piperazine linkages were added to the PMO scaffold. Antiviral activity was assessed in mice following lethal EBOV challenge and in the ZEBOV-GFP assay. Additionally, we evaluated the antiviral activities of two other PMOs, VP24-AUG+4 (complementary to EBOV VP24 mRNA downstream of the start codon) and MARV VP24-AUG (which served as a negative control), as shown in Table 2.
TABLE 2.
EBOV antiviral activities of VP24 PMOs
| PMO | Chemical modificationa | Location of chemical modificationb | In vitro inhibition of EBOV growth (% GFP expression)c | Protective efficacy (%) in C57BL/6 mice at dose ofd:
|
||
|---|---|---|---|---|---|---|
| 50 μg | 5 μg | 1 μg | ||||
| EBOV VP24-AUG | Untagged | 0 | 15 | NDe | ND | |
| (RX)8B | 5′ | 43 | 100 | 60 | 70 | |
| 3′ | 39 | 95 | 95 | 90 | ||
| (RX)4B | 5′ | 11 | 70 | ND | ND | |
| 3′ | 4 | 60 | ND | ND | ||
| (RB)7RXB | 5′ | 34 | 100 | ND | ND | |
| 3′ | 10 | 100 | ND | ND | ||
| Piperazine linkage within | Oligomers 12, 14 | 0 | 40 | ND | ND | |
| PMO backbone | Oligomers 4, 8, 11, 14 | 0 | 70 | ND | ND | |
| Oligomers 8, 10, 12, 14 | 0 | 60 | ND | ND | ||
| EBOV VP24 AUG+4 | Untagged | 0 | 20 | 0 | ||
| (RX)8B | 5′ | 40 | 90 | 55 | 10 | |
| (RX)8B | 3′ | 18 | 100 | 60 | 20 | |
| MARV VP24-AUG | Untagged | 0 | 0 | ND | ND | |
| (RX)8B | 5′ | 2 | 0 | ND | ND | |
| (RX)4B | 3′ | 0 | 0 | ND | ND | |
| (RX)2B | 3′ | 0 | 0 | ND | ND | |
Chemistry designations represent the peptide conjugated to the indicated PMO. R, arginine; X, 6-aminohexanoic acid; B, beta-alanine.
The peptides were covalently conjugated to either the 5′ or the 3′ end. Piperazine was conjugated to the internal PMO backbone at the indicated nucleotide position relative to the 5′ end.
Vero E6 cell monolayers were pretreated for 2 h with 20 μM VP24-5′Term prior to infection (MOI, 1) with ZEBOV-GFP, producing a final PMO concentration of 10 μM. After 48 h, the cells were fixed and the GFP-expressing cells were quantified in duplicate or triplicate wells at nine individual positions per well. Percent inhibition values were calculated as follows: 100 × [1 − (average GFP fluorescence from specific PMO-treated cells)/(average fluorescence from scrambled PMO-treated cells)]. Any negative inhibition values were changed to 0%.
PMOs (at a dose of either 50, 5, or 1 μg) were administered i.p. to male or female C57BL/6 mice (n = 20 to 30) at 24 and 4 h prior to i.p. challenge with 1,000 PFU of mouse-adapted EBOV. Survival was monitored for at least 28 days.
ND, not determined.
Neither unconjugated EBOV VP24-AUG nor the VP24-AUG+4 PMO inhibited the in vitro replication of EBOV-GFP (Table 2). Conjugation of EBOV VP24-AUG with (RX)8B, (RB)7RXB, or (RX)4B generally inhibited the replication of EBOV-GFP, with the levels of inhibition relative to the level for the water-treated controls ranging from 4 to 43%. EBOV VP24-AUG conjugated with (RX)8B at the 5′ end inhibited replication to the greatest degree (43% inhibition), although VP24-AUG+4 conjugated at the 5′ end with the same peptide inhibited replication comparably (40%). The piperazine-modified EBOV VP24-AUG PMO failed to inhibit replication. Consistent with expectations, EBOV-GFP replication was not inhibited by unconjugated MARV VP24-AUG or by (RX)2B-, (RX)4B-, or (RX)8B-conjugated MARV VP24-AUG PMOs, suggesting that the inhibition by conjugated forms of EBOV PMOs observed was mediated by the specificity of the PMO for EBOV VP24 and was not the result of a generalized inhibition conferred by the PMOs.
Administration of the unconjugated EBOV VP24-AUG or VP24-AUG+4 protected 15% and 20% of infected mice, respectively, against lethal EBOV challenge (Table 2). The delivery of arginine-based peptide-conjugated versions of these PMOs conferred an enhanced level of protection relative to that conferred by the unconjugated forms of the PMOs, with protection values ranging from 60 to 100%. Complete protection of challenged mice was observed following administration of four of the conjugated PMOs: EBOV VP24-AUG tagged at either the 5′ or the 3′ end with (RB)7RXB, the same PMO tagged at the 5′ end with (RX)8B, and VP24-AUG+4 conjugated with (RX)8B at the 3′ end. Additionally, high levels of protection (≥90%) were observed in mice treated with 50-, 5-, or 1-μg doses of EBOV VP24-AUG tagged with (RX)8B at the 3′ end. Treatment with EBOV VP24-AUG tagged at the 5′ end with (RX)8B protected 60% and 70% of mice at the 5- and 1-μg doses, respectively. Low to moderate levels of protection (10 to 60% survival) were observed following the administration of (RX)8B-conjugated VP24-AUG+4 at the 5- and 1-μg dose levels. Moderate levels of protection (40 to 70% survival) were also observed in mice treated with 50-μg doses of EBOV VP24-AUG containing multiple piperazine backbone modifications. As expected, the delivery of peptide-conjugated MARV VP24-AUG failed to protect the mice against the lethal EBOV challenge, which suggests that the protective responses observed occur via specific interactions between the PMOs and VP24 mRNA and are not invoked by innate properties of the peptides.
DISCUSSION
The recent emergence of a possible new EBOV species in the Bundibugyo District of Uganda highlights the threat that EBOVs pose to human health in regions where these viruses are endemic. Additionally, EBOV is listed as a category A pathogen on the basis of its potential to be weaponized and the severity of the disease that it causes. While several approaches to therapeutic intervention have shown promise in mouse models of infections, to date, the highest survival rate (50%) in EBOV-infected nonhuman primates following ZEBOV challenge was reported in animals receiving an antisense-based therapeutic regimen consisting of a mixture of PMOs specific to the EBOV L protein, VP35, and VP24 (35). While the function of VP24 in viral replication is poorly understood, we have previously demonstrated that the administration of two 500-μg doses of EBOV VP24-AUG, a PMO complementary to a region of the VP24 mRNA spanning the start codon, nearly completely protected mice against lethal EBOV challenge (35). In the present study, we constructed two new PMOs, VP24-5′Term and EBOV VP24-AUG+4, which also target the VP24 transcript. The antiviral activities of these PMOs were evaluated by a reporter-based in vitro VP24 translation assay, a cell-based ZEBOV-GFP infection assay, and after treatment in EBOV-challenged mice.
While the chemically unmodified VP24-5′Term PMO successfully inhibited the expression of VP24-GFP during a cell-free translation assay, it failed to inhibit the replication of ZEBOV-GFP in Vero E6 cell monolayers and provided no protection to mice following EBOV challenge. Similarly, chemically unmodified versions of EBOV VP24-AUG and VP24-AUG+4 exhibited no detectable antiviral activity against ZEBOV-GFP and conferred only a low level of protection to challenged mice. PMOs do not efficiently penetrate cells (20), and the failure of VP24-5′Term to enter infected cells likely contributed to its inability to inhibit viral replication in cell-based and in vivo evaluations. The conjugation of cell-penetrating peptides with PMOs has been shown to significantly enhance the cellular entry of PMO (2, 22). Various PPMOs have successfully been used to inhibit the replication of several viruses in cell culture and in animal models (4, 5, 7, 9, 18, 25, 41). To evaluate whether the antisense efficacy of VP24-specific PMOs could be enhanced through chemical modifications, we conjugated various peptides to the VP24-specific PMOs, focusing on arginine-rich sequences interspersed with the nonnatural uncharged amino acid 6-aminohexanoic acid. The inclusion of 6-aminohexanoic acid has been suggested to increase the stability of the peptide and enhance endosomal escape (2, 40).
Indeed, conjugation of VP24-specific PMOs with arginine-rich peptides consistently enhanced the antisense efficacy during in vivo EBOV challenge experiments. This effect was observed for all the EBOV-specific PMOs that we evaluated, including the VP24-AUG, VP24-5′Term, and VP24-AUG+4 PMOs. Additionally, we demonstrated that the degree of protection conferred by a PMO in these in vivo evaluations was generally well correlated with the number of arginine-6-aminohexanoic repeats (up to eight) present in the conjugated peptide. These results are consistent with those of our previous evaluations, in which we showed that an R9F2-conjugated EBOV VP35-specific PMO protected mice to a greater degree than an unconjugated version of this PMO when the PMOs were delivered prior to EBOV infection (7). We also observed that addition of peptides containing eight arginine residues enhanced the antisense efficacies of the VP24-specific PMOs relative to those of the unconjugated forms, as measured by their ability to inhibit the in vitro replication of ZEBOV-GFP.
The introduction of positively charged moieties to PMOs, either through conjugations with a peptide or through backbone modification, enhanced the potencies of the PMOs in the cell-free translation assay. The results of these cell-free translation evaluations agree with the findings of Nelson et al. (24), who showed that the conjugation of a PMO to an arginine-rich peptide generally increases its antisense potency. RNA-PMO complexes exhibit greater thermal stability when the PMOs are conjugated with the arginine-rich R9F2 peptide (24), and it has been shown that conjugation of a PMO with an arginine-rich peptide accelerates the association of a DNA oligonucleotide with its DNA target by 164-fold (20). These findings suggest that the addition of arginine-rich peptides to PMOs may enhance the antiviral activity of the PMO by promoting a higher binding efficiency between a PMO and its complementary regions. In support of this hypothesis, we observed that the potency of VP24-5′Term, measured by in vitro translation of a VP24 reporter, was enhanced 10- to 100-fold when the PMO was conjugated with various arginine- and ornithine-rich peptides. The results from this cell-free translation assay demonstrate that conjugated peptides can enhance the suppression of translation from a targeted transcript in a manner independent of the cell entry mechanisms, but whether this is the case in Vero cells and in mice has yet to be determined. Further studies are required to evaluate whether the peptide portions of the conjugates remain intact or are degraded by cellular enzymes, which would likely decrease the potency of the PPMO. Using mass spectrometry, we have shown that the peptide portions of (RXR)4- and (RX)8-conjugated PPMOs are degraded in HeLa cells, and intact conjugates are not detected (40).
The addition of the piperazine modification to the PMO backbone confers a positive charge to the otherwise charge-neutral molecule. While we observed that piperazine-modified VP24-5′Term inhibited the in vitro translation of a VP24 reporter construct to a greater degree than unmodified PMO did, the piperazine modification did not noticeably enhance the ability of VP24-5′Term or VP24-AUG to inhibit the replication of ZEBOV-GFP. However, mice that were treated with piperazine-modified PMOs generally exhibited higher rates of survival following lethal EBOV challenge than mice treated with unmodified PMOs, but piperazine-modified PMOs were less efficacious than peptide-conjugated PMOs.
To more clearly detect the relationships between the data obtained during the in vitro and the in vivo evaluations whose results are presented in Table 1, for each peptide-conjugated version of VP24-5′Term, percent survival was plotted versus either the in vitro translational EC50 (Fig. 7A) or the percent inhibition of ZEBOV-GFP (Fig. 7B). Regions were established within each graph by using cutoff values of 50% for in vivo survival, an EC50 of 20 nM for the in vitro inhibition assay, and 25% inhibition of the ZEBOV-GFP. This evaluation allowed us to set up go or no-go decisions on the basis of the in vitro and in vivo efficacies of individual PMOs. Compounds demonstrating >50% protective efficacy in mice and viral inhibition in vitro will be tested further in guinea pigs and nonhuman primate models. PMOs demonstrating high protective efficacy in mice (>50%) but lower in vitro efficacy will be further refined. PMOs that offer low levels of protection in mice (<50%) will be discarded from further testing. By using these parameters, eight PMOs emerged from the analysis as possible candidates for further evaluation in challenge trials with guinea pigs or nonhuman primates (Fig. 7A). Four of these—(RX)8B-, (RB)8B-, (RXR)4XB-, and (RB)7RXB-tagged VP24-5′Term—were reidentified as promising candidates when the inhibition values from the ZEBOV-GFP assay were plotted against the in vivo efficacy results (Fig. 7B).
FIG. 7.
Efficient prediction of in vivo protective efficacy by activity of PMOs by in vitro translation and ZEBOV-GFP screening assays. (A) To determine the relationship between in vivo protection and in vitro inhibition of viral replication, for each PMO in Table 1, the EC50 derived from the in vitro translation assay was plotted versus the percent survival associated with in vivo challenge. (B) Likewise, to evaluate the relationship between in vivo protection and PMO-mediated inhibition of VP24 translation, percent survival was plotted versus percent inhibition from data presented for each PMO presented in Table 1. NHP, nonhuman primates.
The EBOV mouse model was an obvious choice for use for the testing of multiple candidate PMOs due to the relatively low expense of housing and maintaining this species and the availability of a mouse-adapted EBOV strain (3). Although there are several differences between the pathogenesis of EBOV infection in mice and that in nonhuman primates and humans (11, 12, 14), the mouse model is widely used to provide an additional level of screening before antiviral candidates are tested in guinea pig and nonhuman primate models of infection. Evaluations of therapies that target mouse-specific virus-host interactions or rodent-specific pathological responses to infection can provide results that are poorly predictive of efficacy in nonhuman primates. Thus, we propose that the PMOs that demonstrated antiviral activities during both in vitro screening assays and which showed in vivo efficacy in a mouse model represent the most promising therapeutic candidates and merit consideration for inclusion in more costly in vivo evaluations with guinea pigs or nonhuman primates.
The in vivo evaluations described in this report identified several PMOs that are effective as prophylactic treatments in mice. In humans, PMOs could be licensed for use as prophylactic treatments by first responders to emergency situations and by medical personnel who attend EBOV-infected patients. As the efficacies of these PMOs are evaluated in guinea pig and primate models of infection, it will be important to characterize the therapeutic potential of these agents for use in postexposure scenarios. Additionally, the expression of VP24 in infected laboratory animals will need to be monitored to determine the potential mechanisms of action of the PMOs and to evaluate potential off-target effects. Because the VP24-specific PMOs described in this report were designed with sequence homology to ZEBOV, it is not likely that these PMOs will protect against EBOV Sudan infections because the sequences of these two species lack a high degree of homology. It will be of interest to design new PMOs with specificity to the VP24 transcript of EBOV Sudan to determine whether the findings obtained with ZEBOV can be applied to the treatment of infections caused by other EBOV species.
Acknowledgments
We thank R. Bakken, J. Smith, D. Reed, S. VanTongeren, N. Posten, C. Rice, and J. Stockman for excellent technical assistance and D. A. Stein and M. J. Aman for scientific insight.
The research described herein was sponsored by the Defense Threat Reduction Agency JSTO-CBD and the Medical Research and Material Command.
The opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army.
Footnotes
Published ahead of print on 17 February 2009.
REFERENCES
- 1.Abes, R., A. A. Arzumanov, H. M. Moulton, S. Abes, G. D. Ivanova, P. L. Iversen, M. J. Gait, and B. Lebleu. 2007. Cell-penetrating-peptide-based delivery of oligonucleotides: an overview. Biochem. Soc. Trans. 35:775-779. [DOI] [PubMed] [Google Scholar]
- 2.Abes, S., H. M. Moulton, P. Clair, P. Prevot, D. S. Youngblood, R. P. Wu, P. L. Iversen, and B. Lebleu. 2006. Vectorization of morpholino oligomers by the (R-Ahx-R)4 peptide allows efficient splicing correction in the absence of endosomolytic agents. J. Control. Release 116:304-313. [DOI] [PubMed] [Google Scholar]
- 3.Bray, M., K. Davis, T. Geisbert, C. Schmaljohn, and J. Huggins. 1998. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J. Infect. Dis. 178:651-661. [DOI] [PubMed] [Google Scholar]
- 4.Deas, T. S., C. J. Bennett, S. A. Jones, M. Tilgner, P. Ren, M. J. Behr, D. A. Stein, P. L. Iversen, L. D. Kramer, K. A. Bernard, and P. Y. Shi. 2007. In vitro resistance selection and in vivo efficacy of morpholino oligomers against West Nile virus. Antimicrob. Agents Chemother. 51:2470-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deas, T. S., I. Binduga-Gajewska, M. Tilgner, P. Ren, D. A. Stein, H. M. Moulton, P. L. Iversen, E. B. Kauffman, L. D. Kramer, and P. Y. Shi. 2005. Inhibition of flavivirus infections by antisense oligomers specifically suppressing viral translation and RNA replication. J. Virol. 79:4599-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolnik, O., L. Kolesnikova, and S. Becker. 2008. Filoviruses: interactions with the host cell. Cell. Mol. Life Sci. 65:756-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enterlein, S., K. L. Warfield, D. L. Swenson, D. A. Stein, J. L. Smith, C. S. Gamble, A. D. Kroeker, P. L. Iversen, S. Bavari, and E. Muhlberger. 2006. VP35 knockdown inhibits Ebola virus amplification and protects against lethal infection in mice. Antimicrob. Agents Chemother. 50:984-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldmann, H., S. M. Jones, H. Schnittler, and T. Geisbert. 2005. Therapy and prophylaxis of Ebola virus infections. Curr. Opin. Investig. Drugs 6:823-830. [PubMed] [Google Scholar]
- 9.Ge, Q., M. Pastey, D. Kobasa, P. Puthavathana, C. Lupfer, R. K. Bestwick, P. L. Iversen, J. Chen, and D. A. Stein. 2006. Inhibition of multiple subtypes of influenza A virus in cell cultures with morpholino oligomers. Antimicrob. Agents Chemother. 50:3724-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geisbert, T. W., and L. E. Hensley. 2004. Ebolavirus: new insights into disease aetiopathology and possible therapeutic interventions. Expert Rev. Mol. Med. 6:1-24. [DOI] [PubMed] [Google Scholar]
- 11.Geisbert, T. W., L. E. Hensley, T. Larsen, H. A. Young, D. S. Reed, J. B. Geisbert, D. P. Scott, E. Kagan, P. B. Jahrling, and K. J. Davis. 2003. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am. J. Pathol. 163:2347-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibb, T. R., M. Bray, T. W. Geisbert, K. E. Steele, W. M. Kell, K. J. Davis, and N. K. Jaax. 2001. Pathogenesis of experimental Ebola Zaire virus infection in BALB/c mice. J. Comp. Pathol. 125:233-242. [DOI] [PubMed] [Google Scholar]
- 13.Han, Z., H. Boshra, J. O. Sunyer, S. H. Zwiers, J. Paragas, and R. N. Harty. 2003. Biochemical and functional characterization of the Ebola virus VP24 protein: implications for a role in virus assembly and budding. J. Virol. 77:1793-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart, M. K. 2003. Vaccine research efforts for filoviruses. Int. J. Parasitol. 33:583-595. [DOI] [PubMed] [Google Scholar]
- 15.Iversen, P. L. 2001. Phosphorodiamidate morpholino oligomers, p. 375-389. In S. T. Crooke (ed.), Antisense drug technology. Marcel Dekker, Inc., New York, NY.
- 16.Iversen, P. L. 2001. Phosphorodiamidate morpholino oligomers: favorable properties for sequence-specific gene inactivation. Curr. Opin. Mol. Ther. 3:235-238. [PubMed] [Google Scholar]
- 17.Jahrling, P. B., T. W. Geisbert, J. B. Geisbert, J. R. Swearengen, M. Bray, N. K. Jaax, J. W. Huggins, J. W. LeDuc, and C. J. Peters. 1999. Evaluation of immune globulin and recombinant interferon-alpha2b for treatment of experimental Ebolavirus infections. J. Infect. Dis. 179(Suppl 1):S224-S234. [DOI] [PubMed] [Google Scholar]
- 18.Kinney, R. M., C. Y. Huang, B. C. Rose, A. D. Kroeker, T. W. Dreher, P. L. Iversen, and D. A. Stein. 2005. Inhibition of dengue virus serotypes 1 to 4 in Vero cell cultures with morpholino oligomers. J. Virol. 79:5116-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn, J. H., S. R. Radoshitzky, A. C. Guth, K. L. Warfield, W. Li, M. J. Vincent, J. S. Towner, S. T. Nichol, S. Bavari, H. Choe, M. J. Aman, and M. Farzan. 2006. Conserved receptor-binding domains of Lake Victoria marburgvirus and Zaire ebolavirus bind a shared receptor J. Biol. Chem. 281:15951-15958. [DOI] [PubMed] [Google Scholar]
- 20.Lebleu, B., H. M. Moulton, R. Abes, G. D. Ivanova, S. Abes, D. A. Stein, P. L. Iversen, A. A. Arzumanov, and M. J. Gait. 2008. Cell penetrating peptide conjugates of steric block oligonucleotides. Adv. Drug Deliv. Rev. 60:517-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moulton, H. M., S. Fletcher, B. W. Neuman, G. McClorey, D. A. Stein, S. Abes, S. D. Wilton, M. J. Buchmeier, B. Lebleu, and P. L. Iversen. 2007. Cell-penetrating peptide-morpholino conjugates alter pre-mRNA splicing of DMD (Duchenne muscular dystrophy) and inhibit murine coronavirus replication in vivo. Biochem. Soc. Trans. 35:826-828. [DOI] [PubMed] [Google Scholar]
- 22.Moulton, H. M., M. H. Nelson, S. A. Hatlevig, M. T. Reddy, and P. L. Iversen. 2004. Cellular uptake of antisense morpholino oligomers conjugated to arginine-rich peptides. Bioconjug. Chem. 15:290-299. [DOI] [PubMed] [Google Scholar]
- 23.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 24.Nelson, M. H., D. A. Stein, A. D. Kroeker, S. A. Hatlevig, P. L. Iversen, and H. M. Moulton. 2005. Arginine-rich peptide conjugation to morpholino oligomers: effects on antisense activity and specificity. Bioconjug. Chem. 16:959-966. [DOI] [PubMed] [Google Scholar]
- 25.Neuman, B. W., D. A. Stein, A. D. Kroeker, M. J. Churchill, A. M. Kim, P. Kuhn, P. Dawson, H. M. Moulton, R. K. Bestwick, P. L. Iversen, and M. J. Buchmeier. 2005. Inhibition, escape, and attenuated growth of severe acute respiratory syndrome coronavirus treated with antisense morpholino oligomers. J. Virol. 79:9665-9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuman, B. W., D. A. Stein, A. D. Kroeker, A. D. Paulino, H. M. Moulton, P. L. Iversen, and M. J. Buchmeier. 2004. Antisense morpholino-oligomers directed against the 5′ end of the genome inhibit coronavirus proliferation and growth. J. Virol. 78:5891-5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noda, T., P. Halfmann, H. Sagara, and Y. Kawaoka. 2007. Regions in Ebolavirus VP24 that are important for nucleocapsid formation. J. Infect. Dis. 196(Suppl. 2):S247-S250. [DOI] [PubMed] [Google Scholar]
- 28.Patel, D., T. Opriessnig, D. A. Stein, P. G. Halbur, X. J. Meng, P. L. Iversen, and Y. J. Zhang. 2008. Peptide-conjugated morpholino oligomers inhibit porcine reproductive and respiratory syndrome virus replication. Antivir. Res. 77:95-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reid, S. P., C. Valmas, O. Martinez, F. M. Sanchez, and C. F. Basler. 2007. Ebolavirus VP24 proteins inhibit the interaction of NPI-1 subfamily karyopherin alpha proteins with activated STAT1. J. Virol. 81:13469-13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, A. W., P. L. Iversen, P. D. O'Hanley, D. E. Skilling, J. R. Christensen, S. S. Weaver, K. Longley, M. A. Stone, S. E. Poet, and D. O. Matson. 2008. Virus-specific antiviral treatment for controlling severe and fatal outbreaks of feline calicivirus infection. Am. J. Vet. Res. 69:23-32. [DOI] [PubMed] [Google Scholar]
- 31.Stein, D. A., D. E. Skilling, P. L. Iversen, and A. W. Smith. 2001. Inhibition of Vesivirus infections in mammalian tissue culture with antisense morpholino oligomers. Antisense Nucleic Acid Drug Dev. 11:317-325. [DOI] [PubMed] [Google Scholar]
- 32.Summerton, J., and D. Weller. 1997. Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 7:187-195. [DOI] [PubMed] [Google Scholar]
- 33.Towner, J. S., J. Paragas, J. E. Dover, M. Gupta, C. S. Goldsmith, J. W. Huggins, and S. T. Nichol. 2005. Generation of eGFP expressing recombinant Zaire Ebolavirus for analysis of early pathogenesis events and high-throughput antiviral drug screening. Virology 332:20-27. [DOI] [PubMed] [Google Scholar]
- 34.Warfield, K. L., J. G. Perkins, D. L. Swenson, E. M. Deal, C. M. Bosio, M. J. Aman, W. M. Yokoyama, H. A. Young, and S. Bavari. 2004. Role of natural killer cells in innate protection against lethal Ebolavirus infection. J. Exp. Med. 200:169-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warfield, K. L., D. L. Swenson, G. G. Olinger, D. K. Nichols, W. D. Pratt, R. Blouch, D. A. Stein, M. J. Aman, P. L. Iversen, and S. Bavari. 2006. Gene-specific countermeasures against Ebolavirus based on antisense phosphorodiamidate morpholino oligomers. PLoS Pathog. 2:5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe, S., T. Noda, P. Halfmann, L. Jasenosky, and Y. Kawaoka. 2007. Ebolavirus (EBOV) VP24 inhibits transcription and replication of the EBOV genome. J. Infect. Dis. 196(Suppl. 2):S284-S290. [DOI] [PubMed] [Google Scholar]
- 37.Weller, D. D., and J. N. Hassinger. 2008. Oligonucleotide analogs having cationic intersubunit linkages. Publication no. WO/2008/03612. World Intellectual Property Organization, Geneva, Switzerland.
- 38.World Health Organization. 2008. Outbreak news. Ebola haemorrhagic fever, Uganda—end of the outbreak. Wkly. Epidemiol. Rec. 83:89-90. [PubMed] [Google Scholar]
- 39.Wu, R. P., D. S. Youngblood, J. N. Hassinger, C. E. Lovejoy, M. H. Nelson, P. L. Iversen, and H. M. Moulton. 2007. Cell-penetrating peptides as transporters for morpholino oligomers: effects of amino acid composition on intracellular delivery and cytotoxicity. Nucleic Acids Res. 35:5182-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youngblood, D. S., S. A. Hatlevig, J. N. Hassinger, P. L. Iversen, and H. M. Moulton. 2007. Stability of cell-penetrating peptide-morpholino oligomer conjugates in human serum and in cells. Bioconjug. Chem. 18:50-60. [DOI] [PubMed] [Google Scholar]
- 41.Yuan, J., D. A. Stein, T. Lim, D. Qiu, S. Coughlin, Z. Liu, Y. Wang, R. Blouch, H. M. Moulton, P. L. Iversen, and D. Yang. 2006. Inhibition of coxsackievirus B3 in cell cultures and in mice by peptide-conjugated morpholino oligomers targeting the internal ribosome entry site. J. Virol. 80:11510-11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang, Y. J., K. Y. Wang, D. A. Stein, D. Patel, R. Watkins, H. M. Moulton, P. L. Iversen, and D. O. Matson. 2007. Inhibition of replication and transcription activator and latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus by morpholino oligomers. Antivir. Res. 73:12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]