Abstract
EDP-420 (EP-013420, S-013420) is a first-in-class bicyclolide (bridged bicyclic macrolide) currently in clinical development for the treatment of respiratory tract infections. It has good preclinical pharmacokinetic properties across multiple species and potent in vitro and in vivo activity against respiratory tract infection pathogens, including Haemophilus influenzae, atypical organisms (e.g., Chlamydia pneumoniae, Mycoplasma pneumoniae, and Legionella pneumophila), and multidrug-resistant streptococci. The safety, tolerability, and pharmacokinetics of an orally administered EDP-420 suspension in 40 healthy adult subjects were assessed in a randomized, double-blind, placebo-controlled, ascending single-dose study. Eligible subjects were sequentially randomized into one of five study groups (i.e., 100-, 200-, 400-, 800-, or 1,200-mg dosing groups) consisting of eight subjects (six active and two placebo) each. EDP-420 was well tolerated. There were no serious adverse events reported, nor were there any dose-limiting clinical or laboratory adverse events reported. EDP-420 was rapidly absorbed after a single oral dose. The mean plasma terminal half-life ranged from 15.6 to 20.1 h with low clearance. At the 400-mg dose, the area under the curve was 14.4 μg·h/ml, which well exceeded the required area under the concentration-time curve to cover common respiratory tract infection pathogens based on preclinical pharmacokinetic/pharmacodynamic modeling. The long half-life and high systemic exposure of EDP-420 support once-daily dosing and may allow for shorter treatment durations compared to other macrolide antibiotics. Based on its human pharmacokinetic profiles, taken together with its in vitro/in vivo activity against common respiratory pathogens, EDP-420 warrants efficacy trials for the treatment of respiratory tract infections.
Macrolide antibiotics have been used effectively and safely as first-line treatments for respiratory tract infections (RTI) for more than 50 years (34). However, the incidence of worldwide resistance to antibiotics (including macrolide antibiotics) is increasing. For example, Streptococcus pneumoniae is the most commonly implicated bacterium in RTI, being responsible for 45% of the cases of community-acquired pneumonia and 34% of acute sinusitis (1, 2). Fully 31% of S. pneumoniae infections in the United States have yielded organisms resistant to the macrolide antibiotic erythromycin, and nearly one-third are resistant to penicillin. S. pneumoniae resistance is more common in Asia, where 80% of S. pneumoniae infections are erythromycin resistant, and 53% are penicillin resistant (11, 35, 38). Thus, there is an urgent need to develop new antibiotics with activity against a broad spectrum of pathogens (especially resistant strains) commonly encountered in community-acquired RTI (1, 11, 35, 38).
EDP-420 (formerly EP-013420 and S-013420) is a novel bridged bicyclic macrolide antibiotic (the structure is shown in Fig. 1) currently in clinical development for the treatment of RTI. It was designed specifically to combat atypical and respiratory tract pathogens that have acquired resistance to macrolide antibiotics. EDP-420 has a unique 6,11-O-bridging moiety, with replacement of the cladinose sugar by a 3-keto group (33). RNA footprinting suggests that EDP-420 binds near the entrance to the peptide exit tunnel of the 50S ribosomal subunit from E. coli and forms interactions with both domain V and domain II of 23S rRNA (36). The additional binding motif to the 50S ribosomal subunit (i.e., a second binding site along the 23S rRNA hairpin loop 35 of domain II) provided by the 6,11-O-bridging moiety should help overcome macrolide, lincosamide, and streptogramin B resistance, resulting in an improved structure-activity relationship.
FIG. 1.
Structure of EDP-420.
EDP-420 exhibits potent in vitro activities against RTI pathogens, including multidrug-resistant streptococci and atypical organisms (e.g., Chlamydia pneumoniae, Mycoplasma pneumoniae, and Legionella pneumophila) (21, 28, 31). For example, MIC90 (i.e., the MIC required to inhibit the growth of 90% of organisms) for EDP-420 against 115 strains and 100 clinical isolates of S. pneumoniae, including macrolide-resistant strains carrying mefA and/or ermB genes, was 0.25 μg/ml, which is comparable to that of telithromycin (MIC90 = 0.125 μg/ml) and significantly better than that of clarithromycin, azithromycin, and erythromycin (MIC90 > 64 μg/ml) (37). The antibacterial activity of EDP-420 against S. pyogenes carrying constitutive type ermB was 32 times more potent than that of telithromycin (27). EDP-420 is potent against methicillin-susceptible Staphylococcus aureus, including strains with inducible type ermA/C (27). The MIC90 of EDP-420 against Legionella spp. was 0.031 μg/ml, which is comparable to that of levofloxacin and significantly more active than erythromycin and azithromycin (9, 24). In one study, 80% of S. pneumoniae strains (10 strains, including an erythromycin-resistant strain) and all of the Haemophilus influenzae strains (10 strains) tested showed minimum bactericidal concentration/MIC ratios of <4, and EDP-420 killed more than 99.9% of viable cells of S. pneumoniae and of H. influenzae at 2× the MIC within 4 h (21). The postantibiotic effects of EDP-420 on two strains of H. influenzae, SR24159 and SR1280, lasted 4.3 and 2.2 h, which exceed and were comparable to the corresponding values for clarithromycin (2.0 and 0.2 h) and telithromycin (4.3 and 2.3 h), respectively (21). The growth of H. influenzae in the infected epithelial layers was still inhibited 12 h after the removal of EDP-420; however, bacterial growth resumed almost immediately after the removal of clarithromycin, telithromycin, and levofloxacin (26). EDP-420 showed potent intracellular activity and persistent effects against L. pneumophila both in the alveolar epithelial cells and in the bone marrow macrophages (25).
In vivo, EDP-420 also demonstrated efficacy against S. pneumoniae, S. pyogenes, S. aureus, and Mycobacterium avium in mouse protection tests; against S. pneumoniae and H. influenzae in a rat lung infection model; and against penicillin- and quinolone-resistant pneumococci in a rabbit meningitis model (3-6, 17, 19, 20, 22, 28, 30, 31). The oral mean effective doses of EDP-420 sufficient to protect 50% of the mice (ED50) from lethal infection were 2.93, 3.27, 3.12, and 2.12 mg/kg against S. pneumoniae SR16605, S. pneumoniae SR20946 (ermB), S. pneumoniae SR26113 (ermB), and S. pyogenes C-203, respectively, while telithromycin required higher doses of 3.07, 16.5, 3.65, and 4.24, respectively (31). The in vivo efficacy of EDP-420 against rat lung infection caused by H. influenzae SR11925 and SR1280 was comparable to that of telithromycin (31). EDP-420 nonclinical pharmacokinetics (PK) across multiple species displayed a long half-life, as well as extensive distribution and uptake into respiratory tissue and fluids (12-15, 28, 30). The preclinical safety results and good PK properties, along with the in vivo antimicrobial profiles and results of the efficacy studies in animals, supported the decision to begin phase 1 testing in healthy adult volunteers.
(This study was presented in part at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, in September 2007.)
MATERIALS AND METHODS
Study drugs.
Clinical trial materials, including the investigational product EDP-420 and a placebo (Microcrystalline Cellulose; USP), were manufactured according to current good manufacturing practice procedures. EDP-420 drug substance and the placebo were supplied as a powder for reconstitution. The appropriate amount of EDP-420 or placebo was weighed into a 75-ml high-density polyethylene bottle and reconstituted using Simple Syrup (USP). All study drugs were kept in a secure, limited-access storage area under recommended storage conditions (room temperature) until needed or until returned to the sponsor.
Subjects and study designs.
This was a single-center, randomized, double-blind, placebo-controlled, ascending single-dose safety, tolerability, and PK study of orally administered EDP-420 in healthy adult subjects. The study was conducted in Advanced Biomedical Research (Hackensack, NJ) in accordance with good clinical practice as described in Code of Federal Regulations (Title 21, parts 50, 56, and 312, subpart D) and standard operating procedures for clinical investigation. Each subject was provided a written informed consent form describing the nature and purpose of the study, participation/termination conditions, and risks/benefits of participating in the study. Subjects had read, understood, and signed the written informed consent form.
A total of 40 volunteers aged 18 to 45 years participated in the study. All were in good general health as determined by medical history, physical examination (including vital signs), clinical laboratory tests, and 12-lead electrocardiogram (ECG). Eligible subjects were sequentially enrolled into one of five study groups consisting of eight subjects each. Within each study group of eight subjects; in each group, six subjects were randomly assigned to receive a single oral dose of EDP-420 (suspension), and two were randomly assigned to receive placebo (i.e., microcrystalline cellulose). After each dose level was determined to be safe and well tolerated, new subjects were entered into the next-higher dose group. The doses administered in the present study were: 100, 200, 400, 800, and 1,200 mg (i.e., groups 1 to 5, respectively). All test doses were administered after an 8-h overnight fast, and food was prohibited for 4 h after oral administration.
The study consisted of a screening period, a treatment period, and an end-of-study visit. During days −21 to −2 of the screening period, informed consent was obtained, eligibility for study entry was assessed, and screening evaluations were performed. On day −1, subjects returned to the clinic for final assessments before dosing. Subjects who satisfied all of the inclusion criteria and matched none of the exclusion criteria qualified for the treatment period and were randomized. On the morning of the next day (day 1), predose evaluations were obtained, and study medication was administered. After dosing, subjects in study groups 1 to 4 remained in the clinical research facility for 48 h for postdose study assessments and then were discharged. Subjects returned to the clinic (as outpatients) at 72 and 96 h postdose for vital signs and blood collection for PK assessment and at 120 h postdose for vital signs, blood collection for PK assessment, and safety laboratory tests. Subjects in study group 5 remained in-house for 120 h postdose after which they were released. The subjects in study group 5 underwent the same assessments as those in the other study groups, but as an additional safety precaution, had a full blood chemistry panel run at 72 and 96 h postdose. All subjects returned to the clinical research facility for one end-of-study visit on day 8 to 10 for final clinical evaluation.
The sample size for the present study was not determined by formal statistical methods but was deemed a reasonable size to address the objectives of a “first-in-human” ascending dose study. Groups of six active and two placebo treatments are commonly used for first-in-human trials of a new chemical entity.
Blood and urine sample collection.
Blood samples (∼10 ml) were collected into EDTA-containing tubes during each treatment period at predose (0 h) and at 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 10, 12, 16, 24, 30, 36, 48, 72, 96, and 120 h postdose. The tubes were immediately chilled in ice. Plasma was separated by refrigerated centrifugation at 3,000 rpm for ∼10 min. Plasma was then transferred to appropriately labeled storage tubes and stored at approximately −20°C until analyzed. Exact dosing times and blood collection times were recorded.
Urine samples were collected over the following time intervals: predose (first morning specimen) and at 0 to 2 h, 2 to 4 h, 4 to 6 h, 6 to 8 h, 8 to 12 h, 12 to 24 h, and 24 to 48 h after dosing. Urine volumes were measured, and aliquots were stored at −20°C until analyzed.
Analytical methodology.
The concentrations of EDP-420 in plasma and urine were analyzed using a validated high-performance liquid chromatography with tandem mass spectrometric detection (LC-MS/MS) method. EDP-420 is stable in plasma and urine for at least 6 months under storage at −20°C.
(i) Plasma.
EDP-420 was extracted from human plasma samples by protein precipitation. Briefly, 50 μl of blank plasma, quality control (QC) sample, or plasma sample was added into a clean 2-ml Eppendorf tube, and then 50 μl of acetonitrile (ACN) was pipetted into each tube. For plasma calibration standards, 50 μl of blank plasma and 50 μl of appropriate working standard solution prepared in ACN were added into a 2-ml Eppendorf tube. Then, 400 μl of internal standard (∼25 ng of clarithromycin/ml dissolved in ACN) was added to all tubes except the plasma blank samples. For each plasma blank sample, 400 μl of ACN was added in place of the internal standard. All of the tubes were capped and vortex mixed for 10 min, followed by centrifugation at 10,000 rpm for 5 min to remove the precipitate. Then, 200 μl of supernatant was removed and mixed well with 200 μl of water, and 20 μl of the mixture was then subjected to LC-MS/MS analysis. Chromatography was performed on a reversed-phase C18 column (50 mm by 2.1 mm, 5-μm pore size, Ace RP18; MacMod) and a high-pressure liquid chromatography system consisting of an HP1100 pump and a CTC Analytics HTS PAL Leap Technologies autosampler. The mobile phases were water with 0.1% formic acid (buffer A) and ACN with 0.1% formic acid (buffer B), and chromatography was run in an isocratic mode with 60% buffer A and 40% buffer B with a flow rate of 0.3 ml/min. Detection of EDP-420 and internal standard was performed by using a Micromass Quattro Ultima tandem mass spectrometer in the positive-ion multiple reaction monitoring mode. The mass transitions (m/z) monitored were 841.2→158.4 for EDP-420 and 748.5→157.9 for the internal standard clarithromycin. The line of best-fit for calibration standards was calculated by weighted (1/x2) linear regression based on analyte/internal standard peak-area ratios for two replicates of seven calibration standards using a Watson LIMS system. QC and unknown sample concentrations for the analyte were calculated from the calibration standard curve based on analyte/internal standard peak area ratios. The quantification and calibration ranges were from 1 to 1,000 ng/ml. The interbatch accuracy and precision ranged from 93.1 to 103.2% and 5.0 to 8.2% for calibration standards, and 92.1 to 103.0% and 6.1 to 9.8% for QC samples, respectively. The intrabatch accuracy and precision of validation QC samples ranged from 84.7 to 110.0% and 1.5 to 7.7%, respectively. No significant interference with EDP-420 was found from endogenous components of plasma or other sources.
(ii) Urine.
A total of 25 μl of the internal standard (EP-001304, a core structure of EDP-420, 58.1 μg/ml) was added to 25 μl of the urine QC sample, calibration standard, or urine sample in a 15-ml plastic tube, and then 10 ml of 40% ACN in water was pipetted into each of these tubes. The tubes were capped and vortex mixed on a multitube Glas-Col inverter for 30 min, and then 10 μl of the clear solution was subjected to LC-MS/MS analysis. The LC-MS/MS conditions and data analysis for the detection of analyte were similar to those described for plasma except that the mass transitions (m/z) monitored were 667.4→158.1 for the internal standard. The quantification and calibration ranges were from 0.250 to 250 μg/ml. The interbatch accuracy and precision ranged from 90.2 to 107.2% and 2.4 to 6.1% for the calibration standards and from 90.0 to 111.2% and 3.8 to 6.5% for the QC samples, respectively. The intrabatch accuracy and precision of validation QC samples ranged from 85.7 to 116.8% and 1.0 to 6.9%, respectively.
PK and statistical analysis.
PK analyses were performed for EDP-420 in plasma and urine by using noncompartmental methods with WinNonlin Professional version 4.0.1 (Pharsight Corp., Mountain View, CA). Descriptive statistics were prepared with SAS version 8.2 (SAS Institute, Inc., Cary, NC). Prior to the estimation of the plasma PK parameters, “below-limit-of-quantitation” concentrations were assigned a value of zero if they preceded quantifiable samples prior to the time of maximum plasma concentration (Tmax). A below-limit-of-quantitation concentration that occurred after the time to Tmax was assigned a value of missing. The actual elapsed time from dosing was used in the final PK analysis to estimate all individual PK parameters. The following plasma PK parameters were estimated by noncompartmental methods: both maximum plasma concentration (Cmax) and Tmax were obtained directly from the observed concentration-versus-time data; the area under the plasma concentration-time curve from time zero to the last measurable plasma concentration time point (AUC0-last) was calculated by linear-up/log-down trapezoidal summation; the area under the plasma concentration-time curve from time zero to infinity (AUC0-∞) was calculated by linear-up/log-down trapezoidal summation and extrapolated to infinity by addition of the last quantifiable plasma concentration divided by the elimination rate constant (i.e., AUC0-last + Clast/λz); the elimination rate constant (λz) was determined by linear regression of the terminal points of the log-linear plasma concentration-time curve; the terminal half-life (t1/2) was determined as ln2/λz; the apparent oral clearance (CLpo) was calculated as the dose divided by AUC0-∞; the apparent volume of distribution during the terminal phase (Vz/F) was calculated as CLpo divided by λz; the cumulative amount excreted in urine from time zero to 48 h postdose (Ae0-48) was calculated as the summation of the amounts (product of urine volume times urine concentration) excreted in subsequent collection intervals; the cumulative fraction of the dose excreted unchanged in the urine from time zero to 48 h postdose (fe0-48) was calculated as the Ae0-48/dose × 100%; and the renal clearance (CLR) was calculated as the Ae0-48 divided by the plasma AUC0-48.
To assess dose proportionality, an analysis of covariance was performed on Cmax and AUC using a power model with the log-transformed PK parameters as the dependent variable and log-transformed dose level as a continuous covariate. The 90% confidence intervals were also determined.
Safety assessment.
Safety was assessed throughout the study by physical examination, adverse event monitoring, ECG, clinical laboratory tests, and vital sign measurements.
RESULTS
Subjects.
A total of 30 subjects received EDP-420, and 10 received a placebo. The demographic characteristics of these subjects are summarized in Table 1. One female subject who had a tubal ligation in 1998 was randomized into study group 5 and received 1,200 mg of EDP-420. All other subjects in the study were male. The racial distribution of EDP-420-treated subjects was 13% Caucasian, 33% Black, 47% Hispanic, and 7% of other racial background. The racial distribution was similar to the placebo group. The subjects had a mean age of 28.5 years (range, 18 to 45 years) and a mean weight of 76.5 kg (range, 60 to 92 kg). No subject taking EDP-420 had a known concomitant illness or received concomitant treatments/medicine likely to interfere with the PK or safety of EDP-420 during the study. No subject was withdrawn from the study for any reason.
TABLE 1.
Demographic characteristics of subjectsa
| Variable | Dose
|
Active subjects (n = 30) | |||||
|---|---|---|---|---|---|---|---|
| Placebo (n = 10) | 100 mg (n = 6) | 200 mg (n = 6) | 400 mg (n = 6) | 800 mg (n = 6) | 1,200 mg (n = 6) | ||
| Gender, no. of subjects | |||||||
| Male (%) | 10 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 5 (83) | 29 (97) |
| Female (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 1 (3) |
| Race, no. of subjects | |||||||
| Caucasian (%) | 1 (10) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 3 (50) | 4 (13) |
| Black (%) | 3 (30) | 2 (33) | 4 (67) | 2 (33) | 1 (17) | 2 (33) | 10 (33) |
| Asian (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hispanic (%) | 5 (50) | 4 (67) | 1 (17) | 4 (67) | 4 (67) | 1 (17) | 14 (47) |
| Other (%) | 1 (10) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 0 (0) | 2 (7) |
| Age (yr) | |||||||
| Mean | 27.8 | 32.3 | 31.8 | 25.2 | 27.8 | 25.2 | 28.5 |
| SD | 6.6 | 7.7 | 9.6 | 6.4 | 8.8 | 7.7 | 8.2 |
| Range | 19-37 | 25-42 | 23-45 | 19-33 | 21-41 | 18-38 | 18-45 |
| wt (kg) | |||||||
| Mean | 71.6 | 79.6 | 77.7 | 78.5 | 70.9 | 76.0 | 76.5 |
| SD | 8.4 | 5.9 | 6.9 | 12.6 | 9.0 | 5.7 | 8.4 |
| Range | 58-84 | 72-87 | 67-88 | 60-92 | 62-83 | 69-82 | 60-92 |
n, Number of subjects.
PK analyses.
All 30 subjects who received a single oral dose of EDP-420 had evaluable data and were included in the PK analysis.
(i) Plasma.
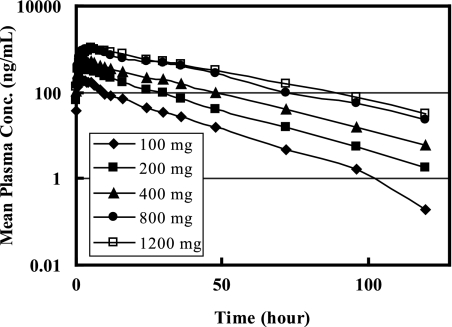
Figure 2 illustrates the concentration-versus-time profiles of EDP-420 in plasma after ascending single oral doses in healthy volunteers. All predose plasma samples did not have quantifiable EDP-420 concentration (<1 ng/ml). After a single-dose oral administration of EDP-420 in a suspension, EDP-420 was rapidly absorbed with concentrations measurable at the first sampling time (0.25 h) postdose. The subjects receiving 200 mg of EDP-420 had measurable plasma concentrations at 96 h postdose, and all of the other subjects taking 400, 800, or 1,200 mg of EDP-420 had quantifiable plasma concentrations at 120 h postdose (the last blood draw of the present study).
FIG. 2.
Semi-log plot of mean plasma EDP-420 concentrations over time for each dose level.
Table 2 illustrates the PK parameters of EDP-420 after a single-dose administration. With a 120-h PK sampling time, the percent AUC obtained by extrapolation for all subjects in the five dose groups was <3%, which suggested a reliable estimation of λz was obtained for calculating AUC0-∞. The mean total systemic exposure was 3.43 μg·h/ml at 100 mg and increased to 38.28 μg·h/ml at 1,200 mg. The mean Cmax of EDP-420 was 0.196 μg/ml at 100 mg, increased with dose to 1.066 μg/ml at 800 mg, and then reached a plateau from 800 to 1,200 mg. The median Tmax increased from 1.75 h to 5.00 h as the dose increased from 100 to 1,200 mg.
TABLE 2.
Plasma PK of EDP-420 following a single oral dose
| Parameter (n = 6) | Mean of CV% | EDP-420 dose
|
||||
|---|---|---|---|---|---|---|
| 100 mg | 200 mg | 400 mg | 800 mg | 1,200 mg | ||
| AUC0-∞ (ng·h/ml) | Mean | 3,432 | 7,713 | 14,400 | 33,890 | 38,280 |
| CV% | 10.6 | 25.5 | 28.9 | 35 | 27.1 | |
| AUC0-last (ng·h/ml) | Mean | 3,400 | 7,654 | 14,270 | 33,370 | 37,400 |
| CV% | 10.7 | 25.5 | 27.8 | 33.4 | 26.7 | |
| Cmax (ng/ml) | Mean | 196 | 360 | 542 | 1,066 | 1,076 |
| CV% | 14.8 | 26.4 | 22.9 | 28.4 | 20.7 | |
| Tmax (h) | Median | 1.75 | 2.50 | 3.25 | 4.26 | 5.00 |
| Range | 1.50-5.00 | 1.50-3.50 | 1.50-5.00 | 2.00-6.00 | 3.50-6.00 | |
| t1/2 (h) | Mean | 15.6 | 15.8 | 15.8 | 17.6 | 20.1 |
| CV% | 18.2 | 17.1 | 18.5 | 26.9 | 12.8 | |
| λz (h−1) | Mean | 0.0444 | 0.0440 | 0.0438 | 0.0393 | 0.0344 |
| CV% | 17.9 | 16.2 | 17.4 | 21.9 | 14.1 | |
| CLpo (liters/h) | Mean | 29.1 | 25.9 | 27.8 | 23.6 | 31.4 |
| CV% | 10.6 | 31.5 | 23.6 | 39.3 | 29.5 | |
| Vz/F (liters) | Mean | 656 | 589 | 635 | 601 | 909 |
| CV% | 24.0 | 18.4 | 24.2 | 30.6 | 23.1 | |
After absorption, EDP-420 was eliminated from plasma with a mean half-life of 15.6 to 20.1 h for the escalating doses (Table 2). The individual estimated t1/2 ranged from 12.6 to 27.2 h across the tested dose ranges (data not shown). The long t1/2 was consistent with the low elimination rate constant λz of ∼0.04 h−1. A slight increase in the mean t1/2 and a slight decrease in the mean λz were observed as the dose increased from 100 to 1,200 mg. The plasma t1/2 of EDP-420 in individual subjects was determined using at least four data points in the terminal phase based on visual inspection.
Apparent oral clearance CLpo was 23.6, 25.9, 27.8, 29.1, and 31.4 liters/h for the doses of 800, 200, 400, 100, and 1,200 mg, respectively, and it was essentially independent from dose across the test range. The apparent volume of distribution Vz/F ranged from 589 to 909 liters in the tested dose range. The intersubject variability of plasma PK parameters, which was characterized by percent coefficient of variation (CV%), was generally in the range of 15 to 30% across the 100- to 1,200-mg dose range.
The AUC0-last-versus-dose profiles of EDP-420 (Fig. 3) shows that the AUC increased linearly with the dose up to 800 mg (with R2 = 0.9941), with a slight further increase from 800 to 1,200 mg. The same phenomenon was observed in the Cmax-versus-dose profile of EDP-420 (Fig. 3). Cmax increased linearly with the dose up to 800 mg (with R2 = 0.9952) and reached a plateau from the 800- to 1,200-mg dose.
FIG. 3.
Mean plasma AUC0-∞ and Cmax (± the standard deviation) of EDP-420 versus dose following a single-dose administration.
Statistical analysis indicated that plasma AUC values were essentially proportional to the dose across the 100- to 1,200-mg dose range. The slope estimates for the effect of dose on AUC0-last and AUC0-∞ were close to unity (0.99 and 0.99, respectively). However, the plasma Cmax was less than proportional over the dose range from 100 to 1,200 mg (Table 2). The point estimate for the slope from the power model for the effect of dose on Cmax was 0.70, and the corresponding 90% confidence intervals did not include the value of 1.
(ii) Urine.
Table 3 and Fig. 4 illustrate urine PK parameters and mean urine Ae0-48-versus-time profile of EDP-420 following a single-dose administration. The mean Ae0-48 increased linearly (R2 = 0.9953) with the dose up to the 800-mg dose and then dropped slightly at the 1,200-mg dose (Fig. 4). Mean fe0-48 values were similar in the dose range of 200 to 800 mg at ca. 10.5 to 12.3%. Lower mean fe0-48 values (<10%) were observed at the 100- and 1,200-mg doses. Mean CLR values were similar in the 200-, 400-, and 800-mg doses at ∼3.5 liters/h. Lower mean CLR values (<3 liters/h) were observed for the 100- and 1,200-mg doses. The intersubject variability of urine PK parameters was in the range of 15 to 35% across all dose groups.
TABLE 3.
Urine PK of EDP-420 following single dose administration
| Parameter (n = 6) | Mean or CV% | EDP-420 dose
|
||||
|---|---|---|---|---|---|---|
| 100 mg | 200 mg | 400 mg | 800 mg | 1,200 mg | ||
| Ae0-48 (mg) | Mean | 8.57 | 24.5 | 41.9 | 91.1 | 70.1 |
| CV% | 15.6 | 29.2 | 12.4 | 27.8 | 34.8 | |
| fe0-48 (%) | Mean | 8.57 | 12.3 | 10.5 | 11.4 | 5.84 |
| CV% | 15.6 | 29.0 | 12.5 | 27.6 | 34.9 | |
| CLR (liters/h) | Mean | 2.78 | 3.62 | 3.48 | 3.45 | 2.48 |
| CV% | 16.9 | 22.7 | 13.8 | 28.3 | 17.7 | |
FIG. 4.
Mean EDP-420 urine Ae0-48 (± the standard deviation) following a single-dose administration.
Safety.
Neither serious adverse events nor any dose-limiting clinical or laboratory adverse events were reported during the study. No subject was discontinued for any reason. EDP-420 was well tolerated. Adverse events were reported in 16.7% of EDP-420-treated subjects and in 30% of placebo-treated subjects. Hepatic transaminase (alanine transaminase or aspartate transaminase) elevations (<2 × the upper limit of normal) were reported in 23% of EDP-420-treated subjects and 20% of placebo-treated subjects. There were no concomitant abnormalities in gamma-glutamyl transpeptidase, direct bilirubin, total bilirubin, or prothrombin time. Drug-related adverse events included one event of diarrhea in the 800-mg dose group and three events of nausea (without vomiting) in the 1,200-mg dose group, all of which resolved the same day as the dosing. No clinically significantly changes in laboratory test results (other than liver function), vital signs, ECG, or physical examinations were noted during the study.
DISCUSSION
EDP-420 is a first-in-class bridged bicyclic macrolide antibiotic with potential indications for community-acquired pneumonia, acute exacerbation of chronic bronchitis, acute sinusitis, tonsillitis/pharyngitis, and otitis media. There has been no prior clinical experience with EDP-420 since this was a first-in-human study. In the present study, single oral doses of EDP-420 or placebo were given to different groups of adult subjects to determine the safety and PK of EDP-420 in healthy adult volunteers.
After a single dose oral administration in a suspension, EDP-420 was rapidly absorbed, with concentrations measurable as early as 15 min postdose, and stayed in the bloodstream as long as 96 h postdose for all dose groups (Fig. 2). The long residence of EDP-420 in plasma was consistent with the low terminal elimination rate λz and the long terminal half-life of EDP-420 observed in the present study. λz and t1/2 ranged from 0.034 to 0.044 h−1 and from 15.6 to 20.1 h, respectively, across the tested dose ranges of 100 to 1,200 mg (Table 2). Compared to other macrolides (such as erythromycin, clarithromycin, and telithromycin), EDP-420 has a much longer t1/2 (Table 4). The t1/2 of erythromycin and clarithromycin were 2.0 and 3.89 h, respectively. The α-phase and terminal t1/2 values of telithromycin (with once-a-day dose regimen) were 2.43 and 7.16, respectively, at the therapeutic dose of 800 mg (23). Azithromycin is known for its long t1/2 of 11 to 40 h (8, 18), which allows for its 1-day short course therapy for the treatment of RTI. Thus, the long t1/2 of EDP-420 should allow for a once-daily dose regimen and a short treatment period.
TABLE 4.
Plasma PK of EDP-420 versus other marketed macrolidesa
| Parameter | EDP-420 | ERY | CLAR | TEL | AZI |
|---|---|---|---|---|---|
| Dose (mg) | 400 | 500 | 400 | 400 | 500 |
| Cmax (μg/ml) | 0.54 | 2.00 | 0.78 | 0.80 | 0.40 |
| AUC0-∞ (μg·h/ml) | 14.40 | 7.70 | 7.52 | 3.09 | 3.39 |
| t1/2 (h) | 15.8 | 2.0 | 3.89 | ||
| t1/2α (h) | 2.13 | 11-14 | |||
| t1/2β (h) | 6.68 | 35-40 |
EDP-420 plasma concentrations declined in a mono-exponential fashion and displayed a single elimination phase (Fig. 2), suggesting the immediate equilibration between the central compartment (e.g., plasma) and all peripheral compartments (e.g., lung tissue). The apparent volume of distribution V/F of >580 liters across the tested dose range was well above 42 liters of total body water for a 70-kg man, which indicates extensive distribution of EDP-420 into the tissues and intracellular space in humans. Indeed, preferential distribution in lung (the targeted site of respiratory tract infections), and penetration into epithelial lining fluid (ELF) and alveolar macrophages (AM) were observed in a study with healthy Japanese subjects (10). After a single oral dose of 400 mg of EDP-420 suspension in humans, the mean Cmax values in ELF and AM were 16.8 and 175.9 μg/ml, respectively, which were much higher than the plasma Cmax of 0.687 μg/ml. AUC0-24 of ELF and AM were 212.4 and 2,559 μg·h/ml, respectively, in comparison with the plasma AUC0-24 of 10.5 μg·h/ml. Thus, the respective ratios of AUC0-24 in ELF and AM over AUC0-24 in plasma were 20.3 and 244.6. There were significant amounts of EDP-420 measurable in the ELF and AM (respective values of 6.5 and 68 μg/ml) even at 24 h postdose. The t1/2 of the ELF and AM were 14.8 and >24 h, respectively (10). Desirable lung distribution of EDP-420 was also observed in rats with a Cmax of 123.1 μg/g and an AUC of 868.8 μg·h/g in lung homogenate (13). The ratio of the AUC in lung homogenate versus the AUC in plasma was 224. A ratio of the AUC in ELF versus the AUC of plasma of 17 was obtained in rats (13).
EDP-420 has higher systemic drug exposure compared to other macrolides at the same or similar oral doses (Table 4). For example, the AUC0-∞ of EDP-420 at the 400 mg oral dose was 14.4 μg·h/ml, which is 4.2-fold that of AUC0-∞ for azithromycin at the therapeutic dose of 500 mg (8, 18). EDP-420 is an AUC/MIC drug (20) and, therefore, the higher systemic exposure of EDP-420 and desirable ELF/AM penetrations in humans, plus its in vitro antimicrobial activity profiles and improved preclinical in vivo efficacy (13, 20, 21, 28, 30, 31), suggest that EDP-420 might be as efficacious at the doses lower (e.g., 400 mg or even 200 mg) than those approved for other macrolide antibiotics. AUC0-last-versus-dose and Cmax-versus-dose profiles of EDP-420 show that both the AUC and the Cmax increased linearly with the dose up to 800 mg. The plateau from the 800- to the 1,200-mg dose observed in the Cmax-versus-dose plot implies that a saturation or delay in the absorption process may occur at doses above 800 mg.
Approximately 6 to 12% of EDP-420 was excreted unchanged in urine over the 48-h period (Table 3). Mean EDP-420 CLR ranged from 2.48 to 3.62 liters/h in the tested dose range of 100 to 1,200 mg, with the majority of the dose groups at ∼3.5 liters/h, and showed no apparent dose dependency. The renal clearance of EDP-420 was lower than the corresponding values for clarithromycin and telithromycin (7, 23). For example, the mean clarithromycin CLR ranged from 5.82 to 11.88 liters/h in the tested dose range of 100 to 1,200 mg, with the majority of the dose groups at ∼7.5 liters/h, which is twofold higher than the EDP-420 CLR. The mean telithromycin CLR was ∼12.3 liters/h for the 400-, 800-, and 1,600-mg doses, which is three- to fourfold higher than the EDP-420 CLR. The lower EDP-420 CLR may also contribute to the relatively high systemic exposure of EDP-420 compared to other macrolides. Similar to plasma AUC0-last-versus-dose and Cmax-versus-dose profiles of EDP-420, the urine Ae0-48-versus-dose profile indicated that the amount of EDP-420 excreted in urine in the 48-h period increased linearly with dose up to 800 mg and then dropped slightly from 800 to 1,200 mg. This suggests that EDP-420 renal elimination is plasma concentration dependent and would follow first-order disposition kinetics.
The PK, safety, and tolerability of EDP-420 after a single ascending oral dose (i.e., 100, 200, 400, 800, and 1,200 mg) was also assessed in healthy Japanese volunteers (29). A long t1/2 of 12.6 to 18.2 h was observed across the oral doses in Japanese subjects, which is consistent with results from the U.S. phase I trial. Due to the lighter mean weight (<60 kg) of Japanese subjects, EDP-420 exposure in terms of both Cmax and AUC was generally higher than that observed in the U.S. subjects. For example, mean Cmax and AUC0-∞ values were 0.63 μg/ml and 15.96 μg·h/ml, respectively, which were 17 and 11% higher than corresponding values observed in the U.S. subjects at the 400-mg dose. The PK values of EDP-420 in Japanese patients with community-acquired pneumonia were also reported (16). A higher exposure in terms of Cmax and AUC0-∞ and longer t1/2 values (∼21 h) of EDP-420 were observed in Japanese patients compared to values obtained with healthy Japanese volunteers at the same oral dose.
The PK parameters of EDP-420 had small intersubject variations with CV% values generally <30%, which might minimize the likelihood of unexpectedly high drug exposure (leading to toxicity), as well as insufficient drug exposure (leading to therapeutic failure). EDP-420 appeared to be safe and well tolerated in a single dose as high as 1,200 mg. The desirable PK profile of EDP-420, together with an improved preclinical activity profile relative to currently marketed macrolides, suggest that EDP-420 may be an effective drug for the treatment of community-acquired respiratory tract infections.
Acknowledgments
We thank the study participants, clinical investigators, study coordinators, and Mike Corrado, Ly Phan, and Andrew Sonderfan, who made this study possible.
Footnotes
Published ahead of print on 17 February 2009.
REFERENCES
- 1.Bartlett, J. G., R. F. Breiman, L. A. Mandell, and T. M. File, Jr. 1998. Community-acquired pneumonia in adults: guidelines for management. Clin. Infect. Dis. 26:811-838. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett, J. G., and L. M. Mundy. 1995. Community-acquired pneumonia. N. Engl. J. Med. 333:1618-1624. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez, L., N. Motamedi, M. Wu, L. S. Young, G. Wang, and L. T. Phan. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-1224.
- 4.Bermudez, L. E., M. D. McNab, L. S. Young, G. Wang, and L. T. Phan. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-2035.
- 5.Bermudez, L. E., N. Motamedi, C. Chee, G. Baimukanova, P. Kolonoski, C. Inderlied, P. Aralar, G. Wang, L. T. Phan, and L. S. Young. 2007. EDP-420, a bicyclolide (bridged bicyclic macrolide), is active against Mycobacterium avium. Antimicrob. Agents Chemother. 51:1666-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chee, C., P. Kolonoski, M. Wu, L. E. Bermudez, L. S. Young, G. Wang, and L. T. Phan. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-2035.
- 7.Chu, S. Y., L. T. Sennello, S. T. Bunnell, L. L. Varga, D. S. Wilson, and R. C. Sonders. 1992. Pharmacokinetics of clarithromycin, a new macrolide, after single ascending oral doses. Antimicrob. Agents Chemother. 36:2447-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coates, P., R. Daniel, A. C. Houston, J. H. Antrobus, and T. Taylor. 1991. An open study to compare the pharmacokinetics, safety and tolerability of a multiple-dose regimen of azithromycin in young and elderly volunteers. Eur. J. Clin. Microbiol. Infect. Dis. 10:850-852. [DOI] [PubMed] [Google Scholar]
- 9.Dubois, J., and M. Paris. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-1859.
- 10.Furuie, H., S. Irie, Y. Saisho, T. Yoshikawa, and J. Shimada. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-798.
- 11.Hoban, D. J., A. K. Wierzbowski, K. Nichol, and G. G. Zhanel. 2001. Macrolide-resistant Streptococcus pneumoniae in Canada during 1998-1999: prevalence of mef(A) and erm(B) and susceptibilities to ketolides. Antimicrob. Agents Chemother. 45:2147-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang, L. J., D. Wachtel, T. Phan, A. Sonderfan, and Y. S. Or. 2007. Abstr. 17th Eur. Cong. Clin. Microbiol. Infect. Dis./25th Int. Cong. Chemother., abstr. F-2091.
- 13.Jiang, L. J., G. L. Drusano, P. Nguyen, T. Murphy, A. Arya, L. T. Phan, A. Sonderfan, and Y. S. Or. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-26.
- 14.Jiang, L. J., G. Wang, L. T. Phan, A. Sonderfan, M. Paris, and Y. S. Or. 2007. Abstr. 17th Eur. Cong. Clin. Microbiol. Infect. Dis./25th Int. Cong. Chemother., abstr. F-2092.
- 15.Jiang, L. J., M. Takeuchi, R. Lewsley, T. Baba, A. Sonderfan, and Y. S. Or. 2007. Abstr. 17th Eur. Cong. Clin. Microbiol. Infect. Dis. and 25th Int. Cong. Chemother., abstr. F-2093.
- 16.Kohno, S., K. Yamaguchi, Y. Tanigawara, A. Watanabe, N. Aoki, Y. Niki, and J. Fujita. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-485.
- 17.Kolonoski, P., C. Chee, G. Baimukanova, L. E. Bermudez, L. S. Young, G. Wang, and L. T. Phan. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-2035.
- 18.Lode, H. 1991. The pharmacokinetics of azithromycin and their clinical significance. Eur. J. Clin. Microbiol. Infect. Dis. 10:807-812. [DOI] [PubMed] [Google Scholar]
- 19.Luo, X., R. Wu, D. Mu, L. T. Phan, G. Wang, A. Polemeropoulos, and Y. S. Or. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-2848.
- 20.Maglio, D., H. K. Sun, T. Patel, M. A. Banevicius, C. H. Nightingale, G. Wang, Z. Chen, L. T. Phan, and D. P. Nicolau. 2004. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1408.
- 21.Maki, H., T. Fujimura, Y. Yamano, J. Shimada, and S. Kuwahara. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-2029.
- 22.Matsuda, H., Y. Kawai, Y. Yamano, and N. Gotoh. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1678.
- 23.Namour, F., D. H. Wessels, M. H. Pascual, D. Reynolds, E. Sultan, and B. Lenfant. 2001. Pharmacokinetics of the new ketolide telithromycin (HMR 3647) administered in ascending single and multiple doses. Antimicrob. Agents Chemother. 45:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato, T., K. Tateda, Y. Ishii, S. Kimura, A. Ohno, S. Miyazaki, and K. Yamaguchi. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-1856.
- 25.Sato, T., K. Tateda, Y. Ishii, S. Kimura, A. Ohno, S. Miyazaki, and K. Yamaguchi. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-1629.
- 26.Sato, T., K. Tateda, Y. Ishii, S. Kimura, A. Ohno, S. Miyazaki, and K. Yamaguchi. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-1630.
- 27.Sato, T., K. Tateda, Y. Ishii, S. Kimura, A. Ohno, S. Miyazaki, and K. Yamaguchi. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-1628.
- 28.Scorneaux, B., A. Arya, A. Polemeropoulos, M. Lillard, F. Han, K. Amsler, A. Sonderfan, G. Wang, Y. Peng, G. Xu, H. Kim, T. Lien, L. T. Phan, and Y. S. Or. 2003. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1191.
- 29.Shimada, J., Y. Saisho, and H. Fukase. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-799.
- 30.Stucki, A., P. Gerber, F. Acosta, M. Cottagnoud, P. Cottagnoud, L. Jiang, P. Nguyen, D. Wachtel, G. Wang, and L. T. Phan. 2008. Effects of EDP-420 on penicillin-resistant and quinolone- and penicillin-resistant pneumococci in the rabbit meningitis model. J. Antimicrob. Chemother. 61:665-669. [DOI] [PubMed] [Google Scholar]
- 31.Tsuji, M., H. Miwa, M. Takema, E. Kanaoka, T. Yoshikawa, J. Shimada, and S. Kuwahara. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-2035.
- 32.Tuominen, R. K., P. T. Mannisto, P. Pohto, A. Solkinen, and A. Vuorela. 1988. Absorption of erythromycin acistrate and erythromycin base in the fasting and non-fasting state. J. Antimicrob. Chemother. 21(Suppl. D):45-55. [DOI] [PubMed] [Google Scholar]
- 33.Wang, G., D. Niu, Y. L. Qiu, L. T. Phan, Z. Chen, A. Polemeropoulos, and Y. S. Or. 2004. Synthesis of novel 6,11-O-bridged bicyclic ketolides via a palladium-catalyzed bis-allylation. Org. Lett. 6:4455-4458. [DOI] [PubMed] [Google Scholar]
- 34.Wierzbowski, A. K., D. J. Hoban, T. Hisanaga, M. Decorby, and G. G. Zhanel. 2005. The use of macrolides in treatment of upper respiratory tract infections. Curr. Infect. Dis. Rep. 7:175-184. [DOI] [PubMed] [Google Scholar]
- 35.Wierzbowski, A. K., K. Nichol, N. Laing, T. Hisanaga, A. Nikulin, J. A. Karlowsky, D. J. Hoban, and G. G. Zhanel. 2007. Macrolide resistance mechanisms among Streptococcus pneumoniae isolated over 6 years of Canadian Respiratory Organism Susceptibility Study (CROSS) (1998-2004). J. Antimicrob. Chemother. 60:733-740. [DOI] [PubMed] [Google Scholar]
- 36.Xiong, L., Y. Korkhin, and A. S. Mankin. 2005. Binding site of the bridged macrolides in the Escherichia coli ribosome. Antimicrob. Agents Chemother. 49:281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamano, Y., T. Fujumura, H. Maki, J. Shimada, and S. Kuwahara. 2006. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-1857.
- 38.Zhanel, G. G., M. DeCorby, N. Laing, B. Weshnoweski, R. Vashisht, F. Tailor, K. A. Nichol, A. Wierzbowski, P. J. Baudry, J. A. Karlowsky, P. Lagace-Wiens, A. Walkty, M. McCracken, M. R. Mulvey, J. Johnson, and D. J. Hoban. 2008. Antimicrobial-resistant pathogens in intensive care units in Canada: results of the Canadian National Intensive Care Unit (CAN-ICU) study, 2005-2006. Antimicrob. Agents Chemother. 52:1430-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]