Abstract
We have evaluated the efficacies of micafungin, amphotericin B, and voriconazole, alone and in double and triple combinations, in a murine model of systemic infection by Scedosporium prolificans. Micafungin combined with voriconazole or amphotericin B was the most effective, these being the only treatments able to prolong survival and to reduce the fungal load in the kidneys and brain. Triple combinations of these drugs did not improve the results obtained with double combinations.
Invasive infections by Scedosporium species are difficult to treat and cause high mortality (7). The outcome of these infections is generally worse when they are caused by Scedosporium prolificans (3, 7), which is refractory to antifungal therapy (18).
Up to now, the only drugs that have shown any beneficial effect in infection of animals by S. prolificans have been liposomal amphotericin B (AMB) at high doses (1, 16), albaconazole (2), and caspofungin (1). However, in the clinical setting, the usefulness of these drugs is questionable (8, 9, 12). It seems reasonable, therefore, to explore the use of new approaches, testing two and even three drugs with different action mechanisms. The in vitro combinations of AMB with different echinocandins or triazoles have shown some degree of synergy against S. prolificans (4, 23). Combinations of more than two drugs have been poorly explored.
We have evaluated the efficacies of micafungin (MFG), AMB, and voriconazole (VRC), alone and in double and triple combinations, in a murine model of disseminated scedosporiosis caused by S. prolificans. Although none of these drugs is active in vitro against this fungus (14, 23), it is not known if the combination of these drugs could be effective in vivo.
A clinical isolate of S. prolificans, FMR 6719, was used. On the day of infection, it was suspended in sterile saline and filtered through sterile gauze to remove clumps of cells or hyphae (17). The resulting suspension, containing ≥95% of viable conidia, was adjusted to the desired inoculum based on the hemocytometer counts and confirmed by culture on potato dextrose agar.
In vitro susceptibilities of the isolate to MFG, AMB, and VRC determined by a reference method (15) are shown in Table 1. Drug interactions were assessed using a checkerboard method (5, 6, 10). All double combinations resulted in indifferent interactions, while the triple combination of MFG plus AMB and VRC was synergistic.
TABLE 1.
In vitro antifungal activities and interactions among antifungal drugs against S. prolificans FMR 6719
| Treatment | MIC(s) (μg/ml) | FICIa |
|---|---|---|
| MFG | 256 | |
| AMB | 32 | |
| VRC | 64 | |
| MFG + AMB | 8, 16 | 0.53 |
| MFG + VRC | 16, 32 | 0.56 |
| AMB + VRC | 2, 32 | 0.56 |
| MFG + AMB + VRC | 0.25, 2, 16 | 0.31 |
FICI, fractional inhibitory concentration index. ≤0.5, synergistic; >0.5 to ≤4, indifferent; >4, antagonistic (8).
Male OF1 mice with a mean weight of 30 g were immunosuppressed with cyclophosphamide 1 day prior to the infection (1). Animal care procedures were approved by the Universitat Rovira i Virgili Animal Welfare Committee. To evaluate the most appropriate infective dose that produced an acute infection, groups of 10 mice were challenged with each of the following three conidial suspensions: 1 × 104 CFU/ml, 7.5 × 104 CFU/ml, and 1 × 105 CFU/ml in 0.2 ml injected into the lateral tail vein. The second inoculum tested was the most appropriate.
The efficacies of the different treatments were evaluated through prolongation of survival and fungal tissue burden reduction in brains and kidneys of infected mice. The different groups (10 mice per group) were treated as follows: MFG at 10 mg/kg of body weight given intraperitoneally once daily (11); AMB at 1.5 mg/kg of body weight given intraperitoneally once daily; VRC at 60 mg/kg of body weight given orally by gavage once daily (19); MFG plus AMB; MFG plus VRC; AMB plus VRC; and MFG plus AMB and VRC. The doses and the routes of administration used in the combined therapies were the same as in the monotherapies. The tests on the control group and the groups treated with MFG alone and in combination with VRC or AMB, which showed the best results, were repeated, and those results were pooled. There were 20 control mice and 20 mice for each treatment. From 3 days prior to infection, the mice that received VRC and the control group were given grapefruit juice instead of water. Control animals received no treatment. All treatments began 1 day after challenge, and the therapy lasted for 10 days. For tissue burden studies, mice were sacrificed on day 7 postinfection and the fungal loads in the kidneys and brain were determined.
Mean survival times were estimated by the Kaplan-Meier method and compared among groups using the log-rank test. Colony counts in tissue burden studies were analyzed using the Kruskal-Wallis test.
An additional group of five mice was similarly infected and treated with VRC (60 mg/kg daily) to determine the level of this drug in serum by bioassay (20), using yeast nitrogen broth and Candida parapsilosis ATCC 22019. The drug level was as expected, 6.71 μg/ml measured on day 5 of therapy, 4 h after dosing (13, 20).
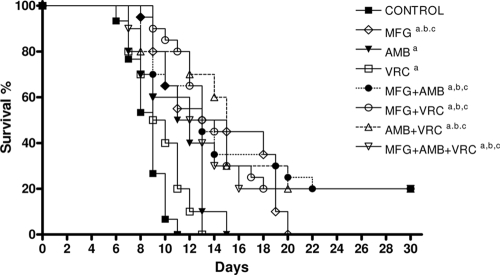
All of the treatments were able to prolong survival. Moreover, MFG and the double and triple combinations prolonged the survival compared to results with the other monotherapies (Fig. 1). In kidneys, only the combination of MFG with VRC or AMB was able to reduce the fungal load with respect to results for the controls and the VRC group (Fig. 2). In brain, MFG alone significantly reduced the fungal load with respect to results for the control group. MFG combined with VRC or AMB was able to reduce the fungal load with respect to those for the control group and the VRC group. The combination of AMB, VRC, and MFG has shown synergy in our in vitro study. However, these results did not correlate with in vivo results because the triple combination did not work as well as the double combinations.
FIG. 1.
Cumulative mortality of mice infected with S. prolificans FMR 6719. a, P < 0.05 versus results for the control; b, P < 0.05 versus results with VRC; c, P < 0.05 versus results with AMB.
FIG. 2.
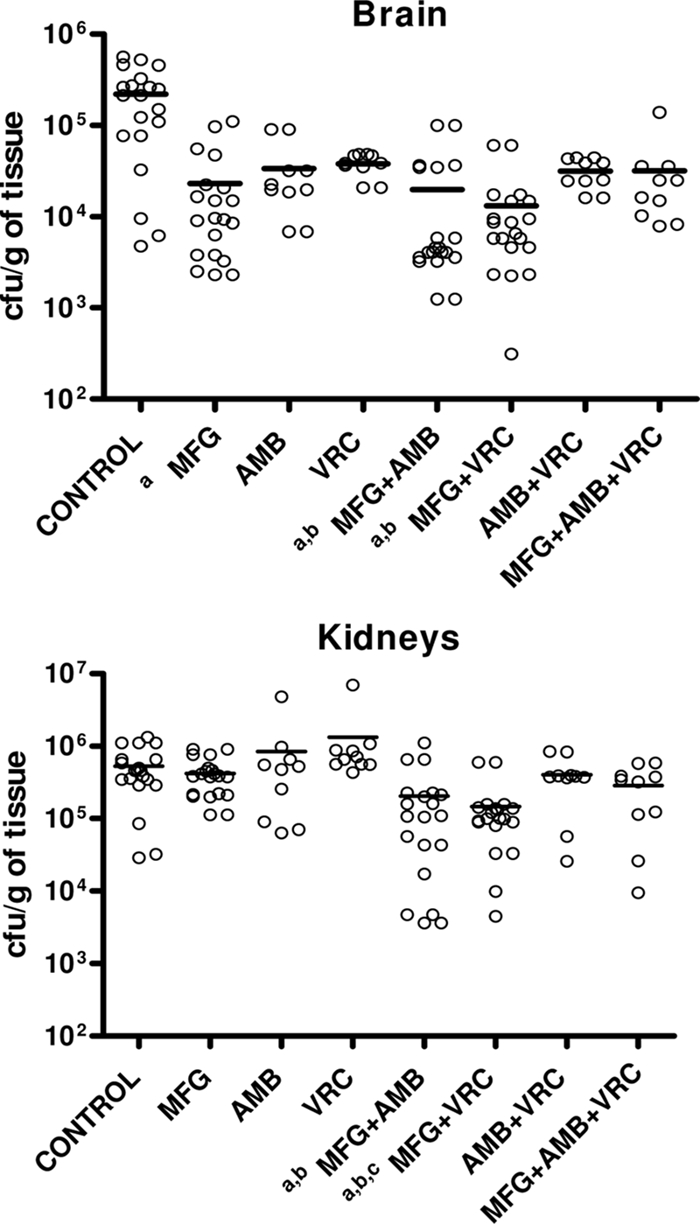
Effects of the antifungal treatment on colony counts of S. prolificans FMR 6719 in the brains and kidneys of mice. a, P < 0.05 versus results for the control; b, P < 0.05 versus results with VRC; c, P < 0.05 versus results with MFG. Horizontal lines indicate mean values.
The mild efficacy of AMB in our murine model agrees with findings of several clinical studies (3, 22). VRC showed benefits in 40% of the patients included in a recent clinical study (21), although their degree of neutropenia was not mentioned. In our study, VRC showed poor efficacy, similar to that of AMB, which correlated with its high in vitro MICs. It is probable that the response to VRC is strain dependent. There are no studies of the use of MFG in the treatment of infections by S. prolificans, but in a murine infection, caspofungin was able to prolong survival, although there was no reduction of tissue burden (1). Our results with MFG agree in part with those results, since in our model this drug alone and principally in combination produced the best results.
The use of combined therapies can be a promising clinical approach for combating infections caused by multiresistant fungi, such as S. prolificans.
Footnotes
Published ahead of print on 17 February 2009.
REFERENCES
- 1.Bocanegra, R., L. K. Najvar, S. Hernandez, D. I. McCarthy, and J. R. Graybill. 2005. Caspofungin and liposomal amphotericin B therapy of experimental murine scedosporiosis. Antimicrob. Agents Chemother. 49:5139-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capilla, J., C. Yustes, E. Mayayo, B. Fernández, M. Ortoneda, F. J. Pastor, and J. Guarro. 2003. Efficacy of albaconazole (UR-9825) in treatment of disseminated Scedosporium prolificans infection in rabbits. Antimicrob. Agents Chemother. 47:1948-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortez, K. J., E. Roilides, F. Quiroz-Telles, J. Meletiadis, C. Antachopoulos, T. Knudsen, W. Buchanan, J. Milanovich, D. A. Sutton, A. Fothergill, M. G. Rinaldi, Y. R. Shea, T. Zaoutis, S. Kottilil, and T. J. Walsh. 2008. Infections caused by Scedosporium spp. Clin. Microbiol. Rev. 21:157-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuenca-Estrella, M., A. Alastruey-Izquierdo, L. Alcazar-Fuoli, L. Bernal-Martinez, A. Gomez-Lopez, M. J. Buitrago, E. Mellado, and J. L. Rodriguez-Tudela. 2008. In vitro activities of 35 double combinations of antifungal agents against Scedosporium apiospermum and Scedosporium prolificans. Antimicrob. Agents Chemother. 52:1136-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dannaoui, E., O. Lortholary, and F. Dromer. 2004. In vitro evaluation of double and triple combinations of antifungal drugs against Aspergillus fumigatus and Aspergillus terreus. Antimicrob. Agents Chemother. 48:970-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eliopoulos, G. M., and R. C. Moellering. 1991. Antimicrobial combinations, p. 432-492. In V. Lorian (ed.), Antibiotics in laboratory medicine, 3rd ed. The Williams & Wilkins Co., Baltimore, MD.
- 7.Guarro, J., A. S. Kantarcioglu, R. Horré, J. L. Rodríguez-Tudela, M. Cuenca-Estrella, J. Berenguer, and G. S. De Hoog. 2006. Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med. Mycol. 44:295-327. [DOI] [PubMed] [Google Scholar]
- 8.Husain, S., P. Muñoz, G. Forrest, B. D. Alexander, J. Somani, K. Brennan, M. M. Wagener, and N. Singh. 2005. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin. Infect. Dis. 40:89-99. [DOI] [PubMed] [Google Scholar]
- 9.Idigoras, P., E. Perez-Trallero, L. Pineiro, J. Larruskain, M. C. Lopez-Lopategui, N. Rodriguez, and J. M. Gonzalez. 2001. Disseminated infection and colonization by Scedosporium prolificans: a review of 18 cases, 1990-1999. Clin. Infect. Dis. 32:158-165. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, M. D., C. MacDougall, L. Ostrosky-Zeichner, J. R. Perfect, and J. H. Rex. 2004. Combination antifungal therapy. Antimicrob. Agents Chemother. 48:693-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis, R. E., N. D. Albert, and D. P. Kontonyiannis. 2008. Comparison of the dose-dependent activity and paradoxical effect of caspofungin and micafungin in a neutropenic murine model of invasive pulmonary aspergillosis. J. Antimicrob. Chemother. 61:1140-1144. [DOI] [PubMed] [Google Scholar]
- 12.Maertens, J., K. Lagrou, H. Deweerdt, I. Surmont, G. E. Verhoef, J. Verhaegen, and M. A. Boogaerts. 2000. Disseminated infection by Scedosporium prolificans: an emerging fatality among hematology patients. Case report and review. Ann. Hematol. 79:340-344. [DOI] [PubMed] [Google Scholar]
- 13.Majithiya, J., A. Sharp, A. Parmar, D. W. Denning, and P. A. Warn. 2009. Efficacy of isavuconazole, voriconazole and fluconazole in temporarily neutropenic murine models of disseminated Candida tropicalis and Candida krusei. J. Antimicrob. Chemother. 63:161-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meletiadis, J., J. F. G. M. Meis, J. W. Mouton, J. L. Rodriguez-Tudela, J. P. Donnelly, P. E. Verweij, and the EUROFUNG Network. 2002. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob. Agents Chemother. 46:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NCCLS/CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard, 2nd ed. Document M38-A2. NCCLS/CLSI, Wayne, PA.
- 16.Ortoneda, M., J. Capilla, F. J. Pastor, C. Serena, and J. Guarro. 2004. Interaction of granulocyte colony-stimulating factor and high doses of liposomal amphotericin B in the treatment of systemic murine scedosporiosis. Diagn. Microbiol. Infect. Dis. 50:247-251. [DOI] [PubMed] [Google Scholar]
- 17.Ortoneda, M., J. Capilla, I. Pujol, F. J. Pastor, E. Mayayo, J. Fernández-Ballart, and J. Guarro. 2002. Liposomal amphotericin B and granulocyte colony-stimulating factor therapy in a murine model of invasive infection by Scedosporium prolificans. J. Antimicrob. Chemother. 49:525-529. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Tudela, J. L., J. Berenguer, J. Guarro, A. S. Kantarcioglu, R. Horre, G. S. De Hoog, and M. Cuenca-Estrella. 2008. Epidemiology and outcome of Scedosporium prolificans infection, a review of 162 cases. Med. Mycol. 1:1-12. [DOI] [PubMed] [Google Scholar]
- 19.Serena, C., F. J. Pastor, M. Mariné, M. M. Rodríguez, and J. Guarro. 2008. Efficacy of voriconazole in a murine model of cryptococcal central nervous system infection. J. Antimicrob. Chemother. 61:877-879. [DOI] [PubMed] [Google Scholar]
- 20.Sugar, A. M., and X. Liu. 2001. Efficacy of voriconazole in treatment of murine pulmonary blastomycosis. Antimicrob. Agents Chemother. 45:601-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troke, P., K. Aguirrebengoa, C. Arteaga, D. Ellis, C. H. Heath, I. Lutsar, M. Rovira, Q. Nguyen, M. Slavin, and S. C. A. Chen. 2008. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob. Agents Chemother. 52:1743-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh, T. J., and A. H. Groll. 1999. Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transplant. Infect. Dis. 1:247-261. [DOI] [PubMed] [Google Scholar]
- 23.Yustes, C., and J. Guarro. 2005. In vitro synergistic interaction between amphotericin B and micafungin against Scedosporium spp. Antimicrob. Agents Chemother. 49:3498-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]