Abstract
In this study, we investigated whether inflammatory responses contribute to oxidative/nitrosative stress in patients with Chagas' disease. We used three tests (enzyme-linked immunosorbent assay, immuno-flow cytometry, and STAT-PAK immunochromatography) to screen human serum samples (n = 1,481) originating from Chiapas, Mexico, for Trypanosoma cruzi-specific antibodies. We identified 121 subjects who were seropositive for T. cruzi-specific antibodies, a finding indicative of an 8.5% seroprevalence in the rural population from Chiapas. Seropositive and seronegative subjects were examined for plasma levels of biomarkers of inflammation, i.e., myeloperoxidase (MPO), inducible nitric oxide synthase (iNOS), and xanthine oxidase (XOD), as well as for oxidative (advanced oxidation protein products [AOPPs]) and nitrosative (3-nitrotyrosine [3NT]) biomarkers. The seropositive subjects exhibited a significant increase in MPO activity and protein level, the indicator of neutrophil activation. Subsequently, a corresponding increase in AOPP contents, formed by MPO-dependent hypochlorous acid and chloramine formation, was noted in seropositive subjects. The plasma level of 3NT was significantly increased in seropositive subjects, yet we observed no change in XOD activity (O2− source) and nitrate/nitrite contents (denotes iNOS activation and NO production), which implied that direct peroxynitrite formation does not contribute to increased nitrosative damage in chagasic subjects. Instead, a positive correlation between increased MPO activity and protein 3NT formation was observed, which suggested to us that MPO-dependent formation of nitrylchloride that occurs in the presence of physiological NO and O2− concentrations contributes to protein nitration. Overall, our data demonstrate that T. cruzi-induced neutrophil activation is pathological and contributes to MPO-mediated collateral protein oxidative and nitrosative damage in human patients with Chagas' disease. Therapies capable of suppressing MPO activity may be useful in controlling the inflammation and oxidative/nitrosative pathology in chagasic cardiomyopathy.
Trypanosoma cruzi, the causative agent of Chagas’ disease, is reported to infect 28 million individuals throughout Latin America (47). Infected individuals, after immune control of acute parasitemia, remain seropositive and clinically asymptomatic. Several years after infection, ∼30% of infected patients develop symptomatic chronic disease, which presents with a progressive cardiac and/or digestive pathology (31). The mechanism(s) of evolution of the clinical form of Chagas’ disease is not known (19).
In vitro and in vivo studies with experimental models have shown that infection by T. cruzi elicits both proinflammatory responses and reactive oxygen species (ROS) that appear essential for control of the parasite (reviewed in references 14 and 48). Reactive oxidants are generated as a consequence of an “oxidative burst” of phagocytic cells (e.g., macrophages and neutrophils) activated by T. cruzi infection (8). NADPH oxidase, activated in all phagocytic cells, produces superoxide (O2−) (1, 11). Endothelial activation of xanthine oxidase (XOD) in response to T. cruzi infection also results in increased O2− production via oxidation of hypoxanthine to xanthine and uric acid (3, 17). Superoxide spontaneously recombines with other molecules to produce other free radicals (e.g., H2O2 and OH) that exert cytotoxic effects via modifications of DNA, protein, and lipids (7, 23). Myeloperoxidase (MPO), a heme enzyme, is released by activated neutrophils into the extracellular milieu, where it uses H2O2 and chloride anion (Cl−), forming hypochlorous acid (HOCl) (20, 44). HOCl is a powerful oxidant and reacts with amines to form chloramines.
Besides ROS, activated macrophages produce nitric oxide (NO) via the inflammatory activation of inducible nitric oxide synthase (iNOS) in response to T. cruzi infection (27). NO, produced in abundance by iNOS, directly reacts with O2− to form peroxynitrite (ONOO−) and peroxynitrous acid (ONOOH), which have been shown to kill T. cruzi (36). Alternatively, nitrite (NO2−), a major product derived from NO, may be oxidized by MPO to nitrogen dioxide (NO2), which may further react with HOCl to form the highly reactive compound nitryl chloride (NO2Cl) (5).
The cytotoxic ROS and reactive nitrogen species cause collateral damage to host cellular components. For example, ONOO−-mediated increased protein 3-nitrotyrosine (3NT) formation is detected in the plasma and heart of mice infected by T. cruzi (13, 27). We have found increased plasma and cardiac levels of protein carbonyls and malonyldialdehydes (MDA; lipid peroxidation markers) in mice and rats infected by T. cruzi (13, 40). A substantial increase in the plasma level of MDA, in association with inefficient glutathione antioxidant defense, has been documented for seropositive patients with Chagas' disease (12, 41).
In this study, we have investigated the role of inflammatory mediators (e.g., MPO, XOD, and iNOS) in the elicitation/sustenance of oxidative/nitrosative stress in human patients with Chagas' disease. Our data demonstrate that the activated expression and activity of MPO are positively correlated with increased protein oxidation and nitration in seropositive subjects and suggest that MPO is the major cause of collateral damage through protein modification in humans with Chagas' disease.
MATERIALS AND METHODS
Study area, population, and sample collection.
This study was conducted in 2006 and 2007 in southern villages of the State of Chiapas, Mexico (Fig. 1). Human serum samples were obtained as part of an ongoing epidemiological study in 18 municipalities located within the coastal regions of Chiapas, Mexico. Samples were collected within the framework of a research project on emerging zoonotic diseases conducted jointly by several institutions, including the Chiapas State University, the Mexican Social Security Institute, the Chiapas Health Institute, and the University of Texas Medical Branch (UTMB) at Galveston. All samples came from clinics located in areas where triatomine infestation and the prevalence of T. cruzi were earlier reported (24).
FIG. 1.
Study area. The present study was conducted in southern villages of the state of Chiapas, Mexico, during 2006 and 2007. (A) Inset showing the state of Chiapas. (B) Highlighted are the municipalities located within the coastal regions of Chiapas from where the samples were collected.
Fasting venous blood samples were collected with K3EDTA (1.5 mg/ml blood) and without anticoagulant to obtain plasma and serum, respectively. Samples were obtained from randomly selected adults (n = 1,481; 45% were male and 55% were female; age, 18 to 73 years). Oral informed consent was obtained from all individuals taking part in the study. All procedures were approved by the institutional review boards at the Chiapas State University and UTMB. Trained medical personnel from Chiapas State Health Services conducted blood sampling by venipuncture.
Serology.
T. cruzi (strain SylvioX10/4) trypomastigotes were propagated in monolayers of C2C12 cells (30). The culture-derived T. cruzi organisms (70% trypomastigote and 30% amastigote) were lysed and used as a source of antigen for the serological detection of T. cruzi-specific antibodies by an enzyme-linked immunosorbent assay (ELISA), as described previously (4). A serum dilution of 1:100 provided the maximum signal/noise ratio and was used for all analyses. Individual and pooled negative samples (1:100 dilution) were analyzed by ELISA, and the mean optical density (OD) plus 2.5 standard deviations (SD) was established as the cutoff value.
Samples positive for T. cruzi-specific antibodies by ELISA were examined by two additional tests. For immuno-flow cytometry, T. cruzi trypomastigotes (1 × 106) were incubated on ice for 30 min each with a serum sample (1:2 dilution) and a fluorescein isothiocyanate-conjugated goat anti-human immunoglobulin G (IgG) antibody (Sigma) (1:50 dilution). Following incubation, parasites were fixed with 2% paraformaldehyde and analyzed by flow cytometry on a FACScan apparatus (BD Biosciences). Parasites stained with anti-major histocompatibility complex antibody (Y3) were used as negative controls. Flow data were analyzed by using Cell Quest software (BD Biosciences) and were expressed as relative percentages of positively fluorescent parasites (4). Chagas Stat-Pak is a rapid immunochromatographic screening test for detection of anti-T. cruzi antibodies and was performed according to the instructions provided by the manufacturer (Chembio Diagnostic Systems, Medford, NY).
MPO activity.
MPO activity was determined by a dianisidine-H2O2 method (6), modified for 96-well plates. Briefly, plasma samples (10 μg protein) were added in triplicate to 0.53 mM o-dianisidine dihydrochloride (Sigma) and 0.15 mM H2O2 in 50 mM potassium phosphate buffer (pH 6.0). After incubation for 5 min at room temperature, the reaction was stopped with 30% sodium azide, and the change in absorbance was measured at 460 nm (ɛ = 11,300 M−1·cm−1). Results were expressed as units of MPO/mg protein, whereby 1 unit of MPO was defined as the amount of enzyme degrading 1 nmol H2O2 per min at 25°C.
MPO protein content was determined by ELISA (9). Briefly, 96-well microtiter plates were coated overnight at 4°C with 100-μl serum samples in 0.1 M carbonate buffer, pH 9.6 (1:10 [vol/vol]), and blocked for 2 h at room temperature with 1% nonfat dry milk. Plates were then sequentially incubated with 100 μl each of anti-MPO monoclonal antibody (Abcam) (1:4,000) for 2 h, horseradish peroxidase-labeled IgG antibody (Sigma) (1:5,000) for 1 h, and Sure Blue TMB substrate for 20 min. The colorimetric change in absorbance was measured at 650 nm on a SpectraMax 190 microplate reader (Molecular Devices).
XOD activity.
XOD activity was measured according to the method of Terada et al. (34). For this assay, plasma samples (10 μg protein) were added in triplicate to 96-well plates, and the reaction was started by adding 0.15 mM xanthine-50 mM phosphate buffer (pH 7.5). The rate of uric acid production was recorded for 5 min at 290 nm (ɛ = 12,200 M−1·cm−1). Results were expressed as units of XOD/mg protein, whereby 1 unit of XOD was defined as the amount of enzyme converting 1.0 μmol xanthine to uric acid at 25°C.
Nitrite level.
The nitrite/nitrate content, indicative of NO production by iNOS, was monitored by the Greiss reagent assay (13). In 96-well plates, reduced plasma samples (∼10 μg protein) were mixed with 100 μl Greiss reagent, consisting of 1% sulfanilamide in 5% phosphoric acid and 0.1% N-(1-napthyl)ethylenediamine dihydrochloride (1:1 ratio [vol/vol]), and incubated for 10 min. The change in absorbance was monitored at 545 nm (standard curve, 0 to 200 μmol sodium nitrite).
AOPPs.
We measured advanced oxidation protein products (AOPPs) in plasma samples by spectrophotometry on a SpectraMax microplate reader calibrated with chloramine-T (Sigma), which absorbs at 340 nm in the presence of potassium iodide (45). Briefly, in 96-well plates, serum samples (1:10 dilution in phosphate-buffered saline [PBS]; 200 μl/well) were mixed in triplicate with 10 μl of 1.16 M potassium iodide and 20 μl of 100% acetic acid. The change in absorbance was immediately read at 340 nm. A standard curve was prepared using chloramine-T (linear range, 0 to 100 μmol), and the AOPP concentration was expressed as μmol chloramine-T equivalents.
3NT.
The plasma level of 3NT was monitored by ELISA as described previously (27). Briefly, 96-well plates were coated overnight at 4°C with 100-μl serum samples (1:100 dilution in 0.1 M carbonate buffer, pH 9.6). Plates were washed and sequentially incubated for 2 h each with 1% gelatin in 1× PBS, anti-3NT polyclonal antibody (Chemicon) (1:4,000 dilution in PBS plus Tween 20), and horseradish peroxidase-conjugated goat anti-rabbit IgG (Bio-Rad) (1:3,000). A colorimetric reaction was initiated with Sure Blue TMB substrate, and the change in absorbance was recorded at 545 nm (standard curve, 0 to 5 nmol 3NT). The positive control was bovine serum albumin (fatty acid-free; Sigma) derivatized with 50 mM NaNO2, 10 mM H2O2, and 100 μM horseradish peroxidase.
Data analysis.
All assays were performed at least twice, and samples were analyzed in triplicate. Data are presented as means ± SD. Results were analyzed using analysis of variance and Student's t test for statistical evaluation of mean values for experimental and control samples. The level of significance was taken as P values of <0.05. Correlation coefficients were calculated to determine the relationships between different parameters.
RESULTS
A total of 1,481 human serum samples, collected from Chiapas, Mexico, were analyzed for T. cruzi-specific antibodies by a trypomastigote-based ELISA. The variation in reactivities of negative and positive sera among different experiments and different plates in the same experiment was <12%. The mean ± SD OD values for the seronegative and seropositive populations were 0.44 ± 0.19 and 1.64 ± 0.45, respectively (Fig. 2). In immuno-flow-cytometry-based detection of serum antibody binding to trypomastigote surface antigens, a mean log fluorescence intensity of 101 to 5 × 102 was observed with trypomastigotes incubated with positive serum samples. Trypomastigotes incubated with negative serum samples exhibited a fluorescence intensity of <101. Seropositive samples were validated by a STAT-PAK immunochromatography assay. Samples positive by at least two methods were considered seropositive. We identified an 8.5% seroprevalence of T. cruzi-specific antibodies in the inhabitants of Chiapas (n = 121 of 1,481 total participants). Equivalent numbers of seronegative samples for further studies were selected by random sample analysis.
FIG. 2.
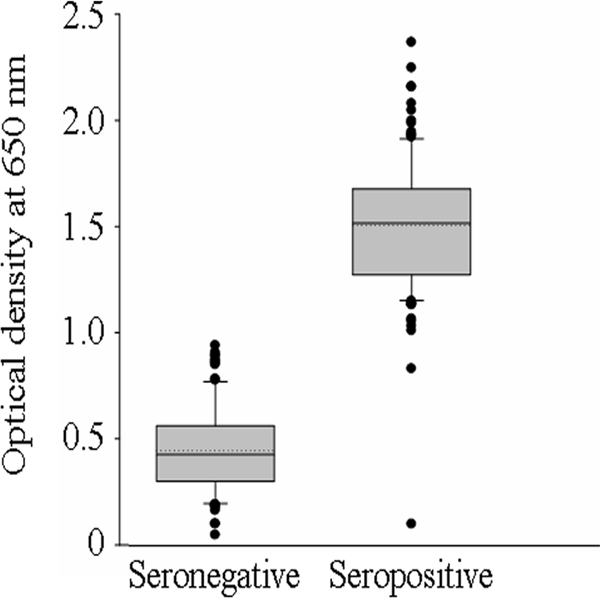
Detection of anti-T. cruzi antibodies in serum samples obtained from inhabitants of Chiapas, Mexico. Culture-derived T. cruzi specimens were used as a source of antigen for the serological detection of T. cruzi-specific antibodies by ELISA. The cutoff was established as the OD plus 2.5 SD derived from the average for known seronegative samples. The standard deviation for triplicate observations for each sample was <12%. Shown is a box plot of ELISA data, graphically depicting the OD values for seronegative and seropositive groups. The horizontal lines of the box (bottom to top) depict the lower quartile (Q1; cuts off the lowest 25% of the data), median (Q2; middle value), and upper quartile (Q3; cuts off the highest 25% of the data). The lower and upper whiskers depict the smallest and largest nonoutlier observations, respectively, and solid dots represent the outliers. The spacing between the different parts of the box indicates the degree of dispersion (spread).
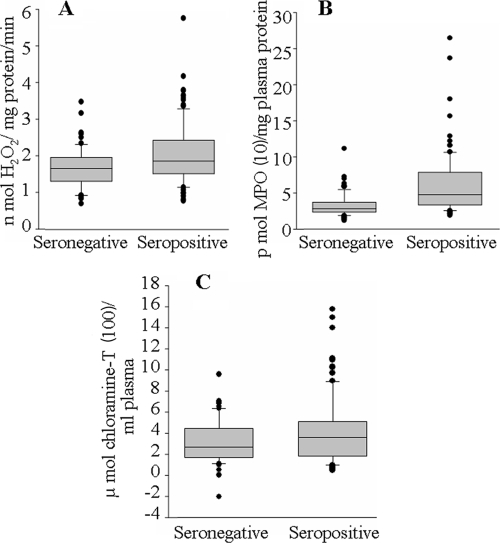
Plasma samples from seropositive and seronegative subjects (n = 121 in each group) were examined for markers of inflammation and oxidative/nitrosative stress. We monitored the enzymatic activity and protein content of MPO as a marker of inflammation. The MPO specific activity, expressed as nmol H2O2 degraded/mg protein/min, was increased 43% in seropositive plasma samples compared to that in seronegative samples (mean, 1.95 ± 0.74 versus 1.36 ± 0.36; range, 1.37 to 5.07 versus 0.61 to 1.99 [seropositive versus seronegative]; P < 0.01). Approximately 40% of the seropositive subjects exhibited MPO activity at or above the mean seropositive level, while <4% of seronegative subjects exhibited MPO activity above the mean seropositive level (Fig. 3A). The overall increase in MPO activity in seropositive subjects was associated with a corresponding increase in MPO protein level, as determined by an ELISA using an anti-MPO antibody (Fig. 3B). We noticed a 79% increase in the plasma level of MPO protein in seropositive subjects compared to that in seronegative controls (mean, 54.51 ± 0.02 versus 30.37 ± 0.02 pmol MPO/mg plasma protein; range, 19.2 to 244.2 versus 10.6 to 66.8 pmol MPO/mg plasma protein [seropositive versus seronegative]; P < 0.01). Approximately 40% of the seropositive subjects exhibited MPO protein levels at or above the mean seropositive level, while <8% of seronegative subjects exhibited MPO protein levels above the mean seropositive level. These data suggest to us that neutrophil-dependent inflammatory responses are activated in seropositive subjects.
FIG. 3.
MPO and AOPPs are increased in subjects with Chagas' disease. The plasma levels of MPO-specific activity (A), MPO protein content (B), and AOPPs (C) were determined for seronegative and seropositive subjects (n = 121 in each group) by assays described in Materials and Methods. Data (means of triplicate observations for each sample) are presented in box plots (see the description in the legend to Fig. 2). The SD for triplicate observations for all samples was <12%.
Dityrosine-containing protein cross-linking products, designated AOPPs, are the products of HOCl-induced chlorination of amines and are considered an excellent marker of MPO activation. Our data showed a 41% increase in AOPP content (measured in chloramine-T equivalents) in seropositive plasma samples compared to that noted in seronegative samples (mean, 401.8 ± 183.6 versus 285.7 ± 124.8 μmol chloramine-T/ml plasma; range, 100 to 1,577 versus 51 to 706 μmol chloramine-T/ml plasma [seropositive versus seronegative]; P < 0.01) (Fig. 3C). Approximately 52% of seropositive subjects exhibited AOPP contents at or above the mean seropositive level, while only 11% of seronegative subjects exhibited AOPP contents above the mean seropositive level. These data suggest that MPO activation results in the formation of deleterious oxidants in seropositive subjects.
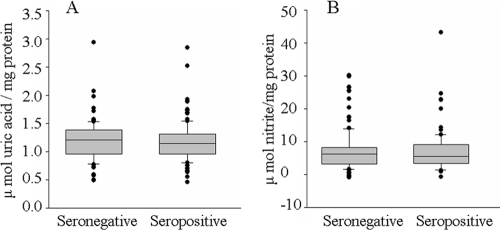
Direct interaction of O2− with NO (when present in excessive amounts) in a nonenzymatic reaction and the MPO-dependent chlorination of NO result in the formation of highly reactive ONOO− and NO2Cl species, respectively, that contribute to protein nitration (5). We observed no significant increase in the activity of ROS producing XOD in seropositive subjects (mean, 1.18 ± 0.35 versus 1.20 ± 0.36 μmol uric acid formed/mg protein; range, 0.81 to 1.30 versus 0.66 to 1.26 μmol uric acid formed/mg protein [seropositive versus seronegative]) (Fig. 4A). Likewise, nitrate/nitrite levels, indicative of iNOS activation and abundant NO production, were not significantly increased in the plasmas of seropositive subjects compared with those in seronegative samples (mean, 7.92 ± 0.71 versus 7.06 ± 0.38 μmol nitrite/mg protein; range, 2.28 to 24.61 versus 2.34 to 30.20 μmol nitrite/mg protein [seropositive versus seronegative]) (Fig. 4B). Yet the plasma level of 3NT, a marker of nitrosative stress, was increased in seropositive subjects (Fig. 5). Overall, we noted a 45% increase in 3NT content in plasma samples obtained from seropositive subjects compared to that noted in seronegative samples (mean, 2.0 ± 0.82 versus 1.27 ± 0.62 nmol 3NT/mg plasma protein; range, 0.82 to 3.94 versus 0.09 to 2.56 nmol 3NT/mg plasma protein [seropositive versus seronegative]). Only 11% of the seronegative subjects and >55% of the seropositive subjects exhibited 3NT contents at or above the mean seropositive level. These data show that despite a lack of iNOS activation, seropositive subjects with Chagas' disease are exposed to increased nitrosative stress.
FIG. 4.
Plasma levels of XOD specific activity and nitrate/nitrite content are not altered in seropositive subjects. (A) XOD activity is an indicator of increased ROS production. (B) The nitrate/nitrite level, an indicator of an increase in iNOS activity and NO formation, was monitored by a Griess reagent assay. Data were derived from the means of triplicate observations/sample. The SD for all samples was <12%.
FIG. 5.
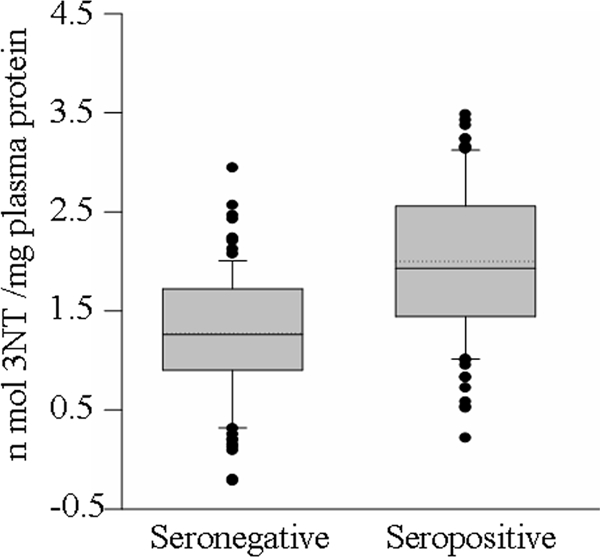
Plasma levels of 3NT, a nitrosative stress biomarker, are increased in seropositive subjects. Data (means of triplicate observations/sample) are presented as a box plot (see the description in the legend to Fig. 2). The SD for all samples was <12%.
Pearson correlation analysis identified a positive correlation between an increase in MPO-specific activity and MPO protein content (R = 0.588; P < 0.01) in seropositive subjects. A direct, positive correlation was also noted between the increase in MPO-specific activity and 3NT content (R = 0.266; P < 0.01) and between an increase in MPO protein and 3NT content (R = 0.317; P < 0.01) in seropositive subjects (Table 1). Likewise, the increase in MPO protein level was positively correlated with an increase in AOPP content in seropositive subjects (R = 0.254; P < 0.05). No direct correlation was observed between other parameters (e.g., NO and XOD) in seropositive subjects. Seronegative subjects exhibited no correlation between any of the parameters tested in this study.
TABLE 1.
Pearson's correlation coefficients for samples from individuals seropositive for Chagas' diseasea
| Parameter | Pearson's correlation coefficientb
|
|||||
|---|---|---|---|---|---|---|
| MPO activity | MPO content | AOPP content | 3NT content | XOD activity | Nitrite level | |
| MPO activity | 1 | 0.588** | 0.064 | 0.266** | 0.071 | 0.043 |
| MPO content | 0.588** | 1 | 0.264* | 0.317** | −0.002 | −0.003 |
| AOPP content | 0.064 | 0.254* | 1 | 0.055 | 0.027 | −0.017 |
| 3NT content | 0.266** | 0.317** | 0.055 | 1 | 0.137 | 0.152 |
| XOD activity | 0.171 | −0.002 | 0.027 | 0.137 | 1 | 0.105 |
| Nitrite level | 0.043 | −0.003 | −0.017 | 0.152 | 0.071 | 1 |
Pearson's correlation analysis was performed for seropositive and seronegative subjects (n = 121 in each group). The plasma level of MPO activity (nmol H2O2/mg protein/min) was positively correlated with MPO protein content (pmol MPO/mg plasma protein) and AOPP content (μmol chloramine-T/ml plasma) for seropositive subjects. The plasma levels of MPO activity and MPO protein contents of seropositive subjects were also positively correlated with 3NT contents (nmol 3NT/mg plasma protein). The XOD activity (μmol uric acid formed/mg protein) and nitrite level (μmol nitrite/mg protein) were not significantly correlated with any of the parameters.
*, P < 0.05; **, P < 0.01.
DISCUSSION
In this study, we report the seroprevalence of T. cruzi-specific antibodies in the inhabitants of Chiapas, Mexico, and demonstrate the significance of inflammatory responses in maintaining oxidative/nitrosative stress in seropositive subjects with Chagas' disease.
Serum samples (n = 1,481) were tested for T. cruzi-specific antibodies by three methods, i.e., an ELISA, immuno-flow cytometry, and a STAT-PAK assay. Following the standard procedures adopted by the Institudo Nacional de Diagnóstico y Referencia Epidemiológica (Mexico City, Mexico), the World Health Organization-recognized reference center in Mexico for the diagnosis of T. cruzi infection, samples found to be positive by at least two tests were considered seropositive. We identified an 8.5% seroprevalence (n = 121 of 1,481 participants) of T. cruzi-specific antibodies in the residents of Chiapas State. Overall, there was a 100% agreement between the trypomastigote-based ELISA and immuno-flow cytometric detection of T. cruzi-specific antibodies. Chagas’ STAT-PAK immunochromatography recognized 81 of the 121 seropositive samples as seropositive, and these findings suggested to us that the antigens included in the STAT-PAK assay might not be expressed by some of the circulating strains in Mexico.
Neutrophils are considered major contributors to the tissue damage that occurs in inflammatory diseases (16). Activated neutrophils produce ROS (O2− and H2O2) via NADPH oxidase as part of their antipathogen response. MPO, a major granule enzyme in neutrophils, accounts for 5% of the total neutrophil protein and is responsible for the production of HOCl oxidant (28). The release of ROS and HOCl by neutrophils may cause damage to important biological structures, such as proteins, carbohydrates, lipids, and nucleic acids, and may enhance inflammatory responses. AOPPs are formed by HOCl-induced chlorination of amines and constitute an excellent marker of MPO activation. AOPPs are found in the extracellular matrix of human atherosclerotic plaques (46), and increased levels of AOPPs have been described as an independent risk factor for coronary artery disease (18) and for several autoimmune inflammatory diseases (2, 35).
In experimental models of T. cruzi infection, neutrophil activation is protective or pathogenic, depending upon the Th1/Th2 dichotomy of the immune system in inbred mice (10). We have shown that T. cruzi infection results in increased carbonyl- and 3NT-modified proteins and MDA in the myocardium and plasma of infected mice (13, 40) and rats (unpublished data) and contributes to mitochondrial electron transport chain dysfunction (39) and altered cardiac hemodynamics in the infected host (unpublished data). The oxidative/nitrosative stress in infected animals was exacerbated due to compromised glutathione antioxidant defense capacity and resulted in an inefficient scavenging of free radicals (42). The extent of protein modification in the heart and plasma of infected animals was correlated with the magnitude of inflammatory responses (13), which was suggestive of a role of inflammatory responses in sustaining oxidative/nitrosative stress during progressive Chagas’ disease. In patients with Chagas' disease, enhanced oxidative stress (e.g., MDA, protein carbonyls, and glutathione disulfide) and a compromised antioxidant response (e.g., glutathione peroxidase and manganese superoxide dismutase activities, glutathione content) are noted in peripheral blood (41) and isolated erythrocytes (12). An increase in inflammatory cytokines (e.g., interleukin-10 [IL-10], IL-13, tumor necrosis factor alpha [TNF-α], and gamma interferon) (15) and MPO activity (21) is also reported for patients with Chagas' disease. Our data in this study validate the observations that MPO activity and protein levels are increased in patients with Chagas' disease. The finding of a positive correlation between the increase in MPO activity (and MPO protein content) and the AOPP level in seropositive subjects suggests that parasite-induced inflammatory responses are pathological and that subjects with Chagas' disease sustain MPO-dependent protein oxidative damage. Our data further point toward the use of plasma levels of MPO and AOPPs as biomarkers of inflammatory oxidative pathology.
In addition to AOPPs, we observed elevated plasma levels of protein 3NT formation in subjects with Chagas' disease. Multiple pathways can participate in tyrosine nitration. For example, iNOS-dependent enhanced NO production results in a direct interaction between NO and O2−, leading to the formation of cytotoxic ONOO− and ONOOH. Moreover, the MPO-dependent formation of a nitrogen dioxide radical (NO2) that may further react with HOCl to form nitryl chloride (NO2Cl) requires physiological levels of NO and O2− that are produced in all mammals, irrespective of disease conditions (e.g., NO from endothelial cells and O2− from the mitochondrial electron transport chain). These reactive nitrogen species result in protein tyrosine nitration (3NT), which is widely recognized as a hallmark of nitrosative stress (32).
In animals with Chagas' disease, increased levels of O2− formation due to an oxidative burst of activated macrophages (25, 26) and an increased leakage of electrons from the respiratory chain to O2 (38) have been documented. NO synthesis by iNOS during the inflammatory response against infectious agents represents a host defense system (36) and is documented for mice infected by T. cruzi (13, 27). Furthermore, mice treated with an NO inhibitor (aminoguanidine) and iNOS−/− mice exhibited increased susceptibility to T. cruzi but were better equipped in handling the nitrosative stress and neuronal and myocardial pathology and exhibited a preservation of cardiac function (22, 29, 33). These studies indicate that in experimental animals, conditions conducive to the formation of peroxynitrite through the direct interaction of NO and O2− exist and contribute to protein nitration and nitrosative stress. In seropositive humans, we observed no significant increase in the plasma level of nitrate/nitrite, measured by the Griess reagent assay. Seropositive subjects exhibited no significant increase in the activity of XOD, a key source of oxidants in T. cruzi-infected mice (13) that is also implicated in the pathogenesis of tissue ischemia-reperfusion injury (37) and atherosclerosis (43). These data suggest that direct peroxynitrite formation does not contribute to increased nitrosative stress in subjects with Chagas' disease. Instead, the increase in MPO activity (and protein level) was positively correlated with 3NT formation and thus suggests that neutrophil-mediated MPO activation causes collateral protein damage in patients with Chagas' disease.
In summary, we demonstrate that (i) MPO activation is a good biomarker of inflammatory responses in patients with Chagas' disease and (ii) MPO activation contributes to enhanced protein oxidation (AOPP) and nitration (3NT) in human Chagas’ disease. Future studies evaluating the usefulness of MPO inhibitors and antioxidants as adjunct therapies in controlling inflammatory oxidative/nitrosative stress and subsequent cardiac pathological processes in chronic Chagas’ disease are warranted.
Acknowledgments
This work was supported in part by grants from the National Heart, Lung, and Blood Institute at the National Institutes of Health (grant HL094802) and the John Sealy Memorial Endowment Fund (CON15420) to N.J.G. J.G.E.F. is supported by National Institutes of Health grant A125489. J.M.P. was a participant in the Summer Undergraduate Research Program (SURP) at the UTMB and the Minority Access to Research Careers (MARC) program at the New Mexico State University, Las Cruces, NM. Field work was supported in part by a grant from Consejo Nacional de Ciencia y Tecnologia (grant no. 69692) to F.J.R.-A.
We thank Mardelle Susman for editing the manuscript.
Footnotes
Published ahead of print on 18 March 2009.
REFERENCES
- 1.Babior, B. M. 1999. NADPH oxidase: an update. Blood 931464-1476. [PubMed] [Google Scholar]
- 2.Baskol, G., H. Demir, M. Baskol, E. Kilic, F. Ates, C. Karakukcu, and M. Ustdal. 2006. Investigation of protein oxidation and lipid peroxidation in patients with rheumatoid arthritis. Cell Biochem. Funct. 24307-311. [DOI] [PubMed] [Google Scholar]
- 3.Berry, C. E., and J. M. Hare. 2004. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J. Physiol. 555589-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia, V., M. Sinha, B. Luxon, and N. Garg. 2004. Utility of Trypanosoma cruzi sequence database for the identification of potential vaccine candidates: in silico and in vitro screening. Infect. Immun. 726245-6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bian, K., Y. Ke, Y. Kamisaki, and F. Murad. 2006. Proteomic modification by nitric oxide. J. Pharmacol. Sci. 101271-279. [DOI] [PubMed] [Google Scholar]
- 6.Bradley, P. P., D. A. Priebat, R. D. Christensen, and G. Rothstein. 1982. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Investig. Dermatol. 78206-209. [DOI] [PubMed] [Google Scholar]
- 7.Butterfield, D. A., T. Koppal, B. Howard, R. Subramaniam, N. Hall, K. Hensley, S. Yatin, K. Allen, M. Aksenov, M. Aksenova, and J. Carney. 1998. Structural and functional changes in proteins induced by free radical-mediated oxidative stress and protective action of the antioxidants N-tert-butyl-alpha-phenylnitrone and vitamin E. Ann. N. Y. Acad. Sci. 854448-462. [DOI] [PubMed] [Google Scholar]
- 8.Cardoni, R. L., M. I. Antunez, C. Morales, and I. R. Nantes. 1997. Release of reactive oxygen species by phagocytic cells in response to live parasites in mice infected with Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 56329-334. [DOI] [PubMed] [Google Scholar]
- 9.Chang, P. Y., T. L. Wu, C. C. Hung, K. C. Tsao, C. F. Sun, L. L. Wu, and J. T. Wu. 2006. Development of an ELISA for myeloperoxidase on microplate: normal reference values and effect of temperature on specimen preparation. Clin. Chim. Acta 373158-163. [DOI] [PubMed] [Google Scholar]
- 10.Chen, L., T. Watanabe, H. Watanabe, and F. Sendo. 2001. Neutrophil depletion exacerbates experimental Chagas' disease in BALB/c, but protects C57BL/6 mice through modulating the Th1/Th2 dichotomy in different directions. Eur. J. Immunol. 31265-275. [DOI] [PubMed] [Google Scholar]
- 11.Cross, A. R., J. L. Yarchover, and J. T. Curnutte. 1994. The superoxide-generating system of human neutrophils possesses a novel diaphorase activity. Evidence for distinct regulation of electron flow within NADPH oxidase by p67-phox and p47-phox. J. Biol. Chem. 26921448-21454. [PubMed] [Google Scholar]
- 12.de Oliveira, T. B., R. C. Pedrosa, and D. W. Filho. 2007. Oxidative stress in chronic cardiopathy associated with Chagas disease. Int. J. Cardiol. 116357-363. [DOI] [PubMed] [Google Scholar]
- 13.Dhiman, M., E. S. Nakayasu, Y. H. Madaiah, J.-J. Wen, I. C. Almeida, and N. J. Garg. 2008. Enhanced nitrosative stress during Trypanosoma cruzi infection causes nitrotyrosine modification of host proteins: implications in Chagas disease. Am. J. Pathol. 173728-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg, N. 2005. Mitochondrial disorders in chagasic cardiomyopathy. Front. Biosci. 101341-1354. [DOI] [PubMed] [Google Scholar]
- 15.Gomes, J. A., L. M. Bahia-Oliveira, M. O. Rocha, O. A. Martins-Filho, G. Gazzinelli, and R. Correa-Oliveira. 2003. Evidence that development of severe cardiomyopathy in human Chagas' disease is due to a Th1-specific immune response. Infect. Immun. 711185-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartl, D., S. Krauss-Etschmann, B. Koller, P. L. Hordijk, T. W. Kuijpers, F. Hoffmann, A. Hector, F. Eber, V. Marcos, I. Bittmann, O. Eickelberg, M. Griese, and D. Roos. 2008. Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. J. Immunol. 818053-8067. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez, S. M., R. A. Kolliker-Frers, M. S. Sanchez, G. Razzitte, R. D. Britos, M. E. Fuentes, and M. N. Schwarcz de Tarlovsky. 2009. Antiproliferative effect of sera from chagasic patients on Trypanosoma cruzi epimastigotes. Involvement of xanthine oxidase. Acta Trop. 109219-225. [DOI] [PubMed] [Google Scholar]
- 18.Kaneda, H., J. Taguchi, K. Ogasawara, T. Aizawa, and M. Ohno. 2002. Increased level of advanced oxidation protein products in patients with coronary artery disease. Atherosclerosis 162221-225. [DOI] [PubMed] [Google Scholar]
- 19.Kirchhoff, L. V., L. M. Weiss, M. Wittner, and H. B. Tanowitz. 2004. Parasitic diseases of the heart. Front. Biosci. 9706-723. [DOI] [PubMed] [Google Scholar]
- 20.Klebanoff, S. J. 1999. Myeloperoxidase. Proc. Assoc. Am. Physicians 111383-389. [DOI] [PubMed] [Google Scholar]
- 21.Macao, L. B., D. W. Filho, R. C. Pedrosa, A. Pereira, P. Backes, M. A. Torres, and T. S. Frode. 2007. Antioxidant therapy attenuates oxidative stress in chronic cardiopathy associated with Chagas' disease. Int. J. Cardiol. 12343-49. [DOI] [PubMed] [Google Scholar]
- 22.Malvezi, A. D., R. Cecchini, F. de Souza, C. E. Tadokoro, L. V. Rizzo, and P. Pinge-Filho. 2004. Involvement of nitric oxide (NO) and TNF-alpha in the oxidative stress associated with anemia in experimental Trypanosoma cruzi infection. FEMS Immunol. Med. Microbiol. 4169-77. [DOI] [PubMed] [Google Scholar]
- 23.Marnett, L. J. 2000. Oxyradicals and DNA damage. Carcinogenesis 21361-370. [DOI] [PubMed] [Google Scholar]
- 24.Mazariego-Arana, M. A., V. M. Monteon, M. A. Ballinas-Verdugo, N. Hernandez-Becerril, R. Alejandre-Aguilar, and P. A. Reyes. 2001. Seroprevalence of human Trypanosoma cruzi infection in different geographic zones of Chiapas, Mexico. Rev. Soc. Bras. Med. Trop. 34453-458. [DOI] [PubMed] [Google Scholar]
- 25.Melo, R. C., D. L. Fabrino, H. D'Avila, H. C. Teixeira, and A. P. Ferreira. 2003. Production of hydrogen peroxide by peripheral blood monocytes and specific macrophages during experimental infection with Trypanosoma cruzi in vivo. Cell Biol. Int. 27853-861. [DOI] [PubMed] [Google Scholar]
- 26.Munoz-Fernandez, M. A., M. A. Fernandez, and M. Fresno. 1992. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-alpha and IFN-gamma through a nitric oxide-dependent mechanism. Immunol. Lett. 3335-40. [DOI] [PubMed] [Google Scholar]
- 27.Naviliat, M., G. Gualco, A. Cayota, and R. Radi. 2005. Protein 3-nitrotyrosine formation during Trypanosoma cruzi infection in mice. Braz. J. Med. Biol. Res. 381825-1834. [DOI] [PubMed] [Google Scholar]
- 28.Noyan, T., A. Güler, M. R. Sekeroğlu, and M. Kamaci. 2006. Serum advanced oxidation protein products, myeloperoxidase and ascorbic acid in pre-eclampsia and eclampsia. Aust. N. Z. J. Obstet. Gynaecol. 46486-491. [DOI] [PubMed] [Google Scholar]
- 29.Pinge-Filho, P., J. P. Peron, T. R. de Moura, R. A. Menolli, V. K. Graça, D. Estevão, C. E. Tadokoro, J. V. Jankevicius, and L. V. Rizzo. 2005. Protective immunity against Trypanosoma cruzi provided by oral immunization with Phytomonas serpens: role of nitric oxide. Immunol. Lett. 96283-290. [DOI] [PubMed] [Google Scholar]
- 30.Plata, F., F. Garcia Pons, and H. Eisen. 1984. Antigenic polymorphism of Trypanosoma cruzi: clonal analysis of trypomastigote surface antigens. Eur. J. Immunol. 14392-409. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez, B., V. Monteón, P. A. Reyes, and B. Espinoza. 2001. Standardization of micro-enzyme-linked immunosorbent assay (ELISA) and Western blot for detection of Trypanosoma cruzi antibodies using extracts from Mexican strains as antigens. Arch. Med. Res. 32382-408. [DOI] [PubMed] [Google Scholar]
- 32.Schopfer, F. J., P. R. Baker, and B. A. Freeman. 2003. NO-dependent protein nitration: a cell signaling event or an oxidative inflammatory response? Trends Biochem. Sci. 28646-654. [DOI] [PubMed] [Google Scholar]
- 33.Silva, J. S., F. S. Machado, and G. A. Martins. 2003. The role of nitric oxide in the pathogenesis of Chagas disease. Front. Biosci. 8s314-s325. [DOI] [PubMed] [Google Scholar]
- 34.Terada, L. S., J. A. Leff, and J. E. Repine. 1990. Measurement of xanthine oxidase in biological tissues. Methods Enzymol. 186651-666. [DOI] [PubMed] [Google Scholar]
- 35.Van Der Vliet, A., M. N. Nguyen, M. K. Shigenaga, J. P. Eiserich, G. P. Marelich, and C. E. Cross. 2000. Myeloperoxidase and protein oxidation in cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 279L537- L546. [DOI] [PubMed] [Google Scholar]
- 36.Vespa, G. N., F. Q. Cunha, and J. S. Silva. 1994. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect. Immun. 625177-5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinbroum, A., V. G. Nielsen, S. Tan, S. Gelman, S. Matalon, K. A. Skinner, E. Jr. Bradley, and D. A. Parks. 1995. Liver ischemia-reperfusion increases pulmonary permeability in rat: role of circulating xanthine oxidase. Am. J. Physiol. 268G988-G996. [DOI] [PubMed] [Google Scholar]
- 38.Wen, J.-J., V. Bhatia, V. L. Popov, and N. J. Garg. 2006. Phenyl-alpha-tert-butyl nitrone reverses mitochondrial decay in acute Chagas disease. Am. J. Pathol. 1691953-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen, J.-J., and N. Garg. 2004. Oxidative modifications of mitochondrial respiratory complexes in response to the stress of Trypanosoma cruzi infection. Free Radic. Biol. Med. 372072-2081. [DOI] [PubMed] [Google Scholar]
- 40.Wen, J.-J., G. Vyatkina, and N. Garg. 2004. Oxidative damage during chagasic cardiomyopathy development: role of mitochondrial oxidant release and inefficient antioxidant defense. Free Radic. Biol. Med. 371821-1833. [DOI] [PubMed] [Google Scholar]
- 41.Wen, J.-J., P. C. Yachelini, A. Sembaj, R. E. Manzur, and N. Garg. 2006. Increased oxidative stress is correlated with mitochondrial dysfunction in chagasic patients. Free Radic. Biol. Med. 41270-276. [DOI] [PubMed] [Google Scholar]
- 42.Wen, J.-J., M. Dhiman, E. B. Whorton, and N. J. Garg. 2008. Tissue-specific oxidative imbalance and mitochondrial dysfunction during Trypanosoma cruzi infection in mice. Microbes Infect. 101201-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White, C. R., V. Darley-Usmar, W. R. Berrington, M. McAdams, J. Z. Gore, J. A. Thompson, D. A. Parks, M. M. Tarpey, and B. A. Freeman. 1996. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc. Natl. Acad. Sci. USA 938745-8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winterbourn, C. C., and A. J. Kettle. 2000. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic. Biol. Med. 29403-409. [DOI] [PubMed] [Google Scholar]
- 45.Witko-Sarsat, V., M. Friedlander, C. Capeillère-Blandin, T. Nguyen-Khoa, A. T. Nguyen, J. Zingraff, P. Jungers, and B. Descamps-Latscha. 1996. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 491304-1313. [DOI] [PubMed] [Google Scholar]
- 46.Woods, A. A., S. M. Linton, and M. J. Davies. 2003. Detection of HOCl mediated protein oxidation products in the extracellular matrix of human atherosclerotic plaques. Biochem. J. 370729-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. 2002. Control of Chagas disease: second report of the W. H. O. expert committee. UNDP/World Bank/WHO, Geneva, Switzerland.
- 48.Zacks, M. A., J.-J. Wen, G. Vyatkina, V. Bhatia, and N. Garg. 2005. An overview of chagasic cardiomyopathy: pathogenic importance of oxidative stress. Ann. Acad. Bras. Cienc. 77695-715. [DOI] [PubMed] [Google Scholar]