Abstract
Current diagnosis of human flaviviral infections relies heavily on serological techniques such as the immunoglobulin M (IgM) antibody capture enzyme-linked immunosorbent assay (MAC-ELISA). Broad application of this assay is hindered by a lack of standardized human positive-control sera that react with the wide variety of flaviviruses that can cause human disease, e.g., dengue virus (DENV), West Nile virus (WNV), yellow fever virus (YFV), Japanese encephalitis virus (JEV), and St. Louis encephalitis virus (SLEV). We have created a human-murine chimeric antibody combining the variable regions of the broadly flavivirus cross-reactive murine monoclonal antibody (MAb) 6B6C-1 and the constant region of human IgM to produce a standardized reagent capable of replacing human positive-control sera in a MAC-ELISA for the diagnosis of all human flaviviral infections. The human-murine chimeric IgM antibody secreted from plasmid-transformed Sp2/0-Ag14 cells had a level of serological activity identical to that of 6B6C-1 as measured by ELISA, immunoblotting, and MAC-ELISA for multiple members of the flavivirus genus, including WNV, SLEV, YFV, DENV, and JEV.
Flaviviruses are positive-stranded RNA viruses, members of the family Flaviviridae, and are responsible for a number of medically important human diseases. Flaviviruses are arthropod-borne viruses (arboviruses) that are most commonly transmitted seasonally and in specific geographic locations. In the United States, flaviviruses are transmitted mainly by mosquitoes (17, 21). More than 70 different flaviviruses are known to exist; however, the majority are not currently associated with human disease (21). The tenuous nature of the status quo was proven by the emergence of West Nile virus (WNV) in the western hemisphere. Prior to the 1999 outbreak of WNV encephalitis in New York City, St. Louis encephalitis virus (SLEV) was the most important agent of epidemic viral encephalitis in North America, last causing a major epidemic in the mid-1970s (22, 24, 28). Since 1999, the distribution of WNV has rapidly expanded from New York to the rest of the United States and into Canada and Central and South America. As of August 2008, 27,841 human WNV cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC) (http://www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount08_detailed.htm). Given the globalization of commerce and travel, virus-infected people, animals, and arthropod vectors are able to move easily between distant locations with great speed (13). Thus, it is likely that other arboviruses will follow the example of WNV, resulting in new or novel disease outbreaks in regions of the world outside their normal geographic ranges. Because of this, a rapid and standardized approach to the identification of arboviral infections is needed, worldwide, for the diagnosis and tracking of current and reemerging arboviral diseases.
The most commonly employed serological technique for the diagnosis of human flaviviral infections is the immunoglobulin M (IgM) antibody capture enzyme-linked immunosorbent assay (MAC-ELISA), which detects virus-reactive IgM, an effective marker of acute viral infection (17, 18, 21), in serum or cerebrospinal fluid from a person with a clinically compatible illness. In the diagnostic setting, the MAC-ELISA and a similar ELISA measuring virus-reactive human IgG are often used in tandem to provide clear diagnostic profiles (9, 13, 18).
Application of the MAC-ELISA in the serodiagnosis of flaviviral infection is hampered by the limited availability of human infection-immune sera for use as virus-reactive, antibody-positive control specimens. For the most part, antibody-positive control sera are derived from small volumes of diagnostic serum specimens. The specimens are typically collected only from the most prevalent flaviviral agents (17, 18). The lot-to-lot variability of these specimens can be high, and constant recalibration of antibody-positive and negative-control sera is necessary to ensure that test parameters remain valid (8, 18). Of even greater concern is the lack of broadly cross-reactive antibody-positive control sera that can be used in the MAC-ELISA for the identification of atypical flaviviral infections (17).
The availability of a flavivirus group-reactive human IgM antibody would be a tremendous asset in the serological diagnosis of flaviviral infections. Although a number of murine monoclonal antibodies (MAbs) demonstrating flavivirus group reactivity exist, they are unsuitable for use in the human MAC-ELISA. Fortunately, advances in the humanization of murine MAbs have made it possible to overcome these limitations (25). One such method, described by Hackett et al., involves the incorporation of the heavy (H)- and light (L)-chain variable (V) regions of a given murine MAb into an expression plasmid (pJH2-24-95B1; referred to below as pJH2) that contains the constant (Cμ) region of human IgM (8). Upon transfection of cells, the resulting plasmid construct expresses a human-murine hybrid (chimeric) IgM molecule that retains the specificity of the “parent” murine MAb but reacts like human IgM in the MAC-ELISA (8, 10).
In this report we describe the development and characterization of such a human-murine chimeric IgM antibody prepared by using the IgM expression plasmid described by Hackett et al. (8). This chimeric IgM was created by incorporating the V regions of the broadly flavivirus cross-reactive murine MAb 6B6C-1 into a plasmid construct containing the human IgM μ chain. The murine MAb 6B6C-1 was originally raised against SLEV and is specific for the flaviviral envelope (E) protein (19, 23). The flavivirus group reactivity of chimeric 6B6C-1 IgM was confirmed, and the chimeric 6B6C-1 IgM was evaluated in the standard MAC-ELISA and shown to be a satisfactory replacement for antibody-positive human control sera against all flaviviruses tested.
MATERIALS AND METHODS
Cell lines.
Sp2/0-Ag14 (Sp2) murine myeloma and 6B6C-1 murine hybridoma cells and their growth conditions have been described previously (7, 10, 23). Cells were propagated in hybridoma growth medium (HGM; high-glucose Dulbecco's modified Eagle medium containing l-glutamine supplemented with 20% fetal bovine serum, 1 mM sodium pyruvate, 2 mM l-glutamine, 0.15% sodium bicarbonate, 100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, and 0.1 mM nonessential amino acids) unless noted otherwise.
Isolation of immunoglobulin V regions.
A Pharmacia QuickPrep mRNA purification kit (Amersham, Pharmacia, Piscataway, NJ) was used according to the manufacturer's specifications to isolate mRNA from 5 × 107 hybridoma cells. PCR cloning of immunoglobulin V regions was performed as previously described (8, 14a) using the first-round PCR primers listed in Table 1. PCR-derived products were isolated with the QIAquick PCR purification kit (Qiagen Inc., Valencia, CA), cloned into pCR2.1-TOPO (Invitrogen, Baltimore, MD), and subsequently used to chemically transform competent Escherichia coli TOP10 cells (Invitrogen) by following the manufacturer's protocol.
TABLE 1.
Oligonucleotide primers for the isolation and modification of V regions
| Primer name | Sequencea |
|---|---|
| First-round primers | |
| M-IgG2a | 5′ ATTCGGATAGATCTAGTGGATAGACCGATGG 3′ |
| MK-REV | 5′ ATTCGGATAGATCTTGGATGGTGGGAAGATG 3′ |
| MHV-9 | 5′ ACTAGTCGACATGGATTGGGTGTGGACCTGCTATTCCTG 3′ |
| MKV-9 | 5′ ACTAGTCGACATGGTATCCACACCTCAGTTCCTTG 3′ |
| Second-round primers | |
| VK-5′ | 5′ TCACGAAGTCTAGACTGGACATGGTATCCCACTCAGTTCCTTG 3′ |
| VK-3′ | 5′ GAATCTATGGATCCTGACACACTTACGTTTCAGCTCCAGCTTGGTCCCAGC 3′ |
| VH-5′ | 5′ ACACTATACTCGAGACATCATGGCTTGGGTGTGGACCTTGCTAT 3′ |
| VH-3′ | 5′ TTCAGATCAAGCTTGACACACTTACCTGAGGAGACGGTGACTGAGGTTCCT 3′ |
Restriction endonuclease sites are underlined.
Modification of V regions.
Variable light/kappa (VK) and heavy (VH) regions of 6B6C-1 were further modified by a second round of PCR (Table 1) in order to add partial 5′ leader sequences, 3′ splice donor junctions, and appropriate restriction sites as previously described (8). In cases where degenerate positions were present in the amplification primers, the sequence was modified, if necessary, based on comparisons to leader sequences in the International ImMunoGeneTics Information System (http://imgt.cines.fr) and Mouse Genome Informatics (http://www.informatics.jax.org/) databases. PCR amplifications were performed using Platinum PCR SuperMix—High Fidelity (Invitrogen) and consisted of a single step of 94°C for 2 min (hot start) followed by 28 cycles of 94°C for 30 s, 55°C for 30 s, and 68°C for 30 s. Reactions were completed after a final elongation step at 72°C for 5 min. Primers (5′ and 3′) were provided by the CDC Biotechnology Core Facility (Atlanta, GA), and each reaction mixture contained 0.1 μM (final concentration) either primer. Plasmid DNA (V regions ligated to pCR2.1-TOPO) was purified from transformed E. coli TOP10 by use of a QIAprep miniprep kit (Qiagen) and served as template DNA (200 ng/reaction). PCR-derived products were isolated and cloned into pCR2.1-TOPO as described above.
Assembly of human-murine chimeric IgM plasmid constructs.
The VH and VK regions of 6B6C-1 were incorporated into the human IgM expression construct pJH2 (provided by Abbott Laboratories, Abbott Park, IL) by ligation as previously described (8), generating plasmid pJH-6M (6B6C-1:IgM). Plasmid pJH-6M was used to transform E. coli DH5αE (Invitrogen) by electroporation according to the manufacturer's protocol.
Sequencing.
V regions were sequenced in triplicate to ensure sequence fidelity after initial isolation and again after PCR modification. Sequencing reactions were performed using the BigDye Terminator cycle sequencing kit (version 3.1; Applied Biosystems, Foster City, CA), and sequence data were analyzed using the ABI 3130xl genetic analyzer (Applied Biosystems).
Transfection of cells with human-murine chimeric Ig plasmid constructs.
Exponentially growing Sp2 cells were harvested and centrifuged at 2,000 rpm for 15 min at 4°C. Cells were resuspended in 10 ml of sterile phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4 [pH 7.2]), and the density was readjusted to 5 × 106 cells/ml. Aliquots (4.5 × 106 cells in 900 μl) of cells were added to prechilled electroporation cuvettes with a 0.4-cm gap (Bio-Rad, Hercules, CA). Circular plasmid DNA (10 to 30 μg) was added to each cuvette and allowed to incubate on ice for 10 min. Cells were electroporated using a Bio-Rad Gene Pulser Xcell system (250 V, 975 μF, 23 to 27 ms) and were subsequently placed on ice for 10 min. Pulsed cells were washed in 10 ml of prechilled HGM (4°C) and resuspended in HGM (at 25°C) at a density of 1 × 105 cells/ml. Cells were dispensed into tissue culture-treated 96-well microtiter plates in 100-μl aliquots (∼1 × 104 cells/well) and incubated at 37°C under 5% CO2. At 48 h postelectroporation (PE), 100 μl of selective medium (HGM supplemented with 0.1 μm methotrexate) was added to each well. At 5 days PE, 100 μl of medium was removed from each well and replaced with 100 μl of fresh selective medium. Wells containing actively growing transfected cells were expanded and screened for the production of human-murine chimeric IgM antibody (pJH-6M IgM) by MAC-ELISA.
Detection of chimeric IgM in cell culture supernatants.
Immulon II HB flat-bottom 96-well plates (Dynatech Industries, Inc., Chantilly, VA) were coated with 75 μl of goat anti-human IgM (Fc5μ) (Jackson Immunoresearch) diluted 1:1,000 in coating buffer (0.015 M sodium carbonate, 0.035 M sodium bicarbonate [pH 9.6]). Plates were washed five times with wash buffer (PBS-T, consisting of PBS and 0.5% Tween 20) and were subsequently blocked with 200 μl of blocking buffer (PBS-T with 5% goat serum) for 1 h at 25°C. The cell culture supernatant containing pJH-6M IgM or purified human IgM (ChromPure human IgM, whole molecule; Jackson Immunoresearch), either at a stock concentration of 4.4 mg/ml or serially diluted twofold in PBS-T, was applied to wells (50 μl/well) and allowed to incubate at 37°C for 1.5 h. A secondary antibody consisting of alkaline phosphatase-conjugated goat anti-human IgM (heavy chain; Jackson Immunoresearch, West Grove, PA) diluted 1:5,000 in PBS-T was added to each well (50 μl/well) and allowed to incubate for 1 h at 37°C. The substrate (Sigma Fast p-nitrophenyl phosphate tablet sets; Sigma Aldrich, St. Louis, MO) was added to each well (75 μl/well) and allowed to incubate for 30 min at 25°C. The A405 was read using an ELx808 absorbance microplate reader (BioTek Instruments Inc., Winooski, VT).
The A405 of the chimeric cell culture supernatant was used to extrapolate the concentration of pJH-6M IgM in the transfected Sp2 cell culture supernatant from a standard curve constructed from A405 values obtained from serially diluted human IgM (ChromPure human IgM, whole molecule) at known concentrations.
Antigens for MAC-ELISA. (i) Viral seed antigen.
Virus-infected cell culture supernatants (virus seed) for multiple flaviviruses were obtained from the Diagnostic and Reference Laboratory (DRL), Arbovirus Diseases Branch (ADB), Division of Vector-Borne Infectious Diseases (DVBID), CDC, Fort Collins, CO. Viral seeds for WNV (strain NY99), dengue virus type 2 (DENV-2) (16681-30 PA), SLEV (MSI-7), Japanese encephalitis virus (JEV) (SA14-14-2), and yellow fever virus (YFV) (17D) were uniformly diluted to 1.0 × 107 PFU/ml in cell culture maintenance medium (HGM supplemented with 2% fetal bovine serum). Viral seeds for WNV, YFV, JEV, and SLEV were originally propagated in Vero cells; DENV-2 was propagated in C636 cells.
(ii) SMB and VLP antigens.
SLEV (strain TBH-28) and YFV (17D) suckling mouse brain (SMB) antigens were prepared as sucrose-acetone extracts of infected murine brain tissue for use in the MAC-ELISA. Lyophilized preparations of DENV-2, WNV, and JEV virus-like particles (VLPs), partially purified from COS-1 cell cultures, were also used as antigens in the MAC-ELISA format. Antigens were independently titrated against an antibody-positive control serum sample using a twofold dilution series and were standardized by selecting a dilution that yielded A450 values between 0.8 and 1.3 for WNV, JEV, and DENV-2 or >0.5 for SLEV and YFV. All viral antigens, as well as corresponding normal antigens (normal SMB or COS-1 antigens), were obtained from the DVBID reference collection and have been described previously (1-3, 11).
Immunoblotting.
The recombinant WNV-VLP antigen was electrophoresed under denaturing conditions on a NuPAGE Novex 12% Bis-Tris gel (Invitrogen) and transferred to a 0.45-μm-pore-size nitrocellulose membrane for use in immunoblotting as previously described (5, 26). Cell culture supernatants containing either pJH-6M IgM or the murine IgG MAb 6B6C-1 were assayed at a dilution of 1:1,500. Goat anti-mouse IgG (heavy and light chains) or goat-anti-human IgM (Fc5μ) (Jackson Immunoresearch) antibodies conjugated to alkaline phosphatase were used as detecting antibodies at dilutions of 1:5,000.
MAC-ELISA screening of transfectants for production of flavivirus-specific chimeric IgM.
To detect the presence of an antiviral IgM antibody in cell culture supernatants or human sera, a modification of the MAC-ELISA protocol, originally described by Martin et al., was used (9, 18, 20). Briefly, Immulon II HB flat-bottom 96-well plates were coated at 4°C overnight with 75 μl of goat anti-human IgM (Fc5μ) diluted 1:1,000 in coating buffer. Wells were blocked with 200 μl of blocking buffer for 1 h at 25°C. Human sera for use as positive or negative antibody controls comprised pooled or individual serum samples obtained from the CDC DRL that are typical of the human serum controls commonly employed by the DRL for arboviral diagnostic testing. Human sera or cell culture supernatants from pJH-6M-transfected cells were diluted in wash buffer, added to wells (50 μl/well), and subsequently incubated at 37°C for 1.5 h. Positive-control sera were diluted in PBS-T to produce dilutions that yielded A450 values between 0.8 and 1.3 for WNV, JEV, and DENV-2 or >0.5 for SLEV and YFV. Human negative-control sera were diluted 1:400 as previously recommended (8). Cell culture supernatants containing human-murine chimeric IgM were diluted 1:50 in PBS-T prior to serial twofold titration in PBS-T. Flaviviral antigens (in the form of VLPs, SMBs, or viral seed) were added to each well (50 μl/well) and incubated overnight at 4°C. A secondary antibody consisting of horseradish peroxidase-conjugated 6B6C-1 diluted 1:3,000 in blocking buffer was added to each well (50 μl/well), and plates were incubated at 37°C for 1 h. The bound conjugate was detected after the addition of 3,3′,5,5′-tetramethylbenzidine (Enhanced K-Blue TMB substrate; Neogen Corp., Lexington, KY) substrate (75 μl/well), followed by incubation at 25°C for 10 min. The substrate reaction was stopped by the addition of 1 N H2SO4 (50 μl/well), and the A450 was measured. All ELISAs were performed in triplicate.
Test validation and calculation of P/N ratios.
Test validation was performed, and positive-to-negative (P/N) ratios were determined, according to the procedure of Martin et al. (18) using internal positive and negative serum controls. The N value for each viral antigen or seed was defined as the average A450 for normal human serum reacted with a given viral antigen or seed. The P value of pJH-6M IgM for each flavivirus antigen or viral seed was determined to be the average A450 at the maximum dilution at which a P/N value of 3 or greater was obtained for a given positive antigen.
Bicinchoninic acid assay.
Protein concentrations were determined using the Pierce bicinchoninic acid protein assay kit (Pierce Biotechnology Inc., Rockford, IL).
CDR analysis.
Complementarity-determining regions (CDRs) were determined using the Kabat method (14).
Nucleotide sequence accession numbers.
The 6B6C-1 V region sequences were assigned GenBank accession numbers as follows: 6B6C-1 VK, FJ234927; 6B6C-1 VH, FJ234928.
RESULTS
Cloning and sequencing of murine MAb 6B6C-1 V regions.
In order to generate a human-murine chimeric IgM, the hybridoma secreting the flavivirus group-reactive murine MAb 6B6C-1 was grown in cell culture. MAb 6B6C-1 has been used extensively in immunoassays for the detection of flaviviruses (12, 13, 18, 22). Prior to cloning, the flavivirus group reactivity of 6B6C-1 was confirmed by an immunofluorescence assay using WNV- or YFV-infected cells (data not shown). The VH and VK regions of 6B6C-1 were cloned by reverse transcription-PCR using a combination of degenerate primers that annealed to conserved VH and VK gene leader sequences and C region-specific primers (8, 14a). Multiple clones of each V gene product were sequenced to avoid possible DNA polymerase-induced errors.
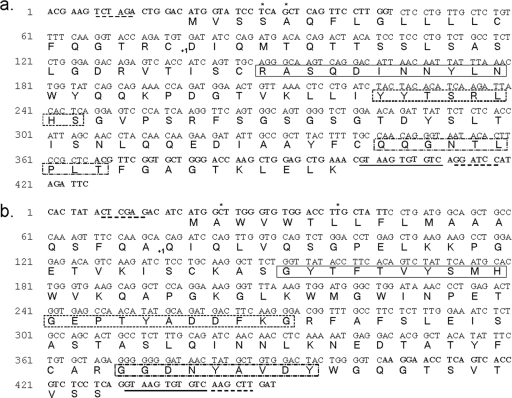
The 6B6C-1 VH and VK cDNAs were sequenced, and a consensus of multiple sequence determinations was derived (Fig. 1a and b, respectively). Consensus sequences for both the VH and VK regions were compared to similar murine genes sharing >90% identity obtained from a “blastn” search of the Mouse Genome Database. Upon sequence comparison, two nucleotide substitutions in the leader sequence of the VK gene were shown to reflect changes incorporated by degenerate primers used during the first round of PCR. A similar nucleotide change and a nucleotide insertion were discovered in the empirically determined VH consensus sequence after sequence homologues provided by the Mouse Genome Database were consulted.
FIG. 1.
Nucleotide and deduced amino acid sequences of the pJH-6M V regions. Restriction endonuclease sites are indicated by dashed underlining; the J chain splicing region has a solid underline. Nucleotides introduced by second-round PCR primers are in boldface; nucleotides introduced by degenerate primers and changed in second-round PCR primers to reflect murine germ line genes are asterisked. +1, start of mature protein; solid-line box, CDR 1; dashed box, CDR 2; dashed-and-dotted box, CDR 3. (a) pJH-6M VK region derived from 6B6C-1. (b) pJH-6M VH region derived from 6B6C-1.
Assembly of the human-murine chimeric IgM plasmid construct (pJH-6M).
The 6B6C-1 V regions were further modified by a second round of PCR to prepare VK and VH cDNAs for insertion into the human IgM expression plasmid pJH2. The primers used in this second-round PCR were designed to correct nucleotide substitutions in the leader sequences introduced by the degenerate primers (see above) used during the first round of PCR and to incorporate 3′ splice donor junctions in order to ensure correct expression of the 6B6C-1 murine V regions with the human Cμ region functional splice acceptor sites located in pJH2. The second-round PCR primers also added appropriate restriction endonuclease sites at either end of each V region to permit subsequent cloning of the finished VK and VH inserts into pJH2. The pJH2 plasmid contains genomic clones of the human kappa (CΚ) and IgM (Cμ) C-region genes, both of which are controlled by a metallothionein I promoter and a mouse immunoglobulin H-chain enhancer. Plasmid pJH2 also contains an altered dihydrofolate reductase gene that allows for selective growth in media containing methotrexate. The 6B6C-1 V regions were cloned into pJH2, forming plasmid pJH-6M.
Expression of a human-murine chimeric IgM antibody.
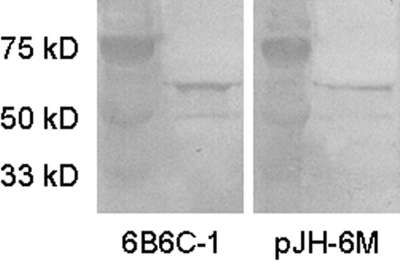
Wells containing Sp2 cells transfected with plasmid pJH-6M that demonstrated signs of growth were expanded beginning approximately 14 days PE. Cell-free supernatants of expanded Sp2 cells were analyzed by MAC-ELISA for the presence of chimeric IgM (pJH-6M IgM). The culture supernatant from a single transfectant, 6ME2, that tested positive for the presence of pJH-6M IgM was assayed by immunoblotting for specific anti-WNV reactivity with a recombinant VLP (WNV-VLP). The 6ME2 supernatant reacts with the E protein of the WNV-VLP in a manner identical to that of the parent murine MAb 6B6C-1 (Fig. 2). Taken together, these results indicate that the 6ME2 supernatant contains an IgM antibody composed of human heavy and Fc5μ regions that mimics the murine 6B6C-1 IgG MAb in WNV E protein reactivity. Quantitative analysis of the 6ME2 supernatant indicated a chimeric IgM concentration of 12.45 μg/ml, approximately 0.11% of the total protein content of the 6ME2 supernatant. Sp2 cells expressing the 6ME2 IgM chimeric antibody that were passaged repeatedly (more than five times) in methotrexate-free growth medium and subjected to multiple freeze-thaw cycles demonstrated excellent plasmid retention and chimeric IgM expression (data not shown).
FIG. 2.
Reactivity of a chimeric antibody with WNV-VLP. A human-murine chimeric IgM antibody from cells transfected with pJH-6M was compared to the “parent” MAb 6B6C-1 for the ability to detect the recombinant WNV COS-1 antigen by immunoblotting. The chimeric IgM antibody reacts with the E glycoprotein (∼55 kDa) of the WNV-VLP in a manner identical to that of 6B6C-1.
Flavivirus group reactivity of the chimeric 6ME2 IgM.
The chimeric 6ME2 IgM was assayed for anti-flavivirus group reactivity by MAC-ELISA using viral seed cultures or viral antigens of five prominent members of the family Flaviviridae. The 6ME2 chimeric IgM supernatant, at a dilution of 1/1,200, reacted positively (P/N ratio, >3.0) to all viral seeds tested (Table 2). The chimeric 6ME2 IgM demonstrated the highest reactivities with YFV and SLEV seed antigens, with P/N ratios greater than 3.0 at dilutions of 1/4,800 and 1/2,400, respectively. The 6ME2 IgM also showed high reactivities with YFV and JEV seed antigens, as measured by the high P/N ratios obtained with the optimally diluted supernatant against YFV seed (18.38) and JEV seed (18.10).
TABLE 2.
Reactivities of 6ME2 IgM with flaviviral seed antigens by MAC-ELISA
| Flavivirus | Strain | Avg A450 for NHSa at an antibody dilution of 1:400 | Maximum dilution giving a P/N ratio of >3.0 | P/N ratio at:
|
Optimum dilution | |
|---|---|---|---|---|---|---|
| 1/1,200 | Optimum dilution | |||||
| WNV | NY99 | 0.135 | 1,200 | 5.07 | 9.78 | 200 |
| SLEV | MSI-7 | 0.123 | 2,400 | 6.02 | 10.06 | 400 |
| DENV-2 | PA30 | 0.122 | 1,200 | 4.34 | 10.51 | 100 |
| JEV | SA-14-14-2 | 0.142 | 1,200 | 3.49 | 18.10 | 100 |
| YFV | 17D | 0.136 | 4,800 | 8.58 | 18.38 | 100 |
NHS, normal human serum.
The MAC-ELISA was also used to examine the activity of 6ME2 IgM against SMB or VLP antigens. The 6ME2 IgM reacted positively (P/N ratio, >3.0) with all flavivirus antigens tested at an antibody dilution of 1:100 (Table 3). The 6ME2 IgM showed the greatest reactivity against the JEV-VLP antigen (P/N ratio, >3.0 at an antibody dilution of 1:1,600) and a high reactivity for the WNV-VLP antigen (P/N ratio, 16.95) when optimally diluted (1:50).
TABLE 3.
Reactivity of 6ME2 IgM with flavivirus SMB or VLP antigens by MAC-ELISA
| Flavivirus | Antigen | Avg A450 for NHSa at an antibody dilution of 1:400 | Maximum dilution giving a P/N ratio of >3.0 | P/N ratio at:
|
Optimum dilution | |
|---|---|---|---|---|---|---|
| 1/100 | Optimum dilution | |||||
| WNV | VLP | 0.187 | 800 | 12.84 | 16.95 | 50 |
| SLEV | SMB | 0.080 | 800 | 5.88 | 6.23 | 50 |
| DENV-2 | VLP | 0.169 | 100 | 3.02 | 3.02 | 100 |
| JEV | VLP | 0.080 | 1600 | 5.02 | 5.45 | 400 |
| YFV | SMB | 0.111 | 800 | 3.54 | 4.59 | 400 |
NHS, normal human serum.
DISCUSSION
The rapid and sensitive MAC-ELISA has been employed to identify antiviral IgM, which appears early in infection, rises rapidly in the disease course, and is generally less virus cross-reactive than antiviral IgG (16, 18). The MAC-ELISA utilizes commercial sources of anti-human IgM capture antibody and broadly reactive murine antiviral MAb-enzyme conjugate detectors. These commercial reagents help to standardize the assay and make the protocols easily transferable (18).
Use of the MAC-ELISA in the diagnosis of flaviviral infections is hindered by the narrow range of flaviviral diseases currently being diagnosed or tracked by disease surveillance systems. This limitation results in the availability of adequate amounts of antibody-positive control sera only for the major flaviviral pathogens. Although the majority of flaviviruses have not yet been associated with human disease, it is obvious that the limited variety of existing control sera provides no avenue for discovering human disease caused by novel or atypical flaviviruses (17, 18).
Murine MAbs cannot be used as IgM serum controls in the human MAC-ELISA; therefore, a single standardized murine-human chimeric or humanized IgM reagent possessing broad flavivirus group reactivity would be an attractive alternative positive-control antibody for this test (8, 17). A number of techniques for engineering human antibodies have recently been described. Transgenic mouse strains carrying human heavy- and light-chain loci, the immortalization of human B cells through viral transformation, and the production of human hybridomas using new human fusion partner cell lines are all methods capable of producing human MAbs (6, 15, 27). Unfortunately, these methods do not facilitate the design of human MAbs of a defined specificity. A considerable amount of additional screening would be required to identify flaviviral group-reactive antibodies. An alternative to producing fully human MAbs is humanizing existing murine MAbs of known specificity. Flavivirus group-reactive murine MAbs, such as 6B6C-1 and 4G2, are already used in serological assays as capture antibodies and antibody-enzyme conjugate detectors, and they are likely candidates for humanization (12, 13, 17, 18, 22). Using the 6B6C-1 hybridoma and the pJH2 IgM expression vector, we prepared a human chimeric 6B6C-1 IgM, designated 6ME2 IgM, for use in the flavivirus human MAC-ELISA.
The chimeric 6ME2 IgM achieved positive P/N ratios with each flaviviral antigen tested in the MAC-ELISA, displaying a strong preference for the WNV-VLP antigen (at antibody dilutions of <1:100) and for JEV-VLP (at antibody dilutions of >1:800). Given the homogeneity of the 6B6C-1 epitope among all flaviviruses (4), the enhanced reactivity of 6ME2 IgM with the WNV or JEV VLP was likely due to the quality and concentration of the specific antigen lots (available from the CDC DRL) used in this assay rather than to the preference of the chimeric antibody for a specific flavivirus. In an attempt to standardize the antigen concentrations in the MAC-ELISA, we replaced the VLP or SMB antigens with a set concentration (1 × 107 PFU/ml) of cell culture flaviviral seed. In this test, the 6ME2 IgM reacted with all viral seeds assayed; however, the replacement of the SMB or VLP antigen with viral seed did result in enhanced reactivity with YFV and JEV at antibody dilutions of 1:100 and in an increase in the maximum antibody dilution capable of giving a P/N ratio of >3.0 for WNV, SLEV, DENV2, and YFV.
Samples of positive human infection-immune control serum were included in each MAC-ELISA performed, and the resulting P/N ratios were compared to those obtained with the chimeric 6ME2 IgM. Neither antibody provided superior reactivity against all flaviviruses tested (data not shown). These results were not unexpected, given the fact that positive human serum controls, typically used in the MAC-ELISA, are polyclonal in nature and naturally contain antibodies against multiple epitopes for each virus/antigen. Therefore, it is quite likely that the signal of a MAb-derived chimeric antibody specific for a single epitope of the E glycoprotein would be unable to quantitatively match the cumulative signal of a polyclonal positive human serum sample.
One possible complication with using a MAb-derived chimeric antibody as a positive-control reagent would be finding a flavivirus with an altered E glycoprotein that renders it nonreactive with the chimeric 6ME2 IgM antibody. The epitope defined by 6B6C-1 and other E protein-specific flavivirus group-reactive murine MAbs has recently been mapped to the E protein fusion loop (4). This sequence is highly conserved among all flaviviruses, probably because of its critical interaction with cell membranes during virus replication. Flaviviruses transmitted by vectors other than mosquitoes, such as tick-borne encephalitis virus and Powassan virus, are also recognized by the murine MAb 6B6C-1 in the MAC-ELISA format (R. S. Lanciotti, CDC DRL, personal communication) and thus should be reactive with the 6ME2 chimeric IgM. Given the group reactivity of 6B6C-1, the identification of new flaviviruses that are nonreactive with 6B6C-1 is unlikely. If such a virus were isolated, however, one solution would be to develop a combination of two different chimeric antibodies sharing flavivirus group reactivity but for separate epitopes. Additionally, since the current MAC-ELISA utilizes a 6B6C-1-enzyme conjugate detector, antibody reactivity with a virus with a mutation in the 6B6C-1 epitope would likely not be detected in the standard assay. The incorporation of human-murine chimeric MAbs in immunoassays that rely on variably reactive human sera as controls will provide diagnostic laboratories with an unlimited supply of control reagents of a set affinity and specificity. Also, the use of a positive control with the specificity of a MAb allows for better characterization of unknown specimens as opposed to the heterogeneous reactivities found in polyclonal sera. Hence, the 6ME2 IgM is a viable alternative to positive-control human sera for flavivirus detection by the MAC-ELISA. Currently we are engineering the 6B6C-1 V region into an expression vector that encodes the human IgG C region to produce a human-murine chimeric IgG 6B6C-1 analog for use as a flavivirus positive-control antibody in the IgG ELISA.
Acknowledgments
This work was supported in part by a postdoctoral fellowship award provided by the American Society for Microbiology and the Coordinating Center for Infectious Diseases.
We thank Amanda Panella (DRL) and Amanda Calvert (ADB) for providing the viral antigens used in this study.
Footnotes
Published ahead of print on 18 March 2009.
REFERENCES
- 1.Burke, D. S., A. Nisalak, D. E. Johnson, and R. M. Scott. 1988. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 38172-180. [DOI] [PubMed] [Google Scholar]
- 2.Chang, G. J., A. R. Hunt, and B. Davis. 2000. A single intramuscular injection of recombinant plasmid DNA induces protective immunity and prevents Japanese encephalitis in mice. J. Virol. 744244-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, G. J., A. R. Hunt, D. A. Holmes, T. Springfield, T. S. Chiueh, J. T. Roehrig, and D. J. Gubler. 2003. Enhancing biosynthesis and secretion of premembrane and envelope proteins by the chimeric plasmid of dengue virus type 2 and Japanese encephalitis virus. Virology 306170-180. [DOI] [PubMed] [Google Scholar]
- 4.Crill, W. D., and G. J. Chang. 2004. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J. Virol. 7813975-13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis, B. S., G. J. Chang, B. Cropp, J. T. Roehrig, D. A. Martin, C. J. Mitchell, R. Bowen, and M. L. Bunning. 2001. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 754040-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, G. C., M. L. Gallo, and J. R. F. Corvalan. 1999. Transgenic mice as a source of fully human antibodies for the treatment of cancer. Cancer Metastasis Rev. 18421-425. [DOI] [PubMed] [Google Scholar]
- 7.Hackett, J., Jr., J. Hoff-Velk, A. Golden, C. Dealwis, D. Ostrow, and W. Mandecki. 1997. The effect of site-specific mutagenesis of a cysteine residue on the stability of a monoclonal antibody, p. 133-157. In W. Hori (ed.), Antibody engineering II, vol. 2. New technology, application and commercialization. International Business Communications, Inc., Southborough, MA. [Google Scholar]
- 8.Hackett., J. Jr., J. Hoff-Velk, A. Golden, J. Brashear, J. Robinson, M. Rapp, M. Klass, D. H. Ostrow, and W. Mandecki. 1998. Recombinant mouse-human chimeric antibodies as calibrators in immunoassays that measure antibodies to Toxoplasma gondii. J. Clin. Microbiol. 361277-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes, D. A., D. E. Purdy, D. Y. Chao, A. J. Noga, and G. J. Chang. 2005. Comparative analysis of immunoglobulin M (IgM) capture enzyme-linked immunosorbent assay using virus-like particles or virus-infected mouse brain antigens to detect IgM antibody in sera from patients with evident flaviviral infections. J. Clin. Microbiol. 433227-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang, T. J., M. E. Reid, G. R. Halverson, and K. Yazdanbakhsh. 2003. Production of recombinant murine-human chimeric IgM and IgG anti-Jsb for use in the clinical laboratory. Transfusion 43758-764. [DOI] [PubMed] [Google Scholar]
- 11.Hunt, A. R., C. B. Cropp, and G. J. Chang. 2001. A recombinant particulate antigen of Japanese encephalitis virus produced in stably-transformed cells is an effective noninfectious antigen and subunit immunogen. J. Virol. Methods 97133-149. [DOI] [PubMed] [Google Scholar]
- 12.Hunt, A. R., R. A. Hall, A. J. Kerst, R. S. Nasci, H. M. Savage, N. A. Panella, K. L. Gottfried, K. L. Burkhalter, and J. T. Roehrig. 2002. Detection of West Nile virus antigen in mosquitoes and avian tissues by a monoclonal antibody-based capture enzyme immunoassay. J. Clin. Microbiol. 402023-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, A. J., D. A. Martin, N. Karabatsos, and J. T. Roehrig. 2000. Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J. Clin. Microbiol. 381827-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, G., and T. T. Wu. 2000. Kabat database and its applications: 30 years after the first variability plot. Nucleic Acids Res. 28214-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Jones, S. T., and M. M. Bendig. 1991. Rapid PCR-cloning of full-length mouse immunoglobulin variable regions. Biotechnology (New York) 9579. [DOI] [PubMed] [Google Scholar]
- 15.Kalantarov, G. F., S. A. Rudchenko, L. Lobel, and I. Trakht. 2002. Development of a fusion partner cell line for efficient production of human monoclonal antibodies from peripheral blood lymphocytes. Hum. Antibodies 1185-96. [PubMed] [Google Scholar]
- 16.Kuby, J. 1997. Antibodies, p. 399. In J. Kuby (ed.), Immunology, 3rd ed. W. H. Freeman and Co., New York, NY.
- 17.Lanciotti, R. S., and J. T. Roehrig. 2006. Arboviruses, p. 757-765. In B. Detrick, R. G. Hamilton, and J. D. Folds (ed.), Manual of molecular and clinical laboratory immunology, 7th ed. ASM Press, Washington, DC.
- 18.Martin, D. A., D. A. Muth, T. Brown, A. J. Johnson, N. Karabatsos, and J. T. Roehrig. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J. Clin. Microbiol. 381823-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathews, J. H., and J. T. Roehrig. 1984. Elucidation of the topography and determination of the protective epitopes on the E glycoprotein of Saint Louis encephalitis virus by passive transfer with monoclonal antibodies. J. Immunol. 1321533-1537. [PubMed] [Google Scholar]
- 20.Purdy, D. E., A. J. Noga, and G.-J. Chang. 2004. Noninfectious recombinant antigen for detection of St. Louis encephalitis virus-specific antibodies in serum by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 424709-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roehrig, J. T. 2000. Arboviruses, p. 356-373. In S. Specter, R. L. Hodinka, and S. A. Young (ed.), Clinical virology manual, 3rd ed. ASM Press, Washington, DC.
- 22.Roehrig, J. T., D. Nash, B. Maldin, A. Labowitz, D. A. Martin, R. S. Lanciotti, and G. L. Campbell. 2003. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg. Infect. Dis. 9376-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roehrig, J. T., J. H. Mathews, and D. W. Trent. 1983. Identification of epitopes on the E glycoprotein of Saint Louis encephalitis virus using monoclonal antibodies. Virology 128118-126. [DOI] [PubMed] [Google Scholar]
- 24.Roehrig, J. T., M. Layton, P. Smith, G. L. Campbell, R. Nasci, and R. S. Lanciotti. 2002. The emergence of West Nile virus in North America: ecology, epidemiology, and surveillance. Curr. Top. Microbiol. Immunol. 267223-240. [DOI] [PubMed] [Google Scholar]
- 25.Shin, S. U., and S. L. Morrison. 1989. Production and properties of chimeric antibody molecules. Methods Enzymol. 178459-476. [DOI] [PubMed] [Google Scholar]
- 26.Thibodeaux, B. A., A. R. Caballero, J. J. Dajcs, M. E. Marquart, L. S. Engel, and R. J. O'Callaghan. 2005. Pseudomonas aeruginosa protease IV: a corneal virulence factor of low immunogenicity. Ocul. Immunol. Inflamm. 13169-182. [DOI] [PubMed] [Google Scholar]
- 27.Traggiai, E., S. Becker, K. Subbarao, L. Kolesnikova, Y. Uematsu, M. R. Gismondo, B. R. Murphy, R. Rappuoli, and A. Lanzavecchia. 2004. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat. Med. 10871-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai, T. F., and T. P. Monath. 1987. Viral diseases in North American transmitted by arthropods from vertebrate reservoirs, p. 1417-1456. In R. D. Feigin and J. D. Cherry, (ed.), Textbook of pediatric infectious diseases. W. B. Saunders Co., Philadelphia, PA.