Abstract
A direct binding Luminex assay has been developed and validated for the detection of human immunoglobulin G (IgG) antibodies to the Staphylococcus aureus iron surface determinant B protein (IsdB) in serum following natural infection or immunization with investigational Saccharomyces cerevisiae-derived IsdB-based vaccines. To ensure that IsdB-specific IgG antibodies are measured following immunization with S. cerevisiae-derived IsdB, an Escherichia coli-produced IsdB antigen is used in the assay. The IsdB antigen is covalently conjugated to maleimide microspheres via an engineered carboxy-terminal cysteine residue. Antibody titers are determined in a direct binding format, where the phycoerythrin-labeled monoclonal antibody (HP6043) specific for IgG1 to IgG4 binds to human serum IgG antibodies. Fluorescent signal emitted from bound HP6043 is directly proportional to an individual's antibody levels. A pooled human reference serum from vaccinees with high titers to IsdB is used to generate a 12-point standard curve. The correlation of mean fluorescent intensity (MFI) units to μg/ml of IsdB-specific IgG is made by interpolating the MFI data through a four-parameter curve-fitting algorithm. The assay is sensitive to 1.06 μg/ml with a dynamic range of 2.1 to 10,625 μg/ml. The overall specificity of the assay is >96% and the linearity (parallelism) of the assay is −4% per 10-fold dilution. The total precision of the assay was 16.6% relative standard deviation across three different IsdB antigen lots, three different microsphere lots, two secondary antibody lots, and three different operators. The assay has proven useful for evaluating the immune response following the administration of different dosages and formulations of investigational IsdB-based vaccines.
Staphylococcus aureus is a gram-positive commensal bacterium of the nares and skin and is a common cause of both community-acquired and nosocomial disease (30). S. aureus infections can cause a spectrum of diseases, including impetigo, respiratory tract infections, toxic shock syndrome, food poisoning, endocarditis, septic arthritis, and scalded skin syndrome (6, 20). Up to 40% of children and adults can have transient, asymptomatic colonization with S. aureus (16). Due to the widespread use of antibiotics, numerous strains of S. aureus exhibit broad-spectrum antibiotic drug resistance. Multiple antibiotic-resistant strains are isolated in approximately 60% of community-acquired infections and upwards of 80% of nosocomial acquired infections (21).
An estimated 2 million patients develop nosocomial infections in the United States annually (31). In 2003 the hospital expenditures associated with S. aureus infections were estimated at $14.5 billion (32). Current estimates indicate that hospital-acquired S. aureus infections have dramatically increased over the past two decades (3, 8, 22, 24, 47). The increase in nosocomial infections has a direct impact on the health care industry in terms of length of stay, total hospitalization costs, and in-hospital mortality. An analysis of an inpatient sample database for the years 2000 and 2001 revealed that approximately 300,000 hospital inpatients were diagnosed with S. aureus infections prior to discharge. Additionally, inpatients diagnosed with S. aureus infections had five times the risk of hospital death (11.2% versus 2.3%) than S. aureus infection-free inpatients. With a continued rise of antibiotic-resistant S. aureus, inpatient diagnosis of infection represents a significant burden to both patients and the health care system.
S. aureus is adept at colonizing wounds and disabling the immune system by expressing factors such as protein A, which binds to the Fc region of antibodies (15). In addition, S. aureus has the ability to adhere to surgical implants, such as catheters and joint replacements, and can form biofilms that are difficult to eradicate. Iron is critical for the survival of S. aureus. The bacteria express several proteins, such as the IsdA to -E family, for the purpose of scavenging iron from heme from its host during the initial stages of infections (39). In addition to extracting iron from heme, the Isd protein cascade transports heme across the bacterium cell wall to the cytoplasm. Many of these iron-scavenging proteins are conserved across numerous S. aureus strains (11) and represent good targets for drug and vaccine development.
Due to the increasing number of antibiotic-resistant strains and the high morbidity and mortality associated with infection, there is a need to develop new and innovative therapies. Immunological approaches, such as prophylactic antibody treatment or vaccination, have the potential to prevent infection and disease. Vaccines against S. aureus have targeted capsular polysaccharides serotypes 5 and 8 (12), adhesion factors such as clumping factor A, fibronectin, and collagen binding proteins (2, 4, 14, 41), toxins such as alpha-toxin, enterotoxins, and toxic shock syndrome toxin (5, 18, 19, 40), and the surface-associated polysaccharide poly-N-acetyl-β-(1-6)-glucosamine (27). We have developed an IsdB-based investigational vaccine for the prevention of S. aureus infections following surgery and for patients with indwelling catheters (25). A truncated form of the IsdB protein is expressed in Saccharomyces cerevisiae and serves as the immunogen for the investigational S. aureus vaccine. Low to high levels of antibodies are generated against IsdB following S. aureus infection, and IsdB-based vaccines have been shown effective in animal models as a single antigen (25) or as a component of a multivalent vaccine (43). An IsdB-based prophylactic vaccine at a dose level of 60 μg is currently being tested in a clinical trial for the prevention of S. aureus disease following cardiothoracic surgery.
To evaluate the immunogenicity of several different formulations of IsdB-based vaccines, we developed and validated a serologic assay to measure serum levels of IsdB-specific IgG antibodies. Because several different assays to measure the immunogenicity of vaccines have been successfully developed using the open platform Luminex xMAP technology (7, 9, 26, 34, 35, 37, 38, 42, 44), we developed and validated this assay to detect antibodies against IsdB using this technology. The assay uses maleimide-modified microspheres conjugated to the IsdB protein via a carboxyl cysteine. The assay was shown to be rugged, with less than a 10% change in antibody concentrations to three different operators, three IsdB antigen lots, three IsdB-microsphere lots, and two secondary detection antibody lots. The assay was also shown to be acceptably specific and precise and is considered fit for its intended purpose of monitoring antibody levels following vaccination. The assay has proven valuable in monitoring immune responses elicited following natural infection and by an IsdB-based experimental vaccine against staphylococcal infections.
MATERIALS AND METHODS
Serum samples.
The IsdB serologic assay was evaluated using 95 human serum samples from healthy male and female adults 19 to 70 years of age. Ten samples were purchased from commercial bio-brokers and 85 samples were acquired from individuals who received either an investigational IsdB yeast-based vaccine or a placebo. Serum samples from vaccinees and placebos were taken prior to immunization and at days 3, 7, 14, 28, 56, or 84 following a single immunization on day 0. Serum samples were stored at −70°C until assayed. All samples were collected in accordance with Institutional Review Board guidelines and informed consent.
Reference standard.
A reference serum (06LC) was created by pooling sera collected from nine subjects from days 14, 28, 56, and 84 following IsdB vaccination. The human reference serum standard was calibrated against a purified IsdB-specific IgG preparation. The purified IgG preparation or “gold standard” was generated from a portion of the 06LC serum pool by isolating the IsdB-specific antibodies over an IsdB-Sepharose column and then isolating the bound IgG antibodies using a protein A-Sepharose column. This purified IgG “gold standard” was quantified by a bicinchoninic acid assay (Pierce, Rockford, IL) and determined to have a protein concentration of 828 μg/ml. By calibrating the 06LC serum reference standard to the purified “gold standard,” the concentration of the IsdB-specific IgG in the serum standard was determined to be 343 μg/ml.
S. aureus iron surface determinant B antigen.
IsdB is a 645-amino-acid protein identified to be reactive with acute human serum in a recombinant immunological screening study (11). IsdB has been identified as a member of the LPXTG protein family associated with the cell wall of gram-positive bacteria and has been reported to be involved with heme iron transport across the plasma membrane (29). A truncated form of the IsdB protein has been expressed in yeast (Saccharomyces cerevisiae) and serves as the investigational vaccine antigen. A truncated form of the IsdB protein expressed in Escherichia coli with a carboxy-terminal cysteine residue serves as the antigen in the IsdB serologic assay.
Covalent conjugation of S. aureus IsdB antigen to maleimide microspheres.
The S. aureus IsdB antigen was covalently conjugated to xMAP maleimide microspheres (Radix BioSolutions, Georgetown, TX) using 50 mM 2-[N-morpholino] ethanesulfonic acid hydrate, pH 6.5 (MES; Sigma, St. Louis, MO). Maleimide microspheres were brought to room temperature (RT), sonicated, and vortexed for approximately 60 s to obtain an equal distribution of microspheres. An aliquot containing 9.4 × 106 microspheres was added to a 1.5-ml copolymer microcentrifuge tube (USA Scientific, Ocala, FL) and pelleted in a microcentrifuge, and the supernatant was removed. To the microcentrifuge tube, 752 μg of E. coli-derived S. aureus IsdB antigen was added and brought to a final volume of 1.5 ml in MES buffer. The IsdB-microsphere mixture was sonicated and then vortexed for 30 s to break up the pellet, covered in foil, and mixed on a rotator for 2 h at RT. Following the 2-h incubation, the IsdB-microspheres were pelleted and the supernatant removed. To prevent alkylation of any unconjugated cysteine residuals, the IsdB-microspheres were blocked with 1 ml of 1 M N-acetyl-l-cysteine for 2 h. Following the blocking step, the IsdB-microspheres were washed three times with phosphate-buffered saline (PBS) and then resuspended in 1 ml of PBS containing 1% bovine serum albumin and 0.05% sodium azide (storage buffer). The IsdB-microspheres were counted on a Beckman-Coulter (Miami, FL) Z series particle counter, diluted to a concentration of 105 microspheres/ml with storage buffer, and stored at 4°C in the dark. To ensure that the IsdB antigen conjugated to the microspheres and retained its conformational integrity, the IsdB-microspheres were checked with three monoclonal antibodies that bind to conformationally sensitive epitopes on the antigen. The mouse monoclonal antibodies used in the quality control assay were 2H2, 1G3, and 13C7, which bind to the amino terminus, middle region, and carboxy terminus, respectively. Binding of the monoclonal antibodies was measured using a polyclonal, phycoerythrin-conjugated, goat anti-mouse serum (Southern Biotechnology, Birmingham, AL) at a 1:10 dilution.
Human IgG secondary detection antibody.
A phycoerythrin-conjugated, mouse anti-human IgG1 to IgG4 (Fc-specific) monoclonal antibody (clone HP6043; Biotrend Chemicals Inc., Destin, FL) diluted to 2.5 μg/ml was used to detect human IgG bound to IsdB. The HP6043 clone reacts with IgG subclasses 1 to 4 and is nonreactive with IgA, IgM, and IgE (17).
IsdB-specific IgG serologic assay.
Serum samples and four control samples (high, medium, low 1, and low 2) were diluted 1:1,250 and 1:12,500 in a 1.2-ml deep-well assay block (Phenix Research Products, Hayward, CA) using an assay buffer comprised of PBS, 0.05% Tween 20, 1% bovine serum albumin, and 0.05% sodium azide. The 06LC reference standard was prediluted to a concentration of 1.715 μg/ml and further diluted in a twofold serial fashion, creating a 12-point standard curve in duplicate with concentrations ranging from 0.837 to 1,715 ng/ml. Fifty-microliter aliquots of the reference standard, control samples, and test samples were added to a low-protein-binding filter plate (Millipore, Billerica, MA) prewashed with 200 μl of wash buffer (PBS-0.05% Tween 20) followed by the addition of 50 μl of the IsdB-microspheres mixture (5,000 IsdB-microspheres/well), resulting in a final dilution of 1:2,500 and 1:25,0000 for each sample. The plates were covered with foil to prevent photobleaching of the microspheres and placed on a shaker set at 600 to 800 rpm for 60 to 90 min at RT. Following the incubation, the plates were washed and filtered three times with 200 μl of wash buffer. To each well, 100 μl of phycoerythrin-labeled HP6043 (0.25 μg) was added and the mixture was incubated for an additional 60 to 90 min at RT. The plates were then washed and filtered three times with 200 μl of wash buffer, resuspended in 120 μl of wash buffer, and analyzed on a Bio-Plex suspension array system and using Bio-Plex manager 4.1.1 (Bio-Rad, Hercules, CA).
Statistical analysis. (i) Precision.
Variability estimates for intra-, inter-, and total precision were obtained using samples from the ruggedness panel and the control samples. Intra-assay precision was estimated using the variability across the four plates within each run [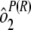 ] and the sample by plate interaction [
] and the sample by plate interaction [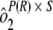 ]. To determine interassay variability, estimates of run-to-run variability (
]. To determine interassay variability, estimates of run-to-run variability (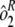 ) and sample-by-run variability (
) and sample-by-run variability (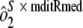 ) were combined. Variability estimates were obtained for the natural log-transformed titers using the MIXED procedure in SAS (SAS Institute, Cary, NC). An estimate of the total assay precision (percent relative standard deviation [RSD]) was calculated as {
) were combined. Variability estimates were obtained for the natural log-transformed titers using the MIXED procedure in SAS (SAS Institute, Cary, NC). An estimate of the total assay precision (percent relative standard deviation [RSD]) was calculated as {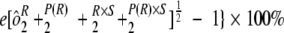 . A statistically meaningful rise in test sample titer was calculated as
. A statistically meaningful rise in test sample titer was calculated as 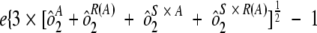 .
.
Standard curve modeling.
The reference standard dilution series mean fluorescence intensity data for duplicate measurements was modeled using the four-parameter logistic function (33) with a weighting factor of 1.6 (indicating that the variation in the signal is between that of Poisson and exponential). The test sample concentrations were interpolated from the fitted standard curve.
Limits of detection and limits of quantitation.
For each of the 108 standard curves generated, the limit of detection (LOD) and limits of quantitation (LOQs) were determined (33). The 99th percentile of all LODs from the 108 standard curves was 0.32 ng/ml, which is less than the lowest concentration tested on the standard curve (0.42 ng/ml); therefore, to be conservative, the LOD of the assay was determined to be 0.42 ng/ml (non-dilution corrected). Similarly, the LOQs were estimated by determining the 99th and 1st percentiles of all 108 lower and upper LOQ estimates. The non-dilution-corrected LOQs were determined to be 0.85 to 425 ng/ml.
Dilutability and parallelism.
For the dilutability (parallelism) experiments the overall dilution effect per twofold dilution was calculated as follows: percent bias per 10-fold dilution = 100% × (10b - 1), where b represents the slope from the linear regression fit of the log10-transformed dilution-corrected antibody values against the log10-transformed dilution factor. Slopes for each sample were pooled and estimated using the mixed procedure in SAS.
Specificity.
The percent specificity of the assay to either IsdB or human papillomavirus (HPV) type 16 virus-like particle (VLP) was estimated by the following equation: 1 − [(IsdBtreated/IsdBmock)] × 100, or [1 − (VLPtreated/VLPmock)] × 100, respectively, where IsdBmock and VLPmock denote the antibody concentrations from serum mock adsorbed with PBS. IsdBtreated and VLPtreated denote the antibody concentrations from samples preadsorbed with either IsdB antigen or HPV type 16 VLPs.
RESULTS
An IsdB-specific IgG assay has been developed to measure antibodies following vaccination with an investigational IsdB-based vaccine. Since this assay will be used to support vaccine clinical trials that could span multiple years, we wanted to validate the operating characteristics of the assay. The objectives of this assay validation were to assess the assay for ruggedness, sensitivity, precision, dilutability (parallelism), specificity, and accuracy. Additionally, the validation established the LOQs, the extravariability criteria (intrasample duplicate variability) for duplicate samples, and the pass-fail plate validity criteria for use in routine testing.
Since multiple reagent and operator changes are likely to occur over the course of a large clinical trial, we first wanted to determine how rugged the assay was to factors that would change during routine testing. To evaluate the overall ruggedness of the assay, we examined multiple conditions with three levels per factor for operator, antigen lot, and microsphere lot and two levels for the detection antibody factor (3 × 3 × 3 × 2 = 54 conditions) over a 3-week period. Representative standard curves from the 108 generated in that assay validation are shown in Fig. 1. To estimate assay ruggedness in this full factorial experimental design, the least square mean antibody concentrations of the test and control samples from all valid test results were determined for each level within each of the ruggedness factors. As summarized in Table 1, there was on average less than a 10% difference in antibody concentrations across the 54 conditions which met the prespecified requirement of <25% across all conditions. These results suggest the assay can be considered acceptably rugged for use in routine clinical testing in support of long-term clinical trials.
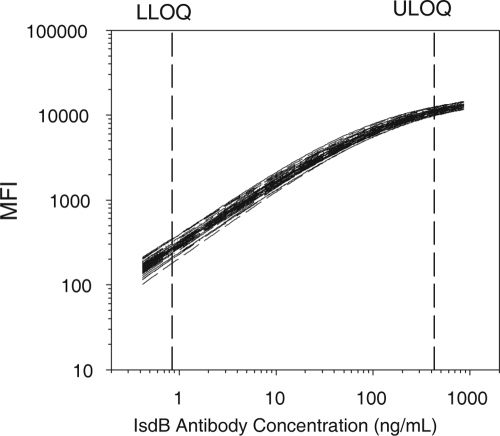
FIG. 1.
Reference standard curves. Shown are 54 representative standard curves of the 108 generated during the assay validation. The mean fluorescence intensity values of two median fluorescence intensity values from duplicate wells are shown. Standard curves were evaluated to determine the LOD, or sensitivity of the assay, and the lower and upper (LLOQs and ULOQ, respectively) or dynamic range of the assay. The unadjusted and adjusted LODs of the assay are 0.425 ng/ml and1.06 μg/ml, respectively, and the unadjusted and adjusted LLOQs and ULOQs are 0.850 to 425 ng/ml and 2.1 to 10,625 μg/ml, respectively. The 108 plates were also used to determine the assay run acceptance criteria. These ranges are shown in Table 2.
TABLE 1.
Assay ruggedness for the 54 separate conditions evaluated in the validation test
| Factor | Level | LS meana (μg/ml) (95% CI) | Comparison | % Difference (95% CI) |
|---|---|---|---|---|
| Secondary MAb | 1 | 162 (158, 166) | 1 vs 2 | 3.0 (−0.7, 6.7) |
| 2 | 157 (153, 162) | |||
| Microsphere | 1 | 156 (152, 161) | 1 vs 2 | −4.2 (−8.3, 0.0) |
| 2 | 163 (158, 168) | 1 vs 3 | −2.8 (−6.3, 0.8) | |
| 3 | 161 (156, 165) | 2 vs 3 | 1.4 (−2.7, 5.8) | |
| IsdB | 1 | 157 (152, 162) | 1 vs 2 | −1.5 (−5.8, 2.9) |
| 2 | 159 (154, 165) | 1 vs 3 | −3.7 (−7.8, 0.6) | |
| 3 | 163 (158, 165) | 2 vs 3 | −2.2 (−6.5, 2.2) | |
| Operator | 1 | 153 (149, 158) | 1 vs 2 | −5.4 (−9.4, −1.2) |
| 2 | 162 (157, 167) | 1 vs 3 | −6.8 (−10.8, −2.6) | |
| 3 | 164 (159, 170) | 2 vs 3 | −1.5 (−5.8, 3.0) |
LS mean, least square mean.
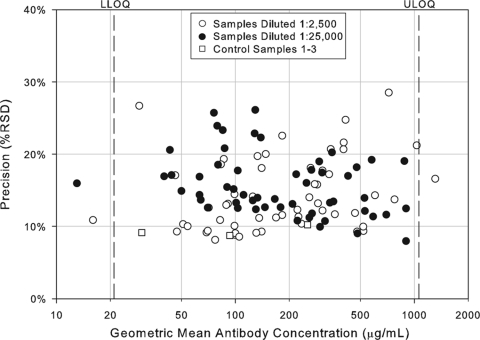
In addition to assessing the ruggedness of the assay, antibody concentrations from test and control samples were also used to determine intra-, inter-, and total assay precision. The precision profiles (percent RSD) for each of the control and test samples across the dynamic range of the assay, including samples with antibody titers outside the LOQs, is shown in Fig. 2. The variability of sample antibody titers was generally less than 25% within the quantifiable range of the assay. Three control samples and three test samples were tested two times per condition, once per plate, to evaluate intra-assay precision, which was determined to be 8% RSD. To determine interassay variability for both test and control samples, estimates of condition-to-condition variability and sample-by-condition variability were combined for an interassay variability estimate of 14.2% RSD. Overall, the assay precision was estimated to be 10.7% RSD for the control samples and 16.6% RSD for test samples. Using these precision estimates, a statistically meaningful change in antibody concentration following infection or vaccination was determined to be 1.9-fold. Assay precision estimates are summarized in Table 2.
FIG. 2.
Precision profile of control and test samples in the IsdB serologic assay. Assay precision was estimated by combining intra- and interassay variability across the 108 assay plates. The precision estimates for each of the 55 samples tested in the IsdB serologic assay at the 1:2,500 and 1:25,000 dilution and the control samples are shown as a function of antibody concentration and are expressed as the percent RSD.
TABLE 2.
IsdB serologic assay summary
| Assay characteristic | Limit(s) |
|---|---|
| EXV limita | Range of absolute MFI of >10 and of transformed MFI of >0.22 |
| LOD (non-dilution corrected, ng/ml) | 0.425 ng/ml |
| LOD (dilution corrected, μg/ml) | 1.06 |
| LOQs (non-dilution corrected, ng/ml) | 0.85-425 |
| LOQs (dilution corrected, μg/ml) | 2.1-10,625 |
| RMSE upper limit (MFI) | 2σ limit, ≤0.30; 3σ limit, ≤0.36 |
| Slope limits (MFI/ng/ml) | 2σ limit, 0.70, 0.83; 3σ limit, 0.67, 0.86 |
| EC50 limits (ng/ml) | 2σ limit, 112, 362; 3σ limit, 83, 486 |
| Control 1 limits (μg/ml) | 2σ limit, 191, 335; 3σ limit, 166, 385 |
| Control 2 limits (μg/ml) | 2σ limit, 70, 123; 3σ limit, 61, 141 |
| Control 3 limits (μg/ml) | 2σ limit, 23, 40; 3σ limit, 20, 46 |
| Control 4 limits (μg/ml) | 2σ limit, ≤3.2; 3σ limit, ≤3.7 |
| Intra-assay precision (% RSD) | 8.0% |
| Interassay precision (% RSD) | 14.2% |
| Total precision (% RSD) | 16.6% |
| Assay ruggedness (% difference between | |
| conditions) | −6.8% to 3.0% |
| Dilutability (% bias per 10-fold dilutions | |
| between 1:2,500 and 1:25,000) | −4.1% |
| Dilutability (% bias per 10-fold dilution | |
| between 1:100 and 1:102,400) | −13.0% |
| Specificity to IsdB | 97.8% |
Limits for untransformed and fifth root-transformed MFIs.
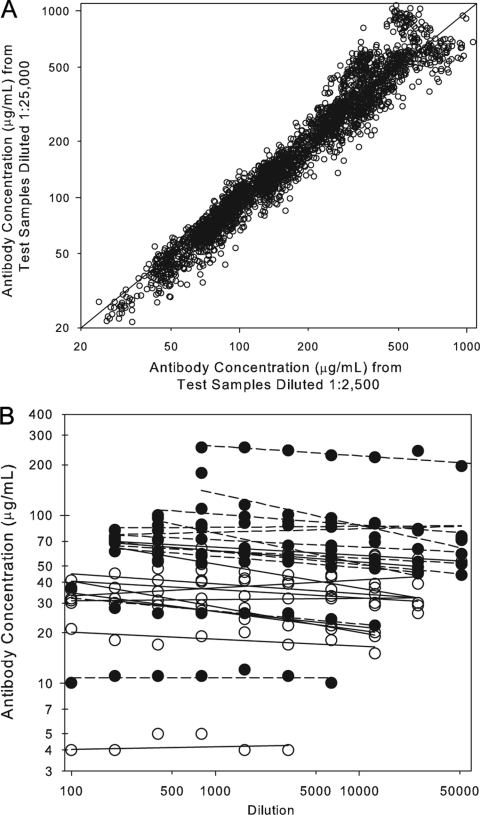
Linearity, also referred to as dilutability or parallelism, is an attribute of a biological assay that demonstrates that a test sample can be diluted through a series, yielding equivalent dilution-corrected antibody concentrations across that series. Two different analyses were performed to estimate the linearity or dilutability of the IsdB serologic assay. In the first experiment, 55 human samples were diluted in assay buffer and tested at the 1:2,500 and 1:25,000 dilutions under each of the 54 different ruggedness conditions. Results from samples with antibody titers at both dilutions were used to determine the difference in antibody concentration between the 2,500 and 25,000 dilutions. The results demonstrated that, on average, there was a 4.1% decrease in antibody concentration between the 2,500 and 25,000 dilution (Fig. 3A and Table 2). In a second experiment, 24 human samples were diluted in assay buffer and tested in a 12-point, twofold dilution series between 1:100 and 1:204,800 dilutions to evaluate the overall linearity of the assay. Data from dilutions with measurable antibody concentrations were used to calculate an estimated 13% decrease in antibody concentrations per 10-fold increase in dilution (Fig. 3B and Table 2). This difference in antibody concentration is considered acceptable and therefore was not taken into consideration when evaluating the response following vaccination.
FIG. 3.
Dilutability (linearity) of the IsdB serologic assay. A. Correlation of sample antibody concentrations obtained from sera at the 1:2,500 and 1:25,000 dilutions. The dilution effect between the 1:2,500 and 1:25,000 dilution was determined by fitting a mixed model containing random terms for sample and run to the natural log-transformed ratio of the 1:25,000 titer divided by the 1:2,500 titer. The overall difference between the 1:2,500 and 1:25,000 dilution was −4.1%. B. Sample antibody concentrations calculated from samples diluted through a twofold series from 1:100 to 1:204,800. The open symbols represent nonvaccinee antibody values and the closed symbols represent vaccinee antibody concentrations. There was an overall decrease of 13% per 10-fold dilution.
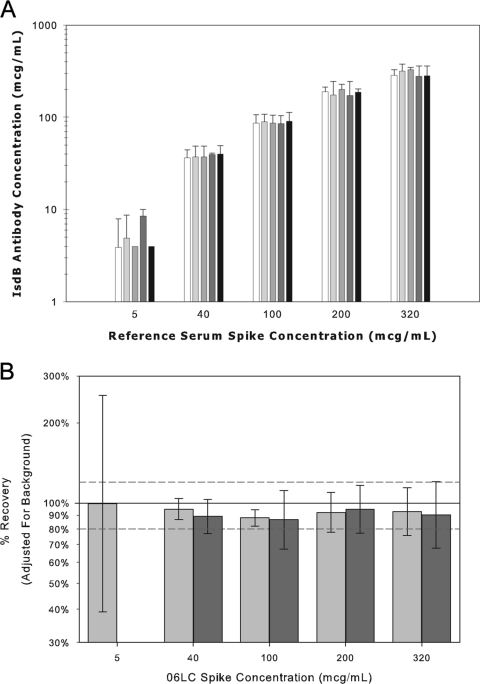
The accuracy of an analytical assay is defined as the closeness of agreement between the value which is accepted either as a conventional true value or an accepted reference value and the reported value (13). Since no international reference standard or reference panels exist for IsdB serology, the accuracy of the serologic assay was assessed by adding the reference standard to five low-titer serum samples to produce final concentrations of 320, 200, 100, 40, and 5 μg/ml. The samples were tested in the assay at the 1:2,500 and 1:25,000 dilutions to quantify the IsdB antibody concentrations. Accuracy or selectivity estimates were computed for each sample by subtracting the background IsdB-specific IgG levels from the measured concentration and determining the percentage of the observed versus expected IsdB-specific IgG levels. Accuracy or selectivity estimates were determined to be 93.4% (95% confidence interval [CI], 87.1% to 100.1%) and 90.1% (95% CI, 84.6% to 96.0%) at 1:2,500 and 1:25,000, respectively (Fig. 4).
FIG. 4.
Accuracy or selectivity of the IsdB serologic assay. A. IsdB antibody concentration for the IsdB human reference serum from vaccinees was added to five IsdB antibody low-concentration sera to evaluate the percent recovery in the assay. The reference standard was added into low anti-IsdB concentration sera at concentration levels of 5, 40, 100, 200, and 320 μg/ml. Background antibody levels were subtracted from the measured levels. Error bars represent 2 standard deviations from the means. B. Percent recovery in the IsdB serologic assay. The percent recovery in the IsdB serologic assay was determined by subtracting the IsdB-specific background antibody levels from the measured values and determining the percent recovery of observed over expected values. White bars represent samples tested at a 1:2,500 dilution and black bars represent samples tested at a 1:25,000 dilution.
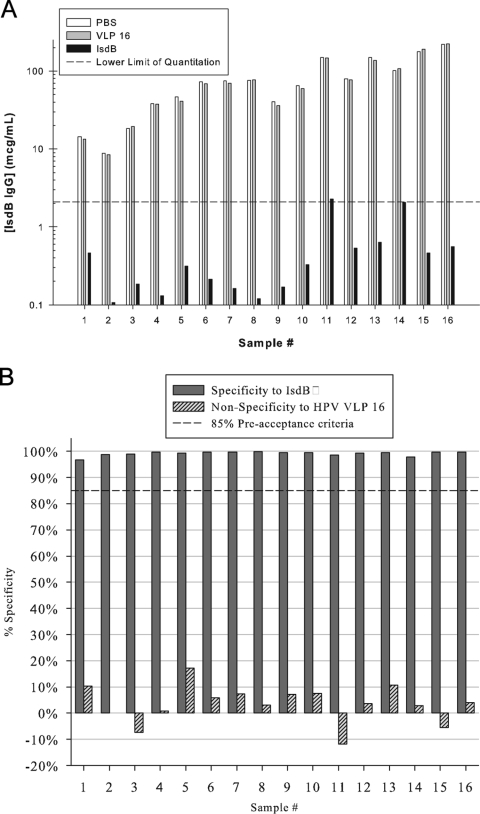
Analytical specificity is the ability of an assay to report only the component it purports to measure or the extent to which an assay measures only the specified analyte and not other substances present in the sample (13). Competition (specificity) experiments were performed with homologous, yeast-derived IsdB antigen and another yeast-derived heterologous antigen, human papillomavirus VLP16, to show analytical specificity of the assay to detect IsdB-specific antibodies. A total of 16 human samples, 8 samples from nonvaccinees and 8 samples from vaccinees, were tested at a dilution of 1:2,500. The samples were preadsorbed with either 0, 0.05, 0.09, 0.19, 0.38, 0.75, 1.50, or 3.00 μg of IsdB or HPV-16 VLPs for 1 h. Postincubation, these samples were assayed for IsdB-specific antibody concentrations (Fig. 5A). For a given sample, the percent specificity of the assay to either IsdB or HPV VLP type 16 was estimated by the equation 1 − [(IsdBtreated/IsdBmock)] × 100, or [1 − (VLPtreated/VLPmock)] × 100, respectively, where IsdBmock and VLPmock denote the antibody concentration from serum mock adsorbed with PBS. IsdBtreated and VLPtreated denote the antibody concentration from samples preadsorbed with either IsdB antigen or HPV type 16 VLPs. Overall, the assay was estimated to be 97.8% specific to IsdB and 3.5% nonspecific to HPV type 16 VLPs (Fig. 5B). Increasing VLP concentration did not significantly impact specificity estimates (P = 0.793), but increasing IsdB concentrations did significantly increase specificity estimates for the IsdB antigen (95.8% to 99.2%; P < 0.001).
FIG. 5.
Specificity of the IsdB serologic assay. A. Adsorption of IsdB-specific IgG antibodies with yeast-derived IsdB or VLP16. Sixteen sera with low to high IsdB-specific antibody titers were mock adsorbed with PBS or adsorbed with 3 μg of HPV VLP 16 or 3 μg of IsdB/y and tested in the IsdB serologic assay at a dilution of 1:2,500. B. Analytical specificity was determined in the IsdB serologic assay by calculating the percentage of antibody adsorbed with either HPV VLP16 or IsdB/y compared to PBS. The dashed line represents 85% specificity. The overall specificity of the IsdB serologic assay is 97.8%.
In addition to evaluating the ruggedness, linearity, accuracy, and specificity of the IsdB serologic assay, preanalytical variables that can negatively influence assay results, such as sample stability and hemolysis, were examined.
An experiment was performed to evaluate the impact of multiple freeze-thaw cycles on 16 serum samples. The results indicated that, on average, there was no statistically significant change in antibody concentrations (2.1% increase; 90% CI, −2.9% to +7.3%) following five freeze-thaw cycles. To assess the degradation of IsdB-specific antibodies over time, antibody concentrations from 28 samples that were stored for up to 4 days at room temperature and 1 to 6 months at 4°C were evaluated. The results demonstrated that sample storage at RT for up to 4 days had a modest impact on IsdB-specific antibody concentrations (9.1% decrease; 90% CI, −11.9% to −6.3%). To evaluate long-term storage at 4°C, a panel of 32 serum samples was assembled, divided into seven aliquots, and subsequently stored at −70°C for 0 to 6 months. Approximately every 30 days, an aliquot was removed from −70°C storage and placed at 4°C storage. An assay performed at the end of 6 months concluded that IsdB-specific antibody concentrations from samples stored at 4°C for 6 months generated results comparable to samples that were tested immediately after being thawed from −70°C (4.8% increase; 90% CI, +1.7% to +7.9%). Assessment of the preanalytical variables and sample processing indicated that the IsdB-specific antibodies elicited following vaccination are stable for short periods at room temperature, can be stored for up to 6 months at 4°C throughout the testing process, and can undergo multiple freeze-thaw cycles.
Hemolysis is another factor that can reduce the accuracy of results by interfering with antigen-antibody interactions or by interfering with the enzyme reactions in enzyme-labeled antibody detection assays (1). Since IsdB is a heme binding protein involved in iron transport across the bacteria cell wall, we examined whether hemolysis of blood samples would negatively impact antibody titers in the assay. To do this, two serum samples were collected from 30 subjects (15 men and 15 women) and the blood was allowed to clot for 30 min at room temperature. One sample was centrifuged and the serum transferred to a tube while the other sample underwent a forced hemolysis by placing the sample at −20°C for 15 min before centrifuging and transferring the serum to a tube. Overall, the antibody titers were 14.8% lower (90% CI, −19.5% to −9.8%) in the hemolyzed samples versus the control samples, suggesting that high levels of hemolysis could negatively impact the antibody titer results.
DISCUSSION
In this report we describe the validation of an IsdB-specific IgG serologic assay to monitor the IgG response to IsdB in sero-epidemiology and vaccine immunization studies. In development we optimized the conjugation reaction by conjugating IsdB via a carboxy-terminal cysteine to reactive maleimide groups on the microsphere. The concentration chosen was 752 μg per 9.4 × 106 microspheres, which should be in excess of the free maleimide groups on the microspheres. Quality control of the conjugation reaction is ensured by testing the IsdB-microsphere with three different monoclonal antibodies that bind to conformationally sensitive epitopes on the antigen. More important than the concentration of the antigen is ensuring that the IsdB antigen is pure and there is lot to lot reproducibility. Overall, we observed a less than 5% difference in antibody titers for samples tested across three IsdB antigen lots and three different microsphere lots (Table 1). In addition to the reproducibility of the conjugation reaction, use of a standard reference helps normalize results across different antigen and microsphere lots. Specificity experiments demonstrated that the assay is more than 97% specific (Fig. 5) and an E. coli adsorbent or non-IsdB antigen adsorbent is not required as in the case for Streptococcus pneumoniae serologic assays (28, 42, 46).
The anti-IsdB reference serum used for quantitation is diluted twofold in a 12-point dilution series. Using this reference standard, the assay was sensitive to 1.06 μg/ml and the dynamic range of the reference standard was from 2.1 to 10,625 μg/ml (Fig. 1). The standard curve is fitted using a four-parameter logistic function and the antibody titers showed good linearity between the 1:2,500 and 1:25,000 dilutions of −4.1% (Fig. 3). These two dilutions capture nearly all the results in both prevaccination and postvaccination samples from adults. Whether the assay is sensitive enough for testing pediatric or adolescent sera will need to be evaluated in the future.
The incidence of both community-acquired and hospital-acquired S. aureus infections has increased dramatically in the last 15 years, paralleling surgical advancements and the rise of antibiotic resistance. S. aureus is an opportunistic microbe, and a large proportion of the human population are asymptomatic carriers. Several reports estimate that approximately 20% of people are long-term S. aureus carriers (23). Our antibody results in the validation study using sera from healthy adults also suggest that exposure to S. aureus is very high, in that all samples tested have detectable levels of IsdB-specific IgG (Fig. 3). Our findings are in agreement with the results from Dryla et al., which showed S. aureus-specific antibodies were present in children as young as 1 year of age and that virtually all adults had moderate to high levels of IsdB-specific IgG. In addition to suggesting very high exposure rates to S. aureus, our data show that a high proportion of circulating antibodies target the IsdB protein. This observation is also consistent with the result from Dryla et al. that showed that 0.1 to 3.0% of circulating antibody can target S. aureus (10). The high level of IsdB-specific IgG in adults is the reason we optimized the assay to test the sera at a starting dilution of 1:2,500.
Several reports have demonstrated that high levels of S. aureus-specific antibodies can provide protection in animal models, and this generally correlates with opsonophagocytic function in in vitro assays. Most notable are the studies showing that IsdB-based vaccines provide protection in mouse models (25, 43) and passive serum transfer experiments showing that anti-ClfA human serum has opsonophagocytic activity and protects in experimental models of infective endocarditis (45). In humans, vaccination with a capsular 5,8-conjugate vaccine also induced opsonophagocytic antibodies (12). Understanding why the immune response in nasopharyngeal carriers is not sufficient to provide protection against invasive disease will provide important insight into guiding vaccine development. The observation that many people suffer recurrent S. aureus infections or from invasive disease despite having S. aureus-specific antibodies (36) suggests that both quantitative and qualitative differences in the levels and types of antibodies elicited by natural immunity are often insufficient to prevent infection. In addition to the quantity of circulating IsdB-specific IgG the amount of IgM and IgA, the different isotypes and subclasses and the avidity of the antibodies may play an important role in inducing opsonophagocytic activity which may correlate with protection. In an epidemiology study examining the IsdB-specific antibody titers following acute S. aureus or other bacterial infections we observed higher IsdB-specific antibodies in the S. aureus cases and observed a significant increase in IsdB-specific IgG over time following infection, suggesting that IsdB is available to the immune system during acute infection (J.-M. Arduino, unpublished data). It is our hope and expectation that directing the immune response to the conserved, less-immunodominant IsdB antigen protein through vaccination will elicit high-titer, functional antibodies that will confer immunity to patients undergoing elective cardiothoracic surgery.
In summary, the IsdB-specific IgG serologic assay we describe is sensitive, specific, reproducible, and rugged for its intended purpose of testing adult serum samples in S. aureus epidemiology studies and vaccine clinical trials. This assay has already proven useful in dose ranging and formulation studies of an experimental IsdB-based vaccine for evaluation in a large-scale clinical efficacy study.
Acknowledgments
We thank Jan ter Meulen for critical review of the manuscript.
Footnotes
Published ahead of print on 25 March 2009.
REFERENCES
- 1.Baer, D. M. 2003. Answering your questions: hemolysis in serologic specimens. Med. Lab. Observer. 35(3). www.mlo-online.com.
- 2.Brouillette, E., P. Lacasse, L. Shkreta, J. Belanger, G. Grondin, M. S. Diarra, S. Fournier, and B. G. Talbot. 2002. DNA immunization against the clumping factor A (ClfA) of Staphylococcus aureus. Vaccine 202348-2357. [DOI] [PubMed] [Google Scholar]
- 3.Cardo, D., T. Horan, M. Andrus, M. Dembinski, J. Edwards, G. Peavy, J. Tolson, and D. Wagner. 2004. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32470-485. [DOI] [PubMed] [Google Scholar]
- 4.Castagliuolo, I., R. Piccinini, E. Beggiao, G. Palu, C. Mengoli, F. Ditadi, G. Vicenzoni, and A. Zecconi. 2006. Mucosal genetic immunization against four adhesins protects against Staphylococcus aureus-induced mastitis in mice. Vaccine 244393-4402. [DOI] [PubMed] [Google Scholar]
- 5.Cui, J. C., D. L. Hu, Y. C. Lin, A. D. Qian, and A. Nakane. 2005. Immunization with glutathione S-transferase and mutant toxic shock syndrome toxin 1 fusion protein protects against Staphylococcus aureus infection. FEMS Immunol. Med. Microbiol. 4545-51. [DOI] [PubMed] [Google Scholar]
- 6.Daum, R. S. 2008. Staphylococcus aureus vaccines, p. 1317. In S. Plotkin, W. A. Orenstein, and P. A. Offit (ed.), Vaccines. Elsevier, Philadelphia, PA.
- 7.de Voer, R. M., F. R. van der Klis, C. W. Engels, G. T. Rijkers, E. A. Sanders, and G. A. Berbers. 2008. Development of a fluorescent-bead-based multiplex immunoassay to determine immunoglobulin G subclass responses to Neisseria meningitidis serogroup A and C polysaccharides. Clin. Vaccine Immunol. 151188-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diekema, D. J., B. J. BootsMiller, T. E. Vaughn, R. F. Woolson, J. W. Yankey, E. J. Ernst, S. D. Flach, M. M. Ward, C. L. Franciscus, M. A. Pfaller, and B. N. Doebbeling. 2004. Antimicrobial resistance trends and outbreak frequency in United States hospitals. Clin. Infect. Dis. 3878-85. [DOI] [PubMed] [Google Scholar]
- 9.Drummond, J. E., E. E. Shaw, J. M. Antonello, T. Green, G. J. Page, C. O. Motley, K. A. Wilson, A. C. Finnefrock, X. Liang, and D. R. Casimiro. 2008. Design and optimization of a multiplex anti-influenza peptide immunoassay. J. Immunol. Methods 33411-20. [DOI] [PubMed] [Google Scholar]
- 10.Dryla, A., S. Prustomersky, D. Gelbmann, M. Hanner, E. Bettinger, B. Kocsis, T. Kustos, T. Henics, A. Meinke, and E. Nagy. 2005. Comparison of antibody repertoires against Staphylococcus aureus in healthy individuals and in acutely infected patients. Clin. Diagn. Lab. Immunol. 12387-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etz, H., D. B. Minh, T. Henics, A. Dryla, B. Winkler, C. Triska, A. P. Boyd, J. Sollner, W. Schmidt, U. von Ahsen, M. Buschle, S. R. Gill, J. Kolonay, H. Khalak, C. M. Fraser, A. von Gabain, E. Nagy, and A. Meinke. 2002. Identification of in vivo expressed vaccine candidate antigens from Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 996573-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fattom, A. I., G. Horwith, S. Fuller, M. Propst, and R. Naso. 2004. Development of StaphVAX, a polysaccharide conjugate vaccine against S. aureus infection: from the lab bench to phase III clinical trials. Vaccine 22880-887. [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration. 2001. Guidance for industry: bioanalytical method validation, 1-25. U.S. Food and Drug Administration, Rockville, MD.
- 14.Gaudreau, M. C., P. Lacasse, and B. G. Talbot. 2007. Protective immune responses to a multi-gene DNA vaccine against Staphylococcus aureus. Vaccine 25814-824. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg, D. P., A. S. Bayer, A. L. Cheung, and J. I. Ward. 1989. Protective efficacy of protein A-specific antibody against bacteremic infection due to Staphylococcus aureus in an infant rat model. Infect. Immun. 571113-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregerson, A., and R. S. Daum. 2004. Staphylococcus aureus vaccine, p. 1347-1355. In S. Plotkin and W. Orenstein (ed.), Vaccines. Saunders, Philadelphia, PA.
- 17.Hamilton, R. G. 1990. Engineered human antibodies as immunologic quality control reagents. Ann. Biol. Clin. (Paris) 48473-477. [PubMed] [Google Scholar]
- 18.Hu, D. L., K. Omoe, K. Narita, J. C. Cui, K. Shinagawa, and A. Nakane. 2006. Intranasal vaccination with a double mutant of staphylococcal enterotoxin C provides protection against Staphylococcus aureus infection. Microbes Infect. 82841-2848. [DOI] [PubMed] [Google Scholar]
- 19.Hume, E. B., J. J. Dajcs, J. M. Moreau, and R. J. O'Callaghan. 2000. Immunization with alpha-toxin toxoid protects the cornea against tissue damage during experimental Staphylococcus aureus keratitis. Infect. Immun. 686052-6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarvis, W. R., R. P. Gaynes, T. C. Horan, J. Alonso-Echanove, T. G. Emori, S. K. Fridkin, R. M. Lawton, M. J. Richards, and G. C. Wright. 1998. National Nosocomial Infections Surveillance (NNIS) system report, data summary from Oct 1986-April 1998. www.cdc.gov.
- 21.Kaplan, S. L., K. G. Hulten, B. E. Gonzalez, W. A. Hammerman, L. Lamberth, J. Versalovic, and E. O. Mason, Jr. 2005. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin. Infect. Dis. 401785-1791. [DOI] [PubMed] [Google Scholar]
- 22.Klevens, R. M., J. R. Edwards, F. C. Tenover, L. C. McDonald, T. Horan, and R. Gaynes. 2006. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992-2003. Clin. Infect. Dis. 42389-391. [DOI] [PubMed] [Google Scholar]
- 23.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuehnert, M. J., H. A. Hill, B. A. Kupronis, J. I. Tokars, S. L. Solomon, and D. B. Jernigan. 2005. Methicillin-resistant Staphylococcus aureus hospitalizations, United States. Emerg. Infect. Dis. 11868-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuklin, N. A., D. J. Clark, S. Secore, J. Cook, L. D. Cope, T. McNeely, L. Noble, M. J. Brown, J. K. Zorman, X. M. Wang, G. Pancari, H. Fan, K. Isett, B. Burgess, J. Bryan, M. Brownlow, H. George, M. Meinz, M. E. Liddell, R. Kelly, L. Schultz, D. Montgomery, J. Onishi, M. Losada, M. Martin, T. Ebert, C. Y. Tan, T. L. Schofield, E. Nagy, A. Meineke, J. G. Joyce, M. B. Kurtz, M. J. Caulfield, K. U. Jansen, W. McClements, and A. S. Anderson. 2006. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect. Immun. 742215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lal, G., P. Balmer, E. Stanford, S. Martin, R. Warrington, and R. Borrow. 2005. Development and validation of a nonaplex assay for the simultaneous quantitation of antibodies to nine Streptococcus pneumoniae serotypes. J. Immunol. Methods 296135-147. [DOI] [PubMed] [Google Scholar]
- 27.Maira-Litran, T., A. Kropec, D. A. Goldmann, and G. B. Pier. 2005. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal Poly-N-acetyl- beta-(1-6)-glucosamine. Infect. Immun. 736752-6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchese, R. D., N. T. Jain, J. Antonello, L. Mallette, K. L. Butterfield-Gerson, J. Raab, P. Burke, C. Schulman, H. Adgate, D. J. Sikkema, and N. Chirmule. 2006. Enzyme-linked immunosorbent assay for measuring antibodies to pneumococcal polysaccharides for the PneumoVax 23 vaccine: assay operating characteristics and correlation to the WHO international assay. Clin. Vaccine Immunol. 13905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazmanian, S. K., E. P. Skaar, A. H. Gaspar, M. Humayun, P. Gornicki, J. Jelenska, A. Joachmiak, D. M. Missiakas, and O. Schneewind. 2003. Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299906-909. [DOI] [PubMed] [Google Scholar]
- 30.Naimi, T. S., K. H. LeDell, K. Como-Sabetti, S. M. Borchardt, D. J. Boxrud, J. Etienne, S. K. Johnson, F. Vandenesch, S. Fridkin, C. O'Boyle, R. N. Danila, and R. Lynfield. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2902976-2984. [DOI] [PubMed] [Google Scholar]
- 31.Noskin, G. A. 2005. The burden of Staphylococcus aureus infections on hospitals in the United States. Arch. Intern. Med. 1651756-1761. [DOI] [PubMed] [Google Scholar]
- 32.Noskin, G. A., R. J. Rubin, J. J. Schentag, J. Kluytmans, E. C. Hedblom, C. Jacobson, M. Smulders, E. Gemmen, and M. Bharmal. 2007. National trends in Staphylococcus aureus infection rates: impact on economic burden and mortality over a 6-year period (1998-2003). Clin. Infect. Dis. 451132-1140. [DOI] [PubMed] [Google Scholar]
- 33.O'Connell, M. A., B. A. Belanger, and P. D. Haaland. 1992. The four parameter logistic model for calibration and assay development. Am. Stat. Assoc. Proc. Biopharm. Section 1992180-185. [Google Scholar]
- 34.Opalka, D., C. E. Lachman, S. A. MacMullen, K. U. Jansen, J. F. Smith, N. Chirmule, and M. T. Esser. 2003. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin. Diagn. Lab. Immunol. 10108-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opalka, D., A. Pessi, E. Bianchi, G. Ciliberto, W. Schleif, M. McElhaugh, R. Danzeisen, R. Geleziunas, M. Miller, D. M. Eckert, D. Bramhill, J. Joyce, J. Cook, W. Magilton, J. Shiver, E. Emini, and M. T. Esser. 2004. Analysis of the HIV-1 gp41 specific immune response using a multiplexed antibody detection assay. J. Immunol. Methods 28749-65. [DOI] [PubMed] [Google Scholar]
- 36.Peacock, S. J., I. de Silva, and F. D. Lowy. 2001. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 9605-610. [DOI] [PubMed] [Google Scholar]
- 37.Pickering, J. W., T. B. Martins, M. C. Schroder, and H. R. Hill. 2002. Comparison of a multiplex flow cytometric assay with enzyme-linked immunosorbent assay for auantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae type b. Clin. Diagn. Lab. Immunol. 9872-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prince, H. E., M. Lape-Nixon, and J. Matud. 2006. Evaluation of a tetraplex microsphere assay for Bordetella pertussis antibodies. Clin. Vaccine Immunol. 13266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rouault, T. A. 2004. Pathogenic bacteria prefer heme. Science 3051577-1578. [DOI] [PubMed] [Google Scholar]
- 40.Savransky, V., D. Pinelis, S. Korolev, B. Ionin, and K. Fegeding. 2004. Immunogenicity of the histidine-to-tyrosine staphylococcal enterotoxin B mutant protein in C3H/HeJ mice. Toxicon 43433-438. [DOI] [PubMed] [Google Scholar]
- 41.Schaffer, A. C., R. M. Solinga, J. Cocchiaro, M. Portoles, K. B. Kiser, A. Risley, S. M. Randall, V. Valtulina, P. Speziale, E. Walsh, T. Foster, and J. C. Lee. 2006. Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect. Immun. 742145-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlottmann, S. A., N. Jain, N. Chirmule, and M. T. Esser. 2006. A novel chemistry for conjugating pneumococcal polysaccharides to Luminex microspheres. J. Immunol. Methods 30975-85. [DOI] [PubMed] [Google Scholar]
- 43.Stranger-Jones, Y. K., T. Bae, and O. Schneewind. 2006. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 10316942-16947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Gageldonk, P. G., F. G. van Schaijk, F. R. van der Klis, and G. A. Berbers. 2008. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J. Immunol. Methods 33579-89. [DOI] [PubMed] [Google Scholar]
- 45.Vernachio, J., A. S. Bayer, T. Le, Y. L. Chai, B. Prater, A. Schneider, B. Ames, P. Syribeys, J. Robbins, and J. M. Patti. 2003. Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrob. Agents Chemother. 473400-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wernette, C. M., C. E. Frasch, D. Madore, G. Carlone, D. Goldblatt, B. Plikaytis, W. Benjamin, S. A. Quataert, S. Hildreth, D. J. Sikkema, H. Kayhty, I. Jonsdottir, and M. H. Nahm. 2003. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin. Diagn. Lab. Immunol. 10514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39309-317. [DOI] [PubMed] [Google Scholar]