Abstract
Moraxella catarrhalis is a common cause of respiratory tract infection in the setting of chronic obstructive pulmonary disease (COPD). Adults with COPD acquire and clear strains of M. catarrhalis from the respiratory tract continuously and develop strain-specific protection following clearance of a strain. In previous work, we identified Hag/MID (Moraxella immunoglobulin D-binding protein), a large multifunctional surface protein that acts as an adhesin and hemagglutinin, as a target of antibody responses in adults with COPD after clearance of M. catarrhalis. The goal of the present study was to characterize the domains of Hag/MID to which humans make antibodies, including both systemic and mucosal antibody responses. Analysis of recombinant peptide constructs, which spanned the M. catarrhalis strain O35E Hag/MID protein, with well-characterized serum and sputum samples revealed that most adults with COPD made antibodies directed toward a region of the molecule bounded by amino acids 706 to 863. Serum immunoglobulin G (IgG) and IgA purified from sputum both recognized the same domain. Some flanking sequence of this fragment was necessary for the epitope(s) in this region to maintain its conformation to bind human antibodies. These results reveal that humans consistently generate both systemic and mucosal antibody responses to an immunodominant region of the Hag/MID molecule, which was previously shown to overlap with several biologically relevant domains, including epithelial cell adherence, IgD binding, collagen binding, and hemagglutination.
Chronic obstructive pulmonary disease (COPD) is a debilitating disorder that is the fourth most common cause of death in the United States (1, 2). The course of the disease is characterized by intermittent exacerbations that result in enormous morbidity, including lost work time, hospital admissions, respiratory failure, and sometimes death (31). Moraxella catarrhalis is the second most common cause of exacerbations of COPD after nontypeable Haemophilus influenzae (30). It is estimated that M. catarrhalis causes 2 to 4 million exacerbations per year in the United States (19).
Adults with COPD acquire and clear strains of M. catarrhalis from the respiratory tract continuously. When an individual acquires M. catarrhalis, the organism is cleared efficiently after a short duration (∼30 days) of carriage. Patients then develop strain-specific protection from reacquisition of the same strain (19). This observation that humans develop apparent protective responses to the organism after clearing it from the respiratory tract provides the opportunity to begin to understand protective immune responses to M. catarrhalis.
The identification of surface antigens that are targets of human antibody responses in the setting of COPD has been investigated recently by several research groups. A hallmark of antibody responses to respiratory tract bacterial pathogens in COPD is variability among individuals. Several surface antigens are the targets of antibody responses in a small proportion of adults with COPD following infection with M. catarrhalis (OMP E, CopB, lipooligosacccharide, Msp22, Msp75, and Msp78) (17, 18, 28). By contrast, selected surface antigens appear to be more consistent targets of antibody responses in a larger proportion of adults with COPD. These antigens include outer membrane protein CD, UspA1, UspA2, transferrin binding protein B, and Hag/MID (Moraxella immunoglobulin D [IgD]-binding protein) (17, 18, 20, 33). The present study focuses on Hag/MID, which was the target for new systemic and mucosal antibody responses in a large proportion of adults with COPD who acquired and cleared M. catarrhalis in our prospective study (17-19).
Approximately 86% of strains of M. catarrhalis contain a hag gene (also called mid) and express its product (4, 7, 16, 24, 25, 34). Hag/MID is a multifunctional protein that acts as an adhesin for human respiratory epithelial cells, a B-cell mitogen, binds IgD, and mediates hemagglutination (3, 4, 6, 9, 12, 22, 24, 26). Hag/MID is an autotransporter protein in the largest known family of virulence factors expressed by gram-negative bacteria (5, 10). The hag gene encodes a protein of ∼2,000 amino acids that exists as a multimer on the bacterial surface. Expression of Hag/MID is subject to translational phase variation via slipped strand mispairing in a homopolymeric guanine track (16).
The goal of the present study was to characterize both the systemic and mucosal antibody responses to Hag/MID in adults with COPD who have acquired and cleared M. catarrhalis from the respiratory tract. Emphasis is placed on identifying the key domains in the Hag/MID protein with regard to both systemic and mucosal antibody responses.
MATERIALS AND METHODS
COPD Study Clinic.
This prospective study has been described previously (19, 30). Patients with COPD were seen at the Buffalo VA Medical Center monthly and whenever they had symptoms suggestive of an exacerbation. At each clinic visit, clinical information and sputum and serum samples were obtained. A clinical evaluation was performed at each visit to determine whether the patient had stable disease or an exacerbation, as previously described.
Serum samples.
Postclearance serum samples were obtained 4 to 8 weeks following clearance of M. catarrhalis from the respiratory tract, based on monthly sputum cultures. Serum samples from patients who were previously demonstrated to have developed a new antibody responses to Hag/MID were studied (18).
Sputum supernatant samples.
Postclearance sputum samples were obtained 4 to 8 weeks following clearance of M. catarrhalis from the respiratory tract based on monthly sputum cultures. After an aliquot of sputum was removed for culture as described previously, sputum supernatants were obtained by centrifugation at 27,000 × g for 30 min at 4°C. The supernatants were saved by storage at −80°C. Sputum supernatant samples from patients who were previously demonstrated to have developed a new sputum antibody responses to Hag were studied (17).
SDS-PAGE and immunoblot assays.
Recombinant proteins were subjected to sodium doceyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 7.5% separating gels. Preparations were heated at 100°C for 5 min in sample buffer containing 0.06 M Tris, 1.2% SDS, 5% β-mercaptoethanol, 11.9% glycerol, and 0.003% bromophenol blue. Electrophoretic transfer to nitrocellulose was carried out in a Hoefer Mighty Small vertical slab gel unit at 100 V for 2 h. The transfer buffer was 0.025 M Tris (pH 8.3), 0.192 M glycine, and 20% methanol.
After transfer the blot was incubated in 3% Blotto (nonfat dry milk) in buffer A (0.01 M Tris, 0.15 M NaCl, pH 7.4) for 1 h at room temperature followed by washing in buffer A. Blots were incubated in serum samples that were diluted in buffer A containing 1% Blotto overnight at room temperature. After washing, blots were incubated with goat anti-human IgG-IgM (KPL, Gaithersburg, MD) diluted in buffer A plus 1% Blotto for 1 h at room temperature. Blots were then developed with horseradish peroxidase color developer (Bio-Rad).
Purification of sputum IgA.
IgA was purified from sputum supernatant samples by affinity chromatography with a streptococcal IgA binding peptide by using a previously described method (17, 29). Briefly, 5 mg of the 50-residue synthetic peptide (purchased from Sigma-Genosys, The Woodlands, TX) was immobilized on a 1-ml HiTrap N-hydroxysuccinimide-activated high-performance column (Amersham Pharmacia Biotech) according to the instructions of the manufacturer. Before purification of IgA, sputum supernatants were centrifuged again at 16,000 × g for 30 min at 4°C and filtered through a 0.45-μm-pore-size filter. A volume of 1 ml of sputum supernatant was applied to the column, which was then washed with phosphate-buffered saline. Bound proteins were eluted with 0.1 M acetate buffer, pH 4, in fractions of 0.32 ml. The pH of the fractions was adjusted immediately by adding 0.32 ml of 1 M Tris, pH 8.3. Fractions were assayed for the presence of IgA by dotting 1 μl of each fraction onto nitrocellulose and probing with peroxidase-conjugated goat anti-human IgA. The fractions that contained IgA (generally the first eight fractions) were pooled and stored at 4°C. The protein concentration was determined by the method of Lowry (Sigma). After use, the column was regenerated with 3 KSCN, washed with phosphate-buffered saline, and stored at 4°C in 0.05 M Na2HPO4, 0.01% NaN3, pH 7.
Construction and expression of recombinant Hag/MID peptides.
A PCR product encoding amino acids 67 to 544 of the M. catarrhalis strain O35E Hag/MID protein (i.e., O35E-Hag/MID) was cloned in the expression vector pETcoco-1 (specifies six N-terminal histidine residues; Novagen) using standard recombinant DNA methods. The resulting plasmid was sequenced to verify that no unwanted mutations were introduced during PCR and to confirm that the Hag/MID fragment was properly joined to its fusion partner (i.e., the His tag). The plasmid was introduced into Escherichia coli strain TUNER (Novagen) for the purpose of overexpressing the recombinant protein (designated His-Hag NT19). Expression was induced by adding isopropyl-β-d-thiogalactopyranoside (final concentration, 1 mM) to broth cultures and incubating for 4 to 8 h at 37°C with agitation. Bacteria were pelleted and the His-Hag NT19 polypeptide was extracted from inclusion bodies using the BugBuster HT protein extraction reagent (Novagen) supplemented with rLysozyme (Novagen) under the manufacturer's recommended conditions. The recombinant protein was then purified under denaturing conditions with the His-Bind resin system (Novagen) per the manufacturer's instructions. The composition of the refolding buffer was determined using the AthenaES protein refolding kit (Athena Enzyme Systems), and urea was gradually removed by dialyzing the His-Hag NT19 preparation at 4°C; protein concentration was determined with the BCA protein assay kit (Pierce). His-tagged recombinant proteins encompassing amino acids (aa) 545 to 1367 (designated His-Hag MID1) as well as residues 1368 to 1964 (designated His-Hag CT77) of O35E-Hag/MID were generated in the same manner. A similar approach was used to purify a series of glutathione S-transferase (GST)-tagged proteins encompassing different regions of the predicted surface-exposed domain of O35E-Hag/MID (i.e., aa 67 to 1865) with minor modifications. Specifically, we used the plasmid pGEX4T-2 (specifies N-terminal GST tag; GE Healthcare Life Sciences) for cloning Hag/MID fragments and Pierce's GST fusion protein purification system to purify recombinant proteins. The various GST-tagged proteins that were generated are illustrated in Fig. 5, below.
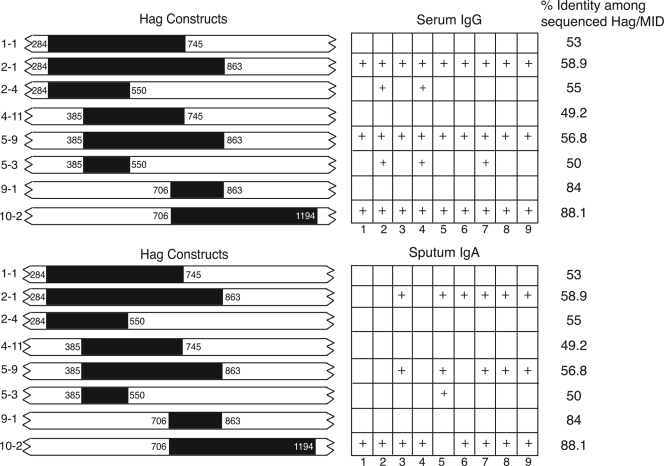
FIG. 5.
Results of immunoassays with eight recombinant peptide constructs of Hag/MID. The black bars on the left indicate the amino acid numbers that comprise each of the GST-tagged recombinant proteins. Construct names are shown to the left of each bar. The right side indicates results of immunoblot assays with each of the constructs assayed for serum IgG (top) and sputum IgA (bottom) from adults with COPD who acquired and cleared M. catarrhalis. A + indicates reactivity in the immunoblot assay, and an empty box indicates absence of reactivity. The numbers at the bottom of the serum IgG panel indicate the results with the following serum samples: 1, 10E67; 2, 19E55; 3, 32E8; 4, 39E34; 5, 11E31; 6, 46E49; 7, 52E4; 8, 63E25; 9, 87E16. The numbers at the bottom of the sputum IgA panel indicate results with IgA purified from sputum supernatants: 1, 3PS68; 2, 12PS75; 3, 7PS95; 4, 10PS92; 5, 19PS55; 6, 66PS6; 7, 29PS25; 8, 44PS24; 9, 1PS45. The percent identities of Hag/MID among seven strains whose hag gene has been sequenced are noted on the right.
RESULTS
Characterization of serum and sputum samples.
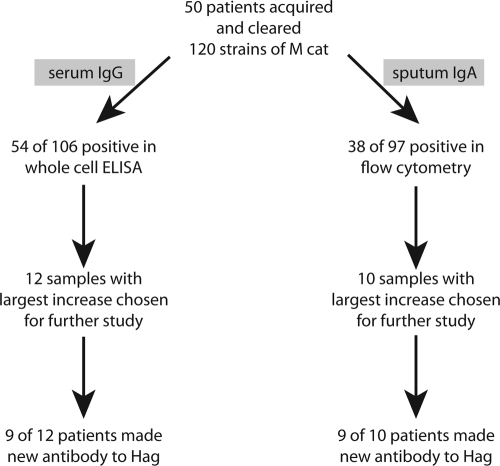
All serum and sputum samples were from adults with COPD who were followed in the COPD Study Clinic at the Buffalo Veterans Affairs Medical Center as part of a prospective study. In previous work, pairs of preacquisition and postclearance serum and sputum samples were studied by whole-cell enzyme-linked immunosorbent assay and flow cytometry, and these studies identified patients who developed new antibodies to surface epitopes on M. catarrhalis (19). Subsequent work utilizing immunoblot assays with isogenic mutants identified nine pairs of serum samples that showed the development of new serum IgG to Hag/MID and nine pairs of sputum samples that showed the development of new sputum IgA to Hag/MID following clearance (Fig. 1) (17, 18). The present study involves analysis of these well-characterized serum and sputum samples to elucidate the regions of Hag/MID that are important in the human immune response.
FIG. 1.
Origin of serum and sputum samples from adults with COPD who made antibody responses to Hag/MID following acquisition and clearance of M. catarrhalis from the respiratory tract as documented in previous studies (17-19).
Identification of Hag/MID domains recognized by human serum antibodies.
To begin to identify the regions of the Hag/MID protein that are recognized by human serum antibodies, recombinant peptide constructs corresponding to three large fragments of the protein were created and studied in immunoblot assays with each of the nine serum samples noted above. All serum samples contained serum IgG that recognized the central (i.e., aa 545 to 1367) and carboxy terminal (i.e., aa 1368 to 1964) fragments of the Hag/MID protein (Table 1). Only two of the nine sera contained antibodies to the amino-terminal fragment.
TABLE 1.
Reactivities of human serum IgG to regions of the Hag/MID molecule
| Serum sample | IgG response to indicated His-Hag region
|
||
|---|---|---|---|
| NT19 (aa 67 to 544) | MID1 (aa 545 to 1367) | CT77 (aa 1368 to 1964) | |
| 10E67 | + | + | |
| 11E31 | + | + | |
| 19E55 | + | + | |
| 32E8 | + | + | + |
| 39E34 | + | + | + |
| 46E49 | + | + | |
| 52E4 | + | + | |
| 63E25 | + | + | |
| 87E16 | + | + | |
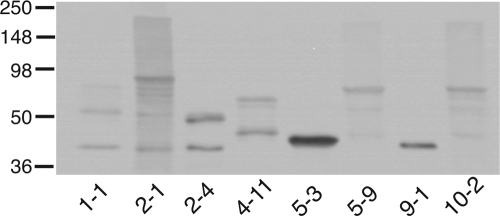
Based on these initial results and on the observation that the central region of the molecule (i.e., aa 545 to 1367) contains adhesin domains for respiratory epithelial cells, type IV collagen, and IgD binding (Fig. 2), additional peptide constructs encompassing most of this biologically relevant area of the molecule were designed and produced as GST-fusion peptides. Figure 3 shows the recombinant proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by Western blotting with an anti-GST antibody.
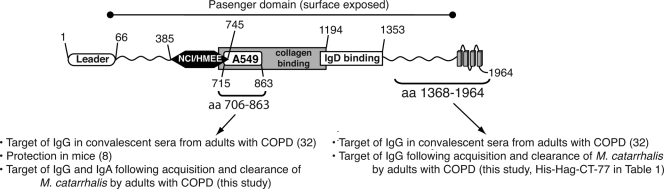
FIG. 2.
Structural features of the M. catarrhalis O35E hag gene product. The positions of residues defining selected domains and regions of antibody binding are shown. NCI/HMEE-CBD, cell binding domain for NCIH292 and HMEE cells; A549-CBD, cell binding domain for A549 cells and erythrocytes.
FIG. 3.
Immunoblot assay of GST-Hag recombinant proteins. Purified polypeptides were resolved by SDS-PAGE, transferred to polyvinylidene difluoride, and probed with an anti-GST antibody. Construct names are shown at the bottom. Molecular mass markers are noted on the left. Refer to Fig. 5 for details regarding what portion of O35E-Hag MID is specified by each construct.
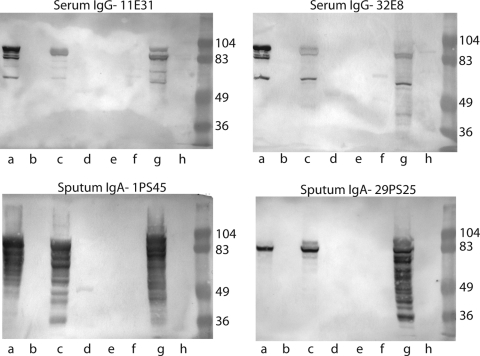
These GST-tagged polypeptides were subjected to immunoblot assays with the human serum samples. Figure 4 (top panels) shows representative immunoblot assays with two serum samples demonstrating that three of the peptide constructs were recognized by serum IgG in the serum of adults with COPD. The region of Hag/MID encompassing these peptides contains a coiled-coil structure. Such peptides characteristically run aberrantly and form aggregates on SDS-PAGE, accounting for the presence of multiple bands recognized by antibodies in some of the serum and sputum samples. Figure 5 shows a summary of results of the nine serum samples with the eight recombinant peptides in the immunoblot assay. Clearly, the region of the Hag/MID molecule corresponding to amino acids 706 to 863, which is common to constructs 2-1, 5-9, and 10-2, is a key domain with regard to binding of human serum IgG. However, based on the absence of reactivity of the fragment corresponding to amino acids 706 to 863 (construct 9-1 in Fig. 5A), this region of the molecule alone is not sufficient to bind human serum IgG antibodies. The presence of flanking sequence, either upstream or downstream, appears to be necessary for the epitope(s) in the 706 to 863 region to bind human serum IgG.
FIG. 4.
Immunoblot assays of recombinant peptides of Hag with human serum samples (top panels) and IgA purified from human sputum samples (bottom panels) from adults with COPD who developed new antibody responses to Hag/MID following acquisition and clearance of M.catarrhalis. Lanes contain Hag/MID peptide constructs as follows: a, 10-2 (aa 706 to 1194); b, 9-1 (aa 706 to 863); c, 5-9 (aa 385 to 863); d, 5-3 (aa 385 to 550); e, 4-11 (aa 385 to 745); f, 2-4 (aa 284 to 550); g, 2-1 (aa 284 to 863); h, 1-1 (aa 284 to 745). Molecular mass markers are noted on the right of each panel in kilodaltons.
Identification of Hag domains recognized by human sputum IgA.
Based on the results of the assays with serum samples that targeted the central region of the molecule and on the limited amounts of purified IgA from sputum samples that were available, purified sputum IgA samples were studied directly with the peptide constructs noted in Fig. 5. Figure 4 (bottom panels) shows representative immunoblot assays with purified human IgA from the sputum of two patients. Although the sputum IgA responses were a bit less homogeneous than serum IgG responses, the same three peptide constructs that were recognized by serum IgG were also the most dominant constructs recognized by samples of sputum IgA (Fig. 5). Thus, the region corresponding to amino acids 706 to 863 of O35E-Hag/MID is also the key region for binding of mucosal IgA.
DISCUSSION
In this study, well-characterized serum and sputum samples from adults with COPD followed prospectively were used to elucidate the regions of the Hag/MID molecule that are important targets of human systemic and mucosal antibody responses. A remarkable degree of consistency was observed among the samples studied. Recombinant peptide fragments of the O35E-Hag/MID protein that contained the 157-amino-acid domain from amino acids 706 to 863 were recognized by IgG in all serum samples studied and by the IgA purified from a majority of sputum samples studied. Of interest, the construct that was comprised of the 157-amino-acid domain itself (aa 706 to 863) was nonreactive in immunoblot assay. However, the three constructs that contained the 157-amino-acid domain along with either upstream or downstream peptide sequence were consistently reactive. The most likely explanation for this observation is that some flanking sequence is required for the epitope(s) that is recognized by human antibodies to maintain the conformation.
In addition to epitopes in the central region of Hag/MID, all nine serum samples bound epitopes in the carboxy region of the molecule (Table 1). By contrast, only two sera contained antibodies to the amino-terminal region. This observation suggests that the amino-terminal region of Hag/MID is less immunogenic. However, another explanation may be sequence heterogeneity in the amino-terminal region of the molecule. Bullard et al. (4) demonstrated that the amino-terminal region of Hag/MID has only 36.8% identity among isolates. The present study was conducted with peptide constructs of strain O35E, thus raising the possibility of strain specificity of antibody responses accounting for the observation that only a small proportion of sera contained antibodies to this region of the molecule. The percent identity among Hag/MID isotypes for the peptides tested in our study is consistent with this hypothesis (Fig. 5). An additional limitation of the present study is that the GST tags that are present on the amino terminus of the recombinant peptides may block the binding of selected antibodies to their corresponding epitopes. The use of overlapping constructs (Fig. 5) minimizes but does not eliminate this limitation.
The hag open reading frame of M. catarrhalis strain O35E is predicted to encode a protein of 1,964 residues with a mass of 201 kDa (24). Previous sequence analysis (4) indicated that this large molecule resembles members of the Oca (oligomeric coiled-coil adhesins) family of autotransporter proteins (11), which includes the well-characterized adhesins Yersinia enterocolitica YadA (11, 23, 27), H. influenzae Hia (13, 32, 35), and M. catarrhalis UspA1 (11, 14). These Oca proteins share structural features, including a C-terminal OM anchor domain composed of four β-strands (also referred to as the transporter domain) connected to a surface-exposed passenger domain that often contains repeated amino acid motifs (5, 10, 11, 15). As depicted in Fig. 2, O35E-Hag/MID possesses these conserved features. Kristian Riesbeck's group at Lund University reported that a recombinant protein corresponding to aa 764 to 913 of the M. catarrhalis strain Bc5 Hag/MID protein binds to A549 cells as well as erythrocytes (6) and that residues 962 to 1200 are responsible for IgD binding (7, 21). Moreover, Bullard and colleagues (3) recently demonstrated that aa 385 to 745 of Hag/MID of strain O35E specify adhesive properties for human lung (NCIH292) and middle ear (HMEE) epithelial cells and that residues 706 to 1194 are necessary for binding to type IV collagen. The regions of Hag/MID of strain O35E corresponding to these biologically relevant domains (i.e., A549-CBD, NCI/HMEE-CBD, collagen binding, and IgD binding) are also shown in Fig. 2. Of note, Hag/MID of strain O35E (1,964 residues) is smaller than Hag/MID of strain Bc5 (2,139 residues). For this reason, some of the numbering in the text does not match that in Fig. 2.
Tan et al. (33) studied the specificity of serum antibodies to Hag/MID in acute- and convalescent-phase sera from adults with COPD. In contrast to our patients, the majority of their patients (17 of 23) had preexisting serum antibodies to Hag. The patient samples identified for analysis in the present study were selected based on the criterion that they developed new antibody responses following acquisition and clearance, allowing us to characterize the specificity of the antibody response specifically to the episode of carriage under consideration. Of interest, the antibody responses of the six patients in the Tan study who developed new antibody responses were directed at aa 764 to 913 of the Hag/MID protein of M. catarrhalis strain Bc5, which is the same general region of O35E-Hag/MID identified in our experiments. Thus, the present study further highlights this region of the molecule as a target of mucosal antibody responses made following acquisition and clearance of M. catarrhalis from the respiratory tract.
A key question raised by the present study is whether antibodies to Hag/MID may mediate protective responses. Based on the results presented here, it is not possible to draw conclusions regarding the potential protective effect of antibodies to Hag/MID. However, the results will facilitate studies to test the hypothesis that human antibodies to Hag/MID mediate a protective effect in COPD. In the context of a prospective study, measuring antibody levels to the key region of Hag/MID and then assessing the subsequent rate of infection relative to antibody level will allow conclusions regarding a potential protective effect. It will be important to assess mucosal antibody responses to Hag/MID in addition to systemic responses, particularly in view of the observation that the development of a mucosal antibody response is associated with fewer symptoms of exacerbation, suggesting that mucosal antibody responses to M. catarrhalis may mediate partial protection in adults with COPD (19).
Hag/MID is a multifunctional molecule that mediates several potential virulence mechanisms, including adherence to respiratory epithelial cells, hemagglutination, IgD binding, B-cell mitogenicity, and collagen binding. In addition, Forsgren et al. have demonstrated that immunization of mice with the same peptide that mediates hemagglutination and adherence to alveolar cells (i.e., A549-CBD) induces enhanced clearance in the mouse pulmonary clearance model (8). Figure 2 shows that regions of the molecule that mediate each of these activities are clustered in the central portion of the Hag/MID molecule. The domain of Hag/MID that was identified in the present study as a key target of human systemic and mucosal antibodies overlaps a portion of all of the domains noted above, suggesting that this region of Hag/MID has the potential to induce protective immune responses in humans. One might speculate that immune responses to this domain block adherence to epithelial cells or mediate enhanced clearance from the respiratory tract. Future work will focus on characterizing the potentially protective effects of human systemic and mucosal antibody responses to this region of Hag/MID.
Acknowledgments
This work was supported by NIH grants AI 051477 (E.R.L.) and AI28304 (T.F.M.) and by the Department of Veterans Affairs.
Footnotes
Published ahead of print on 25 March 2009.
REFERENCES
- 1.American Thoracic Society. 1995. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 152S77-S121. [PubMed] [Google Scholar]
- 2.Barnes, P. J. 2000. Chronic obstructive pulmonary disease. N. Engl. J. Med. 343269-280. [DOI] [PubMed] [Google Scholar]
- 3.Bullard, B., S. Lipski, and E. R. Lafontaine. 2007. Regions important for the adhesin activity of Moraxella catarrhalis Hag. BMC Microbiol. 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullard, B., S. L. Lipski, and E. R. Lafontaine. 2005. Hag directly mediates the adherence of Moraxella catarrhalis to human middle ear cells. Infect. Immun. 735127-5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotter, S. E., N. K. Surana, and J. W. St. Geme III. 2005. Trimeric autotransporters: a distinct subfamily of autotransporter proteins. Trends Microbiol. 13199-205. [DOI] [PubMed] [Google Scholar]
- 6.Forsgren, A., M. Brant, M. Karamehmedovic, and K. Riesbeck. 2003. The immunoglobulin D-binding protein MID from Moraxella catarrhalis is also an adhesin. Infect. Immun. 713302-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forsgren, A., M. Brant, A. Mollenkvist, A. Muyombwe, H. Janson, N. Woin, and K. Riesbeck. 2001. Isolation and characterization of a novel IgD-binding protein from Moraxella catarrhalis. J. Immunol. 1672112-2120. [DOI] [PubMed] [Google Scholar]
- 8.Forsgren, A., M. Brant, and K. Riesbeck. 2004. Immunization with the truncated adhesin Moraxella catarrhalis immunoglobulin D-binding protein (MID764-913) is protective against M. catarrhalis in a mouse model of pulmonary clearance. J. Infect. Dis. 190352-355. [DOI] [PubMed] [Google Scholar]
- 9.Hadzic, R., A. Forsgren, L. O. Cardell, K. Riesbeck, and A. G. Wingren. 2005. The CD19 molecule is crucial for MID-dependent activation of tonsillar B cells from children. Scand. J. Immunol. 61165-172. [DOI] [PubMed] [Google Scholar]
- 10.Henderson, I. R., F. Navarro-Garcia, M. Desvaux, R. C. Fernandez, and D. Ala'Aldeen. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 68692-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoiczyk, E., A. Roggenkamp, M. Reichenbecher, A. Lupas, and J. Heesemann. 2000. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 225989-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holm, M. M., S. L. Vanlerberg, D. D. Sledjeski, and E. R. Lafontaine. 2003. The Hag protein of Moraxella catarrhalis strain O35E is associated with adherence to human lung and middle ear cells. Infect. Immun. 714977-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laarmann, S., D. Cutter, T. Juehne, S. J. Barenkamp, and J. W. St Geme. 2002. The Haemophilus influenzae Hia autotransporter harbours two adhesive pockets that reside in the passenger domain and recognize the same host cell receptor. Mol. Microbiol. 46731-743. [DOI] [PubMed] [Google Scholar]
- 14.Lafontaine, E. R., L. D. Cope, C. Aebi, J. L. Latimer, G. H. McCracken, Jr., and E. J. Hansen. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J. Bacteriol. 1821364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linke, D., T. Riess, I. B. Autenrieth, A. Lupas, and V. A. Kempf. 2006. Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol. 14264-270. [DOI] [PubMed] [Google Scholar]
- 16.Mollenkvist, A., T. Nordstrom, C. Hallden, J. J. Christensen, A. Forsgren, and K. Riesbeck. 2003. The Moraxella catarrhalis immunoglobulin D-binding protein MID has conserved sequences and is regulated by a mechanism corresponding to phase variation. J. Bacteriol. 1852285-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy, T. F., A. L. Brauer, C. Aebi, and S. Sethi. 2005. Antigenic specificity of the mucosal antibody response to Moraxella catarrhalis in chronic obstructive pulmonary disease. Infect. Immun. 738161-8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy, T. F., A. L. Brauer, C. Aebi, and S. Sethi. 2005. Identification of surface antigens of Moraxella catarrhalis as targets of human serum antibody responses in chronic obstructive pulmonary disease. Infect. Immun. 733471-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy, T. F., A. L. Brauer, B. J. Grant, and S. Sethi. 2005. Moraxella catarrhalis in chronic obstructive pulmonary disease. Burden of disease and immune response. Am. J. Respir. Crit. Care Med. 172195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy, T. F., C. Kirkham, D. F. Liu, and S. Sethi. 2003. Human immune response to outer membrane protein CD of Moraxella catarrhalis in adults with chronic obstructive pulmonary disease. Infect. Immun. 711288-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordstrom, T., A. Forsgren, and K. Riesbeck. 2002. The immunoglobulin D-binding part of the outer membrane protein MID from Moraxella catarrhalis comprises 238 amino acids and a tetrameric structure. J. Biol. Chem. 27734692-34699. [DOI] [PubMed] [Google Scholar]
- 22.Nordstrom, T., J. Jendholm, M. Samuelsson, A. Forsgren, and K. Riesbeck. 2006. The IgD-binding domain of the Moraxella IgD-binding protein MID (MID962-1200) activates human B cells in the presence of T cell cytokines. J. Leukoc. Biol. 79319-329. [DOI] [PubMed] [Google Scholar]
- 23.Nummelin, H., M. C. Merckel, J. C. Leo, H. Lankinen, M. Skurnik, and A. Goldman. 2004. The Yersinia adhesin YadA collagen-binding domain structure is a novel left-handed parallel beta-roll. EMBO J. 23701-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson, M. M., E. R. Lafontaine, N. J. Wagner, J. W. St. Geme III, and E. J. Hansen. 2002. A hag mutant of Moraxella catarrhalis strain O35E is deficient in hemagglutination, autoagglutination, and immunoglobulin D-binding activities. Infect. Immun. 704523-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson, M. M., C. A. Laurence, S. E. Guinn, and E. J. Hansen. 2006. Biofilm formation by Moraxella catarrhalis in vitro: roles of the UspA1 adhesin and the Hag hemagglutinin. Infect. Immun. 741588-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riesbeck, K., and T. Nordstrom. 2006. Structure and immunological action of the human pathogen Moraxella catarrhalis IgD-binding protein. Crit. Rev. Immunol. 26353-376. [DOI] [PubMed] [Google Scholar]
- 27.Roggenkamp, A., N. Ackermann, C. A. Jacobi, K. Truelzsch, H. Hoffmann, and J. Heesemann. 2003. Molecular analysis of transport and oligomerization of the Yersinia enterocolitica adhesin YadA. J. Bacteriol. 1853735-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruckdeschel, E. A., C. Kirkham, A. J. Lesse, Z. Hu, and T. F. Murphy. 2008. Mining the Moraxella catarrhalis genome: identification of potential vaccine antigens expressed during human infection. Infect. Immun. 761599-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandin, C., S. Linse, T. Areschoug, J. M. Woof, J. Reinholdt, and G. Lindahl. 2002. Isolation and detection of human IgA using a streptococcal IgA-binding peptide. J. Immunol. 1691357-1364. [DOI] [PubMed] [Google Scholar]
- 30.Sethi, S., N. Evans, B. J. B. Grant, and T. F. Murphy. 2002. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 347465-471. [DOI] [PubMed] [Google Scholar]
- 31.Sethi, S., and T. F. Murphy. 2008. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 3592355-2365. [DOI] [PubMed] [Google Scholar]
- 32.Surana, N. K., D. Cutter, S. J. Barenkamp, and J. W. St Geme, 3rd. 2004. The Haemophilus influenzae Hia autotransporter contains an unusually short trimeric translocator domain. J. Biol. Chem. 27914679-14685. [DOI] [PubMed] [Google Scholar]
- 33.Tan, T. T., J. J. Christensen, M. H. Dziegiel, A. Forsgren, and K. Riesbeck. 2006. Comparison of the serological responses to Moraxella catarrhalis immunoglobulin D-binding outer membrane protein and the ubiquitous surface proteins A1 and A2. Infect. Immun. 746377-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verhaegh, S. J., A. Streefland, J. K. Dewnarain, D. J. Farrell, A. van Belkum, and J. P. Hays. 2008. Age-related genotypic and phenotypic differences in Moraxella catarrhalis isolates from children and adults presenting with respiratory disease in 2001-2002. Microbiology 1541178-1184. [DOI] [PubMed] [Google Scholar]
- 35.Yeo, H. J., S. E. Cotter, S. Laarmann, T. Juehne, J. W. St. Geme III, and G. Waksman. 2004. Structural basis for host recognition by the Haemophilus influenzae Hia autotransporter. EMBO J. 231245-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]