Abstract
Differentiation of latent tuberculosis infection (LTBI) from a healthy, unexposed population plays a vital role in the strategy of controlling and eliminating tuberculosis (TB). Both CFP21 and MPT64, antigens encoded by the RD2 region which are restricted in the Mycobacterium tuberculosis complex, are TB-specific diagnostic candidate antigens. In this study, we designed a fusion protein by linking both CFP21 and MPT64 with a 15-amino-acid peptide, (G4S1)3, and overexpressed the fusion protein in Escherichia coli. A new whole-blood gamma interferon assay based on the recombinant fusion protein, CFP21-MPT64 (rCM-WBIA), was developed and compared with the tuberculin skin test (TST) for screening of LTBI in household contacts of patients with sputum-positive TB. rCM-WBIA had a slightly higher sensitivity (66.7%; 24/36 contacts) than that of the TST (61.1%; 22/36 contacts) for household contacts. We found that rCM-WBIA had a very high sensitivity (90.9%) and specificity (71.4%) for LTBI detection compared with TST. The overall agreement between rCM-WBIA and TST was 83.3% (k = 0.64); rCM-WBIA positivity was associated with a larger TST induration. These results suggest that rCM-WBIA, based on the recombinant fusion protein CFP21-MPT64, is a promising alternative diagnostic tool for detection of LTBI.
Despite progress in the past decades, tuberculosis (TB) remains a major public health problem worldwide. About one-third of the world population is latently infected with Mycobacterium tuberculosis, and 10% of those infected persons develop disease during their lifetime. Therefore, sensitive and specific assays for diagnosis of latent TB infection (LTBI) are important for effective control and prevention of TB (7). In contrast to sputum-positive cases of TB, LTBI is more difficult to diagnose. The tuberculin skin test (TST), based on purified protein derivative (PPD), has been used for diagnosis of LTBI for decades (1). However, the test has a poor specificity among persons vaccinated with the Mycobacterium bovis bacillus Calmette-Guérin (BCG) strain.
The identification of M. tuberculosis-specific antigens by mycobacterial genomic studies allowed the development of a new generation of diagnostic tests. Comparative genomics of M. tuberculosis and M. bovis BCG has led to the identification of several regions of difference (RDs). One region, designated RD1, is present in virulent strains of M. bovis and M. tuberculosis but not in BCG substrains (3) and most environmental mycobacteria (1). A whole-blood gamma interferon (IFN-γ) assay (IGRA or enzyme-linked immunospot [ELISPOT] assay) based on the RD1-encoded antigens early secretory antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10) has been found to be sensitive and specific for the detection of infection with M. tuberculosis (1, 20, 22). Two tests, QuantiFERON-TB Gold (Cellestis, Carnegie, Australia) and T-Spot.TB test (Oxford Immunotec, Oxford, United Kingdom), have been evaluated in clinical studies and showed better performances than that of TST. However, exposure to some nontuberculous mycobacteria that are able to produce ESAT-6 and/or CFP-10, such as M. kansasii, M. marinum, M. leprae, and M. szulgai (1), may cause false-positive results in these assays (2, 12, 13). Additionally, both ESAT-6 and CFP-10 are considered components of many promising vaccine candidates, such as recombinant BCG strains expressing these antigens (10, 24, 25), subunit vaccines (5, 17, 31), and combinations with BCG vaccination (9, 18). Thus, the identification of new targets for the detection of LTBI is a high priority.
The RD2 region is restricted to the M. tuberculosis complex but is absent in BCG substrains derived after 1931 (3, 4). A number of studies confirmed that MPT64, one of the best-characterized antigens from the RD2 region, is able to elicit a strong delayed-type hypersensitivity reaction and to induce high levels of IFN-γ responses in TB patients and their contacts (8, 21, 26, 28). CFP21 is another immunodominant protein encoded in the RD2 region (14) and induces a high level of IFN-γ release from blood cells of TB patients (30). Thus, these two antigens are suitable candidates for T-cell-based TB diagnostic assays. Moreover, the BCG Danish substrain without an RD2 region (3, 4) has been used for neonatal vaccination in China. Therefore, prior BCG vaccination will not interfere with immunological assays based on CFP21 and MPT64 proteins.
In the present study, we designed a fusion protein of CFP21 and MPT64 from M. tuberculosis H37Rv. The fusion protein was expressed in Escherichia coli and purified using Ni-nitrilotriacetic acid (Ni-NTA) affinity chromatography. A whole-blood IFN-γ assay using the CFP21-MPT64 fusion protein (rCM-WBIA) was developed for diagnosis of LTBI. Testing of clinical samples demonstrated that this new assay is a promising alternative diagnostic tool for diagnosis of LTBI.
MATERIALS AND METHODS
Bacterial strains and media.
E. coli strains DH5α and BL21(DE3) were used for cloning and expression, respectively. Both strains were cultured in Luria-Bertani (LB) medium, with or without agar. When required, ampicillin was added to a final concentration of 100 μg/ml. M. tuberculosis strain H37Rv was cultivated in Difco Middlebrook 7H9 medium (BD, Sparks, MD) at 37°C for 3 weeks, as described previously (9).
Plasmid construction.
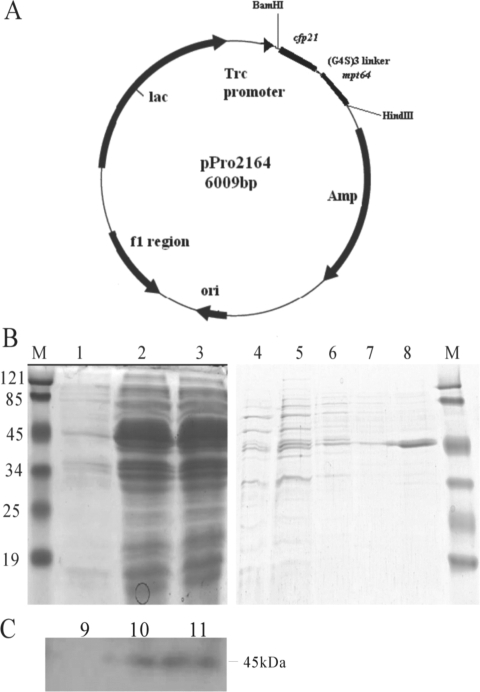
The coding sequences of the mature form of the CFP21 (Rv1984c) and MPT64 (Rv1980c) proteins were amplified by PCRs using the specific primers listed in Table 1 (Sangon Biotech, Shanghai, China), with the chromosomal DNA of M. tuberculosis H37Rv as a template. The PCR fragments encoding the fusion protein of CFP21 and MPT64 were generated by a second PCR according to the method of gene splicing with overlap extension (15). The fusion gene was digested with BamHI and HindIII and inserted into the expression vector pProEXHTb (Invitrogen, Carlsbad, CA), predigested with the same restriction enzymes. This procedure resulted in plasmid pPro2164, with the fusion gene under the control of the Trc promoter (Fig. 1). The correctness of the cloned sequence was confirmed by DNA sequencing.
TABLE 1.
Primers and thermal cycle parameters for cloning of M. tuberculosis antigens
| Antigen | Primer orientation or sequence (5′-3′) | PCR parameters | Amplicon size (bp) |
|---|---|---|---|
| CFP21F | AAGGATCCGATCCGTGTTCGGACATCGCGGTCG | 94°C for 5 min, then 30 cycles at 94°C for | 608 |
| CFP21R | GCTGCCGCCACCGCCGCTTCCGCCACCGCCGCTTCCACCGCCACCTCCGGCGTGATCGAGCCTGTTCGCC | 1 min, 60°C for 1 min, and 72°C for 45 s, then 72°C for 5 min | |
| MPT64F | GGTGGCGGTGGAAGCGGCGGTGGCGGAAGCGGCGGTGGCGGCAGCGCGCCCAAGACCTACTGCGAGGAG | 94°C for 5 min, then 30 cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for | 671 |
| MPT64R | GAAAGCTTCTAGGCCAGCATCGAGTCGATCGC | 45 s, then 72°C for 5 min | |
| rCMF | CFP21F | 94°C for 5 min, then 30 cycles at 94°C for | 1,234 |
| rCMR | MPT64R | 1 min, 60°C for 1 min, and 72°C for 90 s, then 72°C for 10 min |
FIG. 1.
Expression and purification of rCM fusion protein. (A) Map of pPro2164 and the fusion gene encoding the predicted mature forms of CFP21 and MPT64, with a 45-bp linker, which was cloned into the BamHI and HindIII sites of pProExHTb, resulting in the recombinant plasmid pPro2164. E. coli BL21(DE3) harboring pPro2164 was cultured with IPTG. The expression of rCM was confirmed by SDS-PAGE (B) and Western blotting (C) as described in Materials and Methods. Lane M, protein molecular size marker (kDa); lanes 1 and 9, E. coli strain without IPTG; lanes 2 and 10, E. coli strain 4 h after IPTG induction; lanes 3 and 11, E. coli strain 6 h after IPTG induction; lane 4, fraction from Ni-NTA column after wash with denaturing binding buffer; lane 5, fraction after wash with denaturing wash buffer; lanes 6 and 7, fraction after wash with native wash buffer; lane 8, rCM protein eluted with native elution buffer.
Expression and purification of the rCM fusion protein and detection by Western blotting.
E. coli BL21(DE3) harboring plasmid pPro2164 was cultured overnight. Overnight cultures (10 ml) were inoculated into 1 liter of LB medium containing 100 μg/ml ampicillin and incubated at 37°C with shaking until the optical density at 600 nm reached 0.6. The expression of the fusion protein rCM was induced with isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 0.4 mM for 4 to 6 h. E. coli was pelleted by centrifugation at 4°C, and the bacterial pellet was stored at −20°C. Protein samples were prepared in sample buffer and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After SDS-PAGE, proteins were stained with Coomassie blue dye.
Protein purification was performed using a Ni-NTA purification system according to the protocol in the instruction manual (Invitrogen). The purified rCM protein was lyophilized, aliquoted, and stored at −20°C. The preparation of rCM protein was diluted in normal saline, using pyrogen-free reagents, and tested to exclude endotoxin contamination by using a Limulus amebocyte lysate assay (Associates of Cape Cod Inc., East Falmouth, MA). The protein concentration was determined by using an ACTGene protein assay kit (ACTGene Inc., Piscataway, NJ), with bovine serum albumin as the protein standard.
To verify the specificity of purified rCM fusion protein, the protein samples were separated by SDS-PAGE, electrically transferred onto nitrocellulose membranes, and detected with a polyclonal rabbit anti-His6 antibody (GenScript Corp., Piscataway, NJ) as the primary antibody and alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G antibody (Santa Cruz Biotechnology, Santa Cruz, CA) as the secondary antibody.
Testing of clinical samples.
The study protocol was approved by the Ethics Committee of Tongji Medical College. Written informed consent was obtained from all subjects involved in this study. Ten sputum-positive TB patients (mean age, 38 ± 10 years; range, 22 to 55 years; male/female ratio, 4/6) were enrolled by Wuhan TB Hospital (Wuhan, China). Thirty-six household contacts (mean age, 35 ± 18 years; range, 15 to 81 years; male/female ratio, 16/20) of these patients were recruited for the study. Active TB was excluded for all household contacts in this study based on radiologic and clinical examinations, sputum microscopy, and bacteriologic culture. A total of 40 healthy donors (mean age, 20 ± 3 years; range, 17 to 25 years; male/female ratio, 22/18) served as controls. These persons were selected on the basis of a negative TST and no history of recent contact with active TB patients by questionnaire. All subjects included in the study were seronegative for human immunodeficiency virus infection.
TST.
After the collection of blood samples for the IFN-γ assay from persons in this study, TST was performed by trained nursing staff at Wuhan TB Hospital. Briefly, 0.1 ml of BCG PPD (Chengdu Institution of Biological Products, Chengdu, China) containing 5 tuberculin units was injected intradermally on the volar surface of the participant's forearm. After 72 h, the diameter of the area of induration around the injection site was measured across the forearm and was reported in millimeters. Reactions of <5 mm, ≥5 mm, and ≥15 mm were considered TST negative, positive, and strongly positive, respectively.
WBIA based on the rCM fusion protein.
Heparinized whole blood from each donor (1 ml) was seeded in 24-well plates and incubated with 20 μl rCM protein at final concentrations of 0 (normal saline), 5, 20, and 40 μg/ml for 20 to 24 h at 37°C. As a positive control, phytohemagglutinin (PHA) was used at a final concentration of 20 μg/ml. After stimulation, 200 μl of plasma was then taken from each well and stored at −20°C until use. The concentrations of IFN-γ in collected samples were determined in duplicate, using a commercial double-sandwich enzyme-linked immunosorbent assay kit according to the manufacturer's instructions (Dakewei Biotechnology, Shenzhen, China). The difference between the duplicate wells was consistently <10% of the mean. For each subject, the saline control value was subtracted from the values for rCM-stimulated samples.
The cutoff value for a positive response in the rCM-WBIA was established using data from 40 non-M. tuberculosis-exposed control donors, as described previously (6). From a receiver operating characteristic curve analysis based on the highest IFN-γ level, 398.5 pg/ml of IFN-γ was set as the cutoff value for the rCM-WBIA, with a specificity of 97%.
Statistic analysis.
The level of concordance between the TST and rCM-WBIA was assessed and calculated as the agreement between the results of the two assays, using two-by-two contingency tables. The strength of this agreement was examined by using Cohen's kappa coefficient, with values of >0.75 representing excellent agreement beyond chance, 0.40 to 0.75 representing fair to good agreement beyond chance, and <0.40 representing poor agreement beyond chance.
RESULTS
Expression and purification of rCM fusion protein.
The partial sequences of Rv1984c and Rv1980c, encoding the mature proteins CFP21 and MPT64, respectively, were amplified by PCR from the chromosomal DNA of M. tuberculosis strain H37Rv. The PCR fragments, with lengths of 608 bp and 671 bp, were cloned and subjected to DNA sequencing to verify the desired constructs. The fusion gene encoding the rCM protein with a 45-bp linker (encoding three copies of peptide G4S1) was further generated by the method of gene splicing with overlap extension and subcloned into the expression vector pProEXHTb, resulting in pPro2164.
E. coli harboring the plasmid pPro2164 expressed a protein with a molecular mass of approximately 45 kDa upon induction with IPTG. The size of this protein was consistent with the expected molecular mass of the fusion protein rCM (Fig. 1). The expression of the fusion protein reached a maximum after 4 h. Western blotting with an antibody to the hexahistidine tag confirmed that the 45-kDa protein represented the fusion protein rCM (Fig. 1). The fusion protein rCM was not soluble and formed inclusion bodies. Therefore, the rCM protein was extracted by sonication, dissolved in urea, and purified using a Ni-NTA purification system. The purified protein was examined by SDS-PAGE and verified by Western blotting using an anti-hexahistidine-tag antibody (Fig. 1). The yield of the rCM protein was determined to be 200 mg/liter LB broth culture.
IFN-γ assay using whole blood samples stimulated with rCM.
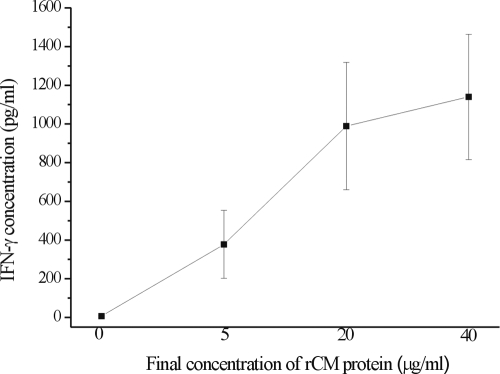
The ability of the rCM fusion protein to stimulate IFN-γ production in whole blood samples was tested. Whole blood samples were collected from eight TST-positive household contacts and stimulated with rCM fusion protein at different concentrations. As shown in Fig. 2, the IFN-γ levels in whole blood samples increased with the concentrations of rCM fusion protein of 5 and 20 μg/ml. An increase of the concentration of rCM to 40 μg/ml only marginally enhanced the IFN-γ production in whole blood samples. Thus, 20 μg/ml of rCM fusion protein was used for further experiments in the present study.
FIG. 2.
Dose-dependent IFN-γ production in whole blood samples from TST-positive household contacts after stimulation with rCM fusion protein. The concentration of IFN-γ in each sample was obtained and expressed as the mean ± standard deviation (n = 8).
Testing of clinical samples with rCM-WBIA.
Whole blood samples were collected from healthy controls, household contacts, and TB patients and subjected to rCM-WBIA (Fig. 3). The levels of IFN-γ in all samples without antigen stimuli were below 30 pg/ml (10.6 ± 5.9 pg/ml) and increased to 5,405 ± 2,518.9 pg/ml after PHA stimulation. The mean IFN-γ levels in healthy donors, household contacts, and patients after PHA stimulation were 5,283.9 ± 2,581.1 pg/ml, 5,451.5 ± 2,644.9 pg/ml, and 5,722.2 ± 2,439.6 pg/ml, respectively. The mean IFN-γ level by rCM-WBIA for samples from 40 healthy donors was 220.7 ± 136.9 pg/ml. The mean IFN-γ level (1,400.1 ± 1,146.9 pg/ml) in samples from the household contacts was significantly higher than that for the healthy control group. The highest IFN-γ levels were detected in whole blood samples from the TB patients (2,291.1 ± 1,143.1 pg/ml), and the levels of IFN-γ of all patients were over the cutoff value.
FIG. 3.
Results of WBIA for rCM and PHA. Each square represents the IFN-γ concentration in a sample, and median values for different groups are indicated by horizontal lines. The dotted line depicts the test cutoff value for a positive result of rCM-WBIA (398.5 pg/ml).
Comparison between TST and rCM-WBIA for detection of LTBI.
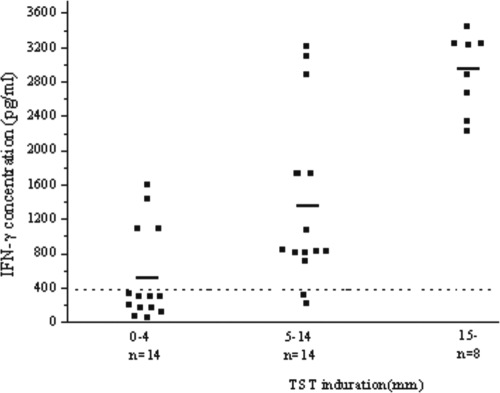
Twenty-two of 36 (61.1%) household contacts were TST positive, and 14 (38.9%) were TST negative. By rCM-WBIA, 24 (66.7%) of 36 household contacts were positive and 12 (33.3%) were negative. Six subjects had discrepant results for TST and rCM-WBIA. Four household contacts were TST negative but rCM-WBIA positive, and two had the reverse status. Among the concordant results, 20 subjects were positive by both TST and rCM-WBIA, and 10 subjects were negative by both TST and rCM-WBIA. Compared with TST, rCM-WBIA had a sensitivity of 90.9% (95% confidence interval, 89.96 to 91.84%) and a specificity of 71.4% (95% confidence interval, 71.25 to 71.55%). This gave an overall agreement between the two tests for household contacts of 83.3% (95% confidence interval, 83.03 to 83.57%), with a k value of 0.64, indicating a good agreement between the two tests. Analysis of the distribution of IFN-γ levels in TST-positive and TST-negative samples showed a significant correlation between TST positivity and IFN-γ level (Fig. 4). The mean levels of IFN-γ were 2,907.5 ± 453.7 pg/ml and 1,387.6 ± 994 pg/ml for samples from the strongly TST-positive and TST-positive groups, respectively, with rCM-WBIA-positive rates of 100% (8/8 subjects) and 85.7% (12/14 subjects), respectively. The median level for TST-negative groups was 552 ± 516.9 pg/ml, with an rCM-WBIA-positive rate of 28.6% (4/14 subjects).
FIG. 4.
Correlation between TST and IFN-γ levels determined by rCM-WBIA. A total of 36 household contacts were tested by TST and rCM-WBIA. Each square represents the IFN-γ concentration in a sample, and median values for different groups are indicated by horizontal lines. The dotted line depicts the test cutoff value for a positive result of rCM-WBIA (398.5 pg/ml).
DISCUSSION
In this study, we established rCM-WBIA as a potential diagnostic assay for LTBI. In testing with defined clinical samples based on TST, rCM-WBIA had a high sensitivity, of 90.9%, and a specificity of 71.4%, and a good agreement was observed between rCM-WBIA and TST (k = 0.64). Whole blood samples from all sputum-positive TB patients also tested positive by rCM-WBIA. This assay is comparable to the IGRA-RD1 or RD1-ELISPOT assay already used in clinical settings and has the same advantages over the TST.
Although the MTP64 antigen was found to induce a strong delayed-type hypersensitivity reaction in guinea pigs (8) and human beings (21, 28), different results were obtained with MTP64 in blood assays. Johnson et al. reported negative blood test results with MPT64 for persons before and after BCG vaccination and for active TB patients (16). In contrast, Roche et al. confirmed that MPB64 was recognized more frequently by TB patients and their contacts than by healthy populations vaccinated with BCG (27). Furthermore, patients with active TB had significant responses to MPT64 in WBIA (26).
The potential usefulness of RD2-based diagnostics in different settings depends mainly on the distribution of different BCG substrains and their use for vaccination (11). The RD2 region has been demonstrated by Behr et al. to be deleted in all BCG substrains derived after 1931 (3, 4). BCG-Danish, BCG-Prague, BCG-Glaxo, BCG-Frappier, BCG-Connaught, BCG-Phipps, BCG-Tice, and BCG-Pasteur substrains do not contain the RD2 region, while this region is present in BCG-Birkhaug, BCG-Japan, BCG-Moreau, BCG-Russia, and BCG-Sweden substrains (3, 4). Thus, the use of rCM-WBIA is limited by the presence of BCG substrains with RD2. However, RD2-based diagnostics may become alternatives to RD1-based WBIAs if the antigens from RD1 are included as vaccine candidates, as done in different studies (17, 25).
In this study, we demonstrated that rCM-WBIA has a slightly higher sensitivity (66.7%) than that of TST (61.1%) for detecting LTBI in household contacts. Several previous studies using IGRA-RD1 or RD1-ELISPOT assay with specific antigen stimuli resulted in similar findings (20). In addition, QFT-G, another commercialized ex vivo IFN-γ assay using PPD as a stimulant, showed a higher sensitivity than that of TST (29). Blood testing and TST measure cell-mediated immune responses to TB antigens by different means (1), and different antigens (PPD and rCM) were used in our study. These may lead to discordant results. TST measures an in vivo multimediator inflammatory response to multiple antigens (19), while blood testing measures ex vivo IFN-γ release by circulating lymphocytes in response to specific or complex antigens (23). Two cases of TST-positive but rCM-WBIA-negative household contacts might be explained by BCG vaccination or environmental mycobacterial infection. However, this is not a plausible explanation for the existence of four household contacts with TST-negative but rCM-WBIA-positive results. Due to the lack of a gold standard for diagnosis of M. tuberculosis infection, a comparison of WBIA and TST is not always possible. Whether the TST-negative, rCM-WBIA-positive contacts have a risk of progression to active TB remains to be investigated by follow-up studies.
In conclusion, our study clearly suggests that the strong correlation between the IFN-γ responses to the rCM fusion protein in a PPD-positive population and the rCM-WBIA results has great potential for use in diagnosis of LTBI in humans, as well as in epidemiologic studies.
Acknowledgments
This work was partly supported by grants from National High Technology Research and Development of China (863 program; grant 2006AA02Z445) and the National Natural Science Foundation of China (grant 30671861).
Footnotes
Published ahead of print on 11 March 2009.
REFERENCES
- 1.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 3561099-1104. [DOI] [PubMed] [Google Scholar]
- 2.Arend, S. M., K. E. van Meijgaarden, K. deBoer, E. C. de Palou, D. van Soolingen, T. H. M. Ottenhoff, and J. T. van Dissel. 2002. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J. Infect. Dis. 1861797-1807. [DOI] [PubMed] [Google Scholar]
- 3.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 2841520-1523. [DOI] [PubMed] [Google Scholar]
- 4.Behr, M. A., and P. M. Small. 1999. A historical and molecular phylogeny of BCG strains. Vaccine 17915-922. [DOI] [PubMed] [Google Scholar]
- 5.Brandt, L., M. Elhay, I. Rosenkrands, E. B. Lindblad, and P. Andersen. 2000. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 68791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brock, I., K. Weldingh, T. Lillebaek, F. Follmann, and P. Andersen. 2004. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am. J. Respir. Crit. Care Med. 17065-69. [DOI] [PubMed] [Google Scholar]
- 7.CDC. 1995. Essential components of a tuberculosis prevention and control program. MMWR Morb. Mortal. Wkly. Rep. 441-17.7799912 [Google Scholar]
- 8.Elhay, M. J., T. Oettinger, and P. Andersen. 1998. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect. Immun. 663454-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan, X., Q. Gao, and R. Fu. 2007. DNA vaccine encoding ESAT-6 enhances the protective efficacy of BCG against Mycobacterium tuberculosis infection in mice. Scand. J. Immunol. 66523-528. [DOI] [PubMed] [Google Scholar]
- 10.Fan, X., T. Yu, Q. Gao, and W. Yao. 2006. Immunological properties of recombinant Mycobacterium bovis bacillus Calmette-Guerin strain expressing fusion protein IL-2-ESAT-6. Acta Biochim. Biophys. Sin. 38683-690. [DOI] [PubMed] [Google Scholar]
- 11.Fine, P. E. M., I. A. M. Carneiro, J. B. Milstien, and C. J. Clements. 1999. Issues relating to the use of BCG in immunization programmes, p. 18-20. WHO, Geneva, Switzerland.
- 12.Geluk, A., K. E. van Meijgaarden, K. L. M. C. Franken, B. Wieles, S. M. Arendz, W. R. Faber, B. Naafsy, and T. H. M. Ottenhoff. 2004. Immunological crossreactivity of the Mycobacterium leprae CFP-10 with its homologue in Mycobacterium tuberculosis. Scand. J. Immunol. 5966-70. [DOI] [PubMed] [Google Scholar]
- 13.Geluk, A., K. E. van Meijgaarden, K. L. M. C. Franken, Y. W. Subronto, B. Wieles, S. M. Arend, E. P. Sampaio, T. de Boer, W. R. Faber, B. Naafs, and T. H. M. Ottenhoff. 2002. Identification and characterization of the ESAT-6 homologue of Mycobacterium leprae and T-cell cross-reactivity with Mycobacterium tuberculosis. Infect. Immun. 702544-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grover, A., M. F. Ahmed, I. Verma, P. Sharma, and G. K. Khuller. 2006. Expression and purification of the Mycobacterium tuberculosis complex-restricted antigen CFP21 to study its immunoprophylactic potential in mouse model. Protein Expr. Purif. 48274-280. [DOI] [PubMed] [Google Scholar]
- 15.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 7761-68. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, P. D. R., R. L. Stuart, M. L. Grayson, D. Olden, A. Clancy, P. Ravn, P. Andersen, W. J. Britton, and J. S. Rothe. 1999. Tuberculin-purified protein derivative-, MPT-64-, and ESAT-6-stimulated gamma interferon responses in medical students before and after Mycobacterium bovis BCG vaccination and in patients with tuberculosis. Clin. Diagn. Lab. Immunol. 6934-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langermans, J. A., T. M. Doherty, R. A. Vervenne, T. van der Laan, K. Lyashchenko, R. Greenwald, E. M. Agger, C. Aagaard, H. Weiler, D. van Soolingen, W. Dalemans, A. W. Thomas, and P. Andersen. 2005. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine 232740-2750. [DOI] [PubMed] [Google Scholar]
- 18.Mauea, A. C., W. R. Water, M. V. Palmer, B. J. Nonnecke, F. C. Minio, W. C. Brown, J. Norimine, M. R. Foote, C. F. Scherer, and D. M. Estes. 2007. An ESAT-6:CFP10 DNA vaccine administered in conjunction with Mycobacterium bovis BCG confers protection to cattle challenged with virulent M. bovis. Vaccine 254735-4746. [DOI] [PubMed] [Google Scholar]
- 19.Menzies, D., and T. M. Doherty. 2006. Diagnosis of latent tuberculosis infection, p. 215-263. In M. C. Raviglione (ed.), Reichman and Hershfield's tuberculosis. A comprehensive international approach. Informa Healthcare USA, New York, NY.
- 20.Menzies, D., M. Pai, and G. Comstock. 2007. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann. Intern. Med. 146340-354. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura, R. M., M. A. Velmonte, K. Kawajiri, C. F. Ang, R. A. Frias, M. T. Mendoza, J. C. Montoya, I. Honda, S. Haga, and I. Toida. 1998. MPB64 mycobacterial antigen: a new skin-test reagent through Mycobacterium tuberculosis infection. Vaccine 231680-1685. [PubMed] [Google Scholar]
- 22.Pai, M., K. Dheda, J. Cunningham, F. Scano, and R. O'Brien. 2007. T-cell assays for the diagnosis of latent tuberculosis infection: moving the research agenda forward. Lancet Infect. Dis. 7428-438. [DOI] [PubMed] [Google Scholar]
- 23.Pai, M., L. W. Riley, and J. M. Colford. 2004. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect. Dis. 4761-776. [DOI] [PubMed] [Google Scholar]
- 24.Palendira, U., J. M. Spratt, W. J. Britton, and J. A. Triccas. 2005. Expanding the antigenic repertoire of BCG improves protective efficacy against aerosol patch method for rapid diagnosis of active tuberculosis. Int. J. Tuberc. Lung Dis. 2541-546. [Google Scholar]
- 25.Pym, A. S., P. Brodin, L. Majlessi, R. Brosch, C. Demangel, A. Williams, K. E. Griffiths, G. Marchal, C. Leclerc, and S. T. Cole. 2003. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 9533-539. [DOI] [PubMed] [Google Scholar]
- 26.Roche, P. W., C. G. Feng, and W. J. Britton. 1996. Human T-cell epitopes on the Mycobacterium tuberculosis secreted protein MPT64. Scand. J. Immunol. 43662-670. [DOI] [PubMed] [Google Scholar]
- 27.Roche, P. W., J. A. Triccas, D. T. Avery, T. Fifis, H. Billman-Jacobe, and W. J. Britton. 1994. Differential T cell responses to mycobacteria-secreted proteins distinguish vaccination with bacille Calmette-Guérin from infection with Mycobacterium tuberculosis. J. Infect. Dis. 1701326-1330. [DOI] [PubMed] [Google Scholar]
- 28.Roche, P. W., N. Winter, J. A. Triccas, C. G. Feng, and W. J. Britton. 1996. Expression of Mycobacterium tuberculosis MPT64 in recombinant Myco. smegmatis: purification, immunogenicity and application to skin tests for tuberculosis. Clin. Exp. Immunol. 103226-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soysal, A., T. Torun, S. Efe, H. Gencer, K. Tahaoglu, and M. Bakir. 2008. Evaluation of cut-off values of interferon-gamma-based assays in the diagnosis of M. tuberculosis infection. Int. J. Tuberc. Lung Dis. 1250-56. [PubMed] [Google Scholar]
- 30.Weldingh, K., I. Rosenkrands, S. Jacobsen, P. B. Rasmussen, M. J. Elhay, and P. Andersen. 1998. Two-dimensional electrophoresis for analysis of Mycobacterium tuberculosis culture filtrate and purification and characterization of six novel proteins. Infect. Immun. 663492-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu, Y., B. Wang, J. Chen, Q. Wang, B. Zhu, H. Shen, Y. Qie, J. Wang, and H. Wang. 2005. Chimeric protein improved immunogenicity compared with fusion protein of Ag85B and ESAT-6 antigens of Mycobacterium tuberculosis. Scand. J. Immunol. 64476-481. [DOI] [PubMed] [Google Scholar]