Abstract
After adolescence, the incidence of meningococcal disease decreases with age as a result of the cumulative immunizing effect of repeated nasopharyngeal colonization. Nevertheless, some adults succumb to meningococcal disease, so we hypothesized that this is due to a subtle functional immunological defect. Peripheral blood lymphocytes derived from survivors of serogroup C meningococcal disease and from age- and sex-matched controls were incubated with a polyclonal B-cell activator containing anti-immunoglobulin D (α-δ-dex) employed to mimic antigen-specific stimuli encountered during immune responses to bacterial polysaccharides, with and without T-cell activation (using anti-CD3/anti-CD28). Subsequent proliferation and activation of T and B lymphocytes were measured. In patients, T-cell responses to polyclonal stimuli and the delivery of T-cell help to B cells were unimpaired. Levels of B-cell proliferation in response to α-δ-dex stimulation alone were low in all samples but were significantly lower in patients than in controls, and these differences were more pronounced with the addition of T-cell help. The data are consistent with the presence of a subtle immunodeficiency in adults who have exhibited susceptibility to meningococcal disease. This defect is manifested as an impaired B-cell response to T-cell-independent type 2 antigens analogous to bacterial capsular polysaccharide.
Meningococcal disease is more common in infants than in adults, likely due to increasing protection over time due to cumulative immunizing episodes of natural nasopharyngeal carriage of Neisseria meningitidis. Several factors are known to determine susceptibility to meningococcal disease, but chief among these is the possession of protective levels of serum bactericidal antibody (SBA) directed against the meningococcus (2). The generation of natural immunity is thought to be the result of recurrent oronasopharyngeal colonization by meningococci. Most colonizing events are transient and result in expulsion of the organism, along with natural generation of protective immunoglobulin G (IgG) antibody against the colonizing strain (23). During this natural exposure to bacteria, responses are generated against the capsular polysaccharide and against T-cell-dependent (TD) protein antigens. Antibodies against both polysaccharides and proteins can exert bactericidal activity in vitro. Carriage of Neisseria spp. is common (14). In older children and adults in the United Kingdom, the prevalence of carriage of N. meningitidis is 25 to 37% (3), and therefore the probability that an adult has never been exposed to the organism is low and cumulative immunizing exposure to the meningococcus should be significant by the time a person reaches adulthood. Nevertheless, there remains a significant disease burden in adults. Goldschneider and colleagues (5) collected serum over the course of an epidemic caused by serogroup C among adult army recruits and demonstrated that patients with meningococcal disease did not have bactericidal antibody against the cognate serogroup prior to the onset of their disease. It was also discovered that patients were relatively deficient of antibody to heterologous serogroup strains. Although this might reflect reduced prior meningococcal exposure for recruits drawn from some geographic regions of the United States with a low incidence of carriage, there remains the possibility that adults who suffer serogroup C meningococcal disease have defective humoral immunity to pathogenic meningococci in general. Although carriage of serogroup C N. meningitidis is infrequent in the general population (<0.5%), most individuals who become colonized by serogroup C do not experience disease (26), despite the fact that adults in an unvaccinated population do not have serum serogroup C bactericidal activity or high concentrations of antipolysaccharide antibody (27).
The chief target for protective immunity against the meningococcus is the polysaccharide capsule. Capsular polysaccharides, as T-cell-independent type 2 (TI-2) antigens, generate relatively weak IgG responses when delivered alone, but the response becomes TD when the polysaccharide is presented with protein, for example, when it is delivered as part of a protein conjugate vaccine (22). Capsular polysaccharides contain multiple repeating epitopes and therefore have the ability to cross-link B-cell receptors and induce B-cell activation directly (16), unlike TD protein antigens. However, immune responses against protein antigens can also contribute to SBA titers, as immunization with outer membrane vesicles (24) or colonization with neisserial species or strains not sharing the same capsular antigen (12) can each induce SBA. Antibody responses against TD antigens such as proteins require T-cell help, but there is also some evidence from model systems that responses against capsular polysaccharides presented on whole organisms can also benefit from T-cell help (10).
We postulated that adults who suffer meningococcal disease have a subtle deficiency of the humoral immune response to colonization by Neisseria, leading to a failure to rapidly generate protective antibody titers. We tested this hypothesis by using a polyclonal TI-2 antigen mimic and polyclonal T-cell activators to stimulate peripheral B and T lymphocytes derived from recovered meningococcal patients and controls.
MATERIALS AND METHODS
Patients and controls.
Patients were recruited from among those who presented to the Sheffield Teaching Hospitals NHS Trust with microbiologically proven meningococcal serogroup C (Men C) disease. Patients were included if N. meningitidis was identified by culture or PCR within a blood or cerebrospinal fluid sample. All patients and controls gave informed consent, and the study was approved by the Central Office for Research Ethics (United Kingdom). Clinical and laboratory data for the seven patients are shown in Table 1. Controls were derived from hospital staff and were matched to the ethnicity, sex, and current age of the patients. The age of the patients at the time of original presentation ranged from 16 years to 32 years. The period of time elapsed from hospitalization to inclusion in the study was 6 to 11 years, and none of the patients or controls had ever received vaccination (either plain polysaccharide or conjugate) against serogroup C disease. None of the patients or controls had a detectable deficiency of complement components C5 to C9 or of mannose binding lectin, deficiencies which are known to enhance susceptibility to meningococcal infection (data not shown). All positive meningococcal isolates were confirmed by the National Meningococcal Reference Unit of the United Kingdom Health Protection Agency.
TABLE 1.
Patient characteristicsc
| Patient no. | Sexa | Current age (yr) | Time (yr) since hospitalization | Serogroupb |
|---|---|---|---|---|
| 1 | M | 41 | 9 | C 2a P1.5 |
| 3 | F | 29 | 8 | C2a P1.5, P1.2 |
| 5 | F | 41 | 8 | C2a P1.5 |
| 7 | F | 22 | 6 | C |
| 8 | M | 26 | 9 | C |
| 9 | M | 28 | 9 | C2b P1.2 |
| 10 | M | 31 | 11 | C 2a P1.5 |
M, male; F, female.
All identifications were made by PCR.
All patients were white.
Assays of B-cell-T-cell cognate interaction and activation.
The first stage of the assay of B-cell-T-cell cognate interaction and activation was designed to assess T-cell responsiveness to polyclonal stimuli designed to mimic signal 1 (through the antigen-specific T-cell receptor [TCR]) and signal 2 (through the activated antigen-presenting cell). Both of these signals are required for maximal T-cell activation and proliferation (4). The assay utilized agonistic antibodies against CD3 (which mimic TCR signaling) and CD28 (which mimic CD80/86 ligation of T-cell CD28). T-cell activation in response to these signals was assessed by measuring expression of the activation marker CD25 on gated CD4 cells. Proliferation was assessed by carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution assay, again on cells gated for CD4 expression.
The second stage of the assay was designed to determine whether B cells from patients are deficient in their responses to TI-2 antigens. This was assessed by measuring B-cell proliferation and activation in response to the polyclonal mimic of TI-2 antigens, anti-IgD conjugated to dextran (α-δ-dex). This anti-IgD antibody cross-links multiple B-cell receptor molecules in a manner analogous to that for capsular polysaccharides, but it does this to all IgD-positive B cells rather than just to the small antigen-specific population (19). Proliferation was assessed using the CFSE assay combined with CD19 staining so that B-cell responses only could be measured. B-cell activation was also assessed by flow cytometric staining for CD19 and CD86.
The third stage of the assay was designed to assess the ability of T cells to become activated and to deliver T-cell help to B cells. B-cell activation and proliferation in response to the stimulation of T cells in the same culture wells were assessed by measuring CD86 expression on B cells (as a marker of activation) and CFSE dilution on CD19-positive cells (for proliferation). The effect of T-cell help on B cells was assessed in both the presence and absence of the mimic TI-2 antigen α-δ-dex, as strong signaling through the B-cell receptor was expected to synergize with T-cell help.
Antibodies and α-δ-dex.
The following fluorescently labeled and unlabeled antibodies for stimulation were obtained from Caltag Laboratories: mouse anti-human CD19-fluorescein isothiocyanate, mouse anti-human CD19-allophycocyanin, mouse anti-human CD4-allophycocyanin, mouse anti-human CD86-tricolor, and mouse anti-human CD25-phycoerythrin. Purified mouse monoclonal antibody to the human CD28 antigen and purified mouse monoclonal antibody to the human CD3 antigen were obtained from BD Pharmingen. Anti-human CD154 antibody was purchased from R&D Systems Inc. α-δ-Dex was prepared by us as described previously (20, 25).
Cell cultivation and activation.
Cell stimulations for all parts of the assay were performed simultaneously in 48-well tissue culture plates (Nunc), and assays for patients were performed simultaneously with assays for their age- and sex-matched controls. Leukocytes were prepared from freshly drawn human blood by centrifugation over Lymphoprep (Axis-Shield PoC AS) and removal of the leukocyte layer by use of a pipette. A proportion of the cells were labeled with 1.0 μM CFSE (Invitrogen), which enables assessment of proliferation by flow cytometry.
Cells were added to 48-well plates at a final concentration of 2 × 106 cells per ml in a volume of 500 μl in RPMI 1640 plus l-glutamine (Gibco) containing 10% autologous serum. Various stimulatory antibodies or α-δ-dex was added as follows: (i) medium alone, (ii) 1 μg/ml α-δ-dex, (iii) well was precoated with 0.1 μg/ml anti-CD3 (purified mouse monoclonal antibody to the human CD3 antigen [Caltag Laboratories]), (iv) well was precoated with 0.1 μg/ml anti-CD3 plus 1 μg/ml α-δ-dex, (v) well was precoated with 0.5 μg/ml anti-CD3 plus 0.5 μg/ml anti-CD28 (purified mouse monoclonal antibody to the human CD28 antigen [Caltag Laboratories]), (vi) well was precoated with 0.5 μg/ml anti-CD3 plus anti-CD28 plus 1 μg/ml α-δ-dex, or (vii) 50 ng/ml phorbol myristate acetate plus 1.0 μM ionomycin as a positive control. Cells were incubated for 4 days in a humidified atmosphere at 37°C and 5% CO2 before being harvested. On day 4, cells were harvested and stained for flow cytometric analysis (see below).
Assessment of cell proliferation by CFSE dilution.
Cells labeled with CFSE were further labeled with a single cell marker to identify B or T lymphocytes. Cells from other wells were labeled with cell markers for B and T lymphocytes plus activation markers of B and T lymphocytes, namely, CD86 and CD25. Cells were washed with fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline containing 0.1% bovine serum albumin) and finally fixed in FACS buffer with 1% paraformaldehyde for analysis within the following 48 h. Cellular proliferation was assessed by marking the peaks of CFSE expression, representing increasing numbers of cell divisions with decreasing fluorescence (8, 13). The number of original parent cells was determined by dividing the number of events at each cell division (n) by 2n. The proliferation index was calculated by dividing the total number of cells at zero to six divisions by the total number of original parent cells. A proliferation index of 1 thus represents no cell proliferation.
Flow cytometric analysis.
A Becton Dickinson FACSCalibur flow cytometer running Cellquest software was used for cell analysis, and FloJo software (Treestar) was used to assess expression of surface activation markers and proliferation of CFSE-labeled B and T cells. Monocytes were excluded from all analyses on the basis of forward- and side-angle light scatter profiles. Preliminary studies on multiple samples indicated no difference in results obtained using this strategy as opposed to preremoving monocytes by plastic adherence prior to culture or gating them out of the analyses on the basis of CD14 expression. Since lymphocyte numbers were limiting, we adopted the former strategy in order to save on the required number of wells in the assay.
For analyses of B-cell activation and proliferation, B cells were positively gated on both forward- and side-angle light scatter and on CD19 expression. Similarly T helper cells were gated on forward- and side-angle light scatter and on CD4 expression. Cellular activation was expressed as the median fluorescence intensity of CD86 or CD25 expression on B or CD4 cells, respectively, or in some cases as the increase in this value compared with that for unstimulated control cells. Proliferation was calculated as described above and then expressed as a proliferation index (with zero proliferation having a value of 1).
SBA assays.
Sera were assayed by SBA assay as described by Maslanka et al. (15), with baby rabbit serum (Pel-Freez Incorporated, Rodgerson, AZ) as an exogenous complement source. The strain used was the C11 strain (phenotype C:16:P1.7-1,1). SBA titers were expressed as the reciprocal of the final serum dilution giving ≥50% killing after 60 min.
Antibody responses against diphtheria toxoid, tetanus toxoid, and Men C polysaccharide.
The diphtheria toxoid and tetanus toxoid IgG titers were determined using a Bio-Plex (Bio-Rad, Hemel Hempstead, United Kingdom) multiplex assay as previously described (21), and Men C-specific IgG was measured as described by Lal et al. (11).
Statistical analyses.
Since cellular assays were always performed on cells from patients simultaneously with cells from their age- and sex-matched controls on the same experimental day, and because a Gaussian distribution could not be assumed, we performed the nonparametric Wilcoxon signed rank test for paired data. Antibody response data for the sera of patients and controls were analyzed using Student's t test, as these titers will almost certainly be normally distributed. In any case, there were no significant differences between patient and control groups by either Student's t test or the Mann-Whitney U test.
In order to avoid confusion, statistical comparisons made in the text and figures are limited to comparisons between patient and control cells in assays. Statistical data on the overall responsiveness of cells to various stimuli are all shown in Table 2.
TABLE 2.
B-cell and CD4-cell activation and proliferation in response to various stimulia
| Comparison |
P valueb
|
|||
|---|---|---|---|---|
| B-cell activation | B-cell proliferation | T-cell activation | T-cell proliferation | |
| α-δ-Dex stimulation vs unstimulated group | 0.0001 | 0.0001 | NS | NS |
| CD3 | ND | ND | 0.0023 | 0.0002 |
| CD3/CD28 vs unstimulated group | 0.0001 | 0.0002 | 0.0002 | 0.0001 |
| CD3/CD28 vs CD3 | ND | ND | 0.0107 | 0.0001 |
| CD3/CD28/α-δ-dex vs CD3/CD28 | 0.0001 | 0.0001 | ND | ND |
Combinations of control and patient samples are shown for simplicity. Both patient and control cells responded to all stimuli. All comparisons were done by the Wilcoxon signed rank test for nonparametric paired data.
ND, not determined; NS, not significant.
RESULTS
Individuals with a history of meningococcal disease have no defect in peripheral T-cell activation via CD3 and CD28.
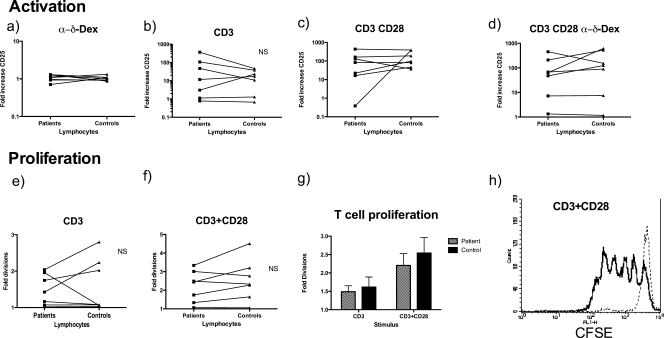
In nature, in response to protein antigens, activated antigen-presenting cells presenting peptides on major histocompatibility complex class II molecules stimulate T cells via both the TCR and CD28, and the T cells are then activated and proliferate. To mimic TCR engagement, T cells were activated with the polyclonal T-cell stimulator anti-CD3, and to mimic CD86/80-CD28 interactions, an agonistic CD28 antibody was used. As expected, α-δ-dex had no effect on T-cell activation, either on its own (Fig. 1a) or in combination with anti-CD3 (data not shown) CD4 T cells were efficiently activated in response to CD3 cross-linking (Fig. 1b), as defined by expression of CD25 (P = 0.023; Wilcoxon signed rank test) (unstimulated cells provide the background reading and would show an increase in CD25 expression of onefold on the y axis). As expected, CD4 cells were further activated by coligation of CD3 and CD28 (P = 0.0107 for CD3 versus CD3 and CD28; Wilcoxon signed rank test) (Fig. 1c), again with α-δ-dex having no effect (Fig. 1d). No defect of CD4 T-cell activation was observed in patients with a history of meningococcal disease in either the presence (Fig. 1a and d) or absence (Fig. 1b and c) of α-δ-dex-activated B cells.
FIG. 1.
T-cell responsiveness to polyclonal stimulation through CD28 and CD3. PBLs derived from patients and their paired controls were incubated with no stimulus or with anti-CD3 or the combination of anti-CD3 and anti-CD28 for 4 days. A proportion of cells were prelabeled with CFSE. After incubation, the non-CFSE-labeled cells were stained with anti-CD4 and anti-CD25 antibodies, and CD25 expression on CD4 cells was used as a measure of activation. (a to d) Mean increases in CD25 expression after stimulation of patient or control cells with either the B-cell TI-2 mimic α-δ-dex (a), anti-CD3 alone (b), anti-CD3 and anti-CD28 antibodies (c), or anti-CD3, anti-CD28, and α-δ-dex (d). There was no significant difference in the ability of patient cells to respond to any stimulus in comparison with controls (Wilcoxon signed rank test). (e to g) CFSE-labeled cells were gated on CD4 expression, and CFSE fluorescence of the CD4-positive population was plotted. (h) Typical CFSE plot showing T-cell proliferation in response to anti-CD3 and anti-CD28 (bold line) versus unstimulated cells (faint line). There was no significant difference in the ability of patient or control T cells to proliferate in response to CD3 or CD3/CD28 stimulation in the presence (e, f, and g) or absence (not shown) of α-δ-dex.
T cells also proliferated appropriately in response to these agonists, with proliferation strongly enhanced by the addition of CD28 monoclonal antibody to the stimulating cocktail. Figure 1e shows individual responses to CD3 activation, and Fig. 1f shows individual responses to stimulation through CD3 and CD28. Figure 1g shows the average responses in Fig. 1e and f, clearly showing the enhancement seen with the addition of CD28 stimulation. Figure 1h shows a typical CFSE plot, in this case of maximally stimulated CD4 cell proliferation via CD3 and CD28. Again, no defect in T-cell proliferation in response to the two polyclonal stimuli was detected in the cohort with a history of meningococcal disease, either in the absence (Fig. 1e to g) or in the presence (not shown) of α-δ-dex.
Peripheral B-cell stimulation by the TI-2 mimic α-δ-dex is defective in individuals with a history of meningococcal disease.
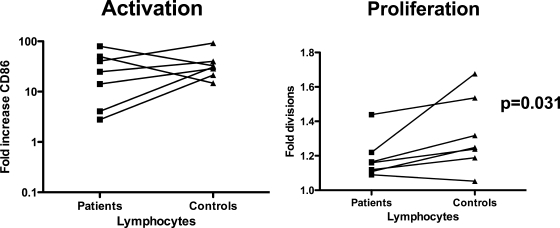
Expression of the CD28 ligand CD86, formally known as B7.2, is a commonly used measure of the activation of B cells and other antigen-presenting cells. B lymphocytes of patients and age-matched controls were activated efficiently by α-δ-dex stimulation (Fig. 2, left panel), with the median fluorescence intensity of CD86 increasing in all cases. Similar to the case for B-cell activation, B cells from both controls and recovered patients responded to α-δ-dex stimulation by proliferating (Fig. 2, right panel). The levels of proliferation in response to α-δ-dex stimulation were low in all samples, but there was significantly less induction of proliferation of B cells from recovered meningococcal patients than that for controls (Wilcoxon signed rank test; P = 0.0312).
FIG. 2.
B-cell responsiveness to the polyclonal TI-2 mimic α-δ-dex. PBLs from patients and their paired controls were incubated for 4 days in the presence of α-δ-dex and then stained with CD19 and CD86 antibodies, and expression of CD86 on CD19-positive gated cells was assessed. Changes in CD86 expression (stimulated/unstimulated) were calculated. (Left) Changes in CD86 expression of paired patient and control cells in response to stimulation with α-δ-dex. There was no significant difference in the levels of activation induced in patient cells versus control cells. In other wells, PBLs were prestained with CFSE prior to incubation and stained for CD19 expression after 4 days. (Right) Average B-cell proliferation, as defined by the proliferation index (with no proliferation having an index of 1), for patient and control samples in response to α-δ-dex. There was a significant difference between patient and control B-cell proliferation in response to α-δ-dex (P = 0.031; Wilcoxon signed rank test).
Patients with a history of meningococcal disease exhibit normal T-cell help to B cells, but enhancement of this help by a TI-2 polyclonal mimic is deficient.
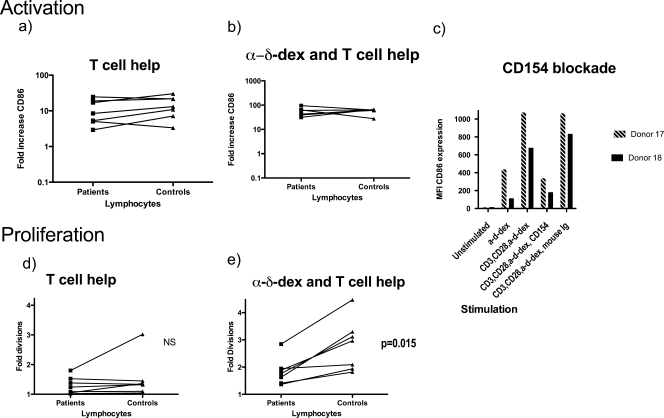
In order to produce a high-affinity class-switched antibody response against TD antigens, B cells must receive cognate help from activated T cells. The ability of activated T cells to deliver help to B cells and the ability of B cells to respond to this help were assessed simultaneously by measuring B-cell activation and proliferation in the presence of T cells stimulated through anti-CD28 and anti-CD3. As expected, in the presence of activated T cells, B-cell CD86 expression was amplified (Fig. 3a and Fig. 2, left panel), the B cells proliferated moderately (Fig. 3d), and there was no difference between the responses of cells from recovered meningococcal patients and controls. Since B cells were efficiently activated in the presence of the T-cell stimuli, we concluded that there was no gross defect in either the ability of patient T cells to provide T-cell help or the ability of patient B cells to respond to such help.
FIG. 3.
T-cell help to B cells. B-cell activation in response to T-cell help was assessed by CD86 expression on cells stimulated with α-δ-dex along with T cells activated through CD3 and CD28. PBLs were incubated in the presence of various stimuli for 4 days, with some having previously been labeled with CFSE. Cells were then stained for CD19 and CD86. Mean increases in CD86 expression are shown for control or patient B cells after incubation of lymphocytes with either anti-CD3/CD28 (a) or both anti-CD3/CD28 and α-δ-dex (b). There was no significant difference in the ability of patient or control B cells to be activated in response to either CD3/CD28 stimulation alone or in response to the combination of α-δ-dex with the two T-cell stimuli. (c) PBLs from two donors were stimulated as described above, but with the addition of a blocking anti-CD154 monoclonal antibody or an isotype control antibody (mouse immunoglobulin) in some of the wells. (d and e) CD19 B-cell proliferation of patient or control B cells after incubation of PBLs with CD3/CD28-activated T cells (d) or with CD3/CD28 and α-δ-dex (b). There was no significant difference in the ability of patient or control B cells to proliferate in response to the T-cell activation signals alone, but additional proliferation in response to α-δ-dex was reduced in the patient cells in comparison with that in controls (P = 0.015; Wilcoxon signed rank test).
It is known that B cells, like T cells, proliferate most strongly in the presence of antigen receptor stimulation (signal 1) and T-cell help (signal 2). Since α-δ-dex stimulates cells through the B-cell receptor, the effect of α-δ-dex with the two T-cell stimuli on B-cell activation and proliferation was assessed. As expected, α-δ-dex with the two T-cell stimuli gave rise to an even stronger amplification of B-cell CD86 expression (Fig. 3b). Again, there was no apparent defect of this activity in patients with a history of meningococcal disease. Similar to activation, B-cell proliferation in the presence of activated T cells was strongly enhanced by concurrent B-cell receptor stimulation through α-δ-dex (Fig. 3e), but the enhancement seen was significantly stronger when the assay was performed on control lymphocytes than when it was performed on patient lymphocytes (P = 0.015). Since there was no defect in patient B-cell responses to bystander help alone (Fig. 3d), it is extremely likely that the defective proliferative response of patients' B cells to the combination of activated T cells and α-δ-dex was due to an aberrant response to the α-δ-dex stimulation, correlating with the defect apparent in Fig. 2.
A major component of T-cell help to B cells is imparted by CD154-CD40 signaling. The bystander activation observed was shown to be negated by antibody blockade of CD154, showing that it was mediated at least in part by CD154-CD40 interactions, probably in conjunction with T-cell cytokines (Fig. 3c).
In order to avoid confusion in the text, statistical comparisons described above and in the figures are largely restricted to comparisons between patient and control results. The positive effects of the various stimuli on cell activation and proliferation are analyzed in Table 2.
Patients with a history of meningococcal disease do not have higher anti-Men C polysaccharide IgG or anti-Men C SBA titers than controls.
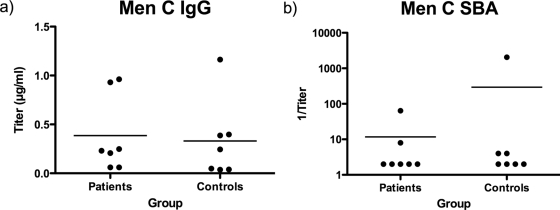
Figure 4 shows anti-Men C polysaccharide IgG and anti-Men C SBA titers in patient and control sera obtained while obtaining blood for peripheral blood lymphocytes (PBLs). Although these sera were obtained several years after the infection, there is no evidence of enhanced Men C titers despite the large antigenic assault these patients have suffered.
FIG. 4.
IgG titers against Men C polysaccharide (a) and Men C SBA titers (b) in sera obtained from patients and controls at the same time that PBLs were obtained.
DISCUSSION
We reasoned that adults who suffer serogroup C meningococcal disease despite the high frequency of natural (and immunizing) colonization by Neisseria throughout childhood and young adulthood must fail to develop optimal acquired immunity to invasive disease following colonization by serogroup C Neisseria meningitidis. We thus postulated that such adults might be poor generators of antibody responses either against serogroup C or related capsular polysaccharides or against bacterial protein components that are sampled during colonization. In vitro assays were set up to test the hypothesis that peripheral lymphocytes derived from a small sample of such patients have impaired responses to polyclonal mimics of TI-2 and/or TD antigens. These revealed a lower response (to TI-2 mimics) of B cells of recovered meningococcal patients, which was observed both with and without T-cell help. Equivalent T-cell responses and unimpaired T-cell help to B cells were observed in the two groups. The results suggest that adults who contract serogroup C meningococcal disease have a defect in their responses to polysaccharide antigens.
It is not apparent that individuals who present with meningococcal disease are widely susceptible to other bacterial infections. However, the possibility of an immune defect being responsible for susceptibility to meningococcal disease has been postulated previously. Whittle et al. assessed responses to group C polysaccharide vaccine of a number of survivors of disease due to serogroup A N. meningitidis and found defective responses in patients and siblings in comparison with controls (28). Our data are consistent with these findings. It has also been shown that humans vary widely in their responses to pneumococcal polysaccharides and that this may be a familial effect (17). If the defect we discovered is a product of gene variation, then it is noteworthy that the patients exhibited no general features of immune deficiency. However, gene defects can result in remarkably specific phenotypes. For example, complete removal of the function of a gene from a mouse sometimes results in a detectable phenotype only under specific challenge conditions (18), and the same is true for humans, for example, homozygosity for the non-sickle-cell form of haemoglobin beta is apparent as a deficiency relative to heterozygosity only in a region where malaria is endemic. Selective immune defects have also been demonstrated in some children who fail to respond serologically to the Haemophilus influenzae type b vaccine yet exhibit normal responses to tetanus toxoid protein (7) and pneumococcal polysaccharides (1).
B-cell proliferation is required not only to provide an expanded pool of antigen-specific cells, either as memory cells or as plasma cells, but also to allow class switching to more effective IgG antibody from IgM (9). While we did not have access to serum samples from patients prior to their disease, serum samples collected from patients after disease diagnosis did not have significantly higher titers of anti-Men C polysaccharide antibody or SBA titers than did sera from controls (Fig. 4). This is surprising in light of the fact that these people had been subjected to a powerful immune stimulus in the form of a systemic infection and fits with the hypothesis that these individuals are defective in their response to polysaccharide antigens after colonization/invasion by meningococci. Patient antibody titers against the TD vaccine antigens diphtheria toxoid and tetanus toxoid were also assessed, and consistent with the normal responses to TD antigen mimics in the cellular assay, these titers were no different from those of controls (not shown).
A number of reservations are attached to our conclusions. The assay we used is a novel one, custom designed to test our hypothesis. It can display only relative differences between patients and controls and falls short of defining the underlying mechanism. Murine α-δ-dex has been used widely in experimental immunology for polyclonal activation of peripheral B cells (19, 20, 25) and likely activates all IgD-positive B cells via their B-cell receptors. It is not appropriate to assess B-cell responses to Men C polysaccharide because of the differential prior exposure (to a heavy bacterial burden in the case of the patients but not in the controls). Also, assessment of B-cell proliferative responses to Men C polysaccharide is not possible because of the exceptionally small number of antigen-specific cells within the peripheral blood. The defect we observed could potentially be due to one or more of multiple steps in cell activation downstream of receptor engagement. The data we report are derived from a small group of individuals, and multiple assays were performed on their cells. The small sample size reflects the difficulty of collecting cohorts of adults with a history of microbiologically proven serogroup C disease, but the sample size was determined prospectively to be adequate for our protocol. We cannot be certain that this defect occurs more generally among adults who have experienced serogroup C meningococcal disease. We limited our study to patients who experienced serogroup C disease because the Men C polysaccharide elicits high concentrations of antibody, in contrast to that of serogroup B meningococci, which is poorly immunogenic. We do not know, therefore, whether this defect is shared by individuals who have experienced serogroup B disease.
In summary, we have discovered that patients who suffer serogroup C meningococcal disease may do so because of a subtle defect of B-cell responsiveness to T-cell-independent antigens which is manifest both in the presence and in the absence of T-cell help. This might explain the occurrence of disease in adults despite their likely lifelong exposure to the immunizing effect of natural colonization by N. meningitidis. It is also consistent with the original findings of Goldschneider and colleagues, who found that adults who suffer disease have relatively weak general immune responses to N. meningitidis compared to nondiseased controls (5, 6), and of Whittle et al. (28), who found defective responses of recovered patients and their siblings to polysaccharide vaccine.
Acknowledgments
We thank Sue Newton of the School of Medicine and Biomedical Science Flow Cytometry Core Facility for help and advice.
R.A.F. was supported by the Binney Fund, J.C. was supported by the Wellcome Trust (061268), and R.C.R., A.W.H., and R.B. were supported by the Meningitis Research Foundation (0604.0).
Footnotes
Published ahead of print on 11 March 2009.
REFERENCES
- 1.Ambrosino, D. M., D. T. Umetsu, G. R. Siber, G. Howie, T. A. Goularte, R. Michaels, P. Martin, P. H. Schur, J. Noyes, G. Schiffman, et al. 1988. Selective defect in the antibody response to Haemophilus influenzae type b in children with recurrent infections and normal serum IgG subclass levels. J. Allergy Clin. Immunol. 811175-1179. [DOI] [PubMed] [Google Scholar]
- 2.Borrow, R., P. Balmer, and E. Miller. 2005. Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine 232222-2227. [DOI] [PubMed] [Google Scholar]
- 3.Cartwright, K. A., J. M. Stuart, D. M. Jones, and N. D. Noah. 1987. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol. Infect. 99591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs, E. 1992. Two signal model of lymphocyte activation. Immunol. Today 13462. [DOI] [PubMed] [Google Scholar]
- 5.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 1291307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 1291327-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granoff, D. M., P. G. Shackelford, B. K. Suarez, M. H. Nahm, K. L. Cates, T. V. Murphy, R. Karasic, M. T. Osterholm, J. P. Pandey, and R. S. Daum. 1986. Haemophilus influenzae type b disease in children vaccinated with type b polysaccharide vaccine. N. Engl. J. Med. 3151584-1590. [DOI] [PubMed] [Google Scholar]
- 8.Hasbold, J., A. V. Gett, J. S. Rush, E. Deenick, D. Avery, J. Jun, and P. D. Hodgkin. 1999. Quantitative analysis of lymphocyte differentiation and proliferation in vitro using carboxyfluorescein diacetate succinimidyl ester. Immunol. Cell Biol. 77516-522. [DOI] [PubMed] [Google Scholar]
- 9.Hasbold, J., A. B. Lyons, M. R. Kehry, and P. D. Hodgkin. 1998. Cell division number regulates IgG1 and IgE switching of B cells following stimulation by CD40 ligand and IL-4. Eur. J. Immunol. 281040-1051. [DOI] [PubMed] [Google Scholar]
- 10.Khan, A. Q., A. Lees, and C. M. Snapper. 2004. Differential regulation of IgG anticapsular polysaccharide and antiprotein responses to intact Streptococcus pneumoniae in the presence of cognate CD4+ T cell help. J. Immunol. 172532-539. [DOI] [PubMed] [Google Scholar]
- 11.Lal, G., P. Balmer, H. Joseph, M. Dawson, and R. Borrow. 2004. Development and evaluation of a tetraplex flow cytometric assay for quantitation of serum antibodies to Neisseria meningitidis serogroups A, C, Y, and W-135. Clin. Diagn. Lab. Immunol. 11272-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, Y., Q. Zhang, M. Winterbotham, E. Mowe, A. Gorringe, and C. M. Tang. 2006. Immunization with live Neisseria lactamica protects mice against meningococcal challenge and can elicit serum bactericidal antibodies. Infect. Immun. 746348-6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyons, A. B., J. Hasbold, and P. D. Hodgkin. 2001. Flow cytometric analysis of cell division history using dilution of carboxyfluorescein diacetate succinimidyl ester, a stably integrated fluorescent probe. Methods Cell Biol. 63375-398. [DOI] [PubMed] [Google Scholar]
- 14.Maiden, M. C., and J. M. Stuart. 2002. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet 3591829-1831. [DOI] [PubMed] [Google Scholar]
- 15.Maslanka, S. E., L. L. Gheesling, D. E. Libutti, K. B. Donaldson, H. S. Harakeh, J. K. Dykes, F. F. Arhin, S. J. Devi, C. E. Frasch, J. C. Huang, P. Kriz-Kuzemenska, R. D. Lemmon, M. Lorange, C. C. Peeters, S. Quataert, J. Y. Tai, G. M. Carlone, et al. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin. Diagn. Lab. Immunol. 4156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mond, J. J., A. Lees, and C. M. Snapper. 1995. T cell-independent antigens type 2. Annu. Rev. Immunol. 13655-692. [DOI] [PubMed] [Google Scholar]
- 17.Musher, D. M., J. E. Groover, D. A. Watson, J. P. Pandey, M. C. Rodriguez-Barradas, R. E. Baughn, M. S. Pollack, E. A. Graviss, M. de Andrade, and C. I. Amos. 1997. Genetic regulation of the capacity to make immunoglobulin G to pneumococcal capsular polysaccharides. J. Investig. Med. 4557-68. [PubMed] [Google Scholar]
- 18.Pearson, H. 2002. Surviving a knockout blow. Nature 4158-9. [DOI] [PubMed] [Google Scholar]
- 19.Pecanha, L. M., C. M. Snapper, F. D. Finkelman, and J. J. Mond. 1991. Dextran-conjugated anti-Ig antibodies as a model for T cell-independent type 2 antigen-mediated stimulation of Ig secretion in vitro. I. Lymphokine dependence. J. Immunol. 146833-839. [PubMed] [Google Scholar]
- 20.Pecanha, L. M., C. M. Snapper, A. Lees, H. Yamaguchi, and J. J. Mond. 1993. IL-10 inhibits T cell-independent but not T cell-dependent responses in vitro. J. Immunol. 1503215-3223. [PubMed] [Google Scholar]
- 21.Pickering, J. W., T. B. Martins, M. C. Schroder, and H. R. Hill. 2002. Comparison of a multiplex flow cytometric assay with enzyme-linked immunosorbent assay for quantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae type b. Clin. Diagn. Lab. Immunol. 9872-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbins, J. B., R. Schneerson, S. C. Szu, A. Fattom, Y. Yang, T. Lagergard, C. Chu, and U. S. Sorensen. 1989. Prevention of invasive bacterial diseases by immunization with polysaccharide-protein conjugates. Curr. Top. Microbiol. Immunol. 146169-180. [DOI] [PubMed] [Google Scholar]
- 23.Robinson, K., K. R. Neal, C. Howard, J. Stockton, K. Atkinson, E. Scarth, J. Moran, A. Robins, I. Todd, E. Kaczmarski, S. Gray, I. Muscat, R. Slack, and D. A. Ala'Aldeen. 2002. Characterization of humoral and cellular immune responses elicited by meningococcal carriage. Infect. Immun. 701301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenqvist, E., E. A. Hoiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Ronnild, G. Bjune, and H. Nokleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 634642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snapper, C. M., T. M. McIntyre, R. Mandler, L. M. Pecanha, F. D. Finkelman, A. Lees, and J. J. Mond. 1992. Induction of IgG3 secretion by interferon gamma: a model for T cell-independent class switching in response to T cell-independent type 2 antigens. J. Exp. Med. 1751367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trotter, C. L., N. J. Gay, and W. J. Edmunds. 2006. The natural history of meningococcal carriage and disease. Epidemiol. Infect. 134556-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welsch, J. A., and D. Granoff. 2004. Naturally acquired passive protective activity against Neisseria meningitidis group C in the absence of serum bactericidal activity. Infect. Immun. 725903-5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whittle, H. C., A. Oduloju, G. Evans-Jones, and B. M. Greenwood. 1976. Evidence for familial immune defect in meningococcal meningitis. Br. Med. J. 11247-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]