Abstract
Amphiphysins are proteins thought to be involved in synaptic vesicle endocytosis. Amphiphysins share a common BAR domain, which can sense and/or bend membranes, and this function is believed to be essential for endocytosis. Saccharomyces cerevisiae cells lacking the amphiphysin ortholog Rvs161 are inviable when starved for glucose. Altering sphingolipid levels in rvs161 cells remediates this defect, but how lipid changes suppress remains to be elucidated. Here, we show that the sugar starvation-induced death of rvs161 cells extends to other fermentable sugar carbon sources, and the loss of sphingolipid metabolism suppresses these defects. In all cases, rvs161 cells respond to the starvation signal, elicit the appropriate transcriptional response, and properly localize the requisite sugar transporter(s). However, Rvs161 is required for transporter endocytosis. rvs161 cells accumulate transporters at the plasma membrane under conditions normally resulting in their endocytosis and degradation. Transporter endocytosis requires the endocytosis (endo) domain of Rvs161. Altering sphingolipid metabolism by deleting the very-long-chain fatty acid elongase SUR4 reinitiates transporter endocytosis in rvs161 and rvs161 endo− cells. The sphingolipid-dependent reinitiation of endocytosis requires the ubiquitin-regulating factors Doa1, Doa4, and Rsp5. In the case of Doa1, the phospholipase A2 family ubiquitin binding motif is dispensable. Moreover, the conserved AAA-ATPase Cdc48 and its accessory proteins Shp1 and Ufd1 are required. Finally, rvs161 cells accumulate monoubiquitin, and this defect is remediated by the loss of SUR4. These results show that defects in sphingolipid metabolism result in the reinitiation of ubiquitin-dependent sugar transporter endocytosis and suggest that this event is necessary for suppressing the nutrient starvation-induced death of rvs161 cells.
The budding yeast Saccharomyces cerevisiae gene RVS161/END6 encodes a helical protein of 265 amino acids that is a member of the N-BAR (for Bin, amphiphysin, Rvs) family of proteins (61). Rvs161 regulates cell polarity (20), actin cytoskeleton polarization (69), endocytosis (50), and secretory vesicle trafficking (7, 25). rvs161 mutant cells die during stationary phase, have mating defects, are sensitive to high concentrations of NaCl, have endocytosis and actin defects, and are unable to grow on nonfermentable carbon sources (8, 13, 15, 50, 63, 69). Mutational studies have revealed two functionally independent Rvs161 domains: an NH2-terminal/BAR domain involved in endocytosis and actin organization and a COOH-terminal domain required for cell fusion during haploid cell mating (8).
The N-BAR family of proteins is constantly growing and includes yeast Rvs161 and Rvs167; human BIN1, BIN2, and BIN3; hob1+ and hob3+ from Saccharomyces pombe; murine Alp1; and human amphiphysins, which are a family of multidomain proteins that are involved in the late steps of clathrin-coated vesicle scission (24, 30, 58, 61). Two genes encode amphiphysin in humans, one expressed in the brain (amphiphysin I) and a second (amphiphysin II) with a broad tissue distribution and a wide array of alternatively spliced isoforms. Both have an N-terminal BAR domain and a C-terminal SH3 domain. The BAR domain interacts with phospholipids, which induces membrane curvature and tubulation, whereas the distal SH3 domain associates with proteins having proline-based motifs. Human amphiphysin has been implicated in endocytosis due to its interaction with dynamin and because of its homology to Rvs161 and Rvs167. It may be important in recycling the plasma membrane at synaptic terminals (14, 28, 42, 68).
The crystal structures of several N-BAR domains have been solved (9, 45, 58, 71, 72). The domain is present in proteins that are critical for the recycling of synaptic vesicles and T-tubule formation in muscle, such as amphiphysins, endophilin, nadrin, beta-centaurins, arfaptin, and oligophrenins. Peter et al. (58) have shown that an N-BAR domain (the BAR domain plus an adjacent amphipathic helix) is capable of inducing three- dimensional membrane curvature. Some BAR proteins have additional interactions with lipids through pleckstrin homology or phox homology domains. These domains may target a protein to a specific membrane compartment, while the BAR domain simultaneously detects or initiates membrane curvature. There are two other BAR domains, F-BAR and I-BAR, which bind membranes and in some cases induce membrane tubulation (10, 35).
rvs161 and rvs167 mutants have common phenotypes (2, 7, 63, 69), and Rvs161 and Rvs167 physically interact (70). However, they have distinct nonoverlapping cell functions and physical interactions with other proteins (8, 26). The BAR domains of each cannot be interchanged, and the overexpression of RVS161 or RVS167 cannot cross-suppress the other's phenotype (2, 70). Defects of rvs cells, including salt sensitivity, cell death during starvation, and the lack of growth on nonfermentable carbon sources, are suppressed by mutations altering the sphingolipid composition. SUR1, SUR2, SUR4, and IPT1 encode inositolphosphorylceramide mannosyltransferase, long-chain-base (LCB) C4-hydroxylase, very-long-chain fatty acid elongase, and mannose diinositolphosphorylceramide synthase, respectively, and are required for the biosynthesis of yeast complex sphingolipids (Fig. 1) (17, 19). Recessive mutations in these genes alter the amount and composition of complex sphingolipids (4, 18, 29, 51) and suppress rvs defects (1, 15). Suppression may function through remediating the actin depolarization/repolarization defects seen in mutant cells in times of stress (1). However, rvs161 sur4 and rvs167 sur4 cells have steady-state actin defects when starved for glucose (26). Thus, the molecular basis of suppression is complex and remains to be uncovered.
FIG. 1.
Sphingolipid biosynthetic pathway in S. cerevisiae. Genes are in italics. FA, fatty acid; VLCFA, very long chain fatty acids.
The HXT family of proteins are mammalian-facilitated glucose transporter (GLUT) orthologs (44, 56, 76). S. cerevisiae has 20 genes encoding proteins similar to hexose transporters (56). Most are bona fide transporters, such as Hxt1 to Hxt17, while others, such as Snf3 and Rgt2, are glucose sensors (54). Snf3 and Rgt2 sense extracellular glucose concentrations and initiate a transcriptional signaling cascade (53, 54), resulting in the expression of high-affinity (Hxt2 and Hxt4) or low-affinity (Hxt3 and Hxt1) transporters (55, 60). What is known about the stability and degradation of glucose transporters is that under specific conditions, components of the high- and low-affinity glucose uptake system are inactivated (6). Studies have examined the stability and degradation pathway for Hxt6 and Hxt7 (40). It is generally thought that glucose transporters are internalized via endocytosis and subsequently degraded.
rvs161 cells die under conditions of glucose starvation. Here, we show that they harbor starvation defects on other fermentable carbon sources and are unable to thrive when galactose, maltose, or melibiose is the available carbon source. Mutant cells can sense a glucose starvation signal, derepress glucose-repressed genes, initiate Snf3- and Rgt2-dependent HXT transcription, and properly localize high- and low-affinity glucose transporters. They also express and properly localize the Gal2 galactose and Mal61 maltose permeases. However, rvs161 cells are unable to endocytose and degrade these sugar transporters. The loss of function of SUR4 suppresses all carbon source growth defects we observed and restores sugar transporter endocytosis and degradation. Doa1, Doa4, and Rsp5 are required for sur4-dependent suppression and for transporter endocytosis and degradation, as is the conserved AAA-ATPase Cdc48 and its accessory factors, Shp1 and Ufd1.
MATERIALS AND METHODS
Media and miscellaneous microbial techniques.
Yeast strains were grown in YEP (1% yeast extract, 2% Bacto-peptone) containing the indicated concentrations of the indicated carbon source in YPG (1% yeast extract, 2% Bacto-peptone, 3% glycerol) or in synthetic minimal medium containing 0.67% yeast nitrogen base (Difco) supplemented with the appropriate amino acids and adenine. Yeast transformations were performed using the procedure described previously (34). For the routine propagation of plasmids, Escherichia coli XL1-Blue cells were used and grown in Luria broth supplemented with ampicillin (200 mg/ml).
Strain and plasmid construction.
The yeast strains used are derived from W303 (YJN17) (MATa ura3-52 leu2 his3 lys2 ade2). hxt1::HXT1-GFP::TRP1, hxt2::HXT2-GFP::TRP1, hxt4::HXT4-GFP::TRP1, gal2::GAL2-GFP::TRP1, and mal61::MAL61-GFP::TRP1 alleles were generated as described previously (43) using the pFA6a-GFP(S65T)-TRP1 module. cdc48::kanr ura3-52::cdc48-3::URA3 and ufd1::kanr ura3-52::ufd1-1::URA3 strains were generated using the diploid strain YJN1 (MATa/α ura3-52/ura3-52 his3/his3 leu2/leu2 TRP1/trp1 LYS2/lys2 ade2/ade2), as the deletion of CDC48 or UFD1 is lethal in haploid strains. First, cdc48::kanr and ufd1::kanr alleles were synthesized by the PCR amplification of the cdc48::kanr or ufd1::kanr allele from heterozygous CDC48/cdc48::kanr and UFD1/ufd1::kanr strains (Research Genetics), respectively. These alleles were transformed into YJN1, and integrants were selected on yeast extract-peptone-dextrose (YEPD) plates containing 250 μg/ml G418. Proper integration was determined by PCR. YIp-cdc48-3 and YIp-ufd1-1 were digested with StuI and integrated at the ura3-52 locus of the CDC48/cdc48::kanr and UFD1/ufd1::kanr strains, respectively. Cells were sporulated, and haploid cdc48::kanr ura3::cdc48-3::URA3 and ufd1::kanr ura3-52::ufd1-1::URA3 strains were obtained by prototrophic amino acid and temperature-sensitive selections. The cdc48-3 and ufd1-1 alleles used to construct YIp-cdc48-3 and YIp-ufd1-1 were generated by the PCR amplification of YJN3151 and YJN3158, respectively. doa1::HIS3, doa1::doa1ΔC::TRP1, and doa1::doa1F417D F434D::TRP1 alleles were generated by PCR amplification using YJN3222, YJN3233, and YJN3225, respectively. shp1::kanr strains were generated by the PCR amplification of the shp1::kanr allele from a haploid shp1::kanr strain (Open Biosystems, Huntsville, AL) and subsequent to the transformation of the PCR product into YJN17 and selection on G418. pGAL-rsp5-1 was used to express the dominant-negative rsp5-1 allele.
Total RNA isolation.
All solutions were prepared with diethyl pyrocarbonate-treated water. Cells were harvested, centrifuged, and pelleted for 30 s. Cell pellets were resuspended in 200 μl of YRL buffer (200 mM Tris, pH 7.5, containing 500 mM NaCl, 10 mM EDTA, 1% sodium dodecyl sulfate [SDS]) and 200 μl PCIAA (phenol, chloroform, isoamyl alcohol). Two hundred microliters of nitric acid-washed beads was added, and cells were vortexed for 2.5 min. Three hundred microliters of YRL buffer and 200 μl PCIAA were added, and cells were vortexed for 2.5 min. Cells were centrifuged for 5 min, and the resulting clear lysate was removed and added to 400 μl PCIAA, vortexed for 2.5 min, and centrifuged for 5 min. The resulting aqueous layer was added to 500 μl of 100% ethanol, and total RNA was precipitated overnight at −20°C. A total RNA pellet was obtained by centrifugation at 13,000 rpm for 15 min at 4°C, washed twice with 70% ethanol, vacuum dried, and resuspended in water. RNA was stable at −20°C for several weeks.
Northern analysis.
Total RNA was resolved using 6% formaldehyde agarose gel electrophoresis. Total RNA (20 μg) in loading buffer (20 mM morpholinepropanesulfonic acid, pH 7.0, containing 10 mM sodium acetate, 2 mM EDTA, 45% formamide, 6% formaldehyde, 1% ethidium bromide, 0.003% bromophenol blue, 0.03% xylene cyanol FF, 1.5% Ficoll) was analyzed. RNA was blotted onto Hybond-N nitrocellulose (Amersham, Arlington Heights, IL) overnight at room temperature using 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Churches buffer (10 mM sodium-phosphate buffer, 1 mM EDTA, 1% bovine serum albumin, 7% SDS) was used for all hybridization procedures. Hybridization was performed overnight at 65°C. Gel-purified radiolabeled probes were boiled in 200 μl salmon sperm DNA prior to use. After hybridization, blots were washed twice in 2× SSC at room temperature, twice in 2× SSC-0.5% SDS at 65°C, and twice in 0.1× SSC at room temperature. Gene expression was determined by autoradiography using Kodak X-OMAT film. The specificity of each HXT probe was checked by Northern analysis using hxt1, hxt2, hxt3, and hxt4 strains. U2 expression was used as a loading control.
Fluorescence microscopy.
Cultures were grown to exponential phase in YEP containing 6% glucose. Cells then were shifted to YEP containing the indicated concentrations of glucose for the specified time. Hxt1-green fluorescent protein (Hxt1-GFP), Hxt2-GFP, and Hxt4-GFP localization was visualized using a Leica DRBME fluorescence microscope, fluorescein isothiocyanate optics, and a PlanAPO ×100 objective. Images were obtained using Open Labs software (version 2.1). Final fluorescence images were generated using Adobe Photoshop (version 7.0).
Western analysis of Hxt1-GFP and Hxt2-GFP stability.
Cultures were grown to exponential phase (optical density at 600 nm of 0.5 to 1.0) in YEPD and then shifted to the appropriate medium to regulate HXT expression and protein synthesis. Twenty-milliliter cultures were collected at various times, and a total cell protein extract was obtained. Briefly, cells were pelleted, washed once with distilled water, and lysed in buffer A [200 mM Tris-HCl, pH 7.9, containing 390 mM (NH4)2SO4, 10 mM MgSO4, 20% (vol/vol) glycerol, 1 mM EDTA] using glass beads. Lysis buffer also contained 15 mM mercaptoethanol, 1 mM AEBSF [4-(2-aminoethyl)-benzenesulfonyl fluoride], 5 μg/ml pepstatin, 5 μg/ml leupeptin, and 10 μl of Sigma protease inhibitor solution (Sigma Chemicals, St. Louis, MO). Five hundred micrograms of total cell protein extract was resuspended in Laemmli buffer (12), and proteins were resolved using 10% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes.
All steps for Western analysis were performed at room temperature. Membranes were blocked for 1 h with 5% nonfat dry milk in buffer C (10 mM Tris-HCl, pH 7.4, containing 150 mM NaCl and 0.05% Tween-20). Incubations with primary and secondary antibodies were performed for 1 h in buffer C. Membranes were washed six times after antibody incubations with buffer C. Blots were incubated with mouse anti-GFP monoclonal antibodies (1:2,000 dilution) (Clontech, Mountain View, CA) and anti-mouse horseradish peroxidase (HRP)-conjugated monoclonal antibodies (1:5,000 dilution) (Amersham Corp., Arlington Heights, IL). Anti-actin polyclonal antibodies (1:1,000) (Santa Cruz Biotechnology, Santa Cruz, CA) were used to determine protein loading. Hxt1-GFP and Hxt2-GFP were detected using chemiluminescence and autoradiography. Anti-Gal2 and anti-Mal61 polyclonal antibodies were used at a 1:500 dilution.
Detection of extracellular invertase protein levels.
Suc2 invertase expression was induced as described previously (57). Secreted Suc2 was determined by Western analysis using anti-Suc2 polyclonal antibodies at a 1:500 dilution.
Western analysis of total cellular ubiquitin.
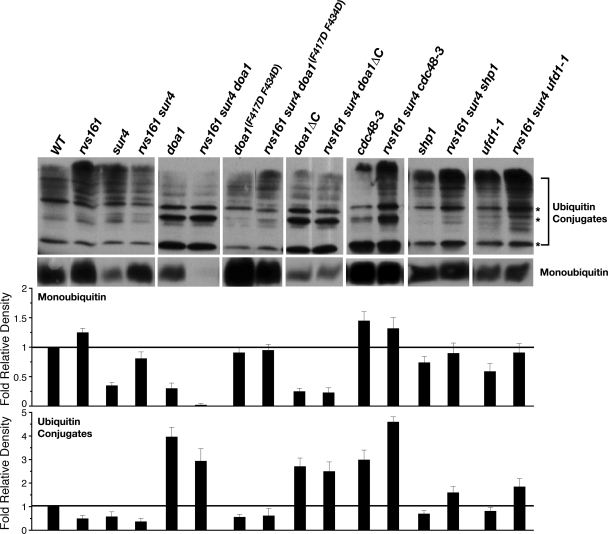
Total cell extracts were obtained using a modified procedure from Mullally et al. (49). Yeast cells grown to late log phase (optical density at 600 nm of 2.0) in synthetic complete medium containing 2% glucose were pelleted and resuspended in whole-cell lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM dithiothreitol, 0.05% Triton X-100) containing complete protease inhibitors (Roche Applied Science, Indianapolis, IN). Cells then were lysed with glass beads by using four cycles of vortexing for 1 min and incubation on ice for 1 min. Cellular extracts were collected after centrifugation, and samples were boiled in SDS loading buffer. Proteins were resolved by SDS-18% PAGE and subsequently transferred to a nitrocellulose membrane that was boiled in distilled deionized water for 10 min prior to blocking. Membranes were treated as described for the detection of Hxt2 and Hxt4. The primary antibody was monoclonal mouse anti-ubiquitin (clone P4D1; 1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), and the secondary antibody was polyclonal goat anti-mouse immunoglobulin G HRP-conjugated antibody (1:1,000 dilution; GE-Amersham, Piscataway, NJ). Proteins were detected using ECL chemiluminescence (GE-Amersham, Piscataway, NJ). For a loading control, actin protein was detected using monoclonal rabbit anti-β-actin primary antibody (clone H-300; 1:500 dilution; Santa Cruz Biotechnology) and polyclonal goat anti-rabbit immunoglobulin G HRP-conjugated antibody (1:1,000 dilution; GE-Amersham). Densitometry was performed using a Bio-Rad GS-800 densitometer and Quantity One software (4.6.6). All relative densitometry values were normalized to total actin levels. The values in Fig. 9 are the averages from three experiments ± standard deviations.
FIG. 9.
rvs161 cells hyperaccumulate monoubiquitin, and the loss of SUR4 suppresses this defect. Whole-cell lysates were resolved by SDS-PAGE, and monoubiquitin and high-molecular-weight polyubiquitin conjugates were visualized immunologically using anti-ubiquitin polyclonal antibodies. The levels of various ubiquitin species were normalized against actin using densitometry analysis. The values are the averages from three experiments ± standard deviations.
RESULTS
rvs161 cells harbor pleiotrophic carbon source growth defects that can be suppressed by altering sphingolipid levels.
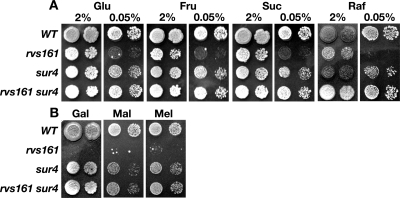
Cells lacking RVS161 cannot grow under conditions of glucose starvation (Fig. 2A). As previously described (15), this starvation defect could be suppressed by altering sphingolipid levels through the loss of SUR4. We asked whether starvation-induced death extended to other fermentable carbon sources. rvs161 cells were unable to grow when starved for fructose, sucrose, or raffinose (Fig. 2A). Interestingly, mutant cells also were incapable of growing on 2% galactose, 2% maltose, or 2% melibiose (Fig. 2B). Deleting SUR4 remediated all carbon source defects we observed (Fig. 2). To uncover the molecular basis for these carbon source defects and their sphingolipid-dependent suppression, we asked whether rvs161 cells could sense changes in glucose levels or distinguish what carbon source they were grown on and regulate gene transcription accordingly.
FIG. 2.
rvs161 cells harbor pleiotrophic carbon source starvation growth defects. Tenfold serial dilutions were spotted onto YEP plates containing the indicated sugar carbon source concentration. The initial cell density was 1 × 105 cells/ml. Cells were grown for 2 days at 30°C. WT, wild type; Glu, glucose; Fru, fructose; Suc, sucrose; Gal, galactose; Mal, maltose; Mel, melibiose.
rvs161 cells are able to derepress glucose-repressed genes as well as activate carbon source-dependent transcription.
Glucose represses the expression of genes that are dispensable under rich growth conditions (e.g., GAL, SUC, and genes encoding cytochromes and tricarboxylic acid cycle enzymes) (54). When glucose levels are depleted, the expression of these and other genes are induced or derepressed, and this transcriptional response is required for sustained growth. We asked whether rvs161 cells were capable of derepressing glucose-repressed genes. As a model for glucose derepression, we examined the expression levels of the invertase gene SUC2.
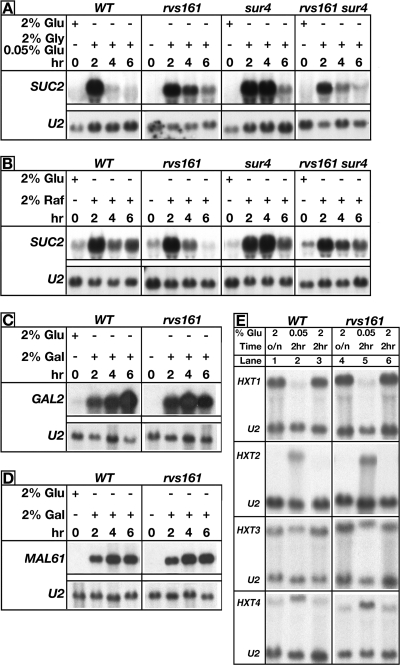
Cells were grown in YEPD and shifted to glycerol-containing medium to induce SUC2 expression. All strains tested were able to derepress and induce the expression of SUC2 (Fig. 3A). We also tested if mutant cells could induce SUC2 expression in raffinose-grown cultures. We reasoned that raffinose utilization (2% raffinose) through invertase-dependent hydrolysis would more closely mimic low-glucose conditions while being less deleterious to cells than examining expression in glucose starvation medium (0.05% glucose), a condition that kills rvs161 cells. The induction of SUC2 expression in raffinose has been demonstrated previously (57). SUC2 expression was induced in all strains tested and was sustained to similar levels (Fig. 3B). rvs161 cells were incapable of growing on galactose and maltose (Fig. 2B). Thus, we examined the galactose- and maltose-induced expression of the GAL2 and MAL61 permease genes, respectively. We found no differences in expression between wild-type and rvs161 cells (Fig. 3C). Finally, the sucrose-induced extracellular excretion of Suc2 invertase that is required for raffinose hydrolysis was normal in rvs161 mutants (data not shown). Based on these results, we conclude that rvs161 cells do not harbor defects in derepressing glucose-repressed genes, in their transcriptional response to growth on alternative fermentable carbon sources, or in secreting enzymes that are required to grow on various carbon sources.
FIG. 3.
Transcriptional responses of rvs161 cells to growth on multiple carbon sources and carbon source starvation are intact. In all cases, cells were grown to exponential phase in YEPD at 30°C prior to shifting cells to the various carbon sources. Gene expression levels were determined by Northern analysis. (A) Cells were shifted to medium containing 2% glycerol-0.05% glucose. (B) Cells were shifted to medium containing 2% raffinose. Cells were shifted to medium containing 2% galactose for GAL2 expression (C) or 2% maltose for MAL61 expression (D). (E) Lane 1, cells were shifted to 2% glucose, and HXT expression levels were determined after 2 h; lane 2, cells were shifted to 0.05% glucose, and HXT expression levels were determined after 2 h; lane 3, cells were shifted back to 2% glucose and HXT expression levels were determined after 2 h. U2 expression was used as a loading control.
rvs161 cells activate the Snf3- and Rgt2-dependent expression of HXT genes.
Glucose utilization in S. cerevisiae begins with the transport of glucose into the cell by specific high- and low-affinity glucose transporters. Hxt2 and Hxt4 are high- affinity glucose transporters and are expressed when glucose levels are low, while the expression of the low-affinity glucose transporter Hxt1 is induced in high glucose concentrations. The expression of HXT3 is moderately induced at all glucose concentrations (55). The glucose-dependent expression of HXT transporters is initiated and terminated by the Snf3 and Rgt2 glucose sensors. We asked whether the lack of proper HXT expression contributed in any way to starvation-induced death by asking if rvs161 cells were capable of initiating and terminating Snf3- and Rgt2-dependent HXT expression. Glucose concentration-dependent HXT expression levels were determined using Northern analysis. The expression patterns of HXT1 to HXT4 in wild-type and rvs161 cells grown under various glucose concentrations were identical (Fig. 3D). Thus, rvs cells are able to initiate Snf3- and Rgt2-dependent transcriptional signaling, resulting in the proper expression of HXT genes.
rvs161 cells are defective in the endocytosis and degradation of multiple sugar transporters.
Normal HXT gene expression observed in rvs161 cells prompted us to determine whether Hxt transporter mislocalization and/or protein instability contributed to starvation-induced death. rvs161 mutants do accumulate vesicles at the cytoplasmic side of the plasma membrane (7, 25). We constructed strains harboring an endogenous GFP-tagged allele of HXT2. Various glucose levels regulated HXT2 gene expression, and fluorescence microscopy was used to visualize localization.
As expected based on our expression data, plasma membrane-associated Hxt2 was not seen in wild-type cells grown in rich medium (data not shown). It localized to the plasma membrane after cells were shifted to starvation media for 2 h (0.05% Glu), and it disappeared after a shift back to rich medium for 5 h (Fig. 4). Thus, wild-type cells properly localized Hxt2 in response to changes in glucose levels. We found that Rvs161 was dispensable for the plasma membrane-associated localization of Hxt2 but was absolutely required for its disappearance (Fig. 4). Hxt2-GFP levels accumulated at the plasma membrane in mutant cells even after being shifted to rich medium for 5 h and persisted for up to 16 h. Importantly, we could reinitiate the loss of Hxt2 at the plasma membrane if we deleted SUR4 in mutant cells (Fig. 4). Interestingly, cells lacking SUR4 showed a fluorescent fragmented vacuolar morphology rather than the large single fluorescent organelle seen in cells with normal Sur4 function. This has been observed previously (39).
FIG. 4.
Aberrant plasma membrane accumulation of Hxt2 in rvs161 cells can be suppressed by the loss of SUR4. Cells were grown to exponential phase at 30°C in YEPD and then sequentially shifted to medium containing the indicated sugar concentrations for the indicated times. Hxt2-GFP localization was visualized by live-cell fluorescence microscopy. WT, wild type; Glu, glucose; hr, hours.
To determine whether the disappearance of Hxtp from the plasma membrane was representative of their endocytosis and degradation, the kinetics of glucose transporter degradation were determined using Western analysis. The high-affinity glucose transporters Hxt2 and Hxt4 were examined first. Hxt2 was detected in wild-type cells after 2 h in 0.05% glucose-containing medium and was almost completely endocytosed and degraded after a 3-h shift to rich medium (Fig. 5A). sur4 mutants showed similar kinetics (Fig. 5A). In contrast, rvs161 cells accumulated Hxt2 (Fig. 5A), while degradation was restored if we deleted SUR4 (Fig. 5A). Identical results were obtained when examining Hxt4 degradation (data not shown).
FIG. 5.
Sugar transporter endocytosis defects of rvs161 cells can be suppressed by the loss of SUR4. Cells were grown to exponential phase at 30°C in YEPD (A and B) or YEP containing 2% galactose (C). They then were sequentially shifted to medium containing the indicated sugar concentrations for the times indicated. Cell lysates were resolved by SDS-PAGE, and sugar transporter levels were determined by Western analysis using anti-GFP polyclonal antibodies (A and B) or anti-Gal2 polyclonal antibodies (C). Act1 (actin) levels were used as a loading control. WT, wild type; Glu, glucose; hr, hours. (A) Hxt2-GFP; (B) Hxt1-GFP; (C) Gal2.
The endocytosis and degradation of low-affinity glucose transporters also were defective in rvs161 cells, as evidenced by the aberrant accumulation of Hxt1. We found that Hxt1 was plasma membrane localized in wild-type cells grown in medium containing 2% glucose (Fig. 5B). After 1 h of being shifted to medium containing 0.05% glucose, wild-type cells completely endocytosed and degraded this transporter (Fig. 5B). rvs161 cells accumulated Hxt1 under these same conditions (Fig. 5B). The loss of SUR4 in rvs161 cells restored Hxt1 endocytosis and degradation levels to those seen in sur4 cells (Fig. 5B). Fluorescence microscopy showed that Hxt1-GFP localized to the plasma membrane in rvs161 cells (data not shown).
rvs161 cells also were defective in endocytosing and degrading the galactose permease Gal2 (Fig. 5C). Once again, deleting SUR4 restored endocytosis (Fig. 5C). Identical results were obtained by examining the endocytosis of the maltose permease Mal61 (data not shown). Both Gal2-GFP and Mal61-GFP localized to the plasma membrane in rvs161 cells (data not shown). Based on our results, we conclude that rvs161 cells harbor a general defect in sugar transporter endocytosis that can be remediated by altering sphingolipid metabolism through the loss of SUR4.
The endocytosis domain of Rvs161 is required for growth under glucose starvation and for the endocytosis of glucose transporters.
The fact that Rvs161 is required for growth on multiple carbon sources and for the endocytosis of multiple sugar transporters prompted us to determine the Rvs161 domain(s) responsible for these functions. Brizzio et al. (8) isolated strains harboring recessive rvs161 alleles giving rise to either endocytosis (End− Fus+) or cell fusion/mating (End+ Fus−) defects, thus delineating the functional domains of Rvs161. R35C, R113K, and P158S alleles cause endocytosis defects, while A175P and P203Q are defective in cell fusion/mating based on several criteria (Fig. 6A) (8).
FIG. 6.
endo domain of Rvs161 is required for growth under conditions of glucose starvation. (A) Schematic of Rvs161 delineating the domains required for endocytosis or cell fusion/mating, indicating the mutations described by Brizzio et al. (8). (B) Tenfold serial dilutions were spotted onto YEP plates containing the indicated sugar carbon source concentration. The initial cell density was 1 × 105 cells/ml. Cells were grown for 2 days at 37°C. WT, wild type; Glu, glucose.
We tested whether the two cell fusion and three endocytosis mutants (P158S, R35C, and R113K) could grow when starved of glucose. Surprisingly, none of these mutants harbored defects at 30°C (data not shown). Only when we grew cells at 37°C did we observe a phenotype (Fig. 6B). rvs161 cells harboring P158S, R35C, or R113K (End− Fus+) alleles were unable to grow. A175P cells (End+ Fus−) grew as well as wild-type cells, but P203Q cells consistently showed an intermediate starvation defect (Fig. 6B). We obtained similar results with the carbon sources and various sugar starvation conditions tested in Fig. 2 (data not shown).
We next asked if the endo domain was required for glucose transporter endocytosis. We examined the localization and endocytosis of Hxt2-GFP by using rvs161 R113K as a representative endo− mutant (Fig. 7). At 30°C, mutant cells localized, endocytosed (with a slight delay), and degraded Hxt2-GFP like wild-type cells. However, at 37°C, rvs161 R113K cells were incapable of endocytosing Hxt2-GFP from the plasma membrane. The loss of SUR4 suppressed this defect (data not shown). Cells harboring the A175P allele were not defective in endocytosis (data not shown). Similar results were observed for Hxt1-GFP, Gal2-GFP, and Mal61-GFP localization and degradation (data not shown). Thus, the endocytosis domain of Rvs161 is required for growth under glucose starvation and for the endocytosis and degradation of multiple sugar transporters.
FIG. 7.
endo domain of Rvs161 is required for sugar transporter endocytosis. Cells were grown to exponential phase at 30°C in YEPD. The culture was split, and cells were incubated at 30 or 37°C in YEP containing the indicated sugar carbon source concentration for the times indicated. Hxt2-GFP localization was visualized by live-cell fluorescence microscopy. WT, wild type; Glu, glucose; hr, hours.
Reinitiation of Hxt2 endocytosis in rvs161 sur4 requires Doa1, Doa4, and Rsp5 functions.
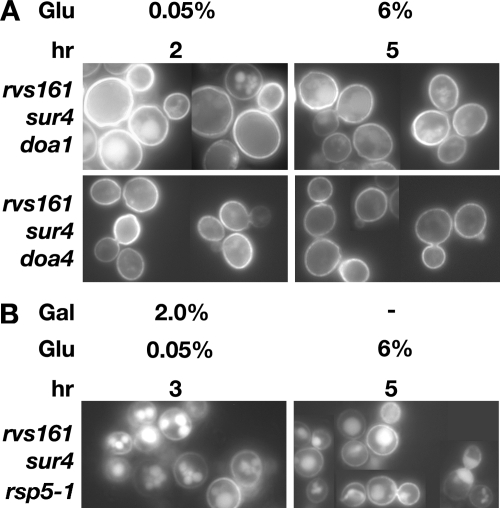
We next asked if factors required for ubiquitin-mediated endocytosis were needed for the sur4-dependent reinitiation of sugar transporter endocytosis. To address this, we determined if Hxt2-GFP endocytosis in rvs161 sur4 cells required Doa1 (which regulates the cellular ubiquitin concentration), Doa4 (ubiquitin hydrolase), and/or Rsp5 (ubiquitin ligase). Fluorescence microscopy revealed a requirement for all three proteins. rvs161 sur4 doa1, rvs161 sur4 doa4, and rvs161 sur4 cells harboring the dominant-negative rsp5-1 allele all accumulated Hxt2-GFP under conditions of high glucose growth (Fig. 8). Thus, the reinitiation of glucose transporter endocytosis in rvs161 cells by the loss of SUR4 requires several factors for ubiquitin-mediated endocytosis.
FIG. 8.
Cell factors regulating ubiquitin levels are required for the loss of SUR4 to suppress the endocytosis defects of rvs161 cells. Cells were grown to exponential phase in YEPD at 30°C. They then were sequentially shifted to medium containing the indicated sugar concentrations for the indicated times. Hxt2-GFP localization was visualized by live-cell fluorescence microscopy. (B) Cells were shifted to YEP containing 2% galactose to induce the expression of the dominant-negative Rsp5-1. WT, wild type; Glu, glucose; hr, hours.
The Cdc48 binding domain of Doa1 is required for the reinitiation of glucose transporter endocytosis in rvs161 sur4 cells.
Doa1 contains a ubiquitin binding domain (PFU) and a second carboxyl-terminal domain (PUL), which binds to the conserved AAA-ATPase Cdc48 (49). Both domains link ubiquitylated substrates to Cdc48 and are thought to be required for Doa1 function (49, 62). We asked if one or both of these domains were required for the sur4-dependent reinitiation of sugar transporter endocytosis in rvs161 cells. We also determined whether Cdc48 itself, and/or the Cdc48 accessory factors Shp1 and Ufd1, also were needed. Shp1 and Ufd1 bind directly to Cdc48 (46). Both Shp1 and Ufd1 bind ubiquitin. Shp1 is necessary for Cdc48 function in membrane fusion and proteosomal degradation, while Ufd1 facilitates endoplasmic reticulum (ER)-dependent degradation and the activation of membrane-associated transcription factors. We examined the glucose concentration-dependent appearance/disappearance of Hxt2-GFP from the plasma membrane, and the data are presented in Table 1.
TABLE 1.
Glucose concentration-dependent appearance/ disappearance of Hxt2-GFP from the plasma membranea
| Strain | % of cells with plasma membrane-associated Hxt2 at glucose concn:
|
||
|---|---|---|---|
| 6% | 0.05% | 6% | |
| Wild type | 1 ± 0.5 | 95 ± 3 | 14 ± 5 |
| rvs161 | 2 ± 1 | 97 ± 3 | 95 ± 4 |
| rvs161 sur4 | 1 ± 1 | 97 ± 5 | 21 ± 4 |
| rvs161 sur4 doa1 | 3 ± 2 | 92 ± 4 | 94 ± 6 |
| rvs161 sur4 doa1F417D F434D | 1 ± 0.5 | 93 ± 3 | 18 ± 4 |
| rvs161 sur4 doa1ΔC | 2 ± 0.5 | 94 ± 3 | 92 ± 4 |
| rvs161 sur4 cdc48-3b | 6 ± 4 | 88 ± 5 | 94 ± 6 |
| rvs161 sur4 ufd1-1b | 1 ± 0.5 | 99 ± 3 | 97 ± 4 |
| rvs161 sur4 shp1 | 1 ± 0.5 | 95 ± 5 | 89 ± 5 |
Cells were sequentially shifted from 6% glucose to 0.05% glucose for 1 h, and then back to 6% glucose for 5 h. Values are the averages from three independent experiments.
Cells were assayed at 30°C.
The Cdc48 binding PUL domain of Doa1 (doa1ΔC allele) was required to reinitiate endocytosis by the loss of SUR4, while the PFU domain (doa1F417D F434D allele) was dispensable. rvs161 sur4 doa1ΔC cells accumulated Hxt2 to the same extent as rvs161 cells shifted from 0.05 to 6% glucose for 5 h, while the accumulation of this transporter in rvs161 sur4 doa1F417D F434D cells was similar to that seen in rvs161 sur4 suppressor cells (Table 1). Cdc48 itself also was required, as rvs161 sur4 cells harboring a cdc48-3 ts allele were incapable of endocytosing Hxt2 at the restrictive temperature. In fact, rvs161 sur4 cdc48-3 cells showed a defect in endocytosis and displayed the characteristic elongated phenotype of cdc48 alleles even at the permissive temperature (62). Both of the Cdc48 binding factors, Shp1 and Ufd1, also were required. rvs161 sur4 cells deleted for SHP1 or harboring a temperature-sensitive ufd1-1 allele were defective in Hxt2 endocytosis (Table 1). Based on these results, we conclude that the sphingolipid-dependent reinitiation of sugar transporter endocytosis in rvs161 cells requires the function of multiple Cdc48 complexes.
Loss of SUR4 remediates the monoubiquitin hyperaccumulation defect of rvs161 cells.
The gene products that are required for the sur4-dependent suppression of rvs161 endocytosis defects bind to and/or regulate ubiquitin levels. Moreover, our attempt to suppress the endocytosis defects of rvs161 cells by overexpressing ubiquitin failed. Thus, we asked whether rvs161 cells have an altered ubiquitin metabolism, and if so, does the loss of SUR4 suppress these defects. rvs161 cells hyperaccumulated monoubiquitin (1.3-fold), and deleting SUR4 in these cells did decrease the level of this ubiquitin species to nearly that seen in wild-type cells (∼80%) (Fig. 9). Interestingly, sur4 cells had a drastically lower level of monoubiquitin than the wild type, but their levels of high-molecular-weight conjugates were normal (Fig. 9). When we looked at those mutations in rvs161 sur4 cells causing the loss of glucose concentration-dependent Hxt2 endocytosis (Table 1), the overall observation was that they all altered ubiquitin metabolism. The common phenotype seen was a decrease in very-high-molecular-weight ubiquitin conjugate levels and the accumulation of several faster-migrating polyubiquitin species, although this phenotype was more subtle in rvs161 sur4 shp1 and rvs161 sur4 ufd1-1 cells. In some cases, the level of monoubiquitin was altered (rvs161 sur4 doa1, rvs161 sur4 doa1ΔC, and rvs161 sur4 cdc48-3 cells). Based on these results, we believe that the synthesis of monoubiquitin, its conversion to high-molecular-weight conjugates, and the subsequent turnover of these conjugates all are necessary for the sur4-dependent suppression of rvs161 endocytosis defects.
DISCUSSION
The rate of glucose transporter endocytosis is drastically reduced in rvs161 cells, and this defect correlates with starvation-induced death under low-glucose conditions. These results suggest that the glucose starvation defect is linked to the improper regulation of hexose transporter trafficking and/or degradation. Viability under starvation is linked to the endo domain of Rvs161, as endo− mutants are inviable when starved and are defective in endocytosing glucose transporters from the membrane. In addition, the loss of SUR4 suppresses the starvation defect and reinitiates glucose transporter endocytosis.
How might the reinitiation of endocytosis remediate starvation-induced death? One possibility is that the accurate endocytosis/degradation of sugar transporters acts as a regulatory step that is necessary to maintain proper stoichiometric transporter ratios, which are critical for growth under nutrient stress conditions; rvs161 mutants accumulate high-affinity transporters in response to glucose starvation, and they lack the ability to endocytose/degrade low-affinity transporters. Glucose transporters in mammalian cells, specifically Glut1, exist in dimeric and tetrameric states, but these oligomerizations are not essential for glucose uptake (48). A nonfunctional chimera consisting of yeast Hxt1 and Hxt4 transporters inhibits the function of wild-type glucose transporters (67). Whether this chimera functions as a dominant-negative mutant, directly interacting with and inhibiting specific glucose transporters, has not been studied. Therefore, is maintaining proper ratios of homo- and heterodimeric transporters necessary for viability under various growth conditions? With that said, we cannot rule out that the reinitiation of global endocytosis itself remediates the starvation defect rather than the specific reinitiation of proper stoichiometric transporter ratios. rvs161 cells are defective for growth under sulfur and nitrogen starvation conditions, and the loss of SUR4 remediates these growth defects as well (15).
An alternative scenario is that rvs161 cells internalize and metabolize too much glucose due to defects in endocytosis and the constitutive accumulation of sugar transporters at the plasma membrane, which depletes cellular ATP stores. The first step in glucose utilization after internalization is a phosphorylation event by hexose kinase, resulting in glucose-6-phosphate production and shunting through the glycolytic pathway (27). rvs161 cells accumulate the high-affinity glucose transporter Hxt2 and may accumulate others, such as Hxt6 and Hxt7. The concentration of glucose (2.7 mM) under starvation conditions is within the Km ranges of Hxt6 and Hxt7 (Km = 1 to 2 mM) as well as those of Hxt2 (Km = 1.5 mM) and Hxt4 (Km ∼ 10 mM) (34). Here again the general reinitiation of endocytosis/degradation would act as a balance in conjunction with the rate of biosynthesis in order to maintain proper transporter ratios at the plasma membrane. Preliminary studies examining the rate of glucose binding and internalization in rvs161 cells thus far have been inconclusive (E. Swain and J. T. Nickels, unpublished data).
In addition to its role in endocytosis, Rvs161 is required for actin repolarization following osmotic salt stress. Wild-type cells depolarize actin following salt stress and repolarize after a period of adaptation, whereas rvs161 mutant cells depolarize actin but are unable to repolarize afterwards (1, 69). The loss of SUR4 suppresses the actin polarization defect of rvs161 cells under conditions that are semipermissive for viability (3.4% NaCl) (1). However, it does not suppress the actin polarization defect of mutant cells grown under glucose starvation conditions (26) or under high salt stress, which results in inviability (6% NaCl) (45a). Thus, how the loss of SUR4 suppresses rvs defects cannot be explained solely through its effects on the actin cytoskeleton. Interestingly, the glucose starvation defect of the endo-deficient Rvs161 point mutants correlates with their ability to form an Rvs161-Rvs167 complex, as the rvs161 R113K allele cannot bind Rvs167, as determined by two-hybrid analyses (P. McCourt, J. Morgan, and J. T. Nickels, unpublished data). These results seem reasonable, as the loss of SUR4 is able to remediate defects of rvs161 rvs167 cells (15). Thus, suppression can occur in the absence of any Rvs161- or Rvs167-associated multiprotein complexes.
The Cdc48 binding domain of Doa1 was required for sugar transporter endocytosis in rvs161 sur4 cells. Moreover, direct roles for Cdc48-Shp1 and Cdc48-Ufd1 were demonstrated. Cdc48-Shp1 regulates several membrane fusion events, including nuclear envelope growth and reforming the ER and Golgi assembly after mitosis (46, 47, 64). During ER-dependent degradation, Cdc48-Ufd1 extracts ubiquitylated substrates from membranes for their degradation. In yeast, Cdc48-Ufd1 mobilizes the transcription factors Spt23 and Mga2 from membranes prior to their moving to the nucleus (33, 59, 66). Based on these and other observations, Meyer and Popp (46) suggest that the fundamental activity of Cdc48 is the energy-dependent removal of ubiquitylated proteins from membranes. Once removed, these proteins are free to be degraded or deubiquitylated. How Cdc48 regulates sugar transporter endocytosis needs to be explored; understanding how it helps in reinitiating sphingolipid-dependent endocytosis should be an excellent model for study.
The level of monoubiquitin was increased in rvs161 cells, and the deletion of SUR4 remediated this defect to some extent. Monoubiquitin serves as a regulatory signal for the intracellular transport of proteins through the late secretory and endocytic pathways (reviewed in references 31 and 32). In yeast, membrane proteins such as amino acid permeases and mating factor receptors are ubiquitylated, and this modification acts as a signal for internalization and/or endosomal sorting (reviewed in references 38 and 65). The mammalian sugar transporters GLUT1 and GLUT4 are modified with ubiquitin as well as the ubiquitin-like protein SUMO (22, 41). Whether yeast glucose transporters exhibit a similar fate is not known. However, the sphingolipid-dependent reinitiation of endocytosis has a strict requirement for factors regulating ubiquitin pools, pointing to the ubiquitylation of glucose transporters being an important regulatory step in their turnover.
The N-BAR domain of the BAR family of proteins plays a role in initiating and/or sensing membrane curvature (58). If yeast Rvs161 and/or Rvs167 sense and are drawn to nascent buds destined for endocytosis, they may act in the formation and function of a large membrane-associated multiprotein assembly complex that is involved in bud fission and subsequent endocytosis (21). Rvs167 does associate with the fission machinery; however, this is a late event in the scission process (37). sur4 cells accumulate the sphingoid LCB phytosphingosine (51). The endogenous addition of this LCB reinitiates endocytosis in cells deficient in sphingolipid biosynthesis (23, 73), and its transient accumulation may be required for ubiquitin-dependent proteolysis following heat stress (11). LCB accumulation may constitutively alter membrane curvature, causing invaginations within specific microdomains. These may give rise to putative pseudonascent buds that normally are initiated or stabilized by Rvs161-Rvs167 complexes, which then can attract factors that are required for fission. An important question remaining is whether LCB-dependent endocytosis requires the general fission machinery to initiate bud scission and release, as it does require ubiquitylation.
Defects in sphingolipid biosynthesis also could activate signaling pathways required to remediate rvs defects; activating these pathways would circumvent the need for any Rvs-dependent events during endocytosis. Sphingolipid intermediates in yeast, as in mammalian cells (16, 36, 52), are important signaling molecules, particularly the sphingoid base phytosphingosine. The accumulation of LCBs in yeast activates the Pkc1-MAP cell wall integrity pathway (74). The accumulation of the mammalian LCB sphingosine 1-phosphate in yeast stimulates Ca2+ accumulation, possibly initiating the LCB-dependent activation of Ca2+/calmodulin-dependent signaling cascades (5).
Whether the sphingolipid mutations suppressing rvs defects all function by activating some LCB-dependent event(s) is not known. sur4 mutants accumulate phytosphingosine but also accumulate C22 fatty acids, are devoid of C26 fatty acids, produce complex sphingolipids that have shorter fatty acid moieties, and hyperaccumulate an inositolphosphorylceramide species (51). Whether any of these changes in lipid metabolism contribute to suppression is not known. Moreover, sur2 cells do not make phytosphingosine; thus, all complex sphingolipids are derived from the LCB dihydrosphingosine, and they lack C4 hydroxylation (29). Whether the loss of SUR2 and the accumulation of dihydrosphingosine activates pathways identical to those regulated by phytosphingosine is not known.
A number of sphingolipid mutations suppressing rvs161 defects alter calcium homeostasis (75). Csg2 and Sur1 are required for the synthesis of mannose inositolphosphorylceramide, and csg2 and sur1 mutants are calcium sensitive (3, 4). Recessive mutations in SUR2 and SUR4 remediate the calcium sensitivity of csg2 and sur1 cells (75). Interestingly, the addition of exogenous phytosphingosine alone to csg2 cells remediates their calcium-sensitive phenotype (75). We have been unsuccessful in suppressing rvs defects through calcium remediation or phytosphingosine supplementation. The endocytosis defect of rvs cells may preclude using exogenous methods; thus, more detailed studies are warranted.
Acknowledgments
We thank Howard Riezman and Michihiro Kasahara for anti-Suc2 and anti-Gal2 antibodies, respectively. We are grateful to Keith Wilkinson, James Mullally, Dale Haines, Valeria Brizzio, and Mark Rose for strains. We thank Martin Adelson and Eli Mordechai for many helpful discussions.
This work was supported in part by NIH grant HL67401 (J.T.N.). We acknowledge and appreciate the financial support of Medical Diagnostics Laboratories, L.L.C.
Footnotes
Published ahead of print on 13 March 2009.
REFERENCES
- 1.Balguerie, A., M. Bagnat, M. Bonneu, M. Aigle, and A. M. Breton. 2002. Rvs161p and sphingolipids are required for actin repolarization following salt stress. Eukaryot. Cell 11021-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer, F., M. Urdaci, M. Aigle, and M. Crouzet. 1993. Alteration of a yeast SH3 protein leads to conditional viability with defects in cytoskeletal and budding patterns. Mol. Cell. Biol. 135070-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beeler, T., K. Gable, C. Zhao, and T. Dunn. 1994. A novel protein, CSG2p, is required for Ca2+ regulation in Saccharomyces cerevisiae. J. Biol. Chem. 2697279-7284. [PubMed] [Google Scholar]
- 4.Beeler, T. J., D. Fu, J. Rivera, E. Monaghan, K. Gable, and T. M. Dunn. 1997. SUR1 (CSG1/BCL21), a gene necessary for growth of Saccharomyces cerevisiae in the presence of high Ca2+ concentrations at 37°C is required for mannosylation of inositolphosphorylceramide. Mol. Gen. Genet. 255570-579. [DOI] [PubMed] [Google Scholar]
- 5.Birchwood, C. J., J. D. Saba, R. C. Dickson, and K. W. Cunningham. 2001. Calcium influx and signaling in yeast stimulated by intracellular sphingosine 1-phosphate accumulation. J. Biol. Chem. 27611712-11718. [DOI] [PubMed] [Google Scholar]
- 6.Boles, E., and C. P. Hollenberg. 1997. The molecular genetics of hexose transport in yeasts. FEMS Microbiol. Rev. 2185-111. [DOI] [PubMed] [Google Scholar]
- 7.Breton, A. M., J. Schaeffer, and M. Aigle. 2001. The yeast Rvs161 and Rvs167 proteins are involved in secretory vesicles targeting the plasma membrane and in cell integrity. Yeast 181053-1068. [DOI] [PubMed] [Google Scholar]
- 8.Brizzio, V., A. E. Gammie, and M. D. Rose. 1998. Rvs161p interacts with Fus2p to promote cell fusion in Saccharomyces cerevisiae. J. Cell Biol. 141567-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casal, E., L. Federici, W. Zhang, J. Fernandez-Recio, E. M. Priego, R. N. Miguel, J. B. DuHadaway, G. C. Prendergast, B. F. Luisi, and E. D. Laue. 2006. The crystal structure of the BAR domain from human Bin1/amphiphysin II and its implications for molecular recognition. Biochemistry 4512917-12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chitu, V., and E. R. Stanley. 2007. Pombe Cdc15 homology (PCH) proteins: coordinators of membrane-cytoskeletal interactions. Trends Cell Biol. 17145-156. [DOI] [PubMed] [Google Scholar]
- 11.Chung, N., G. Jenkins, Y. A. Hannun, J. Heitman, and L. M. Obeid. 2000. Sphingolipids signal heat stress-induced ubiquitin-dependent proteolysis. J. Biol. Chem. 27517229-17232. [DOI] [PubMed] [Google Scholar]
- 12.Cleveland, D. W., S. G. Fischer, M. W. Kirschner, and U. K. Laemmli. 1977. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J. Biol. Chem. 2521102-1106. [PubMed] [Google Scholar]
- 13.Crouzet, M., M. Urdaci, L. Dulau, and M. Aigle. 1991. Yeast mutant affected for viability upon nutrient starvation: characterization and cloning of the RVS161 gene. Yeast 7727-743. [DOI] [PubMed] [Google Scholar]
- 14.David, C., P. S. McPherson, O. Mundigl, and P. de Camilli. 1996. A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc. Natl. Acad. Sci. USA 93331-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desfarges, L., P. Durrens, H. Juguelin, C. Cassagne, M. Bonneu, and M. Aigle. 1993. Yeast mutants affected in viability upon starvation have a modified phospholipid composition. Yeast 9267-277. [DOI] [PubMed] [Google Scholar]
- 16.Dickson, R. C. 1998. Sphingolipid functions in Saccharomyces cerevisiae: comparison to mammals. Annu. Rev. Biochem. 6727-48. [DOI] [PubMed] [Google Scholar]
- 17.Dickson, R. C., and R. L. Lester. 2002. Sphingolipid functions in Saccharomyces cerevisiae. Biochim. Biophys. Acta 158313-25. [DOI] [PubMed] [Google Scholar]
- 18.Dickson, R. C., E. E. Nagiec, G. B. Wells, M. M. Nagiec, and R. L. Lester. 1997. Synthesis of mannose-(inositol-P)2-ceramide, the major sphingolipid in Saccharomyces cerevisiae, requires the IPT1 (YDR072c) gene. J. Biol. Chem. 27229620-29625. [DOI] [PubMed] [Google Scholar]
- 19.Dickson, R. C., C. Sumanasekera, and R. L. Lester. 2006. Functions and metabolism of sphingolipids in Saccharomyces cerevisiae. Prog. Lipid Res. 45447-465. [DOI] [PubMed] [Google Scholar]
- 20.Durrens, P., E. Revardel, M. Bonneu, and M. Aigle. 1995. Evidence for a branched pathway in the polarized cell division of Saccharomyces cerevisiae. Curr. Genet. 27213-216. [DOI] [PubMed] [Google Scholar]
- 21.Engqvist-Goldstein, A. E., and D. G. Drubin. 2003. Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 19287-332. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes, R., A. L. Carvalho, A. Kumagai, R. Seica, K. Hosoya, T. Terasaki, J. Murta, P. Pereira, and C. Faro. 2004. Downregulation of retinal GLUT1 in diabetes by ubiquitinylation. Mol. Vis. 10618-628. [PubMed] [Google Scholar]
- 23.Friant, S., R. Lombardi, T. Schmelzle, M. N. Hall, and H. Riezman. 2001. Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. EMBO J. 206783-6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallop, J. L., and H. T. McMahon. 2005. BAR domains and membrane curvature: bringing your curves to the BAR. Biochem. Soc. Symp. 2005223-231. [DOI] [PubMed] [Google Scholar]
- 25.Gammie, A. E., V. Brizzio, and M. D. Rose. 1998. Distinct morphological phenotypes of cell fusion mutants. Mol. Biol. Cell 91395-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Germann, M., E. Swain, L. Bergman, and J. T. Nickels, Jr. 2005. Characterizing the sphingolipid signaling pathway that remediates defects associated with loss of the yeast amphiphysin-like orthologs, Rvs161p and Rvs167p. J. Biol. Chem. 2804270-4278. [DOI] [PubMed] [Google Scholar]
- 27.Gonçalves, P., and R. J. Planta. 1998. Starting up yeast glycolysis. Trends Microbiol. 6314-319. [DOI] [PubMed] [Google Scholar]
- 28.Grabs, D., V. I. Slepnev, Z. Songyang, C. David, M. Lynch, L. C. Cantley, and P. De Camilli. 1997. The SH3 domain of amphiphysin binds the proline-rich domain of dynamin at a single site that defines a new SH3 binding consensus sequence. J. Biol. Chem. 27213419-13425. [DOI] [PubMed] [Google Scholar]
- 29.Haak, D., K. Gable, T. Beeler, and T. Dunn. 1997. Hydroxylation of Saccharomyces cerevisiae ceramides requires Sur2p and Scs7p. J. Biol. Chem. 27229704-29710. [DOI] [PubMed] [Google Scholar]
- 30.Habermann, B. 2004. The BAR-domain family of proteins: a case of bending and binding? EMBO Rep. 5250-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hicke, L. 2001. Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell Biol. 2195-201. [DOI] [PubMed] [Google Scholar]
- 32.Hicke, L., and R. Dunn. 2003. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19141-172. [DOI] [PubMed] [Google Scholar]
- 33.Hitchcock, A. L., H. Krebber, S. Frietze, A. Lin, M. Latterich, and P. A. Silver. 2001. The conserved npl4 protein complex mediates proteasome-dependent membrane-bound transcription factor activation. Mol. Biol. Cell 123226-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itoh, T., and P. De Camilli. 2006. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim. Biophys. Acta 1761897-912. [DOI] [PubMed] [Google Scholar]
- 36.Jenkins, G. M. 2003. The emerging role for sphingolipids in the eukaryotic heat shock response. Cell Mol. Life Sci. 60701-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaksonen, M., C. P. Toret, and D. G. Drubin. 2005. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 123305-320. [DOI] [PubMed] [Google Scholar]
- 38.Katzmann, D. J., G. Odorizzi, and S. D. Emr. 2002. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 3893-905. [DOI] [PubMed] [Google Scholar]
- 39.Kohlwein, S. D., S. Eder, C. S. Oh, C. E. Martin, K. Gable, D. Bacikova, and T. Dunn. 2001. Tsc13p is required for fatty acid elongation and localizes to a novel structure at the nuclear-vacuolar interface in Saccharomyces cerevisiae. Mol. Cell. Biol. 21109-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krampe, S., O. Stamm, C. P. Hollenberg, and E. Boles. 1998. Catabolite inactivation of the high-affinity hexose transporters Hxt6 and Hxt7 of Saccharomyces cerevisiae occurs in the vacuole after internalization by endocytosis. FEBS Lett. 441343-347. [DOI] [PubMed] [Google Scholar]
- 41.Lalioti, V. S., S. Vergarajauregui, D. Pulido, and I. V. Sandoval. 2002. The insulin-sensitive glucose transporter, GLUT4, interacts physically with Daxx. Two proteins with capacity to bind Ubc9 and conjugated to SUMO1. J. Biol. Chem. 27719783-19791. [DOI] [PubMed] [Google Scholar]
- 42.Lichte, B., R. W. Veh, H. E. Meyer, and M. W. Kilimann. 1992. Amphiphysin, a novel protein associated with synaptic vesicles. EMBO J. 112521-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules of versatile and economical pcr-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14953-961. [DOI] [PubMed] [Google Scholar]
- 44.Manolescu, A. R., K. Witkowska, A. Kinnaird, T. Cessford, and C. Cheeseman. 2007. Facilitated hexose transporters: new perspectives on form and function. Physiology (Bethesda) 22234-240. [DOI] [PubMed] [Google Scholar]
- 45.Masuda, M., S. Takeda, M. Sone, T. Ohki, H. Mori, Y. Kamioka, and N. Mochizuki. 2006. Endophilin BAR domain drives membrane curvature by two newly identified structure-based mechanisms. EMBO J. 252889-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45a.McCourt, P., J. M. Morgan, and J. T. Nickels, Jr. Stress-induced ceramide-activated protein phosphatase can compensate for loss of amphiphysin-like activity in Saccharomyces cerevisiae and functions to reinitiate endocytosis. J. Biol. Chem., in press. [DOI] [PMC free article] [PubMed]
- 46.Meyer, H., and O. Popp. 2008. Role(s) of Cdc48/p97 in mitosis. Biochem. Soc. Trans. 36126-130. [DOI] [PubMed] [Google Scholar]
- 47.Meyer, H. H. 2005. Golgi reassembly after mitosis: the AAA family meets the ubiquitin family. Biochim. Biophys. Acta 1744481-492. [PubMed] [Google Scholar]
- 48.Mueckler, M. 1994. Facilitative glucose transporters. Eur. J. Biochem. 219713-725. [DOI] [PubMed] [Google Scholar]
- 49.Mullally, J. E., T. Chernova, and K. D. Wilkinson. 2006. Doa1 is a Cdc48 adapter that possesses a novel ubiquitin binding domain. Mol. Cell. Biol. 26822-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munn, A. L., B. J. Stevenson, M. I. Geli, and H. Riezman. 1995. end5, end6, and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell 61721-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh, C. S., D. A. Toke, S. Mandala, and C. E. Martin. 1997. ELO2 and ELO3, homologues of the Saccharomyces cerevisiae ELO1 gene, function in fatty acid elongation and are required for sphingolipid formation. J. Biol. Chem. 27217376-17384. [DOI] [PubMed] [Google Scholar]
- 52.Ohanian, J., and V. Ohanian. 2001. Sphingolipids in mammalian cell signalling. Cell Mol. Life Sci. 582053-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozcan, S., J. Dover, and M. Johnston. 1998. Glucose sensing and signaling by two glucose receptors in the yeast Saccharomyces cerevisiae. EMBO J. 172566-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ozcan, S., J. Dover, A. G. Rosenwald, S. Wolfl, and M. Johnston. 1996. Two glucose transporters in Saccharomyces cerevisiae are glucose sensors that generate a signal for induction of gene expression. Proc. Natl. Acad. Sci. USA 9312428-12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ozcan, S., and M. Johnston. 1995. Three different regulatory mechanisms enable yeast hexose transporter (HXT) genes to be induced by different levels of glucose. Mol. Cell. Biol. 151564-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozcan, S., and M. Johnston. 1999. Function and regulation of yeast hexose transporters. Microbiol. Mol. Biol. Rev. 63554-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ozcan, S., L. G. Vallier, J. S. Flick, M. Carlson, and M. Johnston. 1997. Expression of the SUC2 gene of Saccharomyces cerevisiae is induced by low levels of glucose. Yeast 13127-137. [DOI] [PubMed] [Google Scholar]
- 58.Peter, B. J., H. M. Kent, I. G. Mills, Y. Vallis, P. J. Butler, P. R. Evans, and H. T. McMahon. 2004. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303495-499. [DOI] [PubMed] [Google Scholar]
- 59.Rape, M., T. Hoppe, I. Gorr, M. Kalocay, H. Richly, and S. Jentsch. 2001. Mobilization of processed, membrane-tethered SPT23 transcription factor by CDC48(UFD1/NPL4), a ubiquitin-selective chaperone. Cell 107667-677. [DOI] [PubMed] [Google Scholar]
- 60.Reifenberger, E., E. Boles, and M. Ciriacy. 1997. Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur. J. Biochem. 245324-333. [DOI] [PubMed] [Google Scholar]
- 61.Ren, G., P. Vajjhala, J. S. Lee, B. Winsor, and A. L. Munn. 2006. The BAR domain proteins: molding membranes in fission, fusion, and phagy. Microbiol. Mol. Biol. Rev. 7037-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ren, J., N. Pashkova, S. Winistorfer, and R. C. Piper. 2008. DOA1/UFD3 plays a role in sorting ubiquitinated membrane proteins into multivesicular bodies. J. Biol. Chem. 28321599-21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Revardel, E., M. Bonneau, P. Durrens, and M. Aigle. 1995. Characterization of a new gene family developing pleiotropic phenotypes upon mutation in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1263261-265. [DOI] [PubMed] [Google Scholar]
- 64.Römisch, K. 2006. Cdc48p is UBX-linked to ER ubiquitin ligases. Trends Biochem. Sci. 3124-25. [DOI] [PubMed] [Google Scholar]
- 65.Rotin, D., O. Staub, and R. Haguenauer-Tsapis. 2000. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J. Membr. Biol. 1761-17. [DOI] [PubMed] [Google Scholar]
- 66.Shcherbik, N., and D. S. Haines. 2007. Cdc48p(Npl4p/Ufd1p) binds and segregates membrane-anchored/tethered complexes via a polyubiquitin signal present on the anchors. Mol. Cell 25385-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sherwood, P. W., I. Katic, P. Sanz, and M. Carlson. 2000. A glucose transporter chimera confers a dominant negative glucose starvation phenotype in Saccharomyces cerevisiae. Genetics 155989-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shupliakov, O., P. Low, D. Grabs, H. Gad, H. Chen, C. David, K. Takei, P. De Camilli, and L. Brodin. 1997. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science 276259-263. [DOI] [PubMed] [Google Scholar]
- 69.Sivadon, P., F. Bauer, M. Aigle, and M. Crouzet. 1995. Actin cytoskeleton and budding pattern are altered in the yeast rvs161 mutant: the Rvs161 protein shares common domains with the brain protein amphiphysin. Mol. Gen. Genet. 246485-495. [DOI] [PubMed] [Google Scholar]
- 70.Sivadon, P., M. Crouzet, and M. Aigle. 1997. Functional assessment of the yeast Rvs161 and Rvs167 protein domains. FEBS Lett. 41721-27. [DOI] [PubMed] [Google Scholar]
- 71.Wang, Q., H. Y. Kaan, R. N. Hooda, S. L. Goh, and H. Sondermann. 2008. Structure and plasticity of Endophilin and Sorting Nexin 9. Structure 161574-1587. [DOI] [PubMed] [Google Scholar]
- 72.Weissenhorn, W. 2005. Crystal structure of the endophilin-A1 BAR domain. J. Mol. Biol. 351653-661. [DOI] [PubMed] [Google Scholar]
- 73.Zanolari, B., S. Friant, K. Funato, C. Sutterlin, B. J. Stevenson, and H. Riezman. 2000. Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. EMBO J. 192824-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang, X., R. L. Lester, and R. C. Dickson. 2004. Pil1p and Lsp1p negatively regulate the 3-phosphoinositide-dependent protein kinase-like kinase Pkh1p and downstream signaling pathways Pkc1p and Ypk1p. J. Biol. Chem. 27922030-22038. [DOI] [PubMed] [Google Scholar]
- 75.Zhao, C., T. Beeler, and T. Dunn. 1994. Suppressors of the Ca2+-sensitive yeast mutant (csg2) identify genes involved in sphingolipid biosynthesis. Cloning and characterization of SCS1, a gene required for serine palmitoyltransferase activity. J. Biol. Chem. 26921480-21488. [PubMed] [Google Scholar]
- 76.Zhao, F. Q., and A. F. Keating. 2007. Functional properties and genomics of glucose transporters. Curr. Genomics 8113-128. [DOI] [PMC free article] [PubMed] [Google Scholar]