Abstract
AbiV is a chromosomally encoded phage resistance mechanism that is silent in the wild-type phage-sensitive strain Lactococcus lactis subsp. cremoris MG1363. Spontaneous phage-resistant mutants of L. lactis MG1363 were analyzed by reverse transcriptase PCR and shown to express AbiV. This expression was related to a reorganization in the upstream region of abiV. Transfer of abiV between two lactococcal strains, most likely by conjugation, was also demonstrated. To our knowledge, this is the first report of natural transfer of a chromosomally encoded phage resistance mechanism.
Industrial milk fermentation is dependent on the well-characterized metabolic features of commercial starter cultures, which contain strains of lactic acid bacteria (6). However, milk fermentation failures due to virulent phages that are infecting these specialized bacterial cultures is a persistent problem for the dairy industry (6, 8, 9, 26). Decades of research have led to the discovery of a number of natural defense systems in Lactococcus lactis cells, including the inhibition of phage adsorption (12, 21) and DNA entry (25), restriction/modification systems (1, 12), and abortive infection (Abi) mechanisms (5). These antiviral mechanisms have been used extensively in a relatively small number of industrial strains (7), which has favored the emergence of phage mutants that are insensitive to the natural antiphage barriers (8, 12, 26). This viral evolutionary process has led to a lasting search for new ways to protect cultures against phage attacks. Moreover, to avoid the use of genetic engineering technology, the dairy industry is currently depending on the isolation of novel natural phage resistance barriers in a given wild-type L. lactis isolate that can be naturally transferred into industrial starter strains.
Most known lactococcal restriction/modification and Abi systems are plasmid encoded (2, 3, 5, 10, 11, 13, 20, 29, 39), and some of them can be easily transferred from one strain to another through conjugation. This genetic transformation process is universally accepted and has been successfully utilized to create phage-resistant starter cultures (1, 6, 19, 26, 28, 30, 34). Some phage resistance mechanisms are also chromosomally encoded. However, their industrial application is limited because they cannot be transferred into the desired industrial strains without the use of genetic engineering.
Recently, we isolated a novel chromosomally encoded Abi mechanism named AbiV (17) that is active against several lactococcal phages. The abiV gene is silent in the phage-sensitive strain L. lactis subsp. cremoris MG1363, but it can be activated when a promoter is provided (17). Here, we report the isolation of natural bacteriophage-insensitive mutants (BIMs) of L. lactis MG1363 that spontaneously express AbiV. Furthermore, we demonstrate that abiV can be transferred to lactococcal strains with a protocol used for conjugation assays.
Isolation of mutants of L. lactis MB112 spontaneously expressing AbiV.
To investigate whether MG1363 could mutate spontaneously to express AbiV, we isolated mutants that could grow in the presence of the virulent phage sk1 (4). Ten independent cultures of L. lactis MB112 (fluorouracil-resistant L. lactis MG1363, Δupp) (24) were grown exponentially at 30°C in M17 medium (37) supplemented with 0.5% glucose and then mixed with the 936-like phage sk1 at a multiplicity of infection of >1 in the presence of 10 mM CaCl2. The phage-infected bacterial cultures were then incubated for 10 min at room temperature before plating and incubation overnight at 36°C. BIMs that spontaneously gained resistance to sk1 were observed at a frequency of 10−8. Fifty-six colonies were picked randomly among the 10 independent cultures, purified, and cross-streaked (31) against virulent phages sk1, p2 (27), 712 (23), and the AbiV-insensitive mutant p2.1 (18). A BIM expressing AbiV is expected to be resistant to phages sk1 and p2 but sensitive to 712 and p2.1 due to the absence of a functional AbiV target gene (sav) in the latter two phages (18). Of the 56 BIMs, one had the expected efficiency of plaquing (EOP) (32) values. The EOP values for this BIM (named L. lactis JH-80) were 2 × 10−5 for phage p2, 0.75 for phage 712, and 0.8 for phage p2.1 (17). These values correspond to values obtained with L. lactis JH-20 and JH-32 (engineered strains expressing AbiV; see below) (17), suggesting that the natural BIM L. lactis JH-80 may now be expressing AbiV.
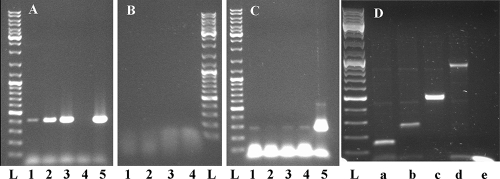
To verify that the phage resistance phenotype was indeed caused by the production of AbiV, the transcription of abiV in L. lactis JH-80 was investigated by using reverse transcriptase PCR (RT-PCR) as described previously (17). The RT-PCR was performed on RNA isolated from L. lactis JH-80 and also on RNA from L. lactis JH-20 (L. lactis MB112 containing abiV cloned into the expression vector pLC5) (17), JH-54 (L. lactis MB112 containing pLC5 without abiV) (17), and JH-32 (L. lactis MB112 expressing abiV due to the integration of pGhost9::ISS1) (17). While the levels of abiV mRNA in L. lactis strains JH-20 and JH-32 were the highest, JH-80 also showed transcription in comparison to undetectable transcription in L. lactis JH-54 (Fig. 1A). No PCR products were obtained in control experiments (omitting the RT enzyme), indicating that the RNA preparations were free of contaminating DNA (Fig. 1B). The above data demonstrate that L. lactis BIMs that spontaneously express AbiV, thereby conferring phage resistance to the cell without artificial genetic modifications, can be isolated.
FIG. 1.
RT-PCR assays were carried out on RNA isolated from various L. lactis strains. (A) Expression of AbiV. (B) Control experiment performed in the absence of RT. (C) Expression of gene tnp981. (A to C) Lane 1, L. lactis JH-80 (spontaneous BIM); lane 2, L. lactis JH-32 (insertional mutant expressing abiV); lane 3, L. lactis JH-20 (abiV gene cloned into an expression vector); lane 4, L. lactis JH-54 (empty vector); lane 5, positive PCR control using genomic DNA from L. lactis MG1363; lane L, GeneRuler ladder (Fermentas). (D) Detection of increasing lengths of abiV transcripts using the same forward primer in abiV and progressively more-distant reverse primers. (D) Lane a, forward and reverse (rev) primers located in abiV; lane b, rev primer 75 bp upstream of abiV; lane c, rev primer 310 bp upstream of abiV; lane d, rev primer 727 bp upstream of abiV; lane e, rev primer 1,079 bp upstream of abiV; lane L, GeneRuler ladder (Fermentas).
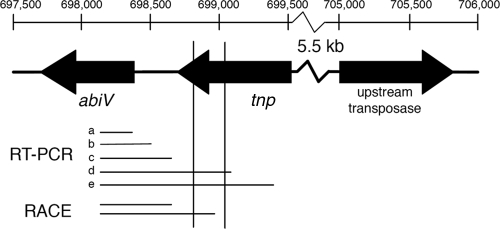
In an attempt to elucidate the mutation(s) in the BIM L. lactis JH-80 (AbiV+), we PCR amplified a 6,320-bp region (nucleotides [nt] 698427 to 704747 in GenBank AM406671) that included the upstream region of abiV. The PCR-amplified DNA fragments of both L. lactis JH-80 and reference strain L. lactis MB112 were sequenced on both strands. The L. lactis MB112 sequence was identical to the L. lactis MG1363 sequence. However, four point mutations were found in JH-80, located between 400 bp and 700 bp upstream of abiV but within the upstream gene tnp981 that codes for a putative transposase for the insertion sequence IS981F (Fig. 2). Analysis of the region containing the mutations revealed 100% nucleotide identity with the 3′ region (500 nt) of a transposase (nt 705075 to 705926 in GenBank AM406671) which shares 99% nucleotide identity with tnp981. This transposase is inversely oriented and located 6,671 bp upstream of abiV (Fig. 2). The exact match of the mutated region in tnp981 to this transposase and the absence of large inversions in the 6-kb region upstream of abiV strongly indicate that the mutations were caused by recombination between the two genes.
FIG. 2.
abiV and its upstream region, with tnp designating tnp981. The abiV transcription start site was shown with RT-PCR and RACE. Lines a to d represent the results of the four positive reactions and line e represents the result of the negative reaction shown in Fig. 1D. Two sizes of transcripts were obtained by using RACE. Vertical lines symbolize the range of observed mutations in tnp981 that were likely caused by a recombination event with the very similar, inversely oriented transposase located 5.5 kb upstream of tnp981. Numbers refer to nucleotide numbers in GenBank AM406671. Figure not drawn to scale.
No terminator structure could be identified between abiV and tnp981, leaving the possibility that promoter activity upstream or within tnp981 caused the increased expression of abiV in JH-80. We therefore determined the transcription level of tnp981 in L. lactis strains JH-80, JH-32, JH-20, and JH-54. We observed faint bands in all cases, indicating that tnp981 was transcribed at low levels in the four strains (Fig. 1C). However, nine other transposases that share 99% nucleotide similarity with tnp981 exist on the genome of L. lactis MG1363, thereby complicating the analysis of the expression of tnp981. Nevertheless, since the transcription levels of tnp981 were similar in all strains tested, we concluded that the increased promoter activity upstream of tnp981 was not the cause of the elevated levels of abiV in L. lactis JH-80.
To test whether the observed mutations in L. lactis JH-80 were involved in abiV transcription, we performed RT-PCR (Fig. 1D and Fig. 2). The resulting data indicate that the abiV transcripts start between 727 nt and 1,079 nt upstream of the gene. Determination of the abiV 5′ mRNA ends was performed by using the rapid amplification of cDNA ends (RACE) method (38). abiV mRNA ends were found at 320 nt, as well as at 583 nt, upstream of the gene. Since the abiV transcription is initiated upstream of the mutations in tnp981, the expression of abiV in L. lactis JH-80 was probably not caused by increased promoter activity. Instead, the mutations may increase the stability or some antitermination of the transcript from an existing weak promoter and hence increase AbiV expression in the BIM L. lactis JH-80. However, our data do not allow us to determine the reason for the weak induction of abiV gene expression in the mutant.
Transfer of abiV between two L. lactis strains.
Most known Abi mechanisms are plasmid encoded, though it has been argued that this overrepresentation could be due to the technical advantages of isolating plasmid-encoded Abi systems (5). As indicated above, the abiV gene is located on the chromosome of L. lactis MG1363 and possibly not readily transferable to another strain, as compared to plasmid-encoded systems. However, conjugation of chromosomal elements, facilitated by the chromosomally encoded sex-factor that is present in, among others, L. lactis MG1363 (35, 36), has been previously observed in L. lactis (14, 16). This genetic element permits the exchange of genetic material between lactococcal strains by chromosomal transfer and subsequent recombination. Therefore, we investigated whether we could take advantage of this ability to transfer an active abiV gene from one strain to another.
We used the donor strain L. lactis JH-32 (AbiV+, erythromycin resistant [Ermr], and fluorouracil resistant [FUr]) and the recipient strain L. lactis MG1614, an MG1363 derivative that is resistant to rifampin (rifampicin) (Rifr) and streptomycin (Strr) (15). In L. lactis JH-32, the abiV gene is activated by the vector pGhost9::ISS1 (22) inserted immediately upstream of abiV on the bacterial chromosome. We envisioned that if pGhost9::ISS1 was successfully transferred to L. lactis MG1614 by conjugation, abiV most likely would be as well, due to their close proximity on the chromosome of L. lactis JH-32 (17). Here, as a proof of concept, we used erythromycin resistance (from pGhost9::ISS1) as a selection marker.
Briefly, donor and recipient cells were grown separately on GM17 plates, subsequently recovered with saline (0.9% NaCl), and then mixed at ratios of 1:1, 1:3, and 1:9. The mixtures were immediately plated (0.1 ml plate−1) on GM17 and incubated in anaerobic jars overnight at 36°C. This incubation temperature was selected to avoid excision of the integrated pGhost9::ISS1 in L. lactis JH-32 (17, 22). Cells were recovered from GM17 plates with saline and incubated again anaerobically (48 h at 36°C) but on GM17 plates containing erythromycin (3 μg ml−1) and rifampin (100 μg ml−1). These two selection markers were used to select for L. lactis MG1614 (rifampin resistant) transconjugants that have acquired pGhost9::ISS1 (erythromycin resistance). The lactococcal colonies that grew on GM17 plates containing erythromycin and rifampin were then tested for their sensitivity to fluorouracil (0.3 μg ml−1) and their resistance to streptomycin (200 μg ml−1) and phages. By using this phage- and streptomycin-free selection approach, we virtually eliminated the risk of isolating false positives due to spontaneous mutations causing the resistance phenotype. Transconjugants with the phenotype, Rifr, Strr, FUs, Ermr, and phage resistance, are expected to be derivatives of MG1614 (Rifr Strr) that have acquired Ermr and phage resistance from JH-32 by chromosomal transfer.
Seven putative transconjugants (Ermr Rifr) were first isolated after two days of anaerobic incubation (36°C). Five of these mutants were derived from the donor and had acquired a spontaneous Rifr resistance. One mutant was derived from the recipient, with a spontaneous Ermr mutation. However, one mutant (L. lactis JH-83) had the expected phenotype (Rifr, Strr, FUs, Ermr, and phage resistance). In fact, phage p2 had an EOP of 10−4 on L. lactis JH-83. These data strongly suggested that L. lactis JH-83 is a transconjugant of L. lactis MG1614 that has acquired an activated abiV gene by conjugation and recombination. Next, we sequenced the rpsL gene of the four strains L. lactis MB112, MG1614, JH-32, and JH-83 and found a specific K-to-R amino acid substitution in rpsL (data not shown) that is known to cause streptomycin resistance in different bacterial species (33). The same mutation was found in L. lactis MG1614 and JH-83, while L. lactis MB112 and JH-32 had the wild-type sequence. Since rpsL was identical in JH-83 and MG1614 and streptomycin was not used as a selection marker, we concluded that indeed MG1614 was the parental origin of JH-83. To our knowledge, this is the first demonstration of a natural transfer of a chromosomally encoded phage resistance mechanism.
Taken altogether, the above data indicate that the chromosomally encoded abiV can be spontaneously activated and also naturally transferred to other lactococcal strains by conjugation. This study suggests that the search for novel chromosomally encoded Abi mechanisms should be revisited and that this may open up new ways to construct naturally phage-resistant strains for large-scale industrial applications.
Acknowledgments
This work was funded in part by a graduate scholarship from the Technical University of Denmark and a grant from the Proof-of-Concept Consortium to J.H. and a strategic grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada to S.M.
Footnotes
Published ahead of print on 13 March 2009.
REFERENCES
- 1.Allison, G. E., and T. R. Klaenhammer. 1998. Phage resistance mechanisms in lactic acid bacteria. Int. Dairy J. 8:207-226. [Google Scholar]
- 2.Bouchard, J. D., E. Dion, F. Bissonnette, and S. Moineau. 2002. Characterization of the two-component abortive phage infection mechanism AbiT from Lactococcus lactis. J. Bacteriol. 184:6325-6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher, I., E. Emond, E. Dion, D. Montpetit, and S. Moineau. 2000. Microbiological and molecular impacts of AbiK on the lytic cycle of Lactococcus lactis phages of the 936 and P335 species. Microbiology 146:445-453. [DOI] [PubMed] [Google Scholar]
- 4.Chandry, P. S., S. C. Moore, J. D. Boyce, B. E. Davidson, and A. J. Hillier. 1997. Analysis of the DNA sequence, gene expression, origin of replication and modular structure of the Lactococcus lactis lytic bacteriophage sk1. Mol. Microbiol. 26:49-64. [DOI] [PubMed] [Google Scholar]
- 5.Chopin, M. C., A. Chopin, and E. Bidnenko. 2005. Phage abortive infection in lactococci: variations on a theme. Curr. Opin. Microbiol. 8:473-479. [DOI] [PubMed] [Google Scholar]
- 6.Coffey, A., and R. P. Ross. 2002. Bacteriophage-resistance systems in dairy starter strains: molecular analysis to application. Antonie van Leeuwenhoek 82:303-321. [PubMed] [Google Scholar]
- 7.Daly, C., G. F. Fitzgerald, L. O'Connor, and R. Davis. 1998. Technology and health benefits of dairy starter cultures. Int. Dairy J. 8:195-205. [Google Scholar]
- 8.Daly, C., G. F. Fitzgerald, and R. Davis. 1996. Biotechnology of lactic acid bacteria with special reference to bacteriophage resistance. Antonie van Leeuwenhoek 70:99-110. [DOI] [PubMed] [Google Scholar]
- 9.Deveau, H., S. J. Labrie, M. C. Chopin, and S. Moineau. 2006. Biodiversity and classification of lactococcal phages. Appl. Environ. Microbiol. 72:4338-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durmaz, E., and T. R. Klaenhammer. 2007. Abortive phage resistance mechanism AbiZ speeds the lysis clock to cause premature lysis of phage-infected Lactococcus lactis. J. Bacteriol. 189:1417-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emond, E., E. Dion, S. A. Walker, E. R. Vedamuthu, J. K. Kondo, and S. Moineau. 1998. AbiQ, an abortive infection mechanism from Lactococcus lactis. Appl. Environ. Microbiol. 64:4748-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forde, A., and G. F. Fitzgerald. 1999. Bacteriophage defence systems in lactic acid bacteria. Antonie van Leeuwenhoek 76:89-113. [PubMed] [Google Scholar]
- 13.Garvey, P., C. Hill, and G. F. Fitzgerald. 1996. The lactococcal plasmid pNP40 encodes a third bacteriophage resistance mechanism, one which affects phage DNA penetration. Appl. Environ. Microbiol. 62:676-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasson, M., J. J. Godon, C. J. Pillidge, T. J. Eaton, K. L. Jury, and C. Shearman. 1995. Characterization and exploitation of conjugation in lactococcus lactis. Int. Dairy J. 5:757-762. [Google Scholar]
- 15.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gireesh, T., B. E. Davidson, and A. J. Hillier. 1992. Conjugal transfer in Lactococcus lactis of a 68-kilobase-pair chromosomal fragment containing the structural gene for the peptide bacteriocin nisin. Appl. Environ. Microbiol. 58:1670-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haaber, J., L. C. Fortier, S. Moineau, and K. Hammer. 2008. AbiV, a novel abortive phage infection mechanism on the chromosome of Lactococcus lactis subsp. cremoris MG1363. Appl. Environ. Microbiol. 74:6528-6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haaber, J., G. M. Rousseau, K. Hammer, and S. Moineau. 2009. Identification and characterization of the phage gene sav, involved in sensitivity to the lactococcal abortive infection mechanism AbiV. Appl. Environ. Microbiol. 75:2484-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrington, A., and C. Hill. 1991. Construction of a bacteriophage-resistant derivative of Lactococcus lactis subsp. lactis 425A by using the conjugal plasmid pNP40. Appl. Environ. Microbiol. 57:3405-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill, C., L. A. Miller, and T. R. Klaenhammer. 1990. Nucleotide sequence and distribution of the pTR2030 resistance determinant (hsp) which aborts bacteriophage infection in lactococci. Appl. Environ. Microbiol. 56:2255-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josephsen, J., and H. Neve. 2004. Bacteriophage and antiphage mechanisms of lactic acid bacteria, p. 295-350. In S. Salminen, A. von Wright, and A. Ouwehand (ed.), Lactic acid bacteria, microbiological and functional aspects. CRC Press, London, United Kingdom.
- 22.Maguin, E., H. Prevost, S. D. Ehrlich, and A. Gruss. 1996. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J. Bacteriol. 178:931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahony, J., H. Deveau, S. McGrath, M. Ventura, C. Canchaya, S. Moineau, G. F. Fitzgerald, and D. van Sinderen. 2006. Sequence and comparative genomic analysis of lactococcal bacteriophages jj50, 712 and P008: evolutionary insights into the 936 phage species. FEMS Microbiol. Lett. 261:253-261. [DOI] [PubMed] [Google Scholar]
- 24.Martinussen, J., and K. Hammer. 1994. Cloning and characterization of upp, a gene encoding uracil phosphoribosyltransferase from Lactococcus lactis. J. Bacteriol. 176:6457-6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGrath, S., G. F. Fitzgerald, and D. van Sinderen. 2002. Identification and characterization of phage-resistance genes in temperate lactococcal bacteriophages. Mol. Microbiol. 43:509-520. [DOI] [PubMed] [Google Scholar]
- 26.Moineau, S. 1999. Applications of phage resistance in lactic acid bacteria. Antonie van Leeuwenhoek 76:377-382. [PubMed] [Google Scholar]
- 27.Moineau, S., S. A. Walker, E. R. Vedamuthu, and P. A. Vandenbergh. 1995. Cloning and sequencing of LlaDCHI [corrected] restriction/modification genes from Lactococcus lactis and relatedness of this system to the Streptococcus pneumoniae DpnII system. Appl. Environ. Microbiol. 61:2193-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neve, H., A. Geis, and M. Teuber. 1987. Conjugation, a common plasmid transfer mechanism in lactic acid streptococci of dairy starter cultures. Syst. Appl. Microbiol. 9:151-157. [Google Scholar]
- 29.O'Connor, L., A. Coffey, C. Daly, and G. F. Fitzgerald. 1996. AbiG, a genotypically novel abortive infection mechanism encoded by plasmid pCI750 of Lactococcus lactis subsp. cremoris UC653. Appl. Environ. Microbiol. 62:3075-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero, D. A., P. Slos, C. Robert, I. Castellino, and A. Mercenier. 1987. Conjugative mobilization as an alternative vector delivery system for lactic streptococci. Appl. Environ. Microbiol. 53:2405-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross, W., S. H. Shore, and M. M. Howe. 1986. Mutants of Escherichia coli defective for replicative transposition of bacteriophage Mu. J. Bacteriol. 167:905-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders, M. E., and T. R. Klaenhammer. 1980. Restriction and modification in group N streptococci: effect of heat on development of modified lytic bacteriophage. Appl. Environ. Microbiol. 40:500-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sreevatsan, S., X. Pan, K. E. Stockbauer, D. L. Williams, B. N. Kreiswirth, and J. M. Musser. 1996. Characterization of rpsL and rrs mutations in streptomycin-resistant Mycobacterium tuberculosis isolates from diverse geographic localities. Antimicrob. Agents Chemother. 40:1024-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steele, J., and L. McKay. 1989. Conjugal transfer of genetic material in lactococci: a review. J. Dairy Sci. 72:3388-3397. [Google Scholar]
- 35.Stentz, R., M. Gasson, and C. Shearman. 2006. The Tra domain of the lactococcal CluA surface protein is a unique domain that contributes to sex factor DNA transfer. J. Bacteriol. 188:2106-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stentz, R., K. Jury, T. Eaton, M. Parker, A. Narbad, M. Gasson, and C. Shearman. 2004. Controlled expression of CluA in Lactococcus lactis and its role in conjugation. Microbiology 150:2503-2512. [DOI] [PubMed] [Google Scholar]
- 37.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tillett, D., B. P. Burns, and B. A. Neilan. 2000. Optimized rapid amplification of cDNA ends (RACE) for mapping bacterial mRNA transcripts. BioTechniques 28:448-456. [DOI] [PubMed] [Google Scholar]
- 39.Twomey, D. P., P. J. De Urraza, L. L. McKay, and D. J. O'Sullivan. 2000. Characterization of AbiR, a novel multicomponent abortive infection mechanism encoded by plasmid pKR223 of Lactococcus lactis subsp. lactis KR2. Appl. Environ. Microbiol. 66:2647-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]