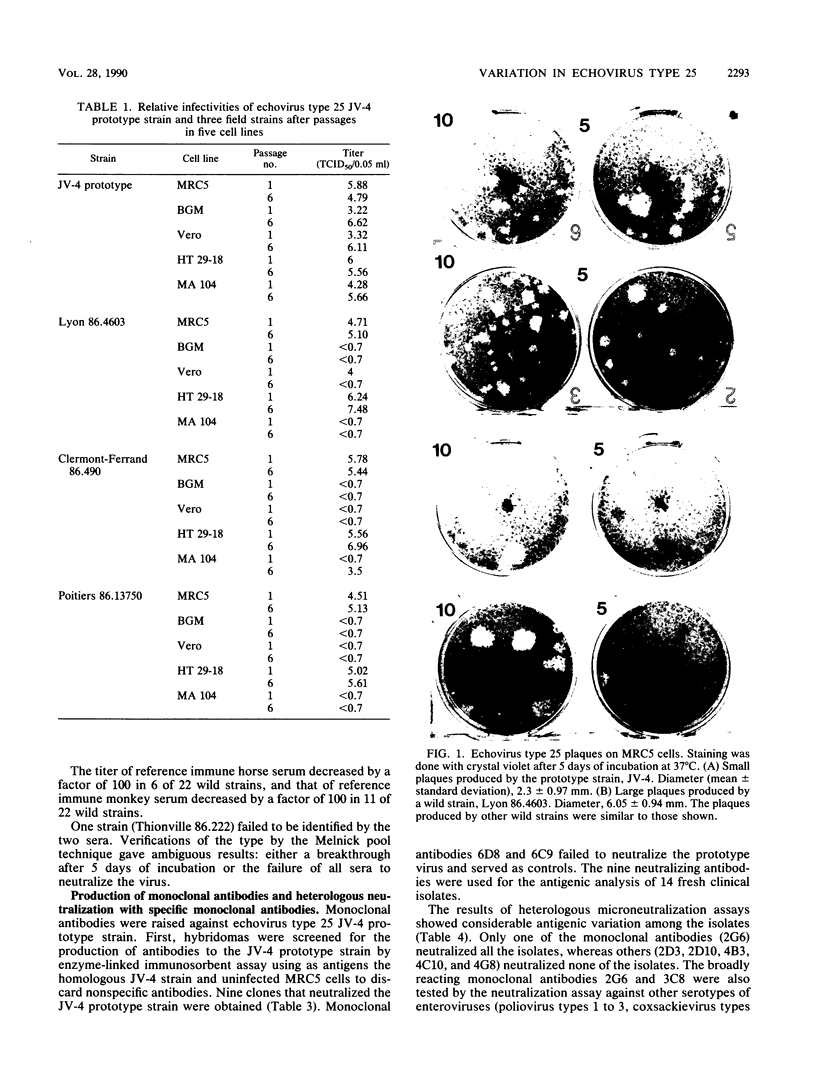
Abstract
We studied the biological and antigenic properties of wild strains of echovirus type 25 isolated in France between 1982 and 1987 and compared them with the JV-4 prototype strains isolated in 1957. The wild strains differed from the prototype strain in their cellular tropism. The prototype strain grew readily in five cell lines (MRC5, MA 104, Vero, BGM, and HT 29-18), while for wild strains MRC5 and HT 29-18 cells were the most sensitive and supported growth to high titres (between 4.5 and 7.4 50% tissue culture infective doses per 0.05 ml). Plaques produced by wild strains were larger (6.05 +/- 0.94 mm in diameter [mean +/- standard deviation]) than those of the prototype strain (2.3 +/- 0.97 mm in diameter) and heterogeneous, even after cloning by three terminal dilution passages, which suggested heterogeneous virus populations. Virus neutralization with polyclonal monovalent sera showed that wild strains were significantly less neutralized by two reference immune sera than the prototype strain was. Monoclonal antibodies were raised against the echovirus type 25 JV-4 prototype strain. Nine clones with neutralizing activity were identified. Heterologous neutralizations of 14 clinical isolates revealed highly conserved, moderately conserved, and poorly conserved epitopes. The natural isolates differed from the prototype strain in two to four epitopes and can be classified into four different groups. We concluded that echovirus type 25, like coxsackie- and polioviruses, consists of heterogeneous viral populations with respect to biological and antigenic properties. In term of viral diagnosis, it may become increasingly difficult to identify recently isolated strains because of their antigenic variation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash P., Leong W. A., Kennett M. L., Schnagl R. D. Neutralization kinetic analysis of echovirus 30 and coxsackievirus B4 strains revealed little antigenic variation amongst the echovirus strains. Aust J Exp Biol Med Sci. 1985 Apr;63(Pt 2):219–221. doi: 10.1038/icb.1985.24. [DOI] [PubMed] [Google Scholar]
- BELL E. J., GRIST N. R. ECHOVIRUS 25 INFECTIONS IN SCOTLAND, 1961-64. Lancet. 1965 Sep 4;2(7410):464–466. doi: 10.1016/s0140-6736(65)91423-6. [DOI] [PubMed] [Google Scholar]
- Cherry J. D., Bobinski J. E., Horvath F. L., Comerci G. D. Acute hemangioma-like lesions associated with ECHO viral infections. Pediatrics. 1969 Oct;44(4):498–502. [PubMed] [Google Scholar]
- Chonmaitree T., Ford C., Sanders C., Lucia H. L. Comparison of cell cultures for rapid isolation of enteroviruses. J Clin Microbiol. 1988 Dec;26(12):2576–2580. doi: 10.1128/jcm.26.12.2576-2580.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi M. Meningite da Echo 25. Minerva Med. 1978 Nov 24;69(57):3941–3943. [PubMed] [Google Scholar]
- Crainic R., Blondel B., Horaud F. Antigenic variation of poliovirus studied by means of monoclonal antibodies. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S535–S539. doi: 10.1093/clinids/6.supplement_2.s535. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Guidotti M. B. An outbreak of skin rash by echovirus 25 in an infant home. J Infect. 1983 Jan;6(1):67–70. doi: 10.1016/s0163-4453(83)95706-7. [DOI] [PubMed] [Google Scholar]
- HENIGST W. [On the occurrence of a variant of type 25 ECHO virus]. Arch Gesamte Virusforsch. 1963 Spring-Fall;12:706–717. [PubMed] [Google Scholar]
- Huet C., Sahuquillo-Merino C., Coudrier E., Louvard D. Absorptive and mucus-secreting subclones isolated from a multipotent intestinal cell line (HT-29) provide new models for cell polarity and terminal differentiation. J Cell Biol. 1987 Jul;105(1):345–357. doi: 10.1083/jcb.105.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELEN A. E., LESIAK J. M., LABZOFFSKY N. A. AN OUTBREAK OF ASEPTIC MENINGITIS DUE TO ECHO 25 VIRUS. Can Med Assoc J. 1964 Jun 13;90:1349–1351. [PMC free article] [PubMed] [Google Scholar]
- Kew O. M., Nottay B. K., Hatch M. H., Nakano J. H., Obijeski J. F. Multiple genetic changes can occur in the oral poliovaccines upon replication in humans. J Gen Virol. 1981 Oct;56(Pt 2):337–347. doi: 10.1099/0022-1317-56-2-337. [DOI] [PubMed] [Google Scholar]
- Kirkwood C. D., Kennett M. L., Schnagl R. D. Neutralization kinetic analysis showed that strains of echovirus types 7, 11 and 17, unlike those of echovirus type 30, varied antigenically over time in Melbourne, Australia. Acta Virol. 1988 May;32(3):267–271. [PubMed] [Google Scholar]
- Kogon A., Spigland I., Frothingham T. E., Elveback L., Williams C., Hall C. E., Fox J. P. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VII. Observations on viral excretion, seroimmunity, intrafamilial spread and illness association in coxsackie and echovirus infections. Am J Epidemiol. 1969 Jan;89(1):51–61. doi: 10.1093/oxfordjournals.aje.a120915. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LIM K. A., BENYESH-MELNICK M. Typing of viruses by combinations of antiserum pools. Application to typing of enteroviruses (Coxsackie and ECHO). J Immunol. 1960 Mar;84:309–317. [PubMed] [Google Scholar]
- MELNICK J. L. Problems associated with viral identification and classification in 1956. Ann N Y Acad Sci. 1957 Apr 19;67(8):363–382. doi: 10.1111/j.1749-6632.1957.tb46060.x. [DOI] [PubMed] [Google Scholar]
- McKinney R. E., Jr, Katz S. L., Wilfert C. M. Chronic enteroviral meningoencephalitis in agammaglobulinemic patients. Rev Infect Dis. 1987 Mar-Apr;9(2):334–356. doi: 10.1093/clinids/9.2.334. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Rennick V., Hampil B., Schmidt N. J., Ho H. H. Lyophilized combination pools of enterovirus equine antisera: preparation and test procedures for the identification of field strains of 42 enteroviruses. Bull World Health Organ. 1973;48(3):263–268. [PMC free article] [PubMed] [Google Scholar]
- Minor P. D., Schild G. C., Ferguson M., Mackay A., Magrath D. I., John A., Yates J. P., Spitz M. Genetic and antigenic variation in type 3 polioviruses: characterization of strains by monoclonal antibodies and T1 oligonucleotide mapping. J Gen Virol. 1982 Aug;61(Pt 2):167–176. doi: 10.1099/0022-1317-61-2-167. [DOI] [PubMed] [Google Scholar]
- Moore M. Centers for Disease Control. Enteroviral disease in the United States, 1970-1979. J Infect Dis. 1982 Jul;146(1):103–108. doi: 10.1093/infdis/146.1.103. [DOI] [PubMed] [Google Scholar]
- Moritsugu Y., Sawada K., Hinohara M., Tsuchiya K., Tagaya I., Hirayama M., Futaki T. An outbreak of type 26 Echovirus infection with exanthem in an infant home near Tokyo. Am J Epidemiol. 1968 May;87(3):599–608. doi: 10.1093/oxfordjournals.aje.a120850. [DOI] [PubMed] [Google Scholar]
- Patel J. R., Daniel J., Mathan V. I. A comparison of the susceptibility of three human gut tumour-derived differentiated epithelial cell lines, primary monkey kidney cells and human rhabdomyosarcoma cell line to 66-prototype strains of human enteroviruses. J Virol Methods. 1985 Dec;12(3-4):209–216. doi: 10.1016/0166-0934(85)90131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar B. S., Haspel M. V., McClintock P. R., Notkins A. L. High frequency of antigenic variants among naturally occurring human Coxsackie B4 virus isolates identified by monoclonal antibodies. Nature. 1982 Nov 25;300(5890):374–376. doi: 10.1038/300374a0. [DOI] [PubMed] [Google Scholar]
- Prabhakar B. S., Menegus M. A., Notkins A. L. Detection of conserved and nonconserved epitopes on Coxsackievirus B4: frequency of antigenic change. Virology. 1985 Oct 30;146(2):302–306. doi: 10.1016/0042-6822(85)90013-3. [DOI] [PubMed] [Google Scholar]
- REILLY C. M., STOKES J., Jr, HAMPARIAN V. V., HILLEMAN M. R. ECHO virus, type 25, in respiratory illness. Case report. J Pediatr. 1963 Apr;62:538–539. doi: 10.1016/s0022-3476(63)80011-6. [DOI] [PubMed] [Google Scholar]
- ROSEN L., JOHNSON J. H., HUEBNER R. J., BELL J. A. Observations on a newly recognized ECHO virus and a description of an outbreak in a nursery. Am J Hyg. 1958 May;67(3):300–310. doi: 10.1093/oxfordjournals.aje.a119936. [DOI] [PubMed] [Google Scholar]
- ROSEN L., KERN J., BELL J. A. OBSERVATIONS ON AN OUTBREAK OF INFECTION WITH A NEWLY RECOGNIZED ENTEROVIRUS (JV-4). Am J Hyg. 1964 Jan;79:1–6. doi: 10.1093/oxfordjournals.aje.a120357. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Merigan T. C. A useful quantitative semimicromethod for viral plaque assay. Proc Soc Exp Biol Med. 1973 Apr;142(4):1174–1179. doi: 10.3181/00379727-142-37202. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Melnick J. L., Wenner H. A., Ho H. H., Burkhardt M. A. Evaluation of enterovirus immune horse serum pools for identification of virus field strains. Bull World Health Organ. 1971;45(3):317–330. [PMC free article] [PubMed] [Google Scholar]
- Spigland I., Fox J. P., Elveback L. R., Wassermann F. E., Ketler A., Brandt C. D., Kogon A. The Virus Watch program: a continuing surveillance of viral infections in metropolitan New York families. II. Laboratory methods and preliminary report on infections revealed by virus isolation. Am J Epidemiol. 1966 May;83(3):413–435. doi: 10.1093/oxfordjournals.aje.a120595. [DOI] [PubMed] [Google Scholar]
- Webster A. D., Tripp J. H., Hayward A. R., Dayan A. D., Doshi R., Macintyre E. H., Tyrrell D. A. Echovirus encephalitis and myositis in primary immunoglobulin deficiency. Arch Dis Child. 1978 Jan;53(1):33–37. doi: 10.1136/adc.53.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]