Abstract
The impacts of 12 common food industry stresses on the single-cell growth probability and single-cell lag time distribution of Listeria monocytogenes were determined in half Fraser broth, the primary enrichment broth of the International Organization for Standardization detection method. First, it was determined that the ability of a cell to multiply in half Fraser broth is conditioned by its history (the probability for a cell to multiply can be decreased to 0.05), meaning that, depending on the stress in question, the risk of false-negative samples can be very high. Second, it was established that when cells are injured, the single-cell lag times increase in mean and in variability and that this increase represents a true risk of not reaching the detection threshold of the method in the enrichment broth. No relationship was observed between the impact on single-cell lag times and that on growth probabilities. These results emphasize the importance of taking into account the physiological state of the cells when evaluating the performance of methods to detect pathogens in food.
Listeria monocytogenes has been involved in severe food-borne outbreaks with high mortality rates. This pathogen is widespread in many environments (16) and can be isolated from a large variety of foods which are the major routes of infection in humans. Ready-to-eat foods that can support the growth of L. monocytogenes may pose a major risk for public health, and the European Union legislation generally requires absence in 25 g at the production stage as a food safety criterion for this type of food (4).
In food, L. monocytogenes is often affected by one or more stresses caused by a variety of processing treatments, including heating, freezing, and exposure to acids and to high osmotic pressures (15, 25, 29, 39). Recovering stressed L. monocytogenes from food is of great importance in food safety since sublethally injured bacteria may repair themselves under suitable conditions and regain or even increase their pathogenicity (19, 30).
The injury of microbial cells has two major consequences for pathogen behavior in enrichment broths. First, injured cells become sensitive to selective components present in enrichment broths to which they normally show resistance (9, 10, 11, 42). Therefore, some cells of the stressed bacterial population do not initiate growth in enrichment broth, eventually resulting in an inefficient detection of pathogenic bacteria in food samples (50). This phenomenon can explain results obtained in several studies showing the effect of inoculum size on the growth limits of bacterial populations (26, 27, 37). Second, due to repair time, stressed cells show a longer lag phase than do healthy cells (5, 7, 37). This situation results in a true risk of not reaching the bacterial concentration necessary for the detection of the pathogen (in the range of 102 to 104 CFU ml−1) within the enrichment duration.
The recent development of gene-based or immunologically based procedures, such as PCR, gene probes, and enzyme-linked immunosorbent assay, has facilitated the development of more-rapid methods which can identify positive samples in considerably shorter time periods. Nevertheless, these relatively rapid tests also require efficient enrichment steps to increase target organism numbers to detectable levels.
At the moment of pathogen detection, low numbers of sublethally injured cells, as often encountered in naturally contaminated foods, show a wide distribution of lag-phase durations (45) and may not be able to multiply in broth containing selective components (11, 42). The challenge of the enrichment stage is to obtain appropriate enrichment conditions (2) which will favor pathogen resuscitation and limit the food microflora growth.
In our study, we have focused on the primary enrichment phase of the International Organization for Standardization 11290-1 L. monocytogenes detection method (3), i.e., the half Fraser broth (1/2FB). The objectives were to investigate the impact of 12 different stresses on the single-cell growth probability and single-cell lag time of L. monocytogenes in 1/2FB. The intraspecific variability and the impact of food components and background microflora on single-cell growth probability were also studied.
MATERIALS AND METHODS
Strains and culture conditions.
Six strains of L. monocytogenes were used in the study. They were maintained at −20°C on cryobeads (AES Laboratoire, Combourg, France). The strains were selected to ensure large-scale biological diversity: (i) LM14 (serotype 4b, meat industry environmental isolate), which is the reference strain for the French program in predictive microbiology Sym'Previus; (ii) INRA101 (serotype 4b, meat industry environmental isolate); (iii) UNIR100 (serotype 4b, dairy product isolate); (iv) ADQP101 (seafood isolate); (v) Scott A (serotype 4b, clinical isolate); and (vi) EGDe (CIP107776, serotype 1/2a, animal isolate).
Prior to each experiment, a cryobead was incubated at 30°C for 24 h in tryptone soy broth supplemented with 0.6% yeast extract (LEB; Oxoid, Unipath Ltd., Basingtoke, United Kingdom) (TSBye). The first bacterial culture was diluted to obtain an initial bacterial concentration of approximately 103 cells ml−1 in TSBye. That initial bacterial suspension was then incubated in TSBye at 25°C for 18 h to obtain 108 cells ml−1 in the exponential growth phase.
Stress experiments.
Twelve different stresses were applied to L. monocytogenes LM14 cultures in the exponential growth phase. First, before each stress, once TSBye had been eliminated by centrifugation (5,000 × g, 10 min, 4°C), L. monocytogenes cells were washed in 0.85% NaCl (Prolabo, Paris, France) (diluent) at 25°C and pH 7 and centrifuged again (5,000 × g, 10 min, 4°C). This stage did not modify the physiological state of the cells since centrifugation did not have any influence on the single-cell lag time or on the single-cell growth probability of L. monocytogenes cells in the exponential growth phase (data not shown). Second, the supernatant was discarded and the cells were treated in the following ways: (i) for mineral acid stress, in diluent at 25°C adjusted to pH 3 with HCl (Prolabo) for 34 min (1:1,000 of a 1 M solution); (ii) for the first organic acid stress with lactic acid, in diluent at 25°C adjusted to pH 4.2 with lactic acid (2.9 × 10−5 M; Prolabo) for 24 h; and (iii) for the second one with acetic acid, in diluent at 25°C adjusted to pH 4.2 with acetic acid (2.3 × 10−4 M; Prolabo) for 24 h; (iv) for alkali stress, in diluent at 25°C adjusted to pH 12 with NaOH (Prolabo) for 25 min (1:100 of a 1 M solution); (v) for heat stress, diluted (1:100) in diluent at 55°C for 2.5 min; (vi) for freezing stress, in diluent dispatched in 1-ml aliquots directly placed at −20°C for 48 h; (vii) for osmotic stress, in 25% (wt/vol) NaCl solution at 30°C for 48 h; (viii) for the first disinfectant stress, in diluent at 25°C supplemented with 10 mg liter−1 benzalkonium chloride (BAC) (Sigma-Aldrich, St. Louis, MO) for 12 min; (ix) for the second one, in diluent at 25°C supplemented with peracetic acid (Acros Organics, Geel, Belgium) at 200 ppm for 15 min; (x) for phenol stress, in diluent at 25°C with phenol at 2.3 ppm for 48 h; (xi) for nitrite stress, in diluent at 25°C with sodium nitrite (NaNO2) (Fisher Scientific, Loughborough, United Kingdom) at 200 ppm for 52 h; (xii) for starvation stress, a second washing in diluent to ensure the complete elimination of nutrients and recovery of the cells in diluent at 25°C for 24 h.
The intraspecific variability of L. monocytogenes was studied by applying the starvation and osmotic stresses to the six strains.
The time of exposure to each stress was the time necessary to obtain a population reduction of 1.5 log10 CFU ml−1 as determined by enumeration on tryptone soy agar supplemented with 0.6% yeast extract (AES Laboratoire) (TSAye). The above population reduction was chosen because of the shape of the inactivation curve observed for the starvation stress. For this stress, a higher reduction would have been more difficult to achieve.
The amount of injury defined by the percentage of injured cells in the bacterial population (% injured) was obtained from the differential enumerations on selective (PALCAM agar; AES Laboratoire) and unselective (TSAye) media.
Determination of single-cell growth probabilities.
Stressed cells were simultaneously diluted in TSBye and in 1/2FB (AES Laboratoire; iron ammonium citrate added just before the experiment). Dilutions were performed to obtain between 10 and 90% of wells showing growth of two 96-microwell plates. The microplates were covered with Parafilm to avoid dehydration and then incubated at 30°C for 48 h. Wells exhibiting turbidity in TSBye and those exhibiting a blackening in 1/2FB due to esculinase activity were considered positive for growth.
Assuming a Poisson distribution of cells in the wells (17), the cell concentration in TSBye, cTSBye, was calculated from the observed proportion of positive wells, wp,TSBye: 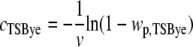 , where v is the volume of broth dispatched in wells. The hypothesis of Poisson distribution of cells in the wells was confirmed for some conditions by inoculating serial dilutions in microplates and observing the number of positive wells (data not shown). TSBye at 30°C was considered the most favorable condition for the growth of stressed cells; thus, the observed concentrations of growing cells in TSBye at 30°C were assumed to be the concentrations of viable cells.
, where v is the volume of broth dispatched in wells. The hypothesis of Poisson distribution of cells in the wells was confirmed for some conditions by inoculating serial dilutions in microplates and observing the number of positive wells (data not shown). TSBye at 30°C was considered the most favorable condition for the growth of stressed cells; thus, the observed concentrations of growing cells in TSBye at 30°C were assumed to be the concentrations of viable cells.
The proportion of positive wells in 1/2FB, wp,1/2FB, depends on the number of cells in the wells (assumed to follow a Poisson distribution) and on the probability for a cell to initiate growth, p:
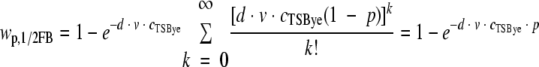 |
where d is the ratio between dilution factors applied to suspensions inoculated in TSBye and 1/2FB (for small cell growth probability, d was higher than 1). The number of cells able to grow in 1/2FB wells follows a Poisson distribution with parameter n·p, where n is the expected number of cells per well.
The single-cell growth probability, p, is thus equal to:
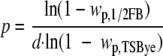 |
Experiments were carried out in at least three replicates. In order to check whether the cells had definitively lost their ability to grow in such conditions, the incubation of microwell plates was continued for 14 days at 30°C. No significant increase of the number of positive wells was observed after this extended incubation period.
Impact of the food indigenous microflora and food components on cell growth probabilities.
The effects of the food microflora and food components on single-cell growth probabilities were explored in two food models: one meat model simulating the enrichment of a fermented meat product, e.g., dried sausages, and a dairy model simulating the enrichment of a red-smear soft cheese (rind excepted).
To simulate dried sausage microflora, the two following predominant bacterial species were used: Lactobacillus sakei cF43K (INRA, Jouy-en-Josas, France) and Staphylococcus xylosus S03-188 (INRA, Theix, France). The two bacterial strains were introduced in 1/2FB in the stationary phase at an initial concentration of approximately 107 CFU ml−1. The main food component capable of modifying L. monocytogenes behavior present in fermented meat products is sodium nitrite. The amount studied was the maximum allowable quantity in this type of product (150 mg kg−1 corresponding to 15 mg liter−1 in 1/2FB). To assess the impact of the food matrices on single-cell growth probability, two stresses linked to the product environment were studied: one with a strong impact on growth probability and one with a low impact (previously determined). For the fermented meat product, the stress with strong impact studied was BAC stress, BAC being used for the disinfection of factories processing meat products. The stress with low impact was starvation stress.
To simulate the microflora present in the core of red-smear soft cheese, the following species of lactic acid bacteria was used: Lactococcus lactis CNRZ 483 (INRA, Grignon, France), at a concentration of approximately 108 CFU ml−1 in 1/2FB. The main component which may influence the growth of L. monocytogenes is the lactic acid produced by bacteria during the fermentation step. The pH of the matrix at the beginning of maturation is 5.0 (38). The stress with strong impact studied was heat stress, representative of the stress encountered during milk pasteurization; the stress with low impact was starvation stress.
We also studied the impact of four additional microorganisms isolated from food samples and able to grow in 1/2FB: (i) Staphylococcus cohnii isolated from garlic sausages, (ii) Pseudomonas fluorescens and (iii) Staphylococcus xylosus isolated from dried sausages, and (iv) Leuconostoc spp. isolated from liver pâté. The identification was performed with the Omnilog ID system (Biolog Inc., Hayward, CA) or with biochemical identification systems (Biomérieux, Marcy l'Etoile, France). Two stresses were studied: one with low impact, the starvation stress, and one with high impact, the osmotic stress.
In order to differentiate L. monocytogenes growth from indigenous microflora growth, the TSBye was supplemented with esculin (Merck, Darmstadt, Germany) and iron(III) citrate ammonium (Sigma-Aldrich) to obtain blackening when Listeria cells multiply. It was assumed that Listeria growth was systematically accompanied by a broth blackening (blackening obtained at approximately 108 cells ml−1). The addition of esculin and iron(III) citrate ammonium to TSBye broth did not modify single-cell growth probability (data not shown).
Microflora and food components were added singly and together in enrichment broths to distinguish the effects of food matrix on single-cell growth probability. Experiments were carried out in at least three replicates.
Determination of single-cell lag times.
Single-cell lag times of cells able to grow in 1/2FB were estimated for strain LM14 and for all stresses except heat stress. Turbidity growth curves were generated with an automatic Bioscreen C (Labsystem France SA, Les Ulis, France) reader. From L. monocytogenes cells in the exponential phase and stressed cells, serial dilutions were performed in tryptone salt broth (AES Laboratoire) in order to reach in the final dilution a maximum concentration of 1.4 growing cells ml−1 in 1/2FB preheated at 30°C. Wells of two Bioscreen plates were inoculated with 300 μl of this suspension to obtain a target value of 0.42 growing cell well−1, increasing the probability of having only one single growing cell in wells showing growth. Assuming a Poisson distribution of the cells in the wells, the maximum concentration of 0.42 growing cell well−1 corresponds to a maximum of 35% of wells showing growth and made it possible to estimate that less than 20% of these wells contained more than one growing cell. The plates were then placed in the Bioscreen C reader at the incubation temperature of 30°C. The optical density was monitored at 600 nm. Measurements were performed every 10 min, and the plates were shaken at medium intensity for 30 s min−1. For each stress experiment, 150 wells were kept for stressed cells and the 50 left over were dedicated to L. monocytogenes in the exponential growth phase. Each experiment was replicated at least three times.
Single-cell lag times were estimated from the detection time, Td, required for the microbial population to generate an 0.05 increase of the initial baseline value of the optical density. Using the standardized procedure described by Guillier et al. (23), the between-experiment variability was corrected and the Td data sets were grouped together. Single-cell lag times of growing stressed cells, τi, were then deduced from Td,i by subtracting the mean of the detection times of wells inoculated with cells in the exponential growth phase, Tde, as described by Guillier and Augustin (22): τi = Td,i − Tde. This method is based on the assumption that the single-cell lag times in different regrowth conditions for cells issued from a culture in the exponential growth phase are not different from zero (6).
Fitting statistical distributions to the observed single-cell lag time distributions.
Four statistical distributions were tested to describe data sets of single-cell lag time distributions. These distributions have already been used in the literature to describe single-cell lag time distributions: the gamma distribution (13, 18, 23, 34, 35), the Weibull distribution (18, 24, 44), the lognormal distribution (22, 28, 31), and the extreme value type II distribution with a shape parameter fixed to 5 (EVIIb) (22). The parameters of the five distributions were computed using maximum likelihood (MLE subroutine of Matlab 7.6; Mathworks). To compare the fitting of theoretical distributions to observed data sets, we used the Wallace-Boulton-Schwarz criterion (BIC criterion). The BIC criterion (41) measures the model efficiency for describing the data in taking into account the degree of parameterization of the distribution and was calculated for each data set using the following equation: BIC = −2·L(y) + q·log(m) with the log maximum likelihood, L(y), equal to
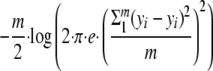 |
where yi are the observed data, ŷi are the adjusted values, m is the number of observations, and q is the number of parameters of the distribution. The best distribution was the one minimizing the BIC value.
RESULTS
Influence of stresses on single-cell growth probability.
The results illustrate the impact of bacterial stresses on single-cell growth probability and show that the proportion of cells able to grow in 1/2FB is dependent on the stress encountered by cells (Table 1 ). By using the single-cell growth probability to compare the impacts of the 12 stresses tested, the following classification was obtained (ascending impact): acetic acid, starvation, nitrite, lactic acid, freezing, HCl, phenol, osmotic stress, NaOH, BAC, heat, and peracetic acid stress.
TABLE 1.
Probability of detecting 25-g food samples as positive for Listeria monocytogenes after an enrichment phase in 1/2FB at 30°C according to the initial contamination, the enrichment length, and the physiological state of contaminating cellsa
| Stress encountered | p | Mean of τ (h) | Probability according to level of food contamination (in 25 g) and length of enrichment
|
|||||
|---|---|---|---|---|---|---|---|---|
| 25 CFU
|
5 CFU
|
1 CFU
|
||||||
| 16 h | 24 h | 16 h | 24 h | 16 h | 24 h | |||
| Exponential growth phase | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Acetic acid (pH 4.2, 25°C, 24 h) | 0.92 | 6.2 | 1 | 1 | 0.81 | 1 | 0.19 | 0.85 |
| Starvation (25°C, 24 h) | 0.91 | 2.3 | 1 | 1 | 1 | 1 | 0.80 | 0.91 |
| Nitrite (200 ppm, 25°C, 52 h) | 0.87 | 2.3 | 1 | 1 | 1 | 1 | 0.77 | 0.87 |
| Lactic acid (pH 4.2, 25°C, 24 h) | 0.52 | 9.6 | 0.69 | 1 | 0.12 | 0.92 | 0.02 | 0.39 |
| Freezing (−20°C, 48 h) | 0.42 | 3.6 | 1 | 1 | 0.81 | 0.93 | 0.27 | 0.42 |
| HCl (pH 3, 25°C, 34 min) | 0.39 | 2.6 | 1 | 1 | 0.86 | 0.91 | 0.32 | 0.39 |
| Phenol (2.3 ppm, 25°C, 48 h) | 0.27 | 4.3 | 0.99 | 1 | 0.52 | 0.78 | 0.13 | 0.26 |
| NaCl (25%, 30°C, 48 h) | 0.24 | 7.9 | 0.59 | 1 | 0.12 | 0.68 | 0.02 | 0.21 |
| NaOH (pH 12, 25°C, 25 min) | 0.15 | 5.7 | 0.73 | 0.98 | 0.19 | 0.54 | 0.04 | 0.14 |
| BAC (10 mg liter−1, 25°C, 12 min) | 0.11 | 1.5 | 0.94 | 0.94 | 0.43 | 0.44 | 0.11 | 0.11 |
| Heat (55°C, 2.5 min) | 0.05 | NDb | ||||||
| Peracetic acid (200 ppm, 25°C, 15 min) | 0.05 | 4.4 | 0.47 | 0.72 | 0.11 | 0.22 | 0.02 | 0.05 |
The detection threshold is set to 103 cells ml−1.
ND, not determined.
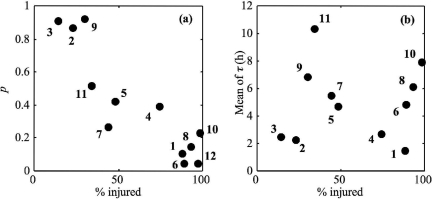
Figure 1a shows that the proportion of cells able to grow in 1/2FB decreased with the amount of cell injury (determined from differential plate counts on TSAye and PALCAM agar): the greater the cell injury, the lower the single-cell growth probability.
FIG. 1.
Plots of single-cell growth probability (a) and of means of single-cell lag times (b) of L. monocytogenes in 1/2FB at 30°C versus the percentages of injury resulting from BAC (1), nitrite (2), starvation (3), HCl (4), freezing (5), peracetic acid (6), phenol (7), NaOH (8), acetic acid (9), NaCl (10), lactic acid (11), and heat (12) stresses.
Intraspecific variability of single-cell growth probability.
The intraspecific variability between different strains of L. monocytogenes was studied for one stress with a high impact on single-cell growth probability, osmotic stress, and one with a low impact, starvation stress. These physiological states were furthermore chosen because they are not specific to a category of food products.
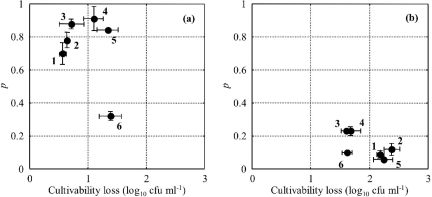
The initial bacterial concentrations obtained before applying the stressing condition were not significantly different between the six strains considered (data not shown). The ability to initiate growth in 1/2FB for cells stressed by starvation was dependent on the strain studied. The results presented in Fig. 2a show that the EGDe strain had a different behavior after starvation injury than did the other strains studied: EGDe cells had a lower probability to grow in 1/2FB (0.32 versus 0.78 on average for other strains). On the other hand, after osmotic stress, the behaviors of the different strains were similar (Fig. 2b). The probability for an L. monocytogenes cell to grow in 1/2FB after osmotic stress was 0.14 on average with a standard deviation of 0.08.
FIG. 2.
Plot of single-cell growth probability in 1/2FB at 30°C versus the loss of cultivability on TSAye for Listeria monocytogenes strains INRA101 (1), ADQP101 (2), UNIR100 (3), LM14 (4), Scott A (5), and EGDe (6) after starvation stress (a) and osmotic stress (b). Points and error bars represent the means and standard deviations of parameters for replicated experiments, respectively.
Impact of indigenous microflora and food components on single-cell growth probability.
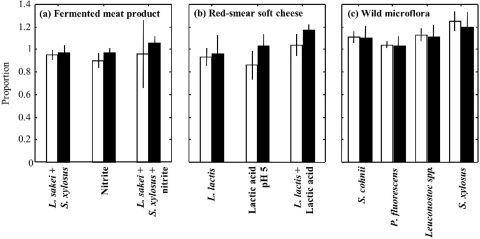
The results obtained in fermented meat product and red-smear soft cheese core models, with or without microflora added, are presented in Fig. 3. The presence of microflora and/or food components during the enrichment phase seemed to have no negative effect on the single-cell growth probability, regardless of the impact of the stress encountered by cells (strong impact for heat and BAC stresses and weak impact for starvation stress).
FIG. 3.
Proportions of Listeria monocytogenes cells able to initiate growth, after a stress with low impact, the starvation stress (black bars), or after one with high impact (white bars)—BAC stress (a), heat stress (b), or osmotic stress (c)—in 1/2FB at 30°C supplemented with microflora and/or food components to simulate the enrichment phase of fermented meat product (a) and core of red-smear soft cheese (b) and to study the effect of wild microflora isolated from various food samples and multiplying in 1/2FB (c). Error bars represent the between-experiment standard deviations.
Influence of stresses on single-cell lag time for cells able to grow in 1/2FB.
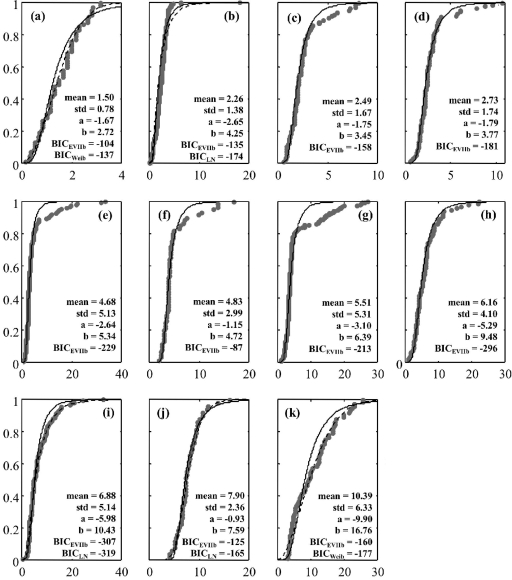
Theoretical distributions were fitted to single-cell lag values. EVIIb seemed to fit well with the major part of lag time distributions of stressed cells. Using the BIC criterion, the EVIIb distribution was the best-fitting distribution for 6 of the 11 physiological states tested (average BIC value of −181 against −142 for the gamma distribution, −124 for the Weibull distribution, and −171 for the lognormal distribution). Figure 4 shows the fitting of this distribution to experimental data. For nitrite, acetic acid, and osmotic stresses, the lognormal distribution was the best fitting, while for BAC and lactic acid stresses the Weibull distribution exhibited the smallest BIC value. For these five stresses, the differences of fitting obtained between EVIIb and the best-fitting distributions were, however, very small (Fig. 4). A good fit was obtained for all stresses with the EVIIb distribution, making it possible to use a distribution with two parameters linked to the mean and the standard deviation of the single-cell lag times (22).
FIG. 4.
Observed cumulative distributions (•) of single-cell lag times of Listeria monocytogenes in 1/2FB at 30°C with fitted EVIIb distributions (solid lines) and best-fitting distributions other than EVIIb (dashed lines for lognormal [LN] or Weibull [Weib] distributions). Cells were previously stressed by BAC (a), nitrite (b), starvation (c), HCl (d), freezing (e), peracetic acid (f), phenol (g), NaOH (h), acetic acid (i), NaCl (j), and lactic acid (k). x axes show τi (h); y axes show cumulative distribution function.
The mean of single-cell lag times was not correlated with the proportion of injured cells, inferred from the differential enumeration between selective and unselective agar media (Fig. 1b), as also observed by Guillier (21).
DISCUSSION
The results obtained in this study confirm the substantial impact of bacterial stresses on single-cell growth probability and show that the proportion of cells which grow is dependent on the stress encountered.
Figure 1a shows that there is a relationship between the single-cell growth probability and the percentage of injured cells in the bacterial population (determined from differential plate counts on TSAye and PALCAM agar). Indeed, the higher the percentage of injured cells, the greater the cell's difficulty in initiating growth in the enrichment broth. We can assume that the inability of cells to multiply both in PALCAM agar and in 1/2FB is due to the presence of the same selective components: acriflavine, lithium chloride, and the antibiotics (ceftazidime and polymyxin B for PALCAM agar and nalidixic acid for 1/2FB).
On the basis of the observed impact of stress on cell growth probability, it can be estimated that, if only a few injured cells of L. monocytogenes are present in a food sample, there is a real risk that these cells may not succeed in initiating growth, resulting in false-negative detection. The probability of detection of L. monocytogenes for a 25-g product analysis has been estimated according to the stress encountered and the initial contamination level (for a detection threshold set to 103 cells ml−1). For example, for a contamination of a 25-g food sample with five cells of the microorganism stressed by peracetic acid, which exhibited one of the strongest impacts on single-cell growth probability, the probability of detecting the sample as positive is only 22% after a 24-h enrichment (Table 1).
Regarding the intraspecific variability of single-cell growth probability, the EGDe strain had a different behavior than that of the other strains after starvation stress. Its single-cell growth probability was significantly lower than those obtained with the five other strains tested, and this result was correlated with the highest loss of cultivability on TSAye (Fig. 2a). Since we observed for other conditions that the greater the loss of cultivability, the lower the single-cell growth probability (data not shown), we can assume that this lower cell growth probability is certainly the consequence of the higher sensitivity to starvation stress. Besides, the origin of this higher sensitivity could be the strain serotype: indeed, strain EGDe was the only serotype 1/2a strain, while the other strains were serotype 4b. For osmotic stress, we observed strong homogeneity for the loss of cultivability and single-cell growth probability between the six strains (Fig. 2b). Adrião et al. (1) observed obvious differences between the responses of four strains of L. monocytogenes to acid and salt stresses. Vermeulen et al. (49) also observed extensive variability in the ability of 11 strains of L. monocytogenes to initiate growth in nutrient broth containing acetic acid near the growth/no growth interface. Some intraspecific variability in response to injury may thus exist for L. monocytogenes.
The presence of food components and indigenous microflora in the primary enrichment broth had no significant impact on the single-cell growth probability of stressed L. monocytogenes cells. The ability of cells to initiate growth even seemed to improve, especially when wild microflora isolated from food enrichment broths was added (Fig. 3c). This observation was somewhat surprising since, in the literature, the study of background microflora effects on Listeria growth has mostly described microbial interactions resulting in either bacteriostatic or bactericidal effects, attributed to the antagonistic actions of metabolic products (e.g., bacteriocins) or to the changes in the physicochemical environment (14, 32). This improvement is perhaps due to a decreasing activity of the selective agents against L. monocytogenes cells when indigenous microorganisms are present at high concentrations in the enrichment broth. The 1/2FB contains nalidixic acid, acriflavine, and lithium chloride as selective agents. These reagents inhibit RNA (12, 36) and DNA (8) synthesis and compete with divalent cations (33, 48). They might have no effect on unstressed bacteria, but some studies show an impact on Listeria growth (9). When cells are injured, their membranes may be disrupted and the entry of antibiotics and selective agents into cells is facilitated. As the introduction of microflora multiplies the number of bacterial cells by 108, the quantity of selective agent molecules by cell is considerably less important. This reduction could explain the improvement in cell growth probability of stressed cells in 1/2FB.
Single-cell lag time distributions of stressed growing cells showed that each treatment causes a specific increase in lag time average and variability. Guillier et al. (23) studied the impact of stress on cell lag time distributions of the same strain of L. monocytogenes, LM14, in TSBye. The sorting obtained in mean or in variability of single-cell lag times was slightly different from our sorting. This difference can be explained by the fact that the stress treatments were slightly different. For example, for lactic acid stress, Guillier et al. (23) used a less acid solution (pH 4.6 versus pH 4.2 in this study) and they classified this stress as a stress with a low impact (in mean and in variability), whereas we observed this stress as the highest-impact stress studied.
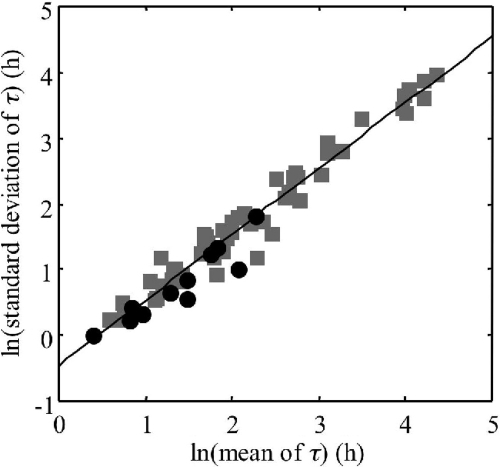
Like Guillier and Augustin (22), we observed that for most of the stresses studied (6 out of 11), the EVIIb was the best distribution to fit the observed single-cell lag time distributions. We also observed that the logarithms of the standard deviations, D(τ), and the means, E(τ), of the single-cell lag times were linearly correlated (Fig. 5). By grouping our data with the 54 data sets obtained by Guillier and Augustin (22) for different physiological states, strains, and growth conditions in TSBye and using linear model II regression (major axis regression) (43), we obtained 1.023 for the slope (95% confidence interval, 0.970 to 1.078) and −0.514 for the intercept.
FIG. 5.
Relationship between means and standard deviations of the single-cell lag times of Listeria monocytogenes. Shown are data obtained in this study in 1/2FB at 30°C and calculated from the EVIIb parameter values (•) and data obtained by Guillier and Augustin (22) (▪) for 54 different physiological states, growth conditions, and strains in TSBye. The solid line is the major axis regression line. (Adapted from reference 22 with permission from Elsevier.)
Assuming that the parameter k, quantifying the physiological state of a cell, which is equal to μmax·τ, is constant for a given physiological state whatever the growth conditions (46), the current relation between E(τ) and D(τ) (slope not different from 1) allowed us to observe, like Guillier and Augustin (22), that the ratio D[k]/E[k] of the standard deviation and the mean of the physiological parameter ki was relatively constant whatever the stress encountered. We obtained a mean value of 0.56 and a standard deviation of 0.12 for this ratio by grouping our 11 values with the 54 values of Guillier and Augustin (22).
This part of our study makes it possible to assess the time required for stressed cells to begin to multiply and to reach the detectable concentration in the enrichment broth. For a low level of food contamination, such as one cell per 25 g, the length of 24 h is sometimes not enough to permit the L. monocytogenes population to reach the detectable concentration (detection threshold set to 103 cells ml−1) when performing a 25-g product analysis. For example, after lactic acid stress, only 75% of the contaminated samples with one growing cell will be detected as positive. Therefore, shortening the enrichment length as proposed in some papers (20, 47) will have a negative impact on the performance of the detection method for strongly stressed bacteria.
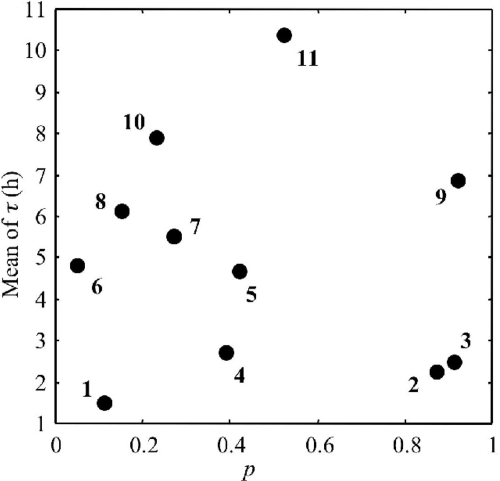
There was no relationship between single-cell growth probability and the mean of single-cell lag time distributions for a certain physiological state (Fig. 6). Thus, it is unfeasible to deduce one parameter from the other. For example, cells stressed with BAC exhibited one of the smallest increases of the mean of single-cell lag times, while a very high impact was observed on the single-cell growth probability with only 11% of cells multiplying in 1/2FB (Table 1). BAC is a disinfectant used in meat industries, whose mechanism of action is thought to be due to the disruption of intermolecular interactions (40). This disinfectant can cause dissociation of membrane bilayers and enzymes, controlling respiratory and metabolic cellular activities, which are particularly susceptible to this deactivation. We can assume that in the bacterial population either cells are affected by the disinfectant, cellular permeability is disrupted, and the cell is unable to multiply in selective broths or cells are not sensitive to this disinfectant and, therefore, the single-cell lag time is almost unchanged.
FIG. 6.
Plot of means of single-cell lag times versus the single-cell growth probability of Listeria monocytogenes in 1/2FB at 30°C resulting from BAC (1), nitrite (2), starvation (3), HCl (4), freezing (5), peracetic acid (6), phenol (7), NaOH (8), acetic acid (9), NaCl (10), lactic acid (11), and heat (12) stresses.
Table 1 illustrates the impact of cell physiological state, including single-cell growth probability and cell lag time distribution, on the probability of detecting contaminated 25-g samples as positive. For lactic acid stress, which had the highest mean for single-cell lag times, a high impact of the enrichment length was observed: for a contamination of five cells per 25 g, the probability of detection was 92% after an enrichment duration of 24 h but only 12% after a 16-h enrichment phase. For BAC stress, only a high impact of the contamination level was observed. This was, in part, resulting from low cell growth probability and also from low impact of this disinfectant treatment on single-cell lag times. Indeed, the enrichment length had no impact on the probability of detection. For osmotic and peracetic acid stresses, a combined effect of the contamination level and the enrichment length was observed.
Conclusion.
This research sought to evaluate the impact of bacterial stress on the performance of the primary enrichment phase of the International Organization for Standardization L. monocytogenes detection method. In order to quantify the impact of stresses, we assessed the single-cell growth probability of stressed cells and the single-cell lag time distributions of growing stressed cells. The results highlight the consequences of food processing for the performance of microbiological detection methods.
We have shown that the physiological state of cells has a great impact on single-cell lag time distribution and growth probability. After an injury with strong impact, single-cell growth probability is decreased and some stressed cells are unable to multiply in 1/2FB. Moreover, the cell lag times may be largely increased, preventing the initial population from reaching the detectable concentration in 1/2FB during the 24-h enrichment period.
The probability of detecting stressed L. monocytogenes cells in food samples depends thus on the number of cells initially present in the samples and on the duration of the enrichment phase as well as on the impact of the stress on single-cell lag times and on single-cell growth probability. These results emphasize the importance of taking into account the physiological state of the cells of pathogenic microorganisms when assessing the performance of pathogen detection methods in foods by independently evaluating single-cell lag times and growth probability.
The methods used and the results obtained in this study can be used for the optimization of the performance of detection methods. Stresses with high impact on single-cell growth probability or single-cell lag times can be chosen as models to assess the performance of existing methods or to improve these methods. Indeed, the impact of the addition or the removal of selective medium constituents can be evaluated for these physiological states throughout the increase of the single-cell growth probability or the decrease of the single-cell lag times.
Acknowledgments
C. Dupont is the recipient of a doctoral fellowship from AES Chemunex and the Association Nationale de la Recherche Technique.
We thank Françoise Irlinger (LGMPA, INRA Grignon, France), Régine Talon (INRA Clermont, France), and Monique Zagorec (INRA Jouy-en-Josas, France) for kindly providing the isolates used as indigenous microflora.
Footnotes
Published ahead of print on 20 March 2009.
REFERENCES
- 1.Adrião, A., M. Vieira, I. Fernandes, M. Barbosa, M. Sol, R. P. Tenreiro, L. Chambel, B. Barata, I. Zilhao, G. Shama, S. Perni, S. J. Jordan, P. W. Andrew, and M. L. Faleiro. 2008. Marked intra-strain variation in response of Listeria monocytogenes dairy isolates to acid or salt stress and the effect of acid or salt adaptation on adherence to abiotic surfaces. Int. J. Food Microbiol. 123:142-150. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, W. H. 1985. A review of culture methods and their relation to rapid methods for the detection of Salmonella in foods. Food Technol. 39:77-82. [Google Scholar]
- 3.Anonymous. 1997. EN ISO 11290-1. Microbiology of food and animal feeding stuffs. Horizontal method for the detection and enumeration of Listeria monocytogenes. Part 1: detection. International Organization for Standardization, Geneva, Switzerland.
- 4.Anonymous. 2005. Commission regulation (EC) no. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union L338/1.
- 5.Augustin, J.-C., A. Brouillaud-Delattre, L. Rosso, and V. Carlier. 2000. Significance of inoculum size in the lag time of Listeria monocytogenes. Appl. Environ. Microbiol. 66:1706-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Augustin, J.-C., L. Rosso, and V. Carlier. 2000. A model describing the effect of temperature history on lag time for Listeria monocytogenes. Int. J. Food Microbiol. 57:169-181. [DOI] [PubMed] [Google Scholar]
- 7.Banada, P. P., Y.-S. Liu, L. Yang, R. Bashir, and A. K. Bhunia. 2006. Performance evaluation of a low conductive growth medium (LCGM) for growth of healthy and stressed Listeria monocytogenes and other common bacterial species. Int. J. Food Microbiol. 111:12-20. [DOI] [PubMed] [Google Scholar]
- 8.Beerens, H., and M. M. Tahon-Castel. 1966. Milieu à l'acide nalidixique pour l'isolement des streptocoques, D. pneumoniae, Listeria, Erysipelothrix. Ann. Inst. Pasteur 111:90-93. [PubMed] [Google Scholar]
- 9.Beumer, R. R., M. C. te Giffel, S. V. R. Anthonie, and L. J. Cox. 1996. The effect of acriflavine and nalidixic acid on the growth of Listeria spp. in enrichment media. Food Microbiol. 13:137-148. [Google Scholar]
- 10.Blackburn, C. W., and J. D. McCarthy. 2000. Modifications to methods for the enumeration and detection of injured Escherichia coli O157:H7. Int. J. Food Microbiol. 55:285-290. [DOI] [PubMed] [Google Scholar]
- 11.Crawford, R. G., C. M. Beliveau, J. T. Peeler, C. W. Donnelly, and V. K. Bunning. 1989. Comparative recovery of uninjured and heat-injured Listeria monocytogenes cells from bovine milk. Appl. Environ. Microbiol. 55:1490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Vries, H., and A. M. Kroon. 1970. Euflavin and ethidium bromide: inhibitors of mitochondriogenesis in regenerating rat liver. FEBS Lett. 7:347-350. [DOI] [PubMed] [Google Scholar]
- 13.Elfwing, A., Y. LeMarc, J. Baranyi, and A. Ballagi. 2004. Observing growth and division of large numbers of individual bacteria by image analysis. Appl. Environ. Microbiol. 70:675-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eppert, I., N. Valdés-Stauber, H. Götz, M. Busse, and S. Scherer. 1997. Growth reduction of Listeria spp. caused by undefined industrial red smear cheese cultures and bacteriocin-producing Brevibacterium linens as evaluated in situ on soft cheese. Appl. Environ. Microbiol. 63:4812-4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farber, J. M., E. Daley, F. Coates, D. B. Emmons, and R. McKellar. 1992. Factors influencing survival of Listeria monocytogenes in milk in a high temperature short-time pasteurizer. J. Food Prot. 55:946-951. [DOI] [PubMed] [Google Scholar]
- 16.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Mol. Biol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francois, K., F. Devlieghere, A. R. Standaert, A. H. Geeraerd, J. F. V. Impe, and J. Debevere. 2003. Modelling the individual cell lag time. Isolating single cells: protocol development. Lett. Appl. Microbiol. 37:26-30. [DOI] [PubMed] [Google Scholar]
- 18.Francois, K., F. Devlieghere, A. R. Standaert, A. H. Geeraerd, J. F. Van Impe, and J. Debevere. 2005. Modelling the effect of environmental parameter (temperature, pH & aw) on the individual cell lag phase of L. monocytogenes. Acta Hortic. 674:39-46. [Google Scholar]
- 19.Gahan, C. G. M., and C. Hill. 1999. The relationship between acid stress responses and virulence in Salmonella typhimurium and Listeria monocytogenes. Int. J. Food Microbiol. 50:93-100. [DOI] [PubMed] [Google Scholar]
- 20.Guerini, M. N., J. M. Bosilevac, and M. Koohmaraie. 2007. Rapid enrichment strategy for isolation of Listeria from bovine hide, carcass, and meat samples. J. Food Prot. 70:53-57. [DOI] [PubMed] [Google Scholar]
- 21.Guillier, L. 2005. Variabilité des temps de latence cellulaires de Listeria monocytogenes en fonction des stress subis et des conditions de re-croissance. Ph.D. thesis. Institut National Agronomique de Paris-Grignon, Paris, France.
- 22.Guillier, L., and J.-C. Augustin. 2006. Modelling the individual cell lag time distributions of Listeria monocytogenes as a function of the physiological state and the growth conditions. Int. J. Food Microbiol. 111:241-251. [DOI] [PubMed] [Google Scholar]
- 23.Guillier, L., P. Pardon, and J.-C. Augustin. 2005. Influence of stress on individual lag time distributions of Listeria monocytogenes. Appl. Environ. Microbiol. 71:2940-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guillier, L., P. Pardon, and J.-C. Augustin. 2006. Automated image analysis of bacterial colony growth as a tool to study individual lag time distributions of immobilized cells. J. Microbiol. Methods 65:324-334. [DOI] [PubMed] [Google Scholar]
- 25.Ko, R., L. T. Smith, and G. M. Smith. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance in Listeria monocytogenes. J. Bacteriol. 176:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koutsoumanis, K. 2008. A study on the variability in the growth limits of individual cells and its effect on the behavior of microbial populations. Int. J. Food Microbiol. 128:116-121. [DOI] [PubMed] [Google Scholar]
- 27.Koutsoumanis, K., and J. N. Sofos. 2005. Effect of inoculum size on the combined temperature, pH and aw limits for growth of Listeria monocytogenes. Int. J. Food Microbiol. 104:83-91. [DOI] [PubMed] [Google Scholar]
- 28.Li, Y., J. A. Odumeru, M. Griffiths, and R. C. McKellar. 2006. Effect of environmental stresses on the mean and distribution of individual cell lag times of Escherichia coli O157:H7. Int. J. Food Microbiol. 110:278-285. [DOI] [PubMed] [Google Scholar]
- 29.Lou, Y., and A. E. Yousef. 1996. Resistance of Listeria monocytogenes to heat after adaptation to environmental stresses. J. Food Prot. 59:465-471. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy, S. A. 1991. Pathogenicity of nonstressed, heat-stressed, and resuscitated Listeria monocytogenes. Appl. Environ. Microbiol. 57:2389-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKellar, R. C., and X. Lu. 2005. Development of a global stochastic model relating the distribution of individual cell and population physiological states. Int. J. Food Microbiol. 100:33-40. [DOI] [PubMed] [Google Scholar]
- 32.Mellefont, L. A., T. McMeekin, and T. Ross. 2008. Effect of relative inoculum concentration on Listeria monocytogenes growth in co-culture. Int. J. Food Microbiol. 121:157-168. [DOI] [PubMed] [Google Scholar]
- 33.Mendonca, A. F., and S. J. Knabel. 1994. A novel strictly anaerobic recovery and enrichment system incorporating lithium for detection of heat-injured Listeria monocytogenes in pasteurized milk containing background microflora. Appl. Environ. Microbiol. 60:4001-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Métris, A., S. M. George, and J. Baranyi. 2006. Use of optical density detection times to assess the effect of acetic acid on single-cell kinetics. Appl. Environ. Microbiol. 72:6674-6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Métris, A., S. M. George, M. W. Peck, and J. Baranyi. 2003. Distribution of turbidity detection times produced by single cell-generated bacterial populations. J. Microbiol. Methods 55:821-827. [DOI] [PubMed] [Google Scholar]
- 36.Meyer, R. R., G. S. Probst, and S. J. Keller. 1972. RNA synthesis by isolated mammalian mitochondria and nuclei: effects of ethidium bromide and acriflavin. Arch. Biochem. Biophys. 148:425-430. [DOI] [PubMed] [Google Scholar]
- 37.Pascual, C., T. P. Robinson, M. J. Ocio, O. O. Aboaba, and B. M. Mackey. 2001. The effect of inoculum size and sublethal injury on the ability of Listeria monocytogenes to initiate growth under suboptimal conditions. Lett. Appl. Microbiol. 33:357-361. [DOI] [PubMed] [Google Scholar]
- 38.Riahi, M. 2006. Modélisation de phénomènes microbiologiques, biochimiques et physico-chimiques intervenant lors de l'affinage d'un fromage de type pâte molle croûte lavée. Ph.D. thesis. Institut National Agronomique de Paris-Grignon, Paris, France.
- 39.Robinson, T. P., M. J. Ocio, F. Lyn, A. Kaloti, and B. M. Mackey. 1997. The use of ethidium bromide to assess a novel injury/recovery phenomenon in Listeria monocytogenes in inhibitory NaCl conditions. Lett. Appl. Microbiol. 25:367-370. [DOI] [PubMed] [Google Scholar]
- 40.Russell, A. D., W. B. Hugo, and A. J. Ayliffe. 1999. Principles and practice of disinfection, preservation, and sterilization, 3rd ed. Blackwell Science, University Press, Cambridge, United Kingdom.
- 41.Schwarz, G. 1978. Estimating the dimension of a model. Annu. Stat. 6:461-464. [Google Scholar]
- 42.Smith, J. L., and D. L. Archer. 1988. Heat induced injury in Listeria monocytogenes. J. Ind. Microbiol. 3:105-110. [Google Scholar]
- 43.Sokal, R. R., and F. J. Rohlf. 1995. Biometry: the principles and practice of statistics in biological research, 3rd ed. Freeman, San Francisco, CA.
- 44.Standaert, A. R., K. François, F. Devlieghere, J. Debevere, J. F. van Impe, and A. H. Geeraerd. 2007. Modeling individual cell lag time distributions for Listeria monocytogenes. Risk Anal. 27:241-254. [DOI] [PubMed] [Google Scholar]
- 45.Stephens, P. J., J. A. Joynson, K. W. Davies, R. Holbrook, H. M. Lapinscott, and T. J. Humphrey. 1997. The use of an automated growth analyser to measure recovery times of single heat-injured Salmonella cells. J. Appl. Microbiol. 83:445-455. [DOI] [PubMed] [Google Scholar]
- 46.Swinnen, I. A. M., K. Bernaerts, E. J. J. Dens, A. H. Geeraerd, and J. F. van Impe. 2004. Predictive modelling of the microbial lag phase: a review. Int. J. Food Microbiol. 96:137-159. [DOI] [PubMed] [Google Scholar]
- 47.Tutenel, A. V., D. Pierard, D. Vandekerchove, J. V. Hoof, and L. D. Zutter. 2003. Sensitivity of methods for the isolation of Escherichia coli O157 from naturally infected bovine faeces. Vet. Microbiol. 94:341-346. [DOI] [PubMed] [Google Scholar]
- 48.Umeda, K., S. Shiota, M. Futai, and T. Tsuchiya. 1984. Inhibitory effect of Li+ on cell growth and pyruvate kinase activity of Escherichia coli. J. Bacteriol. 160:812-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vermeulen, A., N. Smigic, A. Rajkovic, K. Gysemans, K. Bernaerts, A. Geeraerd, J. V. Impe, J. Debevere, and F. Devlieghere. 2007. Performance of a growth-no growth model for Listeria monocytogenes developed for mayonnaise-based salads: influence of strain variability, food matrix, inoculation level, and presence of sorbic and benzoic acid. J. Food Prot. 70:2118-2126. [DOI] [PubMed] [Google Scholar]
- 50.Wu, V. C. H. 2008. A review of bacterial injury and recovery methods in food. Food Microbiol. 25:735-744. [DOI] [PubMed] [Google Scholar]